Abstract
Background
Stakeholders in HIV/AIDS care currently use different programmes for provision of antiretroviral therapy (ART) in Uganda. It is not known which of these represents the best value for money.
Objective
To compare the cost effectiveness of home-based care (HBC), facility-based care (FBC) and mobile clinic care (MCC) for provision of ART in Uganda.
Methods
Incremental cost-effectiveness analysis was performed using decision and Markov modeling of adult AIDS patients in WHO Clinical Stage 3 and 4 from the perspective of the Ugandan healthcare system. The main outcome measures were cost (year 2008 values), life expectancy in life-years (LY) and the incremental cost-effectiveness ratio (ICER) measured as cost per QALY or LY gained over 10 years.
Results
Ten-year mean undiscounted life expectancy was lowest for FBC (3.6 LY), followed by MCC (4.3 LY) and highest for HBC (5.3 LY), while the mean discounted QALYs were also lowest for FBC (2.3), followed by MCC (2.9) and highest for HBC (3.7). The 10-year mean costs per patient were lowest for FBC ($US3212), followed by MCC ($US4782) and highest for HBC ($US7033). The ICER was lower for MCC versus FBC ($US2241 per LY and $US2615 per QALY) than for HBC versus MCC ($US2251 per LY and $US2814 per QALY). FBC remained cost effective in univariate and probabilistic sensitivity analyses.
Conclusions
FBC appears to be the most cost-effective programme for provision of ART in Uganda. This analysis supports the implementation of FBC for scale-up and sustainability of ART in Uganda. HBC and MCC would be competitive only if there is increased access, increased adherence or reduced cost.
Background
In Sub-Saharan Africa, 22.5 million people are living with HIV, comprising 68% of the global total, and 1.7 million new infections occurred in that region in 2007.[1] Although this represents a reduction in new infections,[2] there are indications that prevention may be faltering.[3] Only 31% of the 9.7 million people in need of antiretroviral therapy (ART) received it in 2007,[4] and the need for treatment will only increase due to dramatic reductions in AIDS mortality as a result of ART and steady rates of new infections. Healthcare providers, usually government ministries of health, must develop policies aimed at the efficient use of scarce health resources to sustainably meet this increasing demand for ART. Countries that have achieved high levels of access also need efficient policies; they face increasing pressure on the health workforce and infrastructure.
Uganda’s health system is organized on a facility-based care (FBC) referral model in which patients often have to travel long distances to seek services such as ART. In an effort to improve health outcomes, stakeholders have implemented other types of programmes for ART delivery, such as mobile clinic care (MCC) and home-based care (HBC). MCC, which has been used by the Rakai Health Sciences Program in Western Uganda,[5] is organized around temporary treatment hubs located near patients’ homes to reduce the distance traveled for ART. In HBC, which has been implemented by a partnership between The AIDS Support Organisation and the US Centers for Disease Control and Prevention in eastern Uganda,[6–8] health workers provide ART in patients’ homes, thereby removing the transport barrier to access. HBC leads to improved adherence[9] and reduced mortality[10] and should improve access, given resource availability. MCC would be expected to achieve improved health outcomes compared with FBC but be inferior to HBC. In light of the health outcomes, access and adherence advantages of HBC and MCC over FBC, they would appear to be the best methods for ART provision. However, their implementation involves increased programmatic costs and may be associated with increased overall costs. It is not known whether this potential increase in cost represents good value. Incremental cost-effectiveness analysis considers both costs and outcomes in evaluating the efficiency of programme interventions. The aim of this study was to compare the incremental cost effectiveness of FBC, MCC and HBC for provision of ART in Uganda.
Methods
Decision-Analysis and Markov Model
A decision-analysis model[11] was developed to examine the cost effectiveness of FBC, MCC and HBC for provision of ART to patients with AIDS in Uganda over a 10-year time horizon. This time horizon was chosen because there were no data on long-term adherence to ART, a key parameter in the model. Figure 1 shows a schematic of the methods of ART provision compared in this model. The reference case was a 35-year-old patient in Uganda with stage 3 AIDS at baseline, based on WHO clinical staging.[12] This average age reflects the relative youth of AIDS patients in the country. The Markov model, suited to HIV/AIDS because of the chronic nature of the disease, was used to represent patient transitions over time from one health state to another.[11,13] The model (figure 2) had seven states: (i) stage 3 receiving ART and adherent; (ii) stage 3 receiving ART and non-adherent; (iii) stage 3 not receiving ART; (iv) stage 4 receiving ART and adherent; (v) stage 4 receiving ART and non-adherent; (vi) stage 4 not receiving ART; and (vii) dead. The model was restricted to stages 3 and 4 to capture the majority of patients in need of or receiving ART treatment; those in earlier clinical stages rarely have significant symptoms and must be identified by CD4 cell counts, which are not currently widely available in Uganda. The cycle time was 6 months, consistent with our best estimate of time taken to progress from one WHO clinical stage to another in this setting. The model was validated by varying transition probabilities between 0 and 1, which resulted in logical responses. In addition, setting costs and outcomes to 0 separately resulted in identical expected values.
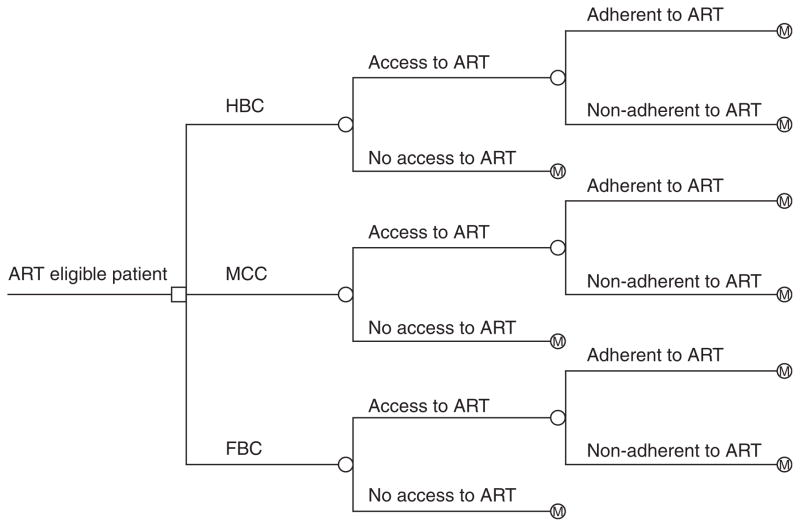
Fig. 1.
Decision tree showing a comparison of the three methods of antiretroviral therapy (ART) provision: home-based care (HBC), mobile clinic care (MCC) and facility-based care (FBC). The square at the root is a decision node; the circles are chance nodes; M denotes transition into the Markov model.
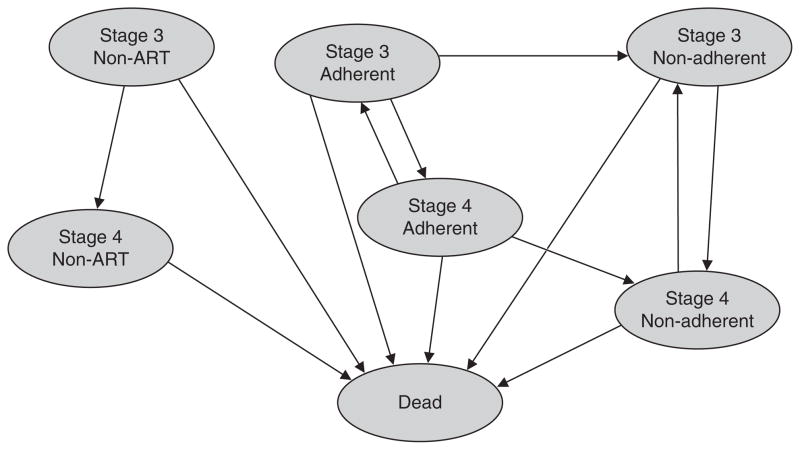
Fig. 2.
Markov model. The model illustrates the different health states through which adult patients with AIDS transition. Each state is associated with a cost and utility. Transitional probabilities from state to state are summarized in the table of parameters (table I). ART= antiretroviral therapy.
The analysis was performed from a governmental Ministry of Health (MOH) perspective and included cost of ART, cost of illness, recurrent costs and capital costs. This perspective is the most relevant because Uganda has a national healthcare system run by the MOH. Costs to patients, including transportation, were excluded. Costs and outcomes were discounted at 3% per year (0–5% in sensitivity analyses) as recommended by the Panel on Cost-Effectiveness and Medicine of the US Public Health Service.[14] Strategies were compared on the basis of costs, life expectancy and incremental cost-effectiveness analysis using QALYs to capture both quality and quantity of life.
The WHO and others have suggested that, because of the lack of a universally accepted standard for a threshold for cost effectiveness, researchers use a GDP-based approach. Suggested thresholds have ranged from 1 to 3 times the per-capita GDP[15–17] per additional QALY or disability-adjusted life-year. Uganda’s GDP per capita was $US350 in 2007.[18] Therefore, ART provision methods were judged to be very cost effective if the incremental cost-effectiveness ratio (ICER) was less than $US350 per QALY (1 times per-capita GDP) and cost effective if the ICER was less than $US1050 per QALY (3 times per-capita GDP).
Rates of Progression and Mortality
WHO divides the natural history of HIV into clinical stages 1–4,[12] a system widely accepted in countries with a high prevalence of HIV. Patients are assigned the presumptive clinical AIDS diagnosis in stages 3 or 4. Data for transition probabilities were obtained from published sources. In the pre-ART era, annual mortality was 16% in stage 3 and 59% in stage 4, while the annual probability of progression from stage 3 to 4 was 18%.[19] These values were used for transition probabilities for patients not receiving ART. We assumed that, in the absence of ART, patients do not return to an earlier clinical stage from a later clinical stage, in line with WHO clinical staging practice. With ART, annual mortality per 100 person-years was 5.9 in stage 3 and 14.1 in stage 4, and the annual rate of progression from stage 3 to 4 was 6.3 per 100 person-years.[20] These estimates were converted into transition probabilities using an exponential survival curve, as is common in Markov models,[13] to obtain annual mortality estimates of 5.7% for stage 3 and 13% for stage 4, and an annual probability of transition from stage 3 to 4 of 6%.
The probability of immune recovery from CD4 count ≤200 to >200 cells/μL was used as a proxy for rate of transition from stage 4 back to stage 3 on ART; WHO clinical staging does not allow the return from a more severe to a less severe stage. However, this transition was allowed in the model to reflect the clinical benefit to patients receiving ART. The annual probabilities of CD4 count increase from ≤200 to >200 cells/μL for two cohorts in the same treatment programme were 0.58 and 0.47, with a mean of 0.53.[21] This estimate was used as the annual transition probability from stage 4 to stage 3 on ART. Adherence to ART has a substantial impact on morbidity and mortality. Compared with adherent individuals, non-adherent individuals have a 3-fold increase in rate of progression,[22] and a 3.87-fold increase in mortality.[23] These estimates were applied to the transition probabilities for non-adherent individuals. We used figures specific to Uganda[24] to create a table of non-AIDS mortality by age.
Access and Adherence to Antiretroviral Therapy
Access estimates were based on the assumption that MCC would improve the absolute rate of access by 20% over FBC, and HBC would improve the absolute rate of access by 20% over MCC. National estimates indicate that 33% of patients who need ART are able to access it.[25] Therefore, the base-case analysis assumed 33% access for FBC, 53% for MCC and 73% for HBC.
Adherence to ART predicts treatment success and is associated with key outcomes such as viral RNA, CD4 counts and mortality.[26] Sub-optimal adherence is also associated with the loss of 1.2 QALYs over a lifetime for patients receiving ART.[27] Therefore, achieved or achievable adherence is important in choosing an optimal strategy for ART provision. A reported 74–81% of patients reported ‘excellent’ adherence while 19–24% reported ‘good’ adherence in the HBC setting in Uganda.[9] We estimated that 85% of patients would be adherent at a level of 95% or greater. This was based on the premise that all 74–81% who reported ‘excellent’ adherence and some of the 19–24% who reported ‘good’ adherence would reach the 95% adherence level. Therefore, the base-case estimate for adherence was assumed to be 85% annually for HBC. We assumed a 10% reduction to 75% adherence for MCC and a further 10% reduction to 65% for FBC. On average, adherence remains stable for the first 2 years and then decreases at an absolute rate of 5% every 6 months,[28] an observation reflected in our model. This rate is the ‘net change’, i.e. some people move from adherent to non-adherent, and a small number of people who were non-adherent become adherent. For simplification, the model only allowed movement from adherent to non-adherent, but at a rate consistent with the ‘net change’ so that overall adherence rates would be consistent with the literature.
Health-Related Quality of Life
The analysis adjusted for health-related quality of life (HR-QOL) based on utility scores obtained from a primary HR-QOL survey of AIDS patients in rural Ugandan clinics using a translated and culturally adapted version of the EuroQol instrument. The results of this study, which included 221 patients in stage 3 and 4 AIDS at four clinics in south-western Uganda, will be reported elsewhere.[29] In the study, the mean utility for stage 3 was 0.69 for patients not receiving ART and 0.84 for patients receiving ART. The mean utility for stage 4 was 0.36 for patients not receiving ART and 0.80 for patients receiving ART. These estimates are close to those obtained in a meta-analysis of utility estimates for HIV/AIDS: 0.44 for AIDS, 0.56–0.82 for symptomatic HIV infection, and 0.68–0.94 for asymptomatic HIV infection.[30] Utilities for non-adherent stage 3 and 4 patients were calculated by assuming a disutility factor of 0.1 for non-adherence to ART (i.e. utilities were reduced by a factor of 0.1).
Costs
Costs were divided into four categories: (i) cost of ART, (ii) cost of illness, (iii) recurrent costs and (iv) capital costs. Cost of ART was assumed to be the cost of first-line drugs, estimated at $US237.50 per patient per year in a recent study in Uganda.[31] Cost of illness included non-ART healthcare resource use, including other drugs, laboratory tests and radiology, which was estimated in a utilization study at $US782 and $US730 per patient per year for stage 3 and 4 disease, respectively.[32] Recurrent costs refer to non-treatment costs of implementing ART programmes, which were obtained from a primary survey of programme accountants performed in August 2005 at two rural Ugandan clinics implementing FBC and MCC.[33] In this survey, we asked the accountants to sum their annual recurrent expenditure (personnel, short-term training, supplies, rent and utilities) and divided this by the number of patients treated during the year. Recurrent costs per patient per year were $US229 for FBC and $US502 for MCC. Recurrent costs of running HBC were not available, so they were estimated by local experts to be $US1000 per patient per year for the first year and $US600 subsequently. Capital costs include vehicles, equipment and long-term training and were estimated from a study of costs of HBC in Rwanda.[34] We assumed that the costs of equipment and long-term training did not differ between programmes and that MCC would use half the cost of vehicles needed for HBC, while FBC would not need vehicles. Capital costs of HBC, FBC and MCC were $US47.60, $US35.50 and $US41.50 per patient per year, respectively. The cost of treatment for non-adherent patients was adjusted by a factor of 0.8 to reflect less medication use. All costs were adjusted to $US, year 2008 values using the consumer price index of Uganda. Table I summarizes the parameters used in the analysis.
Table I.
Parameters of the decision and Markov models
| Parameter | Base case | Sensitivity range | Reference |
|---|---|---|---|
| Transition probabilities | |||
| Stage 3–4 | |||
| Non-ART | 0.18 | 0.10–0.30 | 19 |
| Adherent | 0.06 | 0.03–0.11 | 20 |
| Non-adherent | 0.18 | 0.09–0.33 | 22 a |
| Stage 3–dead | |||
| Non-ART | 0.16 | 0.09–0.28 | 19 |
| Adherent | 0.057 | 0.03–0.10 | 20 |
| Non-adherent | 0.22 | 0.01–0.39 | 23 b |
| Stage 4–3 | |||
| Non-ART | 0 | NA | Assumption |
| Adherent | 0.53 | 0.43–0.63 | 21 c |
| Non-adherent | 0.18 | 0.14–0.21 | 22 a |
| Stage 4–dead | |||
| Non-ART | 0.59 | 0.45–0.74 | 19 |
| Adherent | 0.13 | 0.05–0.24 | 20 |
| Non-adherent | 0.51 | 0.02–0.94 | 23 b |
| Access | |||
| HBC | 0.73 | 0.50–0.76 | Assumption |
| MCC | 0.53 | 0.42–0.64 | Assumption |
| FBC | 0.33 | 0.26–0.39 | 25 |
| Adherence | |||
| HBC | 0.85 | 0.79–0.90 | 9 |
| MCC | 0.75 | 0.70–0.80 | Assumption |
| FBC | 0.65 | 0.60–0.70 | Assumption |
| Utilities | |||
| Stage 3 | |||
| Non-ART | 0.69 | 0.40–0.90 | Primary survey |
| Adherent | 0.84 | 0.65–0.95 | Primary survey |
| Non-adherent | 0.76 | 0.56–0.86 | Imputedd |
| Stage 4 | |||
| Non-ART | 0.36 | 0.20–0.60 | Primary survey |
| Adherent | 0.80 | 0.60–1.00 | Primary survey |
| Non-adherent | 0.72 | 0.54–0.9 | Imputedd |
Used to adjust initial estimate to account for 2.97-fold increase in progression due to non-adherence.
Used to adjust initial estimate to account for 3.87-fold increase in mortality due to non-adherence.
Assumes return from CD4 count ≤200 to >200 cells/μL in 1 year is an appropriate proxy for transition.
Applied a disutility factor of 0.1 for non-adherence (i.e. utilities reduce by a factor of 0.1).
ART= antiretroviral therapy; FBC= facility-based care; HBC= home-based care, MCC= mobile clinic care; NA= not applicable.
Sensitivity Analysis
Sensitivity analyses were performed to determine which variables had substantial impact on cost or outcomes. All parameters in the model were assigned a range of clinically plausible values, using 95% confidence intervals when available (see table I). Costs were halved and doubled. To further test the robustness of our conclusions, we conducted a probabilistic sensitivity analysis. We created probability distributions for all of the parameters in the model. For the annual discount rate, a uniform distribution ranging from 0% to 5% was used. For all other parameters, the base-case value was used for the mean, and the standard error was estimated based on the approximation that the range used for the one-way sensitivity analysis represented a 95% confidence interval, with the range approximately equal to four times the standard error.[35] A Beta distribution was used for probabilities and utilities, and a Gamma distribution was used for cost estimates.[36] Monte Carlo simulation was used to create 1000 samples for which expected values were calculated. The proportion of time for which each strategy was cost effective was then calculated, varying limits of cost effectiveness.
All primary data used in the analysis were collected in a study[37] that was approved by the Case Western Reserve University Institutional Review Board, the Mbarara University of Science and Technology Ethics Review Board and the Uganda National Council of Science and Technology. Data analysis was performed using TreeAge Pro.
Results
Base-case Analysis
Five- and 10-year survival rates were lowest for FBC (21.5% and 8.4%), followed by MCC (31.5% and 14.9%) and highest for HBC (43.6% and 22.9%). The 10-year mean undiscounted life expectancy was lowest for FBC (3.6 years), followed by MCC (4.3 years) and highest for HBC (5.3 years), while the mean discounted QALYs were also lowest for FBC (2.3 QALYs), followed by MCC (2.9 QALYs) and highest for HBC (3.7 QALYs). The 10-year mean costs per patient per year were lowest for FBC ($US3212), followed by MCC ($US4782) and highest for HBC ($US7033). The resulting ICER was lower for MCC versus FBC ($US2241 per LY and $US2615 per QALY) than for HBC versus MCC ($US2251 per LY and $US2814 per QALY). Table II presents the results in detail.
Table II.
Mean and incremental costs ($US, year 2008 values), life expectancy (LE) and cost effectiveness for provision of antiretroviral therapy (ART) in Uganda
| Programmea | Cost | Incremental cost | LE (y) | Incremental LE (y) | ICER ($US/LY) | QALYs | Incremental QALYs | ICER ($US/QALY) | 10-year survival (%) |
|---|---|---|---|---|---|---|---|---|---|
| FBC | 3212 | 3.6 | 2.3 | 8.4 | |||||
| MCC | 4782 | 1569 | 4.3 | 0.7 | 2241 | 2.9 | 0.6 | 2615 | 14.9 |
| HBC | 7033 | 2251 | 5.3 | 1.0 | 2251 | 3.7 | 0.8 | 2814 | 22.9 |
The methods of ART provision are ordered by increasing effectiveness.
FBC= facility-based care; HBC= home-based care; ICER = incremental cost-effectiveness ratio; LY = life-year; MCC= mobile clinic care.
Sensitivity Analysis
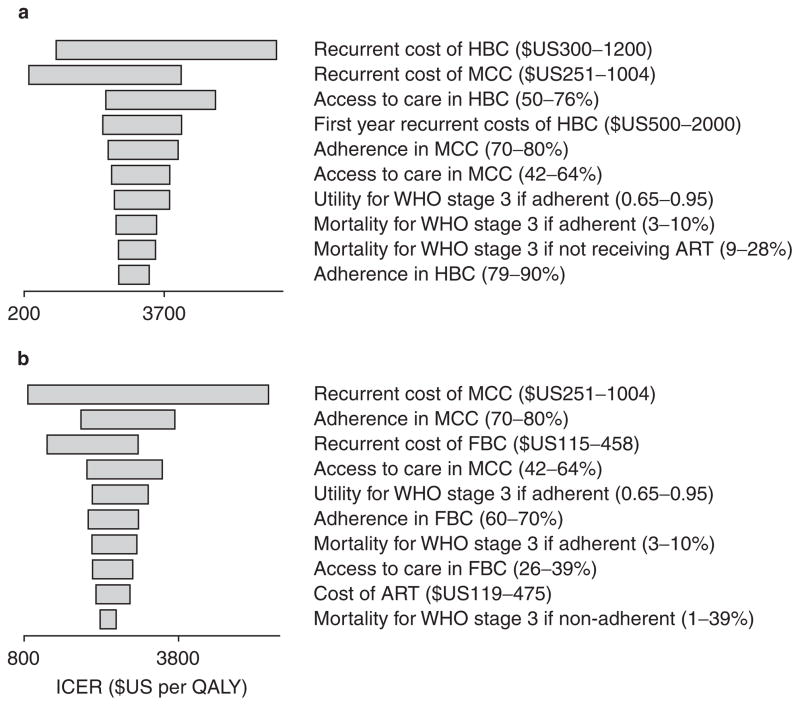
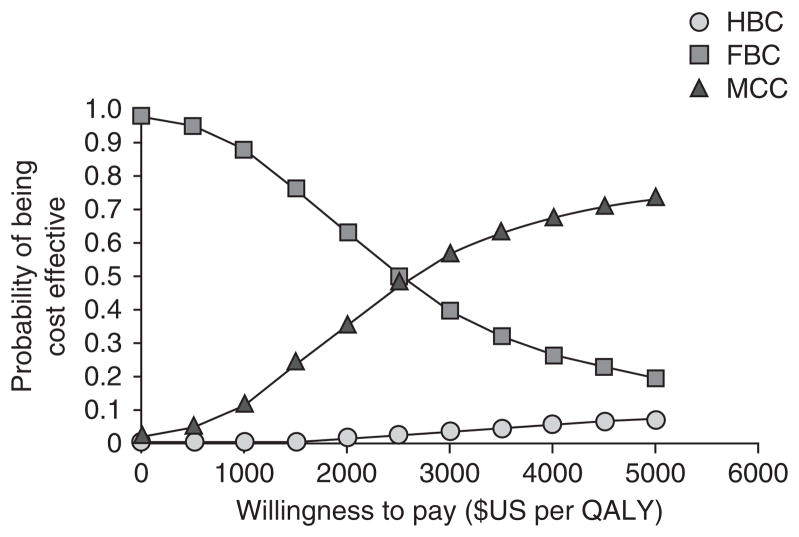
Univariate sensitivity analyses (figure 3) showed that the ICER for HBC versus MCC was most sensitive to the recurrent costs of HBC and MCC, while the ICER for MCC versus FBC was most sensitive to the recurrent costs of MCC and adherence to ART in MCC. Probabilistic sensitivity analysis using Monte Carlo simulation to create 1000 samples (figure 4) showed that FBC and MCC models were equally cost effective when willingness to pay (WTP) was approximately $US2600 per QALY (7.4 times per-capita GDP) and that the probability of HBC being cost effective was always lower than both alternative comparators at a WTP below $US5000. At the most generous threshold for cost effectiveness of $US1050 per QALY (3 times per-capita GDP), FBC was cost effective in approximately 85% of the samples compared with 15% for MCC and 0% for HBC.
Fig. 3.
Tornado diagrams of univariate sensitivity analysis for (a) home-based care (HBC) vs mobile clinic care (MCC) and (b) MCC vs facility-based care (FBC). The ten most influential variables are shown. ART= antiretroviral therapy; ICER = incremental cost-effectiveness ratio.
Fig. 4.
Acceptability curve obtained from probabilistic sensitivity analysis. The curve shows, for each method of antiretroviral therapy provision, the proportion of 1000 simulated samples for which that strategy was cost effective at varying levels of willingness to pay per additional QALY. FBC= facility-based care; HBC= home-based care; MCC= mobile clinic care.
Discussion
Using decision analysis and a WHO clinical stage-based Markov model, this study compared the cost effectiveness of different methods for provision of ART in Uganda and found that FBC was more cost effective than MCC and HBC. This finding was supported by univariate and probabilistic sensitivity analyses.
To our knowledge, this is the first policy model to assess the cost effectiveness of different methods of organizing ART programmes. In countries in which ART has been available for longer, the treatment is transforming the nature of HIV infection into a chronic disease. A strength of this study was its use of a modeling framework focused on patients who were indicated for, or most likely to access, ART, with sensitivity analyses to assess the robustness of conclusions. As this is an important area for health policy in poor countries with high HIV/AIDS burdens, we believe our study has implications for policy development and improvement in access to ART. The model may also be used ‘as is’ or modified to answer similar policy questions using data specific to other countries or settings.
One limitation of this study was the inability to obtain local data on access to ART in programmes implementing MCC and HBC, and adherence estimates for patients attending MCC and FBC. Data specific to this setting are scarce and few studies have been performed. Given these limitations, we made assumptions about the ability of the programmes to achieve different levels of access and adherence, and performed sensitivity analyses on a wide range of parameter values. The sensitivity analyses did not change the relative ranking of the cost effectiveness of the different methods of ART provision. Lack of data also precluded the modeling of long-term adherence and its impact on ART resistance.
The model estimated a survival advantage for HBC of 12% over FBC. This finding was consistent with a previous study conducted in Uganda, which found a significant reduction in HIV/AIDS mortality when co-trimoxazole prophylaxis and ART were delivered in patients’ homes.[10] The present study had the added advantage of including costs and performing a formal assessment of value. A study performed over 20 years ago in Zimbabwe[38] also concluded that costs associated with HBC were prohibitively high. Our study replicates this finding using more contemporary data. While we found no study that compared different programmes of ART provision, the cost effectiveness of HBC and hospital-based care for chronically ill tuberculosis patients has been reported.[39] In this study, investigators found that HBC was more cost effective. Reasons for this discrepancy may include unique disease and treatment differences between HIV/AIDS and tuberculosis or the fact that they measured average cost effectiveness instead of incremental cost effectiveness, and used an intermediate outcome; cost per patient adherent to treatment. We performed an incremental analysis using a mixture of more appropriate endpoints, including morbidity and mortality, and considered adherence as a parameter in the model.
Our findings may be explained by the low costs of implementing FBC compared with MCC and HBC, driven in part by transportation and extra personnel costs. These outweigh the improvements in effectiveness of HBC and MCC (improved survival, better QOL) at the low cost-effectiveness thresholds in poor countries. While HBC and MCC may improve access and adherence enough to be superior to FBC in a specific region or setting, the evidence suggests that FBC is the more efficient national programme from the MOH perspective. This is particularly valid when we consider sustainability. The bulk of ART is implemented via philanthropic interests and non-governmental organizations (NGOs), which are time-bound in funding, yet AIDS is a chronic disease with little hope for a cure. Since national MOHs are expected to take over and run programmes over the long term, implementing the most cost-effective programme from their perspective would be an advantage. From the societal perspective, the situation may be different and is likely to favor HBC.
Another way to look at the results is in terms of budget impact. The modeling framework enables the estimation of national annual cost of implementing HBC, MCC and FBC by multiplying undiscounted mean annual cost per patient by the number of patients receiving ART in Uganda.[40] HBC would cost $US1 billion, MCC $US698 million and FBC $US461 million annually. Uganda’s entire health budget for the fiscal year 2008 was only $US281 million on-budget and $US761 million total;[41] clearly, the implementation and sustainability of large-scale ART using HBC would exceed the total national expenditure on health. While cost effectiveness is not the only consideration for allocation of scarce societal resources[42] and other criteria such as equity or fairness and political considerations often play a part, it should play a significant role in guiding policy and enabling the most efficient use of such a limited budget.
It is not clear how other emerging programme interventions such as task shifting[43] would influence ART provision programmes. For instance, it may be plausible to lower the cost of HBC (using nurses instead of doctors) to levels where it becomes more cost effective than FBC. This is a possible direction for future studies in this area. Future modeling studies will benefit from the increasing volume of clinical and health-services research in poor nations, particularly in the areas of the long-term effect of adherence and its impact on ART resistance. Moreover, the improved precision of input parameters should consequently improve the quality of policy decisions.
Conclusion
FBC provision of ART appears to be more cost effective than HBC and MCC approaches. This implies that poor nations should strengthen existing FBC programmes since they represent the best use of scarce resources and are consistent with the need for constrained maximization in the face of extreme budget constraints. However, other models of care should remain options for alternative strategies. In some hard-to-reach sub-populations (e.g. rural and distant areas, settlements on the other side of physical barriers and highly-mobile groups), FBC may be impractical and HBC or MCC may demonstrate markedly superior access or adherence to ART and have heavily reduced costs. Given the superior life expectancy and QALYs associated with HBC and MCC approaches, programme designers should also continue efforts to reduce the costs associated with these alternatives. Ultimately, governments must decide how best to maximize both access and adherence to ART for their HIV-infected citizens.
Acknowledgments
This project was supported by NIH research grant # D43 TW000011 funded by the Fogarty International Center. The funders had no role in the conduct of the study or preparation of the paper. The authors have no conflicts of interest that are directly relevant to the content of this study.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS epidemic update. Vol. 2007. Geneva: UNAIDS; 2007. [Accessed 2008 Aug 1]. [online]. Available from URL: http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS epidemic update. Vol. 2006. Geneva: UNAIDS; 2006. [Accessed 2009 Aug 31]. [online]. Available from URL: http://data.unaids.org/pub/EpiReport/2006/2006_EpiUpdate_en.pdf. [Google Scholar]
- 3.Heltzer NE. Uganda’s early gains against HIV eroding. AIDS Read. 2007;17:252. [PubMed] [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS), World Health Organization (WHO) and UNICEF. [Accessed 2008 Aug 1];3 million now receiving life-saving HIV drugs: but access to prevention and treatment still lacking for millions [joint news release. 2008 Jun 2; online]. Available from URL: http://www.who.int/mediacentre/news/releases/2008/pr16/en/index.html.
- 5.Johns Hopkins Bloomberg School of Public Health. [Accessed 2009 Jan 26];Rakai Health Sciences Program [online] Available from URL: http://www.jhsph.edu/rakai/about/where_we_work.html.
- 6.Mermin J, Ekwaru JP, Liechty CA, et al. Effect of cotrimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006 Apr 15;367( 9518):1256–61. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 7.Shrestha RK, Marseille E, Kahn JG, et al. Cost-effectiveness of home-based chlorination and safe water storage in reducing diarrhea among HIV-affected households in rural Uganda. Am J Trop Med Hyg. 2006 May;74( 5):884–90. [PubMed] [Google Scholar]
- 8.Pitter C, Kahn JG, Marseille E, et al. Cost-effectiveness of cotrimoxazole prophylaxis among persons with HIV in Uganda. J Acquir Immune Defic Syndr. 2007 Mar 1;44( 3):336–43. doi: 10.1097/QAI.0b013e31802f12b5. [DOI] [PubMed] [Google Scholar]
- 9.Weidle PJ, Wamai N, Solberg P, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006 Nov 4;368( 9547):1587–94. doi: 10.1016/S0140-6736(06)69118-6. [DOI] [PubMed] [Google Scholar]
- 10.Mermin J, Were W, Ekwaru JP, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008 Mar 1;371( 9614):752–9. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- 11.Petitti D. Meta-analysis, decision analysis and cost-effectiveness analysis. New York: Oxford University Press; 1994. [Google Scholar]
- 12.WHO. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance: African region. Geneva: WHO; 2005. [Accessed 2008 Mar 10]. [online]. Available from URL: http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf. [Google Scholar]
- 13.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993 Oct–Dec;13(4):322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 14.Gold MR, Siegel JE, Russell LB, et al. Cost effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 15.Report of the Commission on Macroeconomics and Health. Geneva: WHO; 2001. [Accessed 2008 Jul 6]. Macroeconomics and health: investing in health for economic development. [online]. Available from URL: http://www.paho.org/English/HDP/HDD/Sachs.pdf. [Google Scholar]
- 16.Murray CJ, Lauer JA, Hutubessy RC, et al. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet. 2003 Mar 1;361( 9359):717–25. doi: 10.1016/S0140-6736(03)12655-4. [DOI] [PubMed] [Google Scholar]
- 17.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings: the case of Cote d’Ivoire. N Engl J Med. 2006 Sep 14;355( 11):1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 18.Central Intelligence Agency (CIA) [Accessed 2008 Oct 17];The world factbook: Uganda [online] Available from URL: https://www.cia.gov/library/publications/the-world-factbook/geos/ug.html#Econ.
- 19.Morgan D, Maude GH, Malamba SS, et al. HIV-1 disease progression and AIDS-defining disorders in rural Uganda. Lancet. 1997 Jul 26;350( 9073):245–50. doi: 10.1016/S0140-6736(97)01474-8. [DOI] [PubMed] [Google Scholar]
- 20.Badri M, Bekker LG, Orrell C, et al. Initiating highly active antiretroviral therapy in sub-Saharan Africa: an assessment of the revised World Health Organization scaling-up guidelines. AIDS. 2004 May 21;18( 8):1159–68. doi: 10.1097/00002030-200405210-00009. [DOI] [PubMed] [Google Scholar]
- 21.Médecins Sans Frontières (MSF) Access Campaign and Epicentre. Increased access to HAART in resource-poor settings in Médecins Sans Frontières programmes: outcomes of adults at 18 and 24 months of treatment [abstract]. XV International AIDS Conference; 2004 Jul 11–16; Bangkok. [Accessed 2006 Jul 4]. [online]. Available from URL: http://www.epicentre.msf.org/folder.research/folder.2005-05-04.1929608579/Bangkok_2004_2.pdf. [Google Scholar]
- 22.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002 May 3;16( 7):1051–8. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 23.Garcia de Olalla P, Knobel H, Carmona A, et al. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002 May 1;30( 1):105–10. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 24.Murray C, Lopez A, Ahmad OB, et al. World mortality in 2000: life tables for 191 countries. Geneva: WHO; 2002. [Google Scholar]
- 25.WHO, UNAIDS, UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. [Accessed 2008 Jul 6];Progress report. 2007 April; [online]. Available from URL: http://www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf.
- 26.Bartlett JA. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002 Feb 1;29(Suppl 1):S2–10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- 27.Munakata J, Benner JS, Becker S, et al. Clinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virus. Med Care. 2006 Oct;44( 10):893–9. doi: 10.1097/01.mlr.0000233679.20898.e9. [DOI] [PubMed] [Google Scholar]
- 28.Parruti G, Manzoli L, Toro PM, et al. Long-term adherence to first-line highly active antiretroviral therapy in a hospital-based cohort: predictors and impact on virologic response and relapse. AIDS Patient Care STDS. 2006 Jan;20( 1):48–56. doi: 10.1089/apc.2006.20.48. [DOI] [PubMed] [Google Scholar]
- 29.Data on file. Department of Epidemiology and Biostatistics, School of Medicine, Case Western Reserve University; Cleveland, OH, USA: 2006. [Google Scholar]
- 30.Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002 Nov–Dec;22(6):475–81. doi: 10.1177/0272989X02238300. [DOI] [PubMed] [Google Scholar]
- 31.Schrantz S, Kambugu A, Wandera B, et al. Analysis of antiretroviral therapy in an urban outpatient HIV clinic in Uganda [poster]. 45th Annual Conference of the Infectious Diseases Society of America; 2007 Oct 4–7; San Diego (CA). [Accessed 2008 Feb 22]. [online]. Available from URL: http://www.idsociety.org/Content.aspx?id=8264.2007. [Google Scholar]
- 32.Govender V, McIntyre D, Grimwood A, et al. Bethesda (MD): Partnerships for Health Reform, Abt Associates Inc; 2000. [Accessed 2009 Aug 31]. The costs and perceived quality of care for people living with HIV/AIDS in the Western Cape Province in South Africa [small applied research no. 14] [online]. Available from URL: http://www.abtassociates.com/reports/12-sar14-4-2000.pdf. [Google Scholar]
- 33.Data on file. Department of Epidemiology and Biostatistics, School of Medicine, Case Western Reserve University; Cleveland, OH, USA: 2006. [Google Scholar]
- 34.Chandler R, Decker C, Nziyige B. Estimating the cost of providing home-based care for HIV/AIDS in Rwanda. Bethesda (MD): Partners for Health Reformplus, Abt Associates Inc; 2004. [Google Scholar]
- 35.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000 May;17( 5):479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 36.Del Rio RA, Post AB, Singer ME. Cost-effectiveness of hematologic growth factors for anemia occurring during hepatitis C combination therapy. Hepatology. 2006 Dec;44( 6):1598–606. doi: 10.1002/hep.21409. [DOI] [PubMed] [Google Scholar]
- 37.Data on file. Department of Epidemiology and Biostatistics, School of Medicine, Case Western Reserve University; Cleveland, OH, USA: 2006. [Google Scholar]
- 38.Hansen K, Woelk G, Jackson H, et al. The cost of homebased care for HIV/AIDS patients in Zimbabwe. AIDS Care. 1998 Dec;10( 6):751–9. doi: 10.1080/09540129848361. [DOI] [PubMed] [Google Scholar]
- 39.Moalosi G, Floyd K, Phatshwane J, et al. Cost-effectiveness of home-based care versus hospital care for chronically ill tuberculosis patients, Francistown, Botswana. Int J Tuberc Lung Dis. 2003 Sep;7(9 Suppl 1):S80–5. [PubMed] [Google Scholar]
- 40.Wasswa H. More than half of Ugandan AIDS patients don’t get the drugs they need. BMJ. 2008;336( 7640):348–9. doi: 10.1136/bmj.39486.598044.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Budget Framework Paper, Budget Speech. Ministry of Finance and Ministry of Health; Republic of Uganda: 2008/09. [Google Scholar]
- 42.Musgrove P, Fox-Rushby J. Cost-effectiveness analysis for priority setting. In: Jamison DT, editor. Disease control priorities in developing countries. 2. New York: Oxford University Press; 2006. pp. 271–86. [Google Scholar]
- 43.Samb B, Celletti F, Holloway J, et al. Rapid expansion of the health workforce in response to the HIV epidemic. N Engl J Med. 2007 Dec 13;357( 24):2510–4. doi: 10.1056/NEJMsb071889. [DOI] [PubMed] [Google Scholar]