Abstract
Excessive risk taking is a hallmark of various psychopathological disorders. We have developed a task that models such risky decision making in rats. In this task, rats are given choices between small, safe rewards and large rewards accompanied by a risk of punishment (footshock). The risk of punishment increases throughout the test session, which allows the quantification of risky decision making at different degrees of risk for each subject. Importantly, this task yields a consistently wide degree of reliable individual variability, allowing the characterization of rats as “risk taking” or “risk averse.” This task has been demonstrated to be effective for testing the effects of pharmacological agents on risk taking, and the individual variability (which mimics the human population) allows assessment of neurobiological distinctions between subjects based on risk-taking profile.
Keywords: Decision making, Risk, Cost benefit, Rat, Psychiatric disorders, Behavior, Animal models
1. Introduction
Excessive risk taking is characteristic of several psychopathological disorders, including schizophrenia, ADHD, and drug addiction (1–4). Therefore, animal models of risky decision making have great utility for psychiatric research. We have developed a task for use in rodents based on previous two-choice discrimination tasks (5–8) that assesses preference for safe versus risky rewards. Performance in this “Risky Decision-Making Task” has been demonstrated to be replicable, sensitive, and highly effective for pharmacological testing (9). Importantly, there is a high degree of between-subject variability in this task, which resembles that found in the human population. This reliable variability can be useful for delineating both neurobiological and behavioral differences among subjects, thereby offering insight into mechanisms underlying risky decision making.
In the “Risky Decision-Making Task,” rats are given choices between a small, “safe” food reward and a large food reward associated with the risk of punishment (a mild footshock). Each session consists of five blocks of ten choice trials, with the probability of punishment (risk) accompanying the large reward increasing with each consecutive block (0, 25, 50, 75, 100%). Preference for the large, “risky” reward typically decreases with increased punishment probability (i.e.—the risk of punishment “discounts” the value of the large reward). This task design provides measures of risky choice at varying degrees of risk from each session, allowing for repeated testing using a within-subjects experimental design. Additionally, this task is among the few multichoice animal decision-making tasks that combines rewarding outcomes with the risk of punishment, as opposed to a risk of lost reward opportunity (10–13 , but see ref. 14). This approach captures the ambiguous nature of “real-world” risky decision making, in which choices are often associated with both rewards and risks of adverse consequences which may be physically unrelated to each other (15).
It is important to note that although this task (and this review) is formatted for use with rats, the protocols could be modified for use in mice with some adjustments to the shock parameters and the number and size of food pellets utilized (e.g.—Coulbourn Instruments sells the same apparatus described below scaled for use in mice, with the use of 20-mg instead of 45-mg food pellets).
2. Materials
In order to minimize extraneous factors, all behavioral procedures in this task are fully automated and utilize the equipment listed in Subheading 2 in the configuration detailed in Subheading 3.1.
- Habitest behavioral test system (Coulbourn Instruments, Whitehall, PA)—note that similar systems from other manufacturers should work as well.
- System Power Base with Lincs (can control up to 16 test cages).
- Computer with PCI card and Graphic State software for experiment control and data collection.
- Environmental Connection Board with 1.12 w house light and connector cable.
- Sound-attenuating cubicle.
- Rat test chamber with extra panels, shock floor, and drop pan.
- 45-mg food pellet dispenser.
- Food trough equipped with 1.12-W house light and photobeam to detect head entries (see Note 1).
- Retractable levers (two/test chamber).
- Shock generator and cable.
- Activity monitor (optional, though useful for monitoring shock reactivity).
MLAB rodent tablets (#PJAI-0045, Test Diet, Richmond, IN). These are 45-mg grain-based food pellets, which are compatible with a variety of pellet dispensers (see Note 2). The Risky Decision-Making Task requires up to 270 pellets/session.
Data analysis software. To extract the data from the Coulbourn Instruments files, we use a custom Excel macro written by Dr. Jonathan Lifshitz (University of Kentucky); however, a variety of different data analysis packages (including that which comes bundled with the Graphic State software) will likely suffice.
Nolvasan cleaning solution (2%) in a spray bottle to clean the test chambers between rats. Nolvasan has been deemed safe for use on surfaces that come into contact with animals.
3. Methods
Prior to assessment of risky decision making, it is necessary to train the rats on different aspects of the task in order to ensure optimal task performance. These procedures include magazine training [during which rats learn to associate the food trough with food delivery (Subheading 3.2.2)], lever press shaping (during which rats learn to associate lever presses with food delivery into the food trough (Subheading 3.2.3)), and nose-poke shaping [during which rats learn how to initiate a trial via a nose poke into the food trough and become acclimated to the extension/retraction of the levers (Subheading 3.2.4)]. The methods outlined below describe these shaping procedures as well as the Risky Decision-Making Task itself, followed by the procedures for analyzing data obtained from the task. In addition, a sample design for a within-session pharmacological experiment using the task is provided by way of example.
3.1. Test Chamber Preparation
Place test chamber into sound-attenuating cubicle.
Insert shock floor connected to shock generator into the chamber.
Insert food trough 2 cm above floor grid in the center of the front wall (use combinations of the blank panels to achieve the desired height).
Insert two retractable levers to the right and left of the food trough, each 11 cm above the floor.
Insert food dispenser above the food trough.
Mount 1.12-W house light on the rear wall of the cubicle (in the Coulbourn Instruments system, this light plugs directly into the Environmental Connection Board).
3.2. Shaping Procedures (see Note 3)
3.2.1. Food Restriction
Reduce rats to 85% of their free feeding weight, and maintain as such throughout the duration of behavioral testing. This process typically requires 5–6 days, and food restriction should not begin until at least a week after any invasive treatment (such as stereotaxic surgery). If testing occurs over the course of many weeks (and particularly if young rats are used), the “target weight” should be raised by 10 g every 2 weeks to account for growth.
3.2.2. Magazine Training
Put four of the 45-mg food pellets into the rats’ home cage the day before magazine training begins—this reduces neophobia to the food and facilitates magazine training.
The magazine training session (during which rats learn to associate the sounds that accompany food pellet delivery with food availability within the food trough) lasts 64 min, and consists of 38 discrete deliveries of a single food pellet with an intertrial interval (ITI) of 100 ± 40 s. This generally takes no more than one session, but it is a good idea to check the data from this session to make sure that rats are reliably entering the food trough within a few seconds of food delivery (if not, more sessions can be run as needed).
3.2.3. Lever Press Shaping
Place rat in chamber with single lever extended for 30-min session.
House light is illuminated throughout the session.
Each lever press is reinforced with a single food pellet delivery and illumination of the food trough (the food trough remains illuminated until the rat enters the trough).
After criterion of 50 lever presses is met in a single session, repeat the procedure with the other lever extended.
The order in which levers are presented should be counterbalanced across all subjects (see Note 4).
Lever press shaping can take anywhere from 1 to 4 sessions per lever, depending on the rat (see Note 5).
3.2.4. Nose Poke Shaping
In a 60-min session, the rat is shaped to nose poke into the food trough during simultaneous illumination of the trough and house lights. This light cue lasts 10 s, and the ITI is 40 ± 10 s. If no nose poke is made during this cue, the lights are extinguished for the remainder of the ITI.
Immediately following a nose poke during the cue, the trough light is extinguished and a single lever (either left or right) is extended. The order in which the levers are extended is pseudorandom such that there are no more than two consecutive presentations of the same lever. A lever press results in immediate delivery of a single food pellet, retraction of the lever, and extinction of the house light.
Each session has a maximum of 70 trials. Rats are trained to a criterion of at least 30 presses of each lever within the 60-min session.
3.3. The Risky Decision-Making Task
Each session lasts 60 min and consists of five blocks of 18 trials each.
Each block consists of two trial types: forced choice and choice trials.
Each 40-s trial begins with a 10-s illumination of the food trough and house lights.
A nose poke into the illuminated food trough triggers extension of one of the two levers (on forced choice trials) or both levers simultaneously (on choice trials) for 10 s. Failure to press a lever during this window causes the lights to be extinguished, and the trial proceeds to the ITI and is scored as an omission. The purpose of this nose poke is to position the rat in the center of the chamber in order to avoid a positional (left or right) response bias.
The magnitude of the food pellet reward associated with each lever is fixed throughout the task (i.e.—it is the same in every session). One lever consistently produces a single food pellet (the “safe” lever) while the other lever produces three food pellets (the “risky” lever). The other important distinction between the levers is the associated risk of punishment. Selection of the safe lever is never associated with punishment, whereas selection of the risky lever is accompanied immediately by a possible 1-s scrambled footshock contingent on a preset probability specific to each trial block. Importantly, food pel-lets are delivered following every choice of the risky lever regardless of whether or not footshock occurs.
The probability of footshock accompanying the risky lever is set at 0% during the first 18-trial block. In subsequent 18-trial blocks, the probability of footshock increases to 25, 50, 75, and 100%.
The optimal footshock intensity for this task is typically 0.30–0.35 mA (see Note 6).
Each trial block begins with eight forced choice trials, during which the levers are each presented four times in pseudorandom order. These forced choice trials serve as a reminder of the probability of punishment specific to each block of choice trials.
Following the forced choice trials, there are ten choice trials in which both levers are extended simultaneously.
After selection of a lever (in either the forced choice or choice trials), the lever(s) are immediately retracted. Food delivery is accompanied by reillumination of both the food trough and house lights, which are then extinguished upon entry to the food trough to collect the food or after 10 s, whichever occurs sooner.
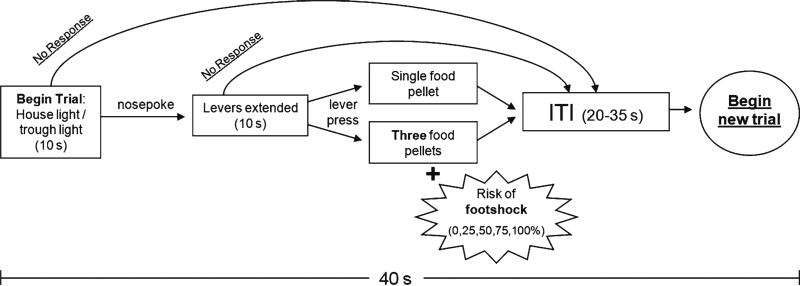
After food trough entry, there is an ITI period ranging from 20 to 35 s (ITI duration is a function of the latency to respond during the light cue, lever extension, and food delivery; however, each full trial lasts 40 s, irrespective of response latencies). See Fig. 1 for task schematic.
Approximately 15–25 sessions are typically required to achieve stable performance (see Subheading 3.4 , step 3) for a large group of rats (n = 12–18) (see Note 7).
Fig. 1.
Schematic of the risky decision-making task. Each trial begins with simultaneous illumination of the house light and trough light, which lasts for 10 s or until the rat performs a nose poke into the food trough. Following the nose poke, either one (forced choice trials) or both (choice trials) levers are extended, and this extension lasts for 10 s or until the rat presses a lever. Selection of one lever (the “safe” lever) causes delivery of a single food pellet, and selection of the other (the “risky” lever) causes delivery of three food pellets. However, the reward following choice of the risky lever is accompanied by the possibility of a 1-s footshock, the probability of which increases throughout the session (0, 25, 50, 75, and 100%). Following an ITI period, the next trial is initiated. Each trial (light cue, lever extension, reward/punishment, ITI) lasts 40 s.
3.4. Risky Decision-Making Task Data Analysis
For each block of choice trials, data are expressed as the percentage of completed trials on which the rat chose the large reward, calculated by dividing the total number of choices of the risky lever by the total number of choice trials completed (excluding omissions). For example, if the risky lever were selected on five trials, the safe lever selected on three trials, and two trials omitted, the percent choice of the risky lever for that block would be 62.5%.
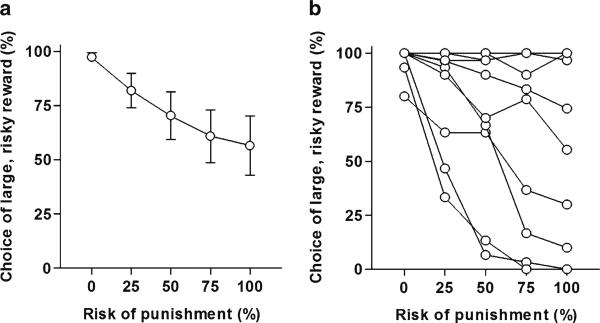
Each rat should produce data from five blocks in each session. These five data points can then be plotted with the percentage of risky lever choices on the Y axis and the risk of punishment (representative of each block) on the X axis (Fig. 2).
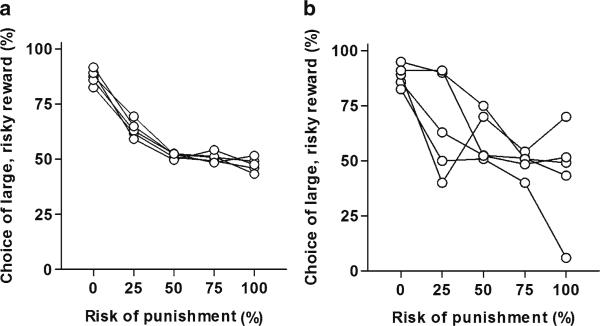
Before data can be interpreted, it is critical that the rats have reached a point at which responding can be considered stable, as performance can fluctuate considerably prior to full acquisition of the task rules. Stable performance can be quantified using a session X trial block repeated measures ANOVA across a series of five consecutive sessions. Stable performance is defined as the absence of a main effect of session or interaction between session and trial block (6). We typically use group sizes of no fewer than 12 rats—however, it is important to note that with smaller group sizes, statistical power is reduced and it becomes less likely that an effect of session or an interaction between session and trial block will be observed. In this case, it is important to make the criteria for stable performance more stringent by either increasing the value of alpha for effects involving the session factor or observing the subjects’ behavior qualitatively across sessions to determine whether it appears consistent from session to session (Fig. 3).
When analyzing performance, it is ideal to utilize the mean performance data across a five-session series rather than an individual session for each subject. This accounts for subtle differences between sessions that occur as a result of acute stressors, day-to-day differences in environmental conditions, and other confounding factors that may influence performance and promote enhanced omissions or slight behavioral biases. However, this approach may not be desirable when using within-subjects experimental designs (see below).
In order to compare two groups of subjects, use a mixed-design repeated measures ANOVA with group as the between-subjects factor, and trial block (risk of punishment) as the repeated measures factor.
Fig. 2.
(a) Performance of a group of rats (n = 10) on the Risky Decision-Making Task (mean ± SEM). (b) Distribution of individual performance on the Risky Decision-Making Task (each line represents data from a single rat). Note the wide degree of variability in performance, allowing rats to be classifi ed according to risk preference.
Fig. 3.
These data help illustrate the criteria for stable performance in the Risky Decision-Making task. Each line represents data from a single session of training. (a) Depiction of stable performance across a fi ve-session span (session X trial block ANOVA, p > 0.05 for effects of session). (b) Depiction of unstable performance across a fi ve-session span (p < 0.05 for main effect of session).
3.5. Repeated Measures Treatment Procedure (Within-Subjects Designs)
Prior to any treatment (e.g.—acute systemic drug administration, intracranial microinjections, acute behavioral manipulations), it is critical that all groups of rats have achieved stable performance in the Risky Decision-Making Task. If performance is unstable, session-to-session fluctuations in behavior could either promote a false-positive effect (type 1 error) or mask an effect of treatment (type 2 error).
We use systemic amphetamine administration as an example of a treatment regimen with four different conditions. For the first session, each rat is given one of three doses of amphetamine (0.33, 1.0, 1.5 mg/kg) or 0.9% saline vehicle prior to testing. In the second session, each subject performs the Risky Decision-Making Task with no treatment. This pattern continues for a total of eight consecutive sessions, with the order in which the treatments are administered counterbalanced across subjects.
After this eight-session experimental schedule, data are available from four treatment sessions (sessions 1, 3, 5, and 7) and four baseline sessions (sessions 2, 4, 6, and 8). The four treatment sessions can be compared using a repeated measures analysis (trial block X treatment) to detect any effects of treatment on risky decision making. The four baseline sessions should also be compared using a similar analysis. This latter test is used to determine whether treatment exerted any long-term effects on behavior that outlasted the individual treatment sessions (i.e.—“carryover effects”). If an effect of session is revealed with this analysis, performance underwent a “baseline shift,” and therefore any effects of treatment may be confounded. If a treatment produces a significant baseline shift, the simple repeated measures statistical design described above may not be an effective method of assessing differences between doses/treatment parameters, and alternative analyses may be necessary (e.g.—normalizing performance in each treatment session to the level of performance in the immediately preceding baseline session). To avoid such confounds, additional baseline sessions may be used between the treatment sessions.
If multiple rounds of experimental manipulations are to be performed, rats should be tested for a minimum of five untreated baseline sessions in between each treatment schedule. These five sessions should be analyzed for stability (see Subheading 3.4); if performance is not stable in five sessions, baseline testing should continue until stability is achieved (see Note 8).
Footnotes
As currently configured by the factory, the mounting position for the photobeam hardware on the Coulbourn Instruments food trough places the photobeam farther back in the trough than is optimal for detecting head entries. To circumvent this problem, we drill holes in the sides of the food trough to allow placement of the photobeam as close as possible to the front (entry) of the trough, and use superglue to affix the photobeam hardware in place on the sides of the trough.
In our experience, the grain-based pellets are readily consumed by food-restricted rats, and are easier to work with than sucrose pellets as they do not as readily absorb moisture and become sticky when exposed to air. They do produce dust that can clog pellet dispensers if not cleaned regularly; however, dispensers can be cleaned easily with a compressed air duster (such as that used for cleaning computer components).
These shaping procedures were adapted from refs. 6, 7 , and can be used for any two-choice decision-making task (8, 9).
The identity and positions of the response levers should be balanced such that for half of the test chambers the left lever is the “large risky reward” lever, and for the other half of the chambers the right lever is the “large risky reward” lever. This can be accomplished most easily in the Coulbourn Instruments system at the hardware level by specifying the same set of inputs/outputs to correspond to the “large risky” and “small safe” lever across all test chambers at the software level, but alternating the left/right configuration of which lever is actually plugged into which set of connections across chambers (e.g.—so that “switch 1” at the software level controls either the left or right lever at the hardware level, depending on the chamber). This ensures that factors, such as proximity to the door of the test chamber, do not bias preference for one or the other levers. In addition, it is important to counterbalance the subjects in different treatment groups across the two different types of chambers (e.g.—so that rats in a given treatment condition do not all have the “large risky” lever on the right).
Different strains of rat seem to shape more rapidly than others. For example, in our experience, Long–Evans and Sprague-Dawley rats typically acquire lever pressing for food reward at a faster rate than Fischer 344 rats (unpublished observations, Simon & Setlow).
While a 0.35-mA footshock typically produces a more robust discounting curve than other intensities (9) , there is considerable variability between rats in sensitivity to shock in this task. For example, some cohorts of rats may be insensitive to 0.35-mA shock, in which case the shock intensity can be increased in small increments (no greater than 0.05-mA increase between sessions). Conversely, some cohorts of rats may avoid this shock intensity, which would require a reduction of intensity between sessions. Note that the 0.35-mA shock value recommended here is optimal for Long–Evans rats; a higher or lower intensity may be optimal for other rat strains. Before performing any experimental manipulations, it is recommended to run a small group of pilot subjects in order to determine ideal foot-shock parameters, as there are differences in shock apparatus, environment, and rat strain/age that may influence shock sensitivity. Importantly, when determining ideal shock intensity, we have found that behavioral performance is typically more consistent if the intensity is begun at a low point and raised until an optimal point is determined (rather than beginning at a higher intensity and lowered).
It is critical to monitor each rat's task performance carefully on a daily basis. Significant changes in the choice distribution from one day to the next (or a reduction in the overall number of choices) can indicate a problem, such as a clogged feeder, inoperable lever, or rats placed in incorrect test chambers.
After extended periods of testing, rats often demonstrate some degree of habituation to the shock. This is behaviorally manifested as a gradual increase in preference for the risky reward across multiple sessions. If this occurs, it may be necessary to increase the footshock intensity by 0.05 mA prior to any subsequent treatment regimen. After any shifts in intensity, it is critical to obtain behavioral stability over a five-session period before conducting further experiments.
References
- 1.Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 2.Drechsler R, Rizzo P, Steinhausen HC. Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. J Neural Transm. 2008;115:201–209. doi: 10.1007/s00702-007-0814-5. [DOI] [PubMed] [Google Scholar]
- 3.Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, et al. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160:1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- 4.Ludewig K, Paulus MP, Vollenweider FX. Behavioural dysregulation of decision-making in deficit but not nondeficit schizophrenia patients. Psychiatry Res. 2003;119:293–306. doi: 10.1016/s0165-1781(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 5.Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 6.Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- 7.Winstanley CA, Dalley JW, Theobald DE, Robbins MJ. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulse choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–31. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- 8.Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long term increases in impulsive choice. Behav Neurosci. 2007;121:543–9. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharm. 2009;34:2208–17. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Den Bos R, Lasthius W, Den Heijer E, Van Der Harst J, Spruijt B. Toward a rodent model of the Iowa gambling task. Behav Res Methods. 2006;38:470–478. doi: 10.3758/bf03192801. [DOI] [PubMed] [Google Scholar]
- 11.Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharm. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- 12.Jentsch JD, Woods JA, Groman SM, Seu E. Behavioral characteristics and neural mechanisms mediating performance in a rodent version of the Balloon Analog Risk Task. Neuropsychopharm. 2010;35:1797–806. doi: 10.1038/npp.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardinal R, Howes N. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negus S. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- 15.Talmi D, Dayan P, Kiebel SJ, Frith CD, Dolan RJ. How humans integrate the prospects of pain and reward during choice. J. Neurosci. 2009;29:14617–14626. doi: 10.1523/JNEUROSCI.2026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]