Abstract
The purpose of this study was to test the hypothesis that administration of epigallocatechin-3-gallate (EGCG), a polyphenol present in abundance in widely consumed tea, inhibits cell proliferation, invasion, and angiogenesis in breast cancer patients. EGCG in 400 mg capsules was orally administered three times daily to breast cancer patients undergoing treatment with radiotherapy. Parameters related to cell proliferation, invasion, and angiogenesis were analyzed while blood samples were collected at different time points to determine efficacy of the EGCG treatment. Compared to patients who received radiotherapy alone, those given radiotherapy plus EGCG for an extended time period (two to eight weeks) showed significantly lower serum levels of vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and reduced activation of metalloproteinase-9 and metalloproteinase-2 (MMP9/MMP2). Addition of sera obtained from patients treated with combination of radiotherapy and EGCG feeding for 2–8 weeks to in vitro cultures of highly-metastatic human MDA-MB-231 breast cancer cells resulted in the following significant changes: (1) suppression of cell proliferation and invasion; (2) arrest of cell cycles at the G0/G1 phase; (3) reduction of activation of MMP9/MMP2, expressions of Bcl-2/Bax, c-Met receptor, NF-κB, and the phosphorylation of Akt. MDA-MB-231 cells exposed to 5–10 µM EGCG also showed significant augmentation of the apoptosis inducing effects of γ-radiation, concomitant with reduced NF-κB protein level and AKT phosphorylation. These results provide hitherto unreported evidence that EGCG potentiated efficacy of radiotherapy in breast cancer patients, and raise the possibility that this tea polyphenol has potential to be a therapeutic adjuvant against human metastatic breast cancer.
Keywords: Adjuvant therapy, breast cancer patients, EGCG, γ-radiation, HGF, MMP9/MMP2, VEGF
INTRODUCTION
Breast cancer is the second leading cause of cancer-related deaths in women in the United States [1]. Population-based investigations have suggested that dietary factors may affect the incidence of breast cancer [2, 3]. For example, epidemiological studies have reported an inverse association between the consumption of green tea and the risk of breast cancer in Asian American [4] and Chinese women [5].
Tea is the most popular beverage in the world, next to water. Among the polyphenols present in green tea (about 30% of the total weight of tea leaves), a major polyphenol, epigallocatechin-3-gallate (EGCG), has shown inhibitory effects on different stages of tumorigenesis based on in vitro experiments and in vivo studies using animal models of carcinogenesis [6–21]. Anti-tumorigenic activities attributed to exposure to EGCG include inhibition of cell proliferation and tumor growth [6, 10–14, 21], induction of apoptosis and cell cycle arrest [7, 11, 12, 17, 21], inhibition of invasion and metastasis [8, 12, 15, 16, 18, 21], and suppression of angiogenesis [20, 21] . At the molecular level, EGCG markedly inhibits the binding of vascular endothelial growth factor (VEGF) with its receptor [22]. Moreover, green tea extract (GTE) or EGCG also significantly decrease the secretion of VEGF into culture media and reduce VEGF mRNA expression in MDA-MB-231 cells [23, 24]. Further, EGCG inhibits HGF/Met signaling in immortalized and tumorigenic breast epithelial cells [25]. Finally, EGCG inhibits the synthesis and activation of tumor invasion-specific MMP2 and MMP9 in human prostate carcinoma DU-145 cells [26]. Nevertheless, the effective concentrations of EGCG used in most of the experiments, including our previous studies, far exceeded plasma concentrations of EGCG observed in humans and animals (Generally, the peak in human plasma concentration of EGCG is in the low-micromolar range after a single oral dose of EGCG, Polyphenon E (a standardized green tea polyphenol preparation), or green tea) [27–29]. This lingering bioavailability issue and the metabolic differences between animals and humans make it challenging in extrapolating results from experiments in vitro to situations in vivo and from animals to human, despite that there have been more than nine hundred papers reporting the effects of EGCG against cancer to date (combining “EGCG” AND “cancer” in PubMed).
To explore the use of EGCG as an adjuvant therapy for carcinogenesis, and to gain further information on its mechanism of action, a pilot clinical study was performed, specifically, to test the hypothesis that EGCG might augment efficacy of radiotherapy in patients diagnosed with breast cancer. As proof of principle, we focussed on parameters related to inhibition of cell proliferation, invasion, and angiogenesis.
MATERIALS AND METHODS
Patients
A total of ten female patients (median age, 46 years; range, 38–55 years old) with locally advanced (T3, T4, and/or N0–N3) noninflammatory breast cancer undergoing radiotherapy were enrolled for this study. Pregnant women were not eligible. Patient selection criteria also included: uncompromised organ (bone marrow, liver, and kidney) functions, a life expectancy of 12 weeks (w), and evidence of bidimensionally measurable lesions as determined by computed tomography, magnetic resonance imaging, or palpation. The Institutional Ethics Review Board (IERB) of Chinese PLA 107 Hospital approved the protocol (Number: 03B006) and the pilot trial was conducted according to the guidelines for good clinical practice and the Declaration of Helsinki. All patients were required to fill-out an IERB-approved informed consent before treatment was initiated. The ten patients (all patients’ breasts were excised by surgery before this study) were randomly assigned to two groups: the group 1 five patients (3 metastasis and 2 relapsed with metastasis) received EGCG treatment and radiotherapy, while the group 2 five patients (3 metastasis and 2 relapsed with metastasis) received a placebo (radiotherapy) instead of EGCG. Specifically, the breast cancer patients were given EGCG orally (400 mg in 2 capsules, with 100 ml of water) 3 times daily or a placebo (empty capsule) during the 5-w (5 weeks, the same hereinafter) radiotherapy cycles and 3-w post radiotherapy cycle. EGCG (>95%, Lot 710291) isolated from green tea was obtained from Taiyo Green Power Co., Ltd., Wuxi, China, and capsules were prepared in School of Pharmacy at Yantai University. Except for radiotherapy or EGCG plus radiotherapy, patients agreed to refrain from self administration of other medications during the 8-w experiment.
Human Sera
Blood samples were collected from patients after an overnight fast except for drinking water. Ten ml blood samples were collected prior to 0 hour (0-h) and 2 hours (2-h) after administration of EGCG or placebo. The serum samples were aliquoted, and stored at −80°C until sample analysis.
Determination of Serum VEGF and HGF Levels by ELISA
Serum samples (0-h and 2-h) from patients given placebo or EGCG were analyzed for changes in VEGF and HGF using ELISA kits purchased from R&D Systems (Minneapolis, MN), according to the instructions provided by the manufacturer. All samples were assayed in triplicate and the mean of the values was calculated. In the present study, we used pg/106 platelets and pg/ml to represent levels of VEGF and HGF in patents’ sera, respectively. It is reported that the platelets and tumor cells, as well as neutrophils, contribute to the serum VEGF level [30–32], which could be affected by radiotherapy. Considering the platelet changes in individual patients during the radiotherapy, we used the relative serum VEGF levels in pg/106 platelets in patients to analyze the VEGF changes.
Cell Culture and Ex Vivo Proliferation/Viability Assays
The highly metastatic MDA-MB-231 human breast cancer cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in DMEM (Sigma, Co., MO) supplemented with 10% serum from breast cancer patients or 10% fetal bovine serum (FBS). Proliferation was assayed at 48- and 72-h using MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) kit (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. The relative cell viability was determined using trypan blue dye exclusion assay after the cells were treated for 36 h with EGCG, γ-radiation, and EGCG plus γ-radiation. To determine the effects of EGCG on γ radiation-treated cell viability and protein expressions in the cells, the cells at 80% confluence were plated in the six-well plate. Twenty-four hours later, the cells were treated with γ radiation at a dose of 8 Gray (Gy). Thirty-six hours later, the γ radiation-treated cells were used for analyses of the cell viability or the protein expressions. Radiation was delivered using a 60Co source with a source-to-flask distance of 40 cm and a dose rate of 1.36 Gy/min. Each experiment was repeated three times.
Analysis of Cell Cycle Phase Transition and Induction of Apoptosis by Flow Cytometry
Cells were detached in PBS/2 mM EDTA, centrifuged at 1,000 rpm for 5 min, and then gently resuspended in 250 µL of hypotonic fluorochrome solution (PBS, 50 µg propidium iodide, 0.1% sodium citrate, and 0.1% Triton X-100) with RNase A (100 units/ml). The DNA content was analyzed by flow cytometry (Becton Dickinson FACS Vantage SE, San Jose, CA). The cell cycle distribution and apoptosis were determined based on DNA content and the sub-G1 cell population, respectively.
Ex Vivo Invasion Assay
To determine cell invasion, modified transwell chambers containing fibronectin- and matrigel-coated polycarbonate filters (Costar, Corning, Inc., NY) were used. Cells (2.5 × 104) were pretreated for 1-h with 10% serum from patients and seeded onto the upper chamber containing 0.2 ml serum-free medium; the lower chamber was filled with 0.66 ml DMEM supplemented with 10% of the autologous serum samples (to serve as a chemoattractant). After incubation for 16 h at 37°C, the cells that invaded to the lower surface of the filter were fixed and stained using propidium iodide. The cells on the upper side of the filter were removed using a rubber scraper. The invaded cells on the underside of the filter were counted and recorded for images by fluorescence microscopy (×10 objective; Nikon, TE2000-U, Japan). Each experiment was repeated three times.
Gelatin Zymography
The serum samples from the patients or the supernatants (serum-free) from the upper chamber of the transwell chamber in the invasion assay, were analyzed for MMP9/MMP2 activation. The patients’ sera or conditioned media were subjected to 10% SDS-PAGE containing 0.1% gelatin under non-reducing conditions. Following electrophoresis, the gels were washed with 2.5% Triton X-100 to remove SDS and then incubated in a developing buffer (50 mM Tris-HCl buffer [pH 7.4], 10 mM CaCl2) overnight at 37°C. Gels were stained with 0.25% Coomassie Brilliant Blue R-250 and de-stained in the same solution without dye. Gelatinase activation was visualized as clear bands against the blue-stained gelatin background. Each experiment was repeated three times.
Western Blot Analysis
MDA-MB-231 cells were treated with sera from the radiotherapy patients taking EGCG or taking placebo, and collected at 48 h. For detection of p-Akt, the cells were treated for 30 minutes with these sera. For analysis of in vitro effects of EGCG on protein level of NF-γB and phosphorylation of Akt in γ-radiation-treated human breast cancer cells, MDA-MB-231 cells were treated with γ-radiation at a dose of 8 Gy of γ rays in the absence or presence of 5–10 µM EGCG, or the inhibitor of NF-κB (Bay = Bay 11–7082) and PI3K/Akt (Ly = Ly294002). Equal amounts of the treated cell extracts (60 µg) were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with primary antibodies (from Cell Signaling Technology, MA) against human Bcl-2, Bax, c-Met, nuclear factor (NF-κB p-65), Akt, p-Akt (Ser473), and β-actin and then horseradish peroxidase-conjugated secondary antibodies, respectively. β-actin was used as a loading control. Detection was done using an enhanced chemiluminescence system (Amersham Life Science, Arlington Heights, IL).
Statistical Analysis
Statistical analysis was done using the one-way or two-way ANOVA followed by the appropriate post hoc test (Bonferroni) and Student’s t-test as well as simple linear regression with the software SPPS 13.0. Data are represented as mean ± SD, an asterisk P<0.05, double asterisk P<0.01, and triple asterisk P<0.001. For all tests, P values less than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
EGCG Reduced Blood VEGF and HGF Levels as well as Decreased MMP9/MMP2 Activation when Orally Administered to Breast Cancer Patients under Radiotherapy
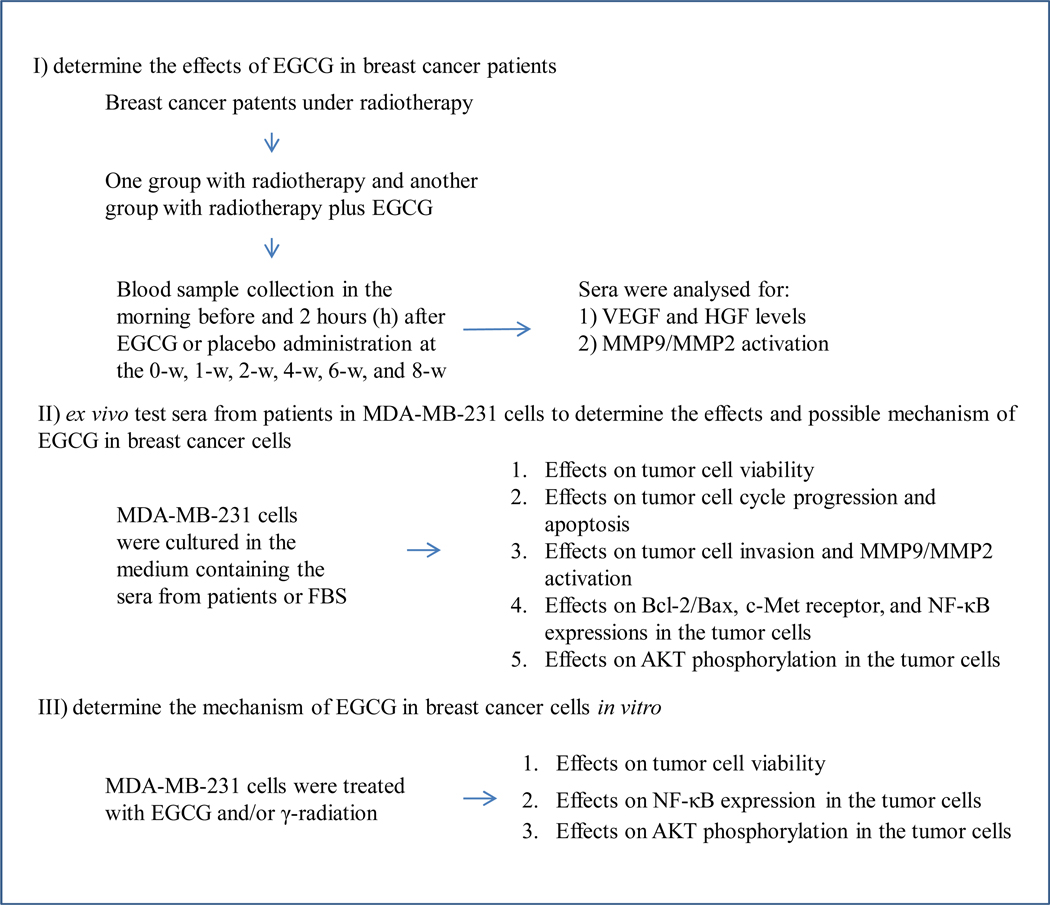
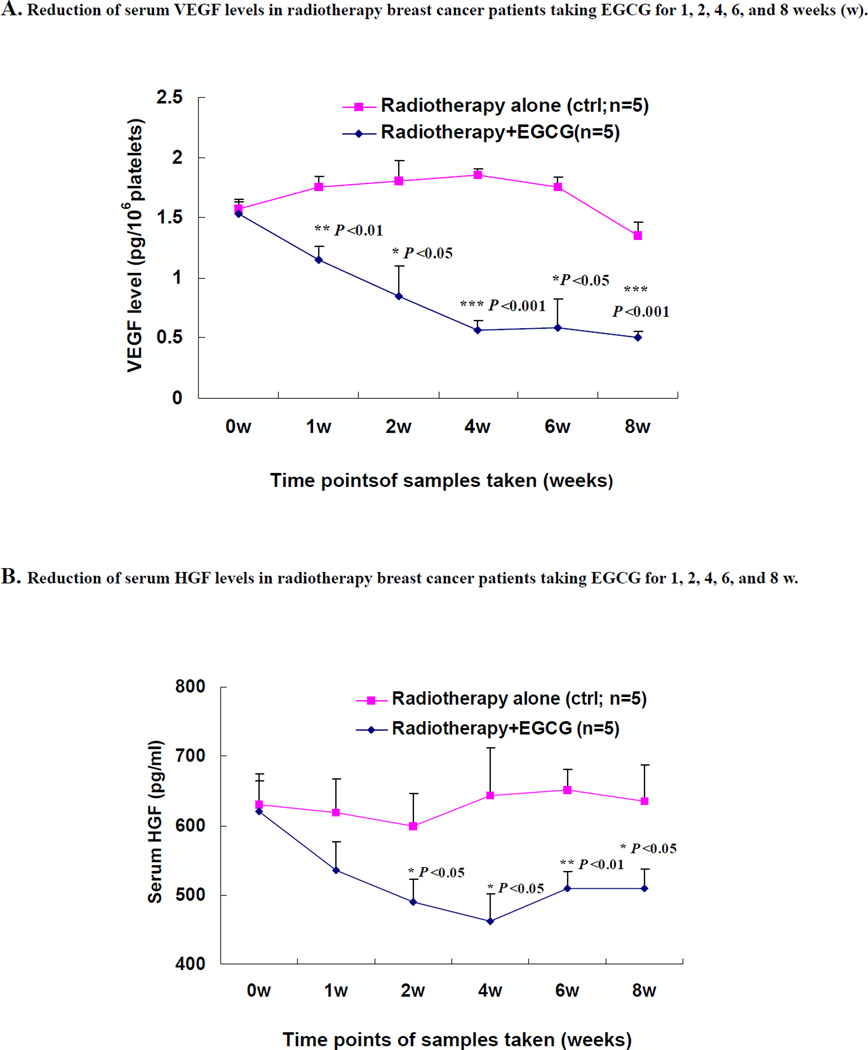
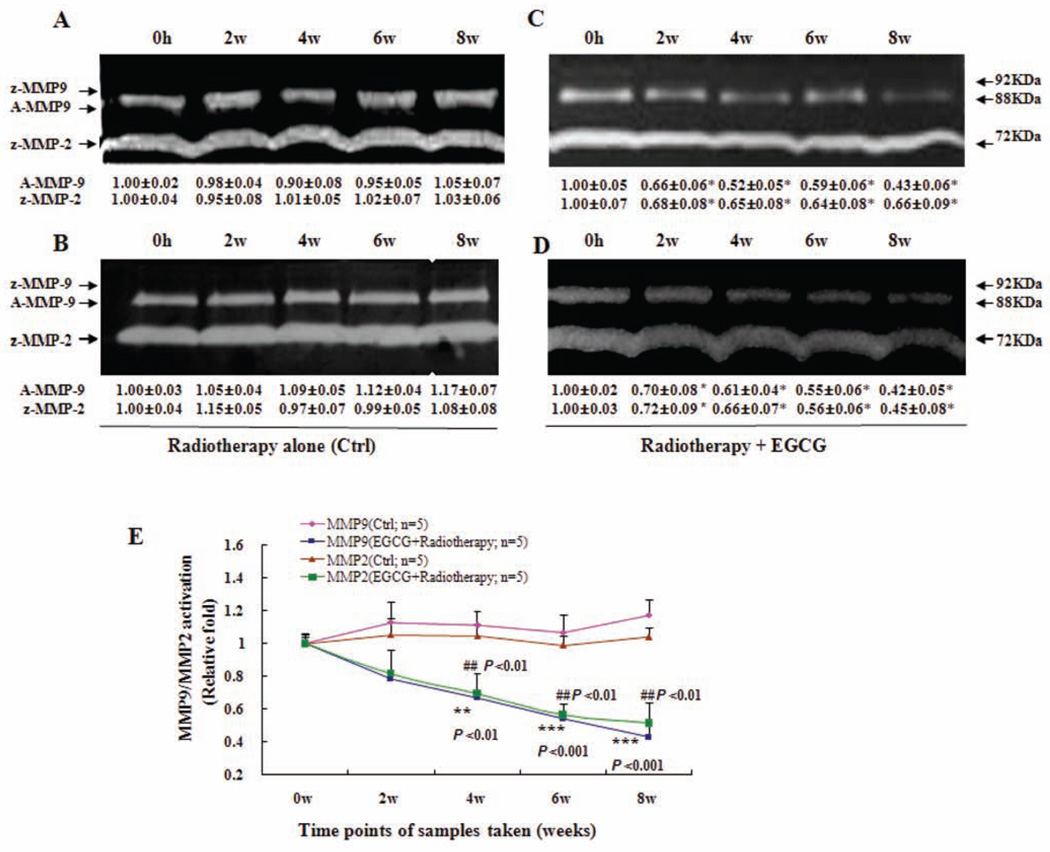
A schematic flow chart illustrating the design of this pilot study is presented in Fig. (1). Of note, breast cancer patients started to receive oral doses of EGCG concurrently with the initiation of radiotherapy. In the group that was given EGCG (n=5) for 8-w, a significant decrease in levels of serum VEGF (Fig. 2A), HGF (Fig. 2B) and MMP9/MMP2 activity (Fig. 3) was observed starting from 2-w, which was maintained for the 8-w duration of the study. Compared with the group who received radiotherapy alone (n=5), serum VEGF levels in the EGCG group decreased by an average of 0.6±0.1 pg/106 platelets (1-w), 1.0±0.1 pg/106 platelets (2-w), 1.3±0.1 pg/106 platelets (4-w), 1.2±0.1 pg/106 platelets (6-w), and 0.9±0.1 pg/106 platelets (8-w), respectively, representing mean inhibition of 34%, 53%, 70%, 67%, and 63% (P < 0.01, P < 0.05, P < 0.001, P < 0.05, and P < 0.001), respectively. Correspondingly, the serum HGF levels in the EGCG group decreased by an average of 84.0±22.0 pg/ml, 109.7±23.5 pg/ml, 180.8±32.2 pg/ml, 145.6±17.5 pg/ml and 125.8±19.8 pg/ml, respectively, representing mean inhibition of 14%, 18%, 28%, 22% and 20% (P < 0.05, P < 0.05, P < 0.01, and P < 0.05) at 1-w, 2-w, 4-w, 6-w and 8-w, respectively. Further, the levels of serum active MMP-9 (A-MMP9) in the EGCG (n=5) group decreased by an average of 0.3±0.1 fold, 0.5±0.1 fold, 0.5±0.1 fold, and 0.7±0.1 fold, respectively, representing mean inhibition of 31%, 40%, 50%, and 55% (P < 0.01, P < 0.001, and P < 0.001) at 2-w, 4-w, 6-w and 8-w, respectively (Fig. 3E), while the levels of serum MMP-2 zymogens (z-MMP2) in the EGCG (n=5) group decreased by an average of 0.2±0.1 fold, 0.4±0.1 fold, 0.43±0.03 fold, and 0.5±0.1 fold, respectively, representing mean inhibition of 22%, 33%, 43%, and 51% (P < 0.01, P < 0.01, and P < 0.01) at 2-w, 4-w, 6-w and 8-w, respectively (Fig. 3E).
Fig. (1).
Schematic flow chart for the experiments.
Fig. (2).
Decrease in serum VEGF (A) and HGF (B) levels by orally administrated EGCG in breast cancer patients under radiotherapy. Breast cancer patients (n=5) received three oral doses of EGCG (400 mg) daily for 8 weeks (w) during the 5-w radiotherapy cycles and 3-w afterwards. The breast cancer patients (n=5) in control group who received radiotherapy alone during the 8-w without EGCG treatment. Blood samples were collected at 0-h, at 0 w (immediately before EGCG administration), and at 1-w, 2-w, 4-w, 6-w and 8-w, at 2-h after the EGCG dose (2-h fasting blood samples). The serum VEGF and HGF levels were determined by ELISA. Values are shown as mean ± SD for each group from 0-h (0-w) to 8-w. All samples were assayed in triplicate and the mean of the values was calculated. Significance levels were determined using the ANOVA and Bonferroni test or Student’s t-test. * P < 0.05; **P < 0.01; and *** P < 0.001.
Fig. (3).
Inhibition of activation of serum MMP9/MMP2 by orally-administrated EGCG in breast cancer patients under radiotherapy. The blood samples were obtained as described in Fig. 2 legend. MMP9/MMP2 activation in sera was analysed using zymography. In (C) and (D), breast cancer patients Z and J received three oral doses of EGCG (400 mg) daily for 8-w during the 5-w radiotherapy cycles and 3-w afterwards. In (A) and (B) The breast cancer patients X, and Y in control group received radiotherapy alone during the 8-w without EGCG treatment. The activation of serum MMP9/MMP2 zymogens (z-MMP9/z-MMP2; Mr 72,000 and Mr 92,000 gelatinase activities) and active MMP9 (A-MMP9; Mr 88,000) was determined by gelatin zymography analysis. Values (the relative activation of serum active MMP9 and MMP2 zymogens) are shown as mean ± SD (Bar) of 3 runs for each sample, only one set of gels is shown. In (E), the activation of serum A-MMP9/z-MMP2 zymogens in the blood samples of all the patients was analyzed by gelatin zymography and statistical analysis. As described above, all the serum samples were from both the breast cancer patients (n=5) received EGCG treatment for 8-w under the radiotherapy and the breast cancer patients (n=5) in control group who received radiotherapy alone during the 8-w without EGCG treatment. Statistical analysis was done using the ANOVA and Bonferroni test. * P < 0.05; ** P < 0.01; *** P < 0.001 (n=3).
Many clinical results have shown that the increase in the blood levels of VEGF and HGF as well as MMP2/MMP9 activity in breast cancer patients are closely associated with the progression and metastasis of breast cancer. A study on 200 women showed that serum VEGF levels were significantly higher in breast cancer patients compared to controls (P < 0.001). Systemic VEGF levels were reduced significantly in the breast cancer patients following tumor excision (P < 0.05) [33]. Another study on 44 patients with breast cancer indicated that surgical operation with other therapy can significantly decrease the VEGF serum level in these patients; there was a close correlation between serum VEGF level and the cancer prognosis [34]. A study on 40 breast cancer patients with metastases also reported that before chemotherapy the median level of VEGF in patients with breast cancer was 496.6 pg/ml, 4.7 times higher than that of healthy controls (P < 0.001). Intensive chemotherapy for breast cancer results in a significant decrease of serum VEGF level [35]. In addition, an investigation on serum HGF levels in 124 consecutive patients with invasive breast cancer reported that the breast cancer patients with more advanced tumor-node-metastasis staging were shown to have higher serum soluble HGF [36]. Other individual investigations on serum HGF levels in 134 and 200 primary breast cancer patients as well as 51 patients with breast cancer metastases also indicated that the serum HGF levels were significantly increased in breast cancer patients [37–39] and associated with the clinical course of metastatic breast cancer patients such as tumor size, nodal status, and histological evidence of venous invasion and metastasis. There have been reports showing that average values of MMP-2 and MMP-9 activity levels were significantly higher in the investigated 80 breast cancer patients than in control (22 healthy volunteers) sera [40]; a strong decrease of pro-MMP-9 serum levels was found in 88 breast cancer patients after 1 month (P <0.05) and after 6 months (P <0.01) after surgery [41]. Evidently, all these clinical evidences indicate that the reduction of blood levels of VEGF, HGF, MMP-2 and MMP-9 suggests a good clinical prognosis for the breast cancer patients with progression and/or metastasis after chemotherapy, radiotherapy, and/or surgical operation. In the present study, we have demonstrated that oral administration of EGCG significantly reduced the blood levels of VEGF (Fig. 2A) and HGF (Fig. 2B) in breast cancer patients under radiotherapy compared to the patients without EGCG treatment. The effect was seen at 2-w or 4-w and remained for 8-w after the patients took EGCG. In the control patients, who did not receive EGCG treatment under radiotherapy, the blood levels of VEGF and HGF did not change significantly. It is reported that the platelets and tumor cells, as well as neutrophils, contribute to the serum VEGF level [30–32], which could be affected by radiotherapy. In the present study, we used pg/106platelets and pg/ml to represent levels of VEGF and HGF in patents’ sera, respectively. Considering the platelet changes in individual patients during the radiotherapy, we used the relative serum VEGF levels in pg/106 platelets in patients to analyze the VEGF changes (Fig. 2A). In addition, we analyzed the neutrophils of blood samples and did not observe any significant change of the % neutrophils in blood samples between the control group and EGCG group during the experiment. EGCG reduced the VEGF secretion and mRNA expression in MDA-MB-231 cells in vitro and ex vivo which were confirmed by our tests (data not shown) and other research [23, 24]. We believe that oral administration of EGCG could reduce the tumor-associated blood VEGF levels. We also demonstrated that compared to the breast cancer patients without EGCG treatment, the activation of serum active MMP9 and MMP2 zymogens was significantly reduced in breast cancer patients after taking EGCG for 4-w, 6-w and 8-w (Fig. 3). All these clinical results show that the breast cancer patients who took EGCG under radiotherapy have received beneficial therapy in reduction of the factors related to breast cancer progression.
Ex Vivo Inhibition of Proliferation and Invasion in Human Breast Cancer Cells with Sera from Patients who Received EGCG and Undergoing Radiotherapy
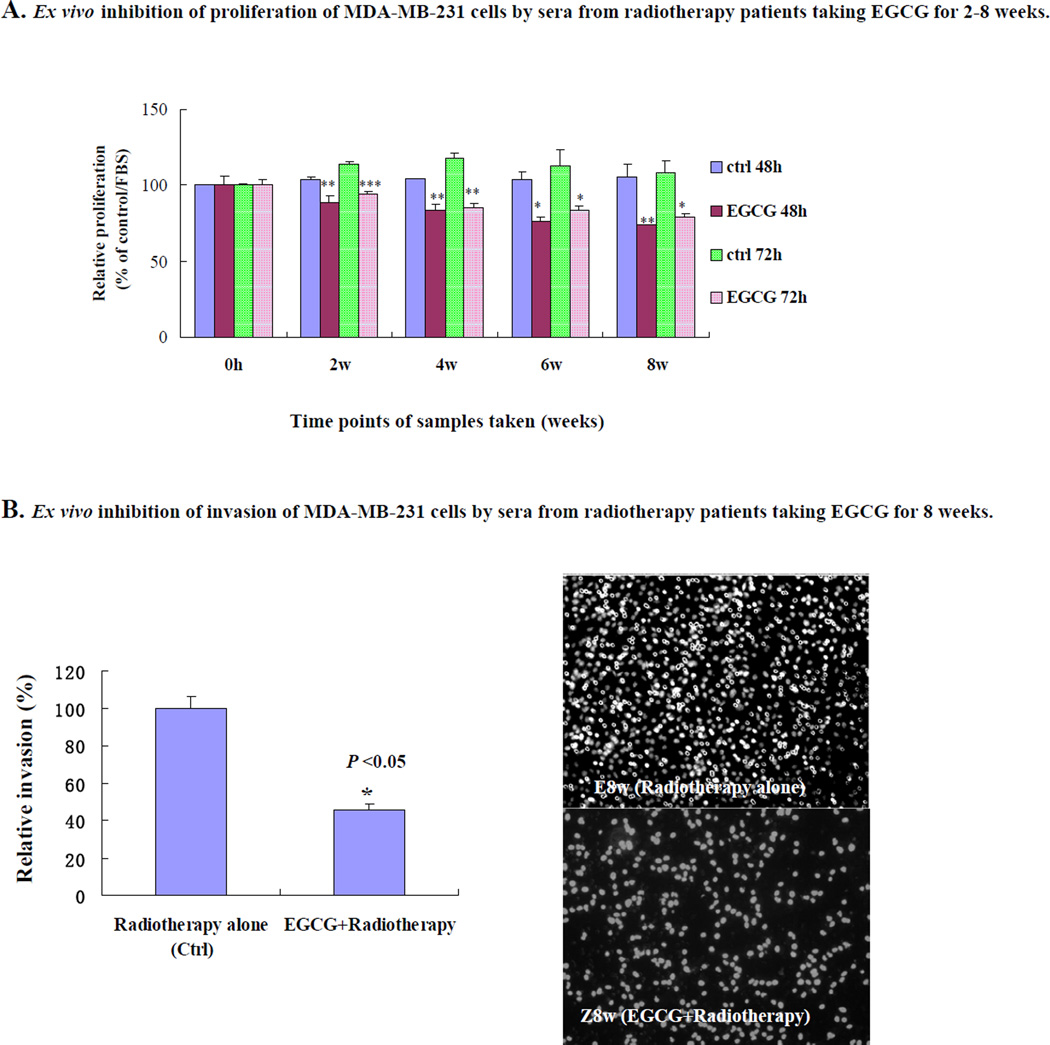
We next compared the ex vivo effects of sera from breast cancer patients using MDA-MB-231 breast cancer cells. The sera were taken at 0-h (0-w) to 8-w, from the group who received radiotherapy alone, and from the group who received EGCG and radiotherapy (n=5, for each group). Compared with the control group (radiotherapy alone), the 2-h sera at 8-w from EGCG-treated group (EGCG+radiotherapy) significantly and time-dependently suppressed the proliferation of MDA-MB-231 (Fig. 4A). In addition, the 2-h sera at 8-w from the EGCG-treated group significantly suppressed the invasion of MDA-MB-231 cells by 49% (P <0.05) compared with the control group (Fig. 4B). In addition, we have also found that the sera from the radiotherapy patients who received EGCG have the similar inhibitory effects on the proliferation and invasion in human breast cancer cell lines MDA-MB-435S and MDA-MB-468 cells (data not shown), indicating there is no cell line bias for the effects on breast cancer.
Fig. (4).
Ex vivo inhibition of proliferation and invasion of highly-metastatic human breast cancer cells by sera from radiotherapy breast cancer patients taking EGCG for 8-w. Breast cancer patients (n=5) received three oral doses of EGCG (400 mg) daily for 8-w during the 5-w radiotherapy cycles and 3-w afterwards. The breast cancer patients (n=5) in control group who received radiotherapy alone during the 8-w without EGCG treatment. Blood samples were collected at 0-h, at 0 w (immediately before EGCG administration), and at 2-w, 4-w, 6-w and 8-w, at 2-h after the EGCG dose (2-h fasting blood samples). The serum samples from the patients were also used for ex vivo assays for proliferation and invasion. (A) Inhibition of proliferation of MDA-MB-231 cells by treatment for 48-h and 72-h with the 0–8 w sera. Values are shown as mean ± SD (Bar) for each group (n=5). (B) Inhibition of invasion of MDA-MB-231 cells by treatment for 16-h with the sera from the radiotherapy patients J, Q, Y, X and Z taking EGCG for 8-w (n=5) or C, D, E, F and G not taking EGCG (n=5) (left panel). The right panel shows the propidium iodide-stained MDA-MB-231 cells invading through matrigel-coated transwell chamber. The cells were treated 16-h respectively with the sera at 8-w from the control patients E who received radiotherapy alone and the radiotherapy patient Z who also took EGCG for 8-w. Statistical analysis was carried out using the ANOVA and Bonferroni test or Student’s t-test. (A, B). * P < 0.05; ** P < 0.01; and *** P <0.001.
Ex Vivo Induction of Cell Cycle Arrest and Apoptosis, Suppression of Activation of MMP-9/MMP-2, Increase in Bax Level, and Decrease in Expressions of Bcl-2, c-Met and NF-γB as well as Phosphorylation of Akt in Human Breast Cancer Cells by Sera from Radiotherapy Patients who Received EGCG
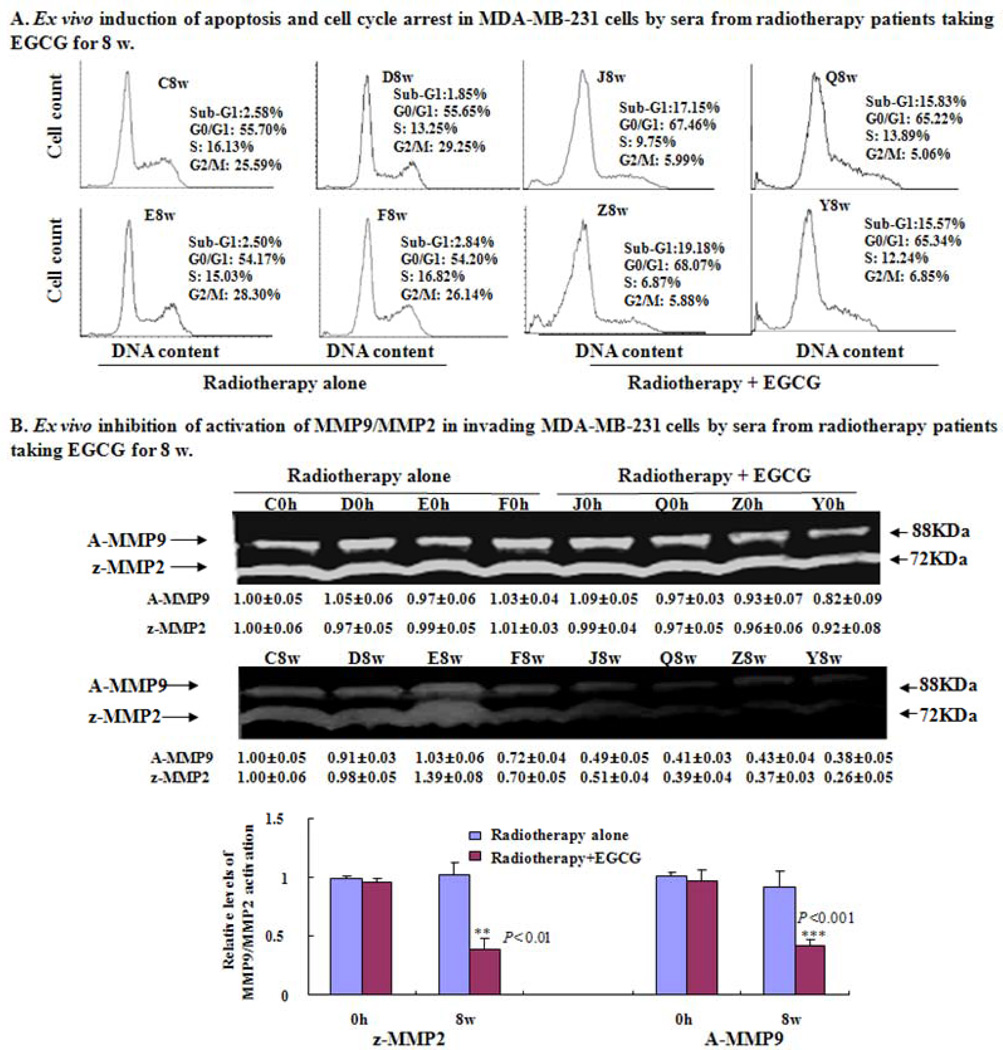
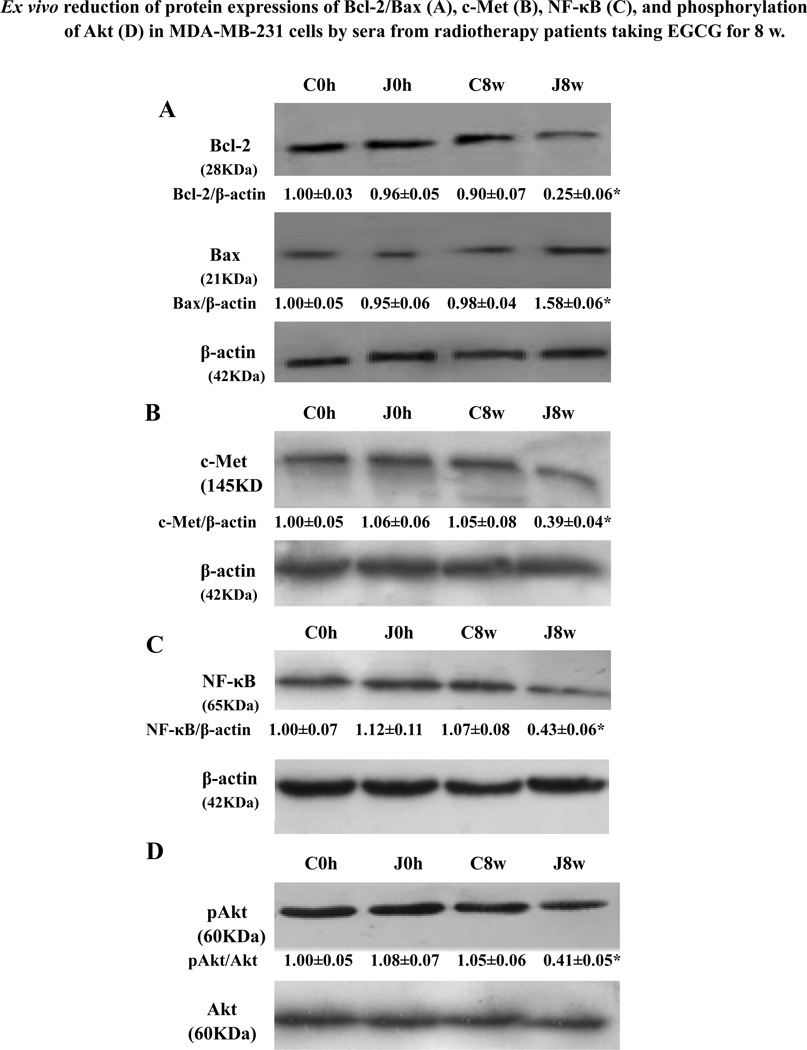
Further study showed that compared to the 2-h sera at 8-w from the patients C, D, E, and F in the control group, the 2-h sera at 8-w from the radiotherapy patients J, Q, Y and Z in the EGCG-treated group showed the following properties: i) induced apoptosis and cell cycle arrest at the G0/G1 phase in MDA-MB-231 cells (Fig. 5A); (ii) reduced activation of active MMP9 and MMP2 zymogens in the supernatants of invading MDA-MB-231 cells (Fig. 5B). In addition, in comparison to the 2-h serum at 8-w from the representative control patient C, the 2-h serum at 8-w from the representative patient J in EGCG-treated group reduced the expression of anti-apoptotic protein Bcl-2 and enhanced the expression of pro-apoptotic protein Bax in MDA-MB-231 cells (Fig. 6A), leading to reduction the ratio of Bcl-2 and Bax. The same 2-h serum also reduced the protein expression of c-Met receptor (Fig. 6B) and NF-κB (Fig. 6C) and suppressed the phosphorylation (Ser473) of Akt (Fig. 6D) in the cancer cells.
Fig. (5).
Induction of cell cycle arrest and apoptosis and inhibition of MMP9/MMP2 activation in supernatants of MDA-MB-231 cells by sera from radiotherapy patients taking EGCG for 8-w. The radiotherapy breast cancer patients J, Q, Z and Y received oral doses of EGCG for 8-w. The breast cancer patients C, D, E and F in control group received radiotherapy alone. The blood samples were collected at 0-h at 0 w (immediately before EGCG administration), and 2-h after EGCG administration on the 8th w. (A) The cells treated for 48-h with the sera were analyzed for DNA content by flow cytometry. The cell samples treated with 8-w sera from the patients J, Q, Z and Y (J8w, Q8w, Z8w and Y8w) were more effective than the control samples (C8w, D8w, E8w and F8w) in inducing apoptosis (cells in sub-G1 phase) and G0/G1 phase arrest. (B) MDA-MB-231 cells were cultured for 16-h in fibronectin- and matrigel-coated transwell chamber (serum-free) after pretreated for 1-h with DMEM medium containing 10% of the 0-h or 8-w sera from the patients J, Q, Z and Y in EGCG group and the patients C, D, E and F in control group, respectively. The activation of active MMP9 (A-MMP9) and MMP2 zymogens (z-MMP2) was analyzed in the supernatants of invading MDA-MB-231 cells. Values (the relative activation of active MMP9 and MMP2 zymogens) are shown as mean ± SD (Bar) for 3 assays of each sample and only one set of gels is shown. Statistical analysis was done using the ANOVA and Student’s t-test. * P < 0.05 (n=4).
Fig. (6).
Ex vivo reduction of protein expressions of Bcl-2/Bax, c-Met, and NF-κB p-65 as well as phosphorylation (Ser473) of Akt in human breast cancer cells by serum from radiotherapy patient J taking EGCG for 8-w. Radiotherapy breast cancer patient J received oral dose of EGCG for 8-w, whereas radiotherapy patient C in control group did not receive EGCG. The blood samples were collected at 0-h at 0 w (immediately before EGCG administration), and at 2-h after EGCG administration on the 8th w. After MDA-MB-231 cells were treated with the 0 h sera (C0h, J0h) at 0 w, and 2-h sera (C8w, J8w) at 8-w from the patients, respectively, equal amounts of protein from total cell lysates were separated by SDS-PAGE, and Western blot analysis of Bcl-2/Bax (A), c-Met (B), NF-κB p-65 (C) and p-Akt (D) were done. For analysis of p-Akt, MDA-MB-231 cells were treated with each 0 h serum (C0h, J0h) at 0-w or 2-h serum at 8-w (C8w, J8w) from the patients, respectively. In the (A, B and C), the density of the band (normalized to β-actin) shown as mean ± SD is relative to that of the control C0h (designated as 1.00). In the (D), the density of the band (normalized to Akt) shown as mean ± SD is relative to that of the control C0h (designated as 1.00). For one experiment, 3 assays were carried out and only one set of gels is shown. In the (A, B and C), compared to the cells treated with the 0 h and 8-w sera from patient C in the control (C0h and C8w), the expression of Bax was significantly enhanced and the expressions of Bcl-2, c-Met and NF-κB were significantly reduced in MDA-MB-231 cells by the 8-w serum from the EGCG-treated patient J (J8w). In the (D), compared to the cells treated with the C0h and C8w sera from patient C in the control group, the phosphorylation (Ser473) of Akt in MDA-MB-231 cells was significantly reduced by the J8w serum from EGCG-treated patient J. Statistical analysis was carried out using the ANOVA and Bonferroni test. * P < 0.05 (n=3).
One of the main regulatory steps of apoptotic cell death is controlled by the ratio of anti-apoptotic and pro-apoptotic members of the Bcl-2 family of proteins, which determines the susceptibility to apoptosis. Bcl-2 and its dominant inhibitor Bax are key regulators of cell growth and apoptosis. Overexpression of Bcl-2 enhances cell survival by suppressing apoptosis, but overexpression of Bax accelerates cell death. NF-κB is a nuclear transcription regulator with a specific motif for Bcl-2 transcription [42–44]. The PI3K/Akt pathway acts as a survival (anti-apoptotic) signal and plays a key role in the regulation of apoptotic change in breast cancer cells. Akt can exert its anti-apoptotic effects in several different ways, such as negatively regulating proapoptotic factors, and stimulating the NF-κB survival pathway [45–48]. Activation of Akt and the NF-κB/Bcl-2 pathway leads to inhibition of chemotherapy/radiotherapy-induced apoptosis, which results in treatment resistance [47]. The Bax, Bcl-2, p-Akt and NF-κB have become the important targets of action by anticancer agents [44, 47, 49–52]. Here, our results have shown that the sera from the patients who took EGCG enhanced the apoptosis ratio, up-regulated the Bax level, and down-regulated the levels of Bcl-2, p-Akt, and NF-κB in the breast cancer cells. Our results suggest that suppression of the p-Akt and the NF-κB/Bcl-2 pathway by reducing the phosphorylation of Akt and NF-κB expression in the breast cancer cells would greatly contribute to the augmentation of apoptosis of the breast cancer cells and may be one of the important mechanisms of action for the EGCG-treated patients’ sera against the proliferation and invasion of breast cancer cells.
In Vitro Effects of EGCG on Cell Viability and NF-κB Expression and Phosphorylation of Akt in ³-Radiation-Treated Human Breast Cancer Cells
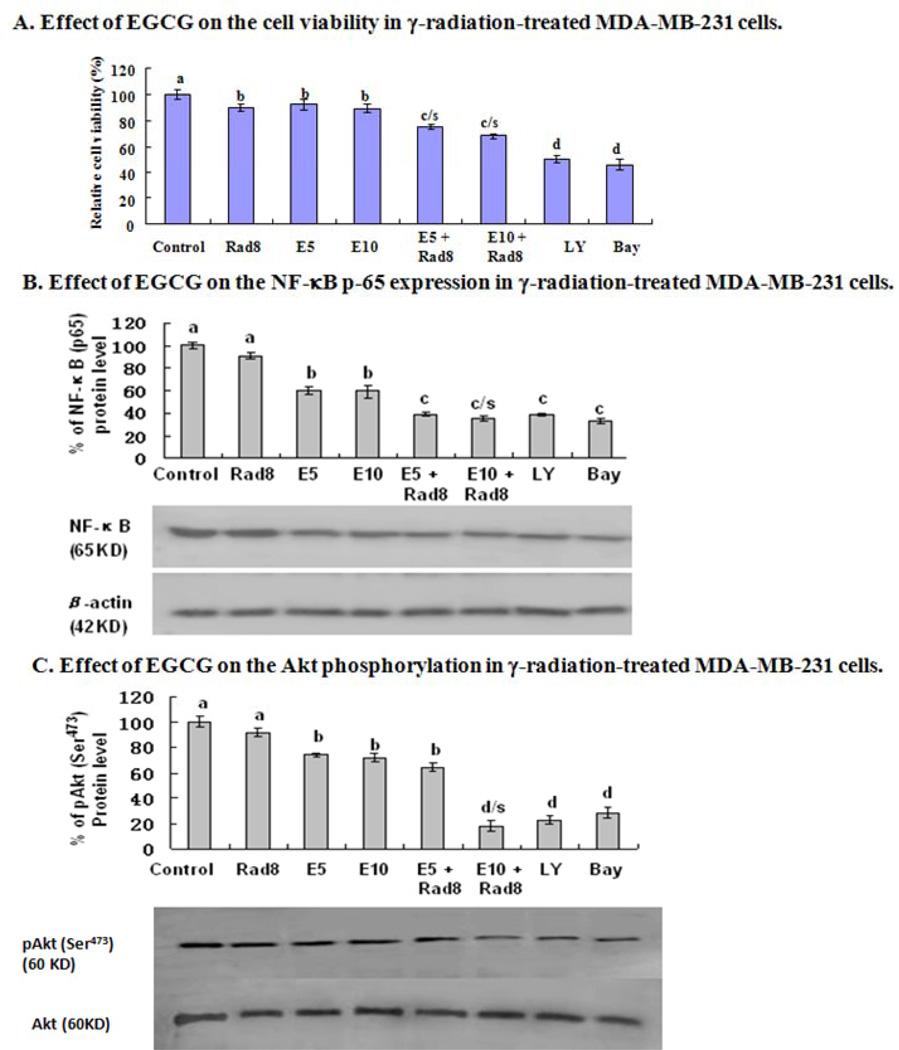
In vitro assay indicated that the relative cell viability in MDA-MB-231 cells was reduced after the cells were treated either with γ-radiation at a dose of 8 Gy or EGCG at concentrations of 5 µM to 10 µM. Such concentrations of EGCG significantly up-regulated the reduction of the cell viability in the dose of γ-radiation–treated MDA-MB-231 cells (Fig. 7A). Further, EGCG at 5 µM and 10 µM dramatically down-regulated the NF-κB p65 protein level and phosphorylation (Ser473) of Akt in γ-radiation–treated MDA-MB-231 cells although the treatment with EGCG alone reduced the NF-κB p65 protein level (Fig. 7B) and phosphorylation (Ser473) of Akt in the cancer cells (Fig. 7C).
Fig. (7).
In vitro effects of EGCG on cell viability and protein level of NF-κB p65 as well as phosphorylation (Ser473) of Akt in γ-radiation-treated human breast cancer cells. MDA-MB-231 cells were treated with γ-radiation at a dose of 8 Gy (Rad8) in the absence or presence of EGCG at concentrations of 5 µM (E5) or 10 µM (E10). After the treated cells were cultured for 36 h, the relative cell viability was determined using a trypan blue dye exclusion assay (A); the equal amounts of protein from total cell lysates were separated by SDS-PAGE, and Western blot analysis of NF-κ B p-65 (B) and p-Akt (C) were done. Bay 11–7082 (BAY), the inhibitor of NF-κB, and Ly294002 (LY), the inhibitor of PI3K/Akt were used as a positive control, respectively. The density of the band (normalized to β-actin for NF-κB or to Akt for p-Akt) shown as mean ± SD is relative to that of the control (designated as 100%). Statistical analysis was carried out using the two-way ANOVA followed by Bonferroni post hoc test to establish whether significant differences existed between the groups. Values with different letters (a–d) differ significantly (P < 0.05). c/s and d/s represent significantly enhanced γ-radiation effects by EGCG treatment compared with the treatment either with γ-radiation or EGCG alone (c/s, P < 0.001, two-way ANOVA; d/s, P < 0.0001, two-way ANOVA).
Increased levels of VEGF and HGF as well as MMP9/MMP2 activities are associated with cancer cell proliferation, invasion, and metastasis as well as angiogenesis [53–59]. Our present results show that the blood VEGF and HGF levels and MMP9/MMP2 activation in breast cancer patients under radiotherapy were significantly reduced or inhibited by oral administration of EGCG. The inhibitory effects on these factors may result from the direct and indirect actions of EGCG. It was reported that HGF, acting through its c-Met receptor, plays an important role in most human solid tumors [60]. HGF induces VEGF expression in cancer cells and is a key switch for turning on angiogenesis [61]. It has been reported that adenovirus-mediated inhibition of MMP-9 (Ad-MMP-9) with radiotherapy decreased levels of NF-κB and activator protein 1, both of which contribute to the radioresistance of MDA-MB-231 cells in vitro and in vivo; treatment with Ad-MMP-9 plus radiation completely regressed tumor growth in orthotopic breast cancer model [62]. Our present results of reduction of MMP-9 in the radiotherapy patients who received EGCG may contribute to the decrease in NF-κB level and inhibition of proliferation in breast cancer cells. In our work, we demonstrated that the sera from the patients who took EGCG reduced the expressions of Bcl-2/Bax and NF-κB, and suppressed the phosphorylation of Akt as well as inhibited activation of active MMP9 and MMP2 zymogens in invading MDA-MB-231 cells. These anti-tumor effects of sera from the EGCG-treated patients could be caused by EGCG- and its in vivo metabolites-induced reduction of VEGF, HGF, and MMP9/MMP2. Indeed, Yang et al. [27, 63] and Chow et al. [28, 29] have reported the pharmacokinetics of EGCG and its metabolites in human sera although there may be some unknown metabolites in human sera and their anti-tumor effects to probe. Chow et al. also reported that the average maximum plasma concentration (Cmax) of EGCG-treated humans (n=8) was about 3.4 µg/ml (equal to 7.4 µM) after oral administration of EGCG [29]. Here, we have confirmed that EGCG at 5 µM significantly reduces the cell viability, NF-κB protein expression, and the phosphorylation of Akt in the breast cancer cells; such concentration of EGCG significantly enhances the γ-radiation effects on the breast cancer cells. These activities could play the important role in the anti-tumor effects of the sera from the EGCG-treated patients as we have observed in the present study. Our findings suggest that EGCG may have extensive adjuvant therapeutic effects against human metastatic breast cancer, which may be involved in the signal transduction pathway of VEGF/HGF-c-Met-PI3K/Akt-NF-κB.
ACKNOWLEDGEMENTS
This work is supported by grants from the Ministry of Education of the People’s Republic of China to G.Z., from the Ministry of Human Resources and Social Security of the People’s Republic of China to G.Z., from the Department of Science and Technology of Shandong Province to G.Z. (Y2008C71; 2009GG10002087), Projects from Yantai University to G.Z., a grant from the National Natural Science Foundation of China to G.Z. (No.30973553), a grant from the PLA and Research Fund of Medicine and Health to Y.W. (06G034), NDSU Faculty Research Funds (NDSU and ND EPSCoR) (E.W.), and Pilot Project Grant (E.W.) from the Centers of Biomedical Research Excellence (COBRE) grant NIH P20 RR020151 from the National Center for Research Resources (NCRR). NCRR is a component of the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH or NCRR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to extend their thanks to Dr. Arthur Pardee (Dana-Farber Cancer Institute) and Dr. CS Yang (The State University of New Jersey) for great help in writing, Dr. Eric T. Wong (Beth Israel Deaconess Medical Center), Dr. Mehdi A. Fini (University of Colorado), Ronald E. Vincent (BIOCON Scientific), and Enrico Sassi (North Dakota State University) for their thoughtful reading.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40(1):43–68. [PubMed] [Google Scholar]
- 3.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 4.Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106(4):574–579. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis. 2007;28(5):1074–1078. doi: 10.1093/carcin/bgl252. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G, Miura Y, Yagasaki K. Effects of green, oolong and black teas and related components on the proliferation and invasion of hepatoma cells in culture. Cytotechnology. 1999;31(1–2):37–44. doi: 10.1023/A:1008076306672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, Miura Y, Yagasaki K. Induction of apoptosis and cell cycle arrest in cancer cells by in vivo metabolites of teas. Nutr Cancer. 2000;38(2):265–273. doi: 10.1207/S15327914NC382_16. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Miura Y, Yagasaki K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000;159(2):169–173. doi: 10.1016/s0304-3835(00)00545-0. [DOI] [PubMed] [Google Scholar]
- 9.Yang CS. Inhibition of carcinogenesis by tea. Nature. 1997;389(6647):134–135. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- 10.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66(23):11494–11501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 11.Lu YP, Lou YR, Xie JG, et al. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci USA. 2002;99(19):12455–12460. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thangapazham RL, Singh AK, Sharma A, Warren J, Gaddipati JP, Maheshwari RK. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007;245(1–2):232–241. doi: 10.1016/j.canlet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64(23):8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZY, Huang MT, Ho CT, et al. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992;52(23):6657–6665. [PubMed] [Google Scholar]
- 15.Garbisa S, Biggin S, Cavallarin N, Sartor L, Benelli R, Albini A. Tumor invasion: molecular shears blunted by green tea. Nat Med. 1999;5(11):1216. doi: 10.1038/15145. [DOI] [PubMed] [Google Scholar]
- 16.Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer. 2001;91(4):822–832. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89(24):1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 18.Sazuka M, Murakami S, Isemura M, Satoh K, Nukiwa T. Inhibitory effects of green tea infusion on in vitro invasion and in vivo metastasis of mouse lung carcinoma cells. Cancer Lett. 1995;98(1):27–31. [PubMed] [Google Scholar]
- 19.Taniguchi S, Fujiki H, Kobayashi H, et al. Effect of (−)-epigallocatechin gallate, the main constituent of green, tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992;65(1):51–54. doi: 10.1016/0304-3835(92)90212-e. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398(6726):381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 21.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nature Rev. 2009;9(6):429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez SK, Guo W, Liu L, Band MA, Paulson EK, Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int J Cancer. 2006;118(7):1635–1644. doi: 10.1002/ijc.21545. [DOI] [PubMed] [Google Scholar]
- 23.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2(6):350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.Sartippour MR, Shao ZM, Heber D, et al. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132(8):2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 25.Bigelow RL, Cardelli JA. The green tea catechins, (−)-Epigallocatechin-3-gallate (EGCG) and (−)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene. 2006;25(13):1922–1930. doi: 10.1038/sj.onc.1209227. [DOI] [PubMed] [Google Scholar]
- 26.Vayalil PK, Katiyar SK. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and-9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. The Prostate. 2004;59(1):33–42. doi: 10.1002/pros.10352. [DOI] [PubMed] [Google Scholar]
- 27.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7(4):351–354. [PubMed] [Google Scholar]
- 28.Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9(9):3312–3319. [PubMed] [Google Scholar]
- 29.Chow HH, Hakim IA, Vining DR, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11(12):4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 30.Senger DR, Van de Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12(34):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 31.Taichman NS, Young S, Cruchley AT, Taylor P, Paleolog E. Human neutrophils secrete vascular endothelial growth factor. J Leukocyte Biol. 1997;62(3):397–400. doi: 10.1002/jlb.62.3.397. [DOI] [PubMed] [Google Scholar]
- 32.Verheul HM, Hoekman K, Luykx-de Bakker S, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3(12 Pt 1):2187–2190. [PubMed] [Google Scholar]
- 33.Lowery AJ, Sweeney KJ, Molloy AP, Hennessy E, Curran C, Kerin MJ. The effect of menopause and hysterectomy on systemic vascular endothelial growth factor in women undergoing surgery for breast cancer. BMC Cancer. 2008;8:279. doi: 10.1186/1471-2407-8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jing J, Zhao YB, Li HJ, Lu Q. Change and clinic significance of serum VEGF level before and after breast cancer patients treated. Sichuan da xue xue bao Yi xue ban. 2006;37(6):889–892. [PubMed] [Google Scholar]
- 35.Tang JH, Zhao JH, Gong JP, Qin JW, Pan LQ, Xu ZY. Effects of chemotherapy on circulating angiogenic factor levels in patients with breast cancer. Zhonghua Zhong Liu Za Zhi. 2007;29(3):210–214. [PubMed] [Google Scholar]
- 36.Sheen-Chen SM, Liu YW, Eng HL, Chou FF. Serum levels of hepatocyte growth factor in patients with breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(3):715–717. doi: 10.1158/1055-9965.EPI-04-0340. [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi T, Toi M, Inada K, Imazawa T, Yamamoto Y, Tominaga T. Serum concentrations of hepatocyte growth factor in breast cancer patients. Clin Cancer Res. 1995;1(9):1031–1034. [PubMed] [Google Scholar]
- 38.Toi M, Taniguchi T, Ueno T, et al. Significance of circulating hepatocyte growth factor level as a prognostic indicator in primary breast cancer. Clin Cancer Res. 1998;4(3):659–664. [PubMed] [Google Scholar]
- 39.Eichbaum MH, de Rossi TM, Kaul S, Bruckner T, Schneeweiss A, Sohn C. Serum levels of hepatocyte growth factor/scatter factor in patients with liver metastases from breast cancer. Tumour Biol. 2007;28(1):36–44. doi: 10.1159/000097701. [DOI] [PubMed] [Google Scholar]
- 40.La Rocca G, Pucci-Minafra I, Marrazzo A, Taormina P, Minafra S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. Br J Cancer. 2004;90(7):1414–1421. doi: 10.1038/sj.bjc.6601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quaranta M, Daniele A, Coviello M, et al. MMP-2, MMP-9, VEGF and CA 15.3 in breast cancer. Anticancer Res. 2007;27(5B):3593–3600. [PubMed] [Google Scholar]
- 42.Viatour P, Bentires-Alj M, Chariot A, et al. NF- kappa B2/p100 induces Bcl-2 expression. Leukemia. 2003;17(7):1349–1356. doi: 10.1038/sj.leu.2402982. [DOI] [PubMed] [Google Scholar]
- 43.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsden VS, O'Connor L, O'Reilly LA, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419(6907):634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 45.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19(7):4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401(6748):82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 47.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274(5288):784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 48.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27(9):462–467. doi: 10.1016/s0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- 49.Patel JB, Mehta J, Belosay A, et al. Novel retinoic acid metabolism blocking agents have potent inhibitory activities on human breast cancer cells and tumour growth. Br J Cancer. 2007;96(8):1204–1215. doi: 10.1038/sj.bjc.6603705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21(23):3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- 51.Emi M, Kim R, Tanabe K, Uchida Y, Toge T. Targeted therapy against Bcl-2-related proteins in breast cancer cells. Breast Cancer Res. 2005;7(6):R940–R952. doi: 10.1186/bcr1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Parr C, Watkins G, Mansel RE, Jiang WG. The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res. 2004;10(1 Pt 1):202–211. doi: 10.1158/1078-0432.ccr-0553-3. [DOI] [PubMed] [Google Scholar]
- 54.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98(22):12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta VK, Jaskowiak NT, Beckett MA, et al. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer journal. 2002;8(1):47–54. doi: 10.1097/00130404-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis. via autocrine and paracrine mechanisms. Cancer Res. 2008;68(1):152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- 58.Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met) J Cell Biochem. 2000;78(3):465–475. doi: 10.1002/1097-4644(20000901)78:3<465::aid-jcb11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki M, Mose E, Galloy C, Tarin D. Osteopontin gene expression determines spontaneous metastatic performance of orthotopic human breast cancer xenografts. Am J Pathol. 2007;171(2):682–692. doi: 10.2353/ajpath.2007.070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nature Rev Cancer. 2002;2(4):289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 61.Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA. 2003;100(22):12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kunigal S, Lakka SS, Joseph P, Estes N, Rao JS. Matrix metalloproteinase-9 inhibition down-regulates radiation-induced nuclear factor-kappa B activity leading to apoptosis in breast tumors. Clin Cancer Res. 2008;14(11):3617–3626. doi: 10.1158/1078-0432.CCR-07-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol. 2005;69(10):1523–1531. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]