Abstract
Although hepatitis C virus (HCV) can be cleared, very few infected persons complete the treatment, resulting in disease progression and transmission. Motivational interventions effectively address health and substance-use-related conditions in many cultures. The research team piloted an HCV treatment motivational enhancement training and supervision for four counselors treating four patients in one (of 11) large methadone programs in Israel between 2007 and 2008. The counselors received a 3-day training followed by seven supervision sessions. Training included cultural and language adaptation from the original United States version to practice in Israel. Feasibility was assessed and demonstrated through training field notes and questionnaire feedback, review of taped intervention sessions for counselor proficiency and patient engagement, and patient completion of intervention sessions and piloted measures. While positive feasibility outcomes were noted, future studies should employ larger numbers of counselors and patients to assess the effectiveness of motivational enhancement in promoting HCV treatment in methadone patients.
Keywords: hepatitis C virus (HCV), motivation, self-determination, methadone maintenance program, illicit drug use, addiction, Israel
BACKGROUND
Hepatitis C virus (HCV) is the most common chronic blood-borne infection in much of the world, with about 200 million people infected across the globe or 3% of the world population (Marcellin, 2009; Shepard, Finelli, & Alter, 2005). Up to 90% of the 3–4 million annual new infections worldwide occur among injecting drug users (IDUs), with prevalence from 40% to 90% throughout East and West Europe, Asia, North America, Latin America, and Australia (Aceijas & Rhodes, 2007; Dore, Law, MacDonald, & Kaldor, 2003; Edlin et al., 2005; George et al., 2009; McCarthy & Flynn, 2001; Van den Berg et al., 2007). In Israel, where we conducted our study, HCV prevalence among IDUs has been found to be 35%–45% (Cohen-Moreno et al., 2010; Loebstein et al., 2008).
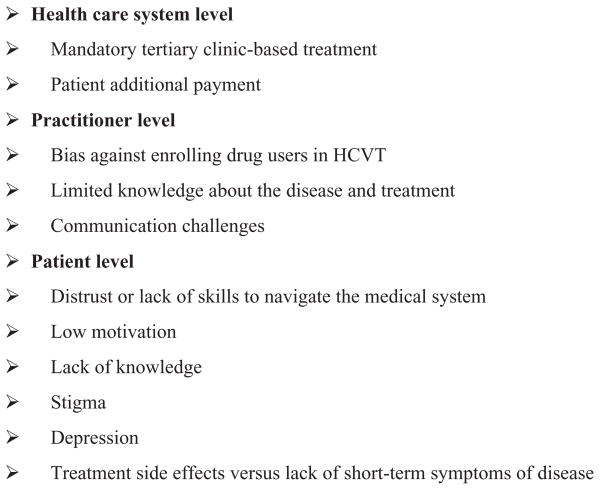
One-quarter to one-half of those infected with HCV develop severe liver disease (Lavanchy, 2009). Viral clearance can be attained and maintained by 60%–90% of those treated with pegylated interferon and ribavirin-based therapies, depending on genotype, viral load, age, and alcohol use (Deutsch & Hadziyannis, 2008; Edlin et al., 2005; Fried et al., 2002; Loguercio et al., 2000). Yet, an effective, widespread treatment is elusive, as up to 63% refuse or do not complete the treatment (Narasimhan et al., 2006). In the United States and elsewhere, drug users face health insurance barriers to access HCV treatment (HCVT) (Doab, Treloar, & Dore, 2005; Edlin et al., 2005; Grebely et al., 2008). Israeli citizens are offered HCVT at reduced cost ($80/month of the $1,000/month actual cost) through government-subsidized health care services since 2005, yet less than 100 (7%) of the estimated 1,500 HCV-infected methadone patients in the country have undergone treatment (Cohen-Moreno et al., 2010). Internationally, multiple barriers to HCVT exist at system, practitioner, and patient levels (Figure 1; Edlin et al., 2005; Fischer et al., 2006; Fishbein, Lo, Reinus, Gourevitch, & Klein, 2004; Hallinan, Byrne, Agho, & Dore, 2007). These include, among others, limited HCV knowledge among IDUs (Cohen-Moreno et al., 2010; O’Brien, Day, Black, & Dolan, 2008; Strauss et al., 2007) and substance abuse treatment staff (Litwin et al., 2007; Zickmund, Ho, Masuda, Ippolito, & LaBrecque, 2003), stigma regarding the disease which can be inadvertently reinforced by treatment providers (Crockett & Gifford, 2004; Fraenkel, McGraw, Wongcharatrawee, & GarciaTsao, 2006), and immediacy of incapacitating side effects of treatment versus potential or distal effects of the disease (Khokhar & Lewis, 2007; Zickmund et al., 2006).
FIGURE 1.
Multiple HCVT barriers.
Patients must have the resilience to overcome multiple barriers at different levels: personal, systemic, and provider based (Evon et al., 2010). It has been hypothesized that patients could be assisted in strengthening the skills and determination to address barriers (Fraenkel et al., 2006; Groessl et al., 2008; Strathdee et al., 2005; Strauss et al., 2007), while providing information and collaborative treatment that is empowering and does not reinforce patient stigma (Tiffen & Sheridan, 2002). A recent intervention addressed some HCVT barriers at an Israeli methadone maintenance program among otherwise abstinent patients (Malnick, Sheidvasser, Basevitz, & Levit, 2009). Patients who scored very low in HCV knowledge received an intervention to remedy this deficit, which when combined with an on-site hepatologist and financial coverage of that portion of the medication not covered by health insurance, increased treatment uptake from 7% to 23% (Malnick et al., 2009). However, this still leaves room for improvement in HCVT uptake.
We present herein a pilot training program for a motivational enhancement (ME) approach to HCVT in a methadone maintenance center in Jerusalem, Israel.
Motivational Enhancement
Motivational interviewing (MI) is a directive, patient-centered approach for facilitating behavior change by assisting patients to explore and resolve ambivalence (Miller & Rollnick, 2002). MI utilizes acceptance, reflection, and empathy and has been proven more beneficial in changing behaviors than a confrontational or blaming approach (Miller, 1983). The theoretical basis for MI has been described as self-determination theory (SDT), wherein the counselor supports the client’s autonomous self-regulation (a feeling of volition or willingness for the targeted behavior) and perceived competence (a feeling of being able to attain the targeted goal) to energize or motivate the behavior (Miller & Brown, 1991). The traditional exhortations of health care providers are more likely to engender controlled motivation (behavior contrary to one’s values or interests or due to someone else’s values or interests; Deci, Eghrari, Patrick, & Leone, 1994; Ryan & Deci, 2008). Our intervention was designed as a synthesis of both the MI manual (Miller & National Institute on Drug Abuse, 1995) and a successful six-session SDT-based intervention for tobacco dependence (Williams et al., 2006a). Hence, we entitle our intervention with the generic term, motivational enhancement (ME). Similarly, our measures of patient self-regulation and perceived competence are SDT based. In HCVT, extrinsic motivation could be a patient taking medication because he believes his physician wants him to do so rather than because he believes it is in his interest. Amotivation could be characterized as a patient having no interest in HCVT. Perceived competence, in the domain of HCVT, could be described as the extent to which a patient believes he can manage the complex challenges of treatment completion (Williams et al., 2006b).
Although ME is known in Israel and was recommended to help patients refrain from illicit drug use (Israel Anti Drug Authority, personal communication, February 4, 2008), there is little supportive evidence in Israel. We found only two descriptive reports of MI interventions addressing the misuse of drugs (Epelboym, 1995) and alcohol (Friedman & Marchevsky, 1995).
SDT has proven applicable across cultures (Chirkov, Ryan, Youngmee, & Kaplan, 2003), as has MI (Miller, Villanueva, Tonigan, & Cuzmar, 2007). Importantly, ME interventions have not been prospectively studied to address patient utilization of HCVT in any population, despite the success of the model across continents and disease processes.
Our goals for this exploratory pilot study were to assess the following: (1) the feasibility of training Israeli methadone maintenance staff in ME to be demonstrated by observing their intervention skills; (2) the applicability of ME for patients considering HCVT to be demonstrated by patient engagement in HCVT discussion during the ME sessions; and (3) the preliminary feasibility of implementing the intervention among Israeli methadone patients as demonstrated by completion of the intervention sessions and performance of the piloted translated motivational measures.
METHODS
The methods are presented in Table 1 according to the stated goals. For Goal 1—training Israeli methadone maintenance staff in ME, the principal investigator (PI) adapted a previously developed (manuscript in preparation) 66-page ME manual into a six-session intervention, specifically targeting HCVT behaviors. The manual incorporated components from an MI substance abuse manual, as others have done (Carels et al., 2007; Channon et al., 2007; Miller & National Institute on Drug Abuse, 1995). The six-session design and its content (see Table 2) were based upon a validated SDT-based intervention for tobacco dependence (Williams et al., 2006a). The investigators held a 3-day training workshop that included brief didactic presentations, demonstration video, and extensive skills practice. These methods were based upon ME facilitator trainings, and materials were adapted with permission (Miller & Deci, personal communication, November 12, 2007). All staff members of two methadone maintenance centers (in the cities of Jerusalem and Ashdod) attended the workshop. The supervision component of the training was continued only for the four counselors in the Jerusalem center chosen to perform the intervention, based upon English proficiency, clinical skill level, and degree of rapport with the PI. Those in supervised training received an ME training manual, which was revised iteratively according to the counselors’ input, and review of audio or videotaped sessions. In all of the seven supervision sessions over 8 weeks, the counselors were instructed to enhance autonomous self-regulation through building rapport and exploring advantages and disadvantages of health-promoting behaviors. The counselors were instructed to offer information about the consequences and treatment of HCV as follows: ask patients what they know already about HCV course and treatment, supplement knowledge as needed and desired, and then solicit patient responses (Williams, Gagné, Ryan, & Deci, 2002). Materials regarding HCV and depression were written in patient-friendly language by relevant specialists. The counselors were instructed to explore patients’ perceived personal and systemic barriers to facilitate HCV health behaviors, while providing support for patients’ autonomy and perceived competence. Individualized HCV health plans were developed according to patients’ readiness using a template plan on which patients highlighted self-chosen behaviors. The counselors were also instructed in the use of empathy, reflection, and confrontation avoidance in resistant patients, which are counseling strategies common to SDT and MI and are expected to increase autonomous self-regulation.
TABLE 1.
Study goals and method of assessment
| Assessed goal | Method utilized |
|---|---|
| (1) The feasibility of training Israeli methadone maintenance staff in ME | Adapted previously developed ME manual and ME workshop to HCVT. |
| Developed and assessed 3-day ME training workshop and supervision at methadone center, integrating HCVT issues. | |
| Training materials translated or subtitled into Hebrew, and live translation at trainings. | |
| (2) The applicability of ME to HCVT | Field notes evaluating the intervention sessions of patient engagement in discussion of HCVT. |
| (3) The feasibility of implementing the intervention among Israeli methadone patients | Pilot intervention on four selected HCV patients. |
| Pilot intervention in native language (Hebrew or Russian). |
TABLE 2.
Overview of ME sessions
|
Session 1: Introduction and self-care history Inquire about past and current self-care: “So to start, I’d like to explore how things are going in terms of Hepatitis C?” Example questions follow, in case the client does not respond to the open-ended strategy, e.g., “How long have you known you have Hepatitis C?” Explore the pros and cons of getting hepatitis C treatment: “You’ve stopped using heroin and maintained that, which is a very important example of a healthy behavior, and I wonder if there are other behaviors that may help you increase your Hepatitis C health, now or in the future?” Explore values or life goals: “What are your goals for the future/your life?” “How has/will the Hepatitis C affect/ed your achievement of those goals?” Explore the pros and cons of not getting hepatitis C treatment: “If you didn’t make further changes to increase your Hepatitis C health, what’s the best thing that would happen?” “What is worst thing that could happen if you don’t make any changes increasing or implementing Hepatitis C health behaviors?” Reinforce self-efficacy: “How have you coped with stopping heroin (other healthy behaviors)?” “It sounds like you’ve been working really hard on this situation.” |
|
Session 2: Hepatitis-related information exchange and education Discuss depression and scale results: Discuss results of client’s depression scores in a personalized way, giving the client control of the information and opportunity to respond, e.g., “What have you been thinking about your depression results? Are you ready to talk about them now? Your results show that you have no/mild/moderate /severe depression markers. What are your reactions to that? Does that surprise you?” Solicit client knowledge and questions regarding hepatitis C: Share relevant information, based upon client responses above and level of knowledge she/he demonstrates, attending to client’s emotional state as well as existing knowledge. “Ok, I’d like to share additional information with you about Hepatitis C if that is ok with you.” Discuss consequences for patient and family regarding HCVT: “What do you know about treatments for hepatitis C? Where did you learn about it?” “Would you like to hear more about treatments for Hepatitis C?” |
|
Session 3: Hepatitis C health plan and self-efficacy Discuss past successful and unsuccessful attempts to increase health: Encourage and praise active involvement of client in the process. “Quitting [heroin/alcohol/smoking/ exercising and eating well-pick one] are major health behaviors and you’ve done that, can you share with me what influenced your decision to make that change?” “Can you share with me how other health behaviors have worked for you in the past?” Inquire regarding client’s thoughts on developing a plan to increase hepatitis C health Develop hepatitis C health plan if ready: “The steps I plan to take in changing are . . ..” How does the client plan to achieve his/her goals? How could the desired change be accomplished? Within the general plan and strategies described, what are some specific, concrete first steps that the client can take? When, where, and how will these steps be taken? Reinforce autonomy and self-efficacy. “Given how resourceful and successful you were before in [quitting heroin/ getting into the methadone program/addressing alcohol/seeking help/taking care of yourself], I’m sure that you can continue to make changes in your life that will help you be healthier.” Explore psychological, physical, systemic barriers to increasing hepatitis C health behaviors: “It sounds like you’re not ready to make a decision related to your health behaviors now and due to the difficulties you’ve experienced and/or the complexities of treatment, that’s understandable.” Discuss behavioral strategies for coping with barriers: “Let’s discuss ways you can overcome these obstacles.” “What practical and emotional support might help? How might you go about getting it?” |
|
Session 4: Hepatitis C health plan and self-efficacy, continued Reestablish rapport, inquire regarding questions Discuss hepatitis C health plan, including utilized and unutilized components as applicable: “Now that we’ve discussed your confidence in being able to implement these behaviors, can you share the positives of engaging in these behaviors?” “Can you share the negatives of engaging in these behaviors?” “Can you share any fears or concerns you have?” Explore importance of and confidence in making specific hepatitis C health changes Explore the pros and cons of the targeted hepatitis C health behaviors Explore and normalize ambivalence Reinforce autonomy and self-efficacy: “On a scale of 1–10 with 10 being very important, how important is it to you to make this change/implement this behavior?” “Why not lower?” “What would be helpful for you to make this change?” “What things would make it difficult for you to succeed in making this change/implement this behavior?” “On a scale of 1–10 with 10 being very confident, how confident are you in your ability to make this change/implement this behavior?” “Why not lower?” |
|
Sessions 5 & 6: Termination Discuss the intervention and continue to normalize ambivalence Review reasons for implementing the targeted hepatitis C health behaviors Review the successful and unsuccessful use of the hepatitis C health plan: Give praise. “Have you utilized these behaviors to increase your Hepatitis C health?” “Were any of those behaviors successful?” “Can you identify how/why these behaviors were successful?” Elicit examples, no matter how small. Positively reframe any lack of success Support autonomy and self-efficacy |
Instruction was in English and Hebrew, while the intervention was held in Hebrew and Russian. An interpreter facilitated mutual understanding between the Hebrew-speaking counselors and the English-speaking PI at all training sessions. Training materials were culturally adapted and translated or subtitled into Hebrew by the investigator (MS) and the translator (a social work graduate student)—both fluent in written and spoken Hebrew and English. The PI met with a Hebrew or Russian translator to preview all the taped intervention sessions for the four counselors prior to group supervision. The PI and one of the trainee counselors independently recorded process notes. Investigators administered an anonymous questionnaire in Hebrew to workshop participants to evaluate the training. The evaluation addressed general satisfaction (1 question); internalization of ME tactics (10 questions) including empathy, discrepancy, rolling with resistance, supporting self-efficacy, open-ended questions, reflective listening, affirmation, summarizing, response to change talk, and response to resistance talk (Cronbach’s alpha = 0.93); utility of learning methods (6 questions) including manual, lecture, exercises, video examples, skills practice in small groups, and discussion in the large group (Cronbach’s alpha = 0.88); and plans for the future incorporation of lessons learned (2 questions) regarding using ME spirit/components and working according to the ME manual (Cronbach’s alpha = 0.75). All questions were rated on a 10-point Likert-type scale ranging from 1 “not at all” to 10 “to a great extent.”
For Goal 2—applying ME to HCVT, the research team held a discussion about HCVT barriers and facilitators with five methadone program clients, who recently completed HCVT, and their spouses. Their input informed subsequent HCV-oriented ME strategies and was integrated into the ME training. The PI and coinvestigators adapted the treatment self-regulation questionnaire (TSRQ) and perceived competence scale (PCS; Williams et al., 2006b) to measure autonomous regulation and competence regarding HCVT for the preliminary estimation of reliability and face validity.
For Goal 3—implementing the intervention among Israeli methadone patients, four pilot HCV patients, who were not then undergoing HCVT, received a six-session intervention in their native language (Hebrew or Russian), using ME to address pretreatment fears and ambivalence about HCVT and its physical and psychological side effects. The written TSRQ and PCS measures were also piloted among 30 patients.
This project was approved by the institutional review board of the School of Social Work and Social Welfare, Hebrew University, and conducted in 2007–2008.
Participant Selection
Of 321 Jerusalem methadone center patients, 146 (45.5%) were HCV positive. Of 102 available patients, we randomly chose 30 to perform the written measures. From those 30, we purposefully chose 4 for the intervention based upon their not having taken any medical action regarding their HCV and having engaged in a therapeutic alliance with a social worker at the center.
Data Analysis
We computed first-order descriptive analyses [means and standard deviations (SDs)] to assess participants’ satisfaction with the workshop. We reviewed field notes from the counselor supervision sessions for their reports of client engagement, counselor adherence to the ME approach, and needed cultural adaptation. We computed Cronbach’s alpha coefficients on the TSRQ and PCS measures to assess the internal reliability of the scale items.
RESULTS
Feasibility of Training
A total of 30 staff members (social workers, physicians, physician assistants, and administrative staff) attended the first 2 days of workshop training, of whom 23 completed feedback questionnaires. On a 10-point scale, the mean satisfaction score was 8.00 (SD = 1.76), the mean self-reported internalization of ME tactics score was 7.58 (SD=1.62), and the mean utility of learning methods was 7.93 (SD = 1.54). Self-predicted implementation of ME by counselors was somewhat less than expected (mean score = 6.67, SD = 1.64), prompting the decision to hold a 1-day reinforcement workshop.
We defined cultural feedback as a consensus by trainees that elements of the manual were discordant with patients’ culture. For example, Russian counselors caring for Russian patients indicated that some Hebrew phrases did not meaningfully translate, and that a manual in Russian with sample phrases would be helpful. These counselors devised some relevant statements, which were back translated into English and deemed consistent with ME style.
Initial counselor resistance to ME was evident. Some counselors voiced concern that supporting patient’s autonomy about taking medications instead of pressuring patients to do so gave too much power to the patient. This was a paradoxical complaint by the counselors since the patient does ultimately make the choice of whether or not to take medications. Early intervention tapes demonstrated frequent lapses into controlling and even sarcastic statements made by these counselors, as in the example below.
An example of resistant counselor (C)/resistant patient (P):
C: What do you think caused you not to go and take this blood test again?
P: Nothing is stopping me from doing that.
C: I remember the doctor told you 2–3 weeks ago that you should take the test.
P: I understood that I have time and it’s not urgent.
C: Well, yes; you’re right. You really do have a lot of time. In about 10 years, you’ll have cirrhosis or cancer. You’ll have plenty of time to get the disease.
By the end of the supervision, all the four counselors demonstrated subjective proficiency (according to the trainer knowledgeable in ME) with autonomy-supportive tactics (Table 3).
TABLE 3.
Examples of elicited interest in change or self-efficacy talk
| Counselor–patient statement | |
|---|---|
| 1 | C: It sounds like you’re saying it would take a lot of effort to go to the specialist. P: Yes. It would take a lot of time, but it would be worth it if it’s a good doctor. |
| 2 | C: I see that it is important that someone help you . . . remind you of your appointment in the morning. Your wife is not that person and your father—you don’t want to worry him. P: He knows that there is a problem but not exactly what. I can tell him, “Father I’m starting a preventive treatment and I want to give you the dates of every appointment I have, and you will remind me.” That won’t be a problem. |
| 3 | C: Let’s try and think. Do you think there is a way for you to solve this [transportation] problem? P: I need to just sit down and talk to my family doctor. I need to talk to him about the location of treatment. Even if I have to take a bus, I don’t think I’ll have to go every day or every other day because it’s just one shot a week. I could get a month’s supply of pills. |
| 4 | C: How do you get away from the [drugs] in the neighborhood. P: I just go somewhere. I have good friends who don’t do drugs . . . I go with them. |
The research team adapted the manual based upon PI and counselor supervision process notes. For example, patient HCV and depression information sheets addressed the likely effects of HCVT on the ability to work, similarities of HCVT side effects to opiate withdrawal symptoms, and a flowchart for HCVT steps. Also, since one client found it useful to ask another patient questions about his HCVT, this option was added. Lastly, since one patient asked that the counselor assists in making a hepatologist appointment and accompanies him to the visit, this option was also added to the manual with explicit confidentiality guidelines.
Applicability of ME to HCVT
The discussion with HCV-treated patients and their spouses confirmed the presence of depression that impacted negatively on relationships with spouse and children and on employment. Consequently, the manual explicitly addressed potential depression.
The process notes and audio or videotapes of the intervention sessions revealed that all four clients engaged in discussions during the intervention sessions of whether or not to start HCVT. This is notable since patients were not pressured to discuss HCVT and utilized the sessions in self-directed ways.
Questionnaire Function
We administered the TSRQ and PCS to 30 patients (as described above), demonstrating face validity, in that all understood the content sufficiently to complete them, and reliability: Cronbach’s alpha coefficients were adequate for the TSRQ autonomous motivation subscale (0.77) and the controlled motivation subscale (0.79). Perceived competence as measured for 30 patients by the PCS yielded a Cronbach’s alpha value of 0.82 for the competence subscale.
Feasibility of Implementing the Intervention Among Israeli Methadone Patients
All four patients completed the six-session intervention, and tape reviews revealed that change talk regarding HCVT had occurred (see Table 3). All patients reported depressive symptoms, minimal healthy behaviors, low self-efficacy, and barriers to HCVT. Demonstrating the potential utility of the intervention, three of the four patients subsequently went for HCV testing. For one of them, the test results indicated the patient did not need treatment. After testing, the other two decided not to go for treatment—one of the two was found to have HCV type 1, which is less responsive to treatment (Zeuzem, 2004).
DISCUSSION
This pilot study applies a novel structured ME intervention to HCVT. We describe the feasibility of training Israeli methadone maintenance counselors in ME, the applicability of ME to patients considering HCVT, and the feasibility of implementing an experimental ME intervention targeting HCVT among Israeli methadone patients. This was a Phase 1 study, attempting to establish whether this could be a promising treatment approach (Rounsaville, Carroll, & Onksen, 2001).
There were a number of preliminary indications of feasibility. Dialogues revealed the increasing use of ME tactics by the trained counselors. Additionally, all clients completed the intervention, and tape review indicated that patients willingly engaged in discussing HCVT. Since all the methadone patients attend clinic regularly, a six-session weekly intervention would not be difficult. Training methadone staff was appropriate, as they regularly encounter these patients.
Although HCVT improves outcomes for many patients, side effects and treatment challenges complicate the decision even when the cost of treatment is low. Hence, it is ethically appropriate for counselors to remain neutral and support patients to decide for themselves, providing information regarding the risks and benefits of treating or not treating (Braddock, Edwards, Hasenberg, Laidley, & Levinson, 1999). ME has been successfully applied to HIV treatment adherence, systemically complex with a long latent disease, multiple treatment side effects, and a similar patient population, supporting its potential role in HCVT (Kennedy, Goggin, & Nollen, 2004; Parsons, Golub, Rosof, & Holder, 2007).
We demonstrated the feasibility of training Israeli counselors despite initial language and cultural barriers. Similarly, the translated ME measures (TSRQ and PCS) showed promise in this new intervention. Other populations have successfully translated and evaluated ME in a variety of domains (Chirkov et al., 2003; Roth, Assor, Kanat-Maymon, & Kaplan, 2006). Although only 4 staff participated in the intervention, all 23 of the 30 staff who gave feedback reported nearly 80% satisfaction, learning, and internalization of tactics. Counselor criteria for this pilot study included the ability to achieve a therapeutic alliance, key for therapeutic change and for provision of autonomy support in ME (Castonguay, Constantino, & Holtforth, 2006; Julius, Novitsky, & Dubin, 2009; Kennedy et al., 2004).
Introducing an intervention into this methadone maintenance program setting was complex. We were open to culturally informed feedback (Chirkov et al., 2003). We performed a microlevel intervention for patients to address their own HCVT barriers. Four measured domains of stigma are social isolation, social rejection, financial insecurity, and internalizing shame (Fife & Wright, 2000). For a microintervention to be effective, it would need to address intrapersonal and interpersonal treatment barriers and help patients cope with stigma from health care providers, family, and others. Acceptance and empathy are integral to ME, which has improved health services utilization in substance abusing populations (Shanahan, Beers, Alford, Brigandi, & Samet, 2010); hence, it seems possible that ME could be shown to decrease stigma in future studies.
Similar to others (Miller & Mount, 2001), our work indicates that strong supervision is needed after a long workshop introduction to develop and maintain counselor fidelity in ME interventions. This sort of ongoing supervision and regular training opportunities would also be needed to address staff turnover.
STUDY LIMITATIONS
Potential limitations of this project warrant mention. First, we did not measure counselor fidelity in ME according to standards such as that measured by the Motivational Interviewing Treatment Integrity scale (Miller&Mount, 2001) or the Health Care Climate (Williams & Deci, 2001). However, this pilot study aimed to assess the feasibility of training as one of its goals. Future studies could more precisely examine fidelity and effectiveness. Other studies have rigorously applied and validated the translated measures (Brueck et al., 2009), and such a study could be applied in Hebrew and/or Russian in Israel. Second, our study is based on a purposeful sample of those willing to participate and with engaged counseling relationships, so it did not investigate those who could most benefit from a motivational intervention. It was also a purposeful sample of counselors and treatment programs, so did not indicate what conditions or qualities would promote counselor performance or program success. As a Phase 1 pilot study (Rounsaville et al., 2001), numbers were small and the study was not a randomized clinical trial. Post-ME motivation and satisfaction outcomes were not measured for this small group, although we did assess whether they engaged in subsequent HCVT. Potential patient and counselor process and outcome measures for future studies are presented in Table 4. Wider implementation would also require programmatic support, which we did have for this project, but on a larger scale. Finally, this pilot study does not aim to demonstrate generalizability or large-scale feasibility. There is evidence that Israeli and US methadone patients share much in common (Peles, Linzy, Kreek, & Adelson, 2008), and our small sample of four patients had diverse stages of HCV and ethnicities. This pilot study of the intervention and measures on a small scale suggests preliminary feasibility for a larger-scale study.
TABLE 4.
Criteria for assessing outcomes in future studies
| Type of measurement | |
|---|---|
| (1) Behavioral outcomes |
|
| (2) Process measures: patient |
|
| (3) Process measures: Quantitative assessment of counselor fidelity regarding ME implementation | Motivational Interviewing Treatment Integrity scale (Miller & Mount, 2001). |
| Health Care Climate Questionnaire strategy (Williams & Deci, 2001). |
CONCLUSION
The pilot community-based six-session ME intervention addressing HCVT services utilization demonstrated preliminary feasibility regarding training and supervising counselors, face validity of the translated measures in this population, applicability to HCVT, transferability to Israeli culture in content and translation, and acceptability. Counselors subjectively demonstrated the ability to support patient autonomy by the end of the supervision period. Future studies could evaluate ME in larger randomized trials with Israeli methadone patients considering HCVT.
Acknowledgments
The authors wish to acknowledge the patients, counselors, and staff of the methadone centers in Ashdod and Jerusalem, Israel, for their participation in this study. We also gratefully acknowledge the assistance of Samah Salaime-Egbariya MSW for her assistance in translating the measures and manual. We gratefully acknowledge Kelly Cava, Caroline Chen, Heather Clifford, Meaghan Bernstein, and Peter Tran for technical assistance.
GLOSSARY
- HCV
Hepatitis C Virus
- HIV
Human Immunodeficiency Virus
- Methadone Maintenance Treatment Program (MMTP)
supervised administration of long-acting opiate as a treatment for opiate addiction
- Self-Determination Theory (SDT)
an empiric theory and practice of motivation in which human motivation is explained by autonomous regulation, autonomy support, and perceived competence
- Motivational Interviewing (MI)
a particular motivational intervention utilizing specific tactics and methods
- Motivational Enhancement
the general class of motivational interventions
Biographies
Dr. Diane S. Morse, MD, is an Assistant Professor of medicine and psychiatry at the School of Medicine and Dentistry, University of Rochester. She is an internist whose present research focuses on the application of self-determination theory to comorbid HIV and HCV treatment utilization. She has also published research on sequelae of family violence history, including childhood abuse and intimate partner violence, and on empathy and selfdisclosure in patient-physician communication. Her clinical work currently focuses on medical care of patients with psychiatric comorbidities. Her activities during the period of this project were funded by the National Institute of Mental Health (NIMH T32 MH18911, PI Eric Caine, MD) and the Fulbright Scholar Program, US Department of State.

Dr. Miriam Schiff, Ph.D., MSW, is a Senior Lecturer and the Head of the MSW program at the Paul Baerwald School of Social Work and Social Welfare, Hebrew University. She is also a Licensed School Psychologist and a Social Worker. Her research and publications focus on the associations between man-made trauma and substance use as well as mental health in general among youth and women. She has published several articles on the aftereffects of collective trauma and on the helping components in social work services and interventions. Her clinical activity includes working as a volunteer with drug-addicted women in a methadone clinic in Jerusalem, providing them individual and group treatment.

Dr. Shabtay Levit, Ph.D., MSW, IL, is the Chairman of Directors of Israel’s Methadone Clinics. Dr. Levit created and directs both the methadone treatment programs and the needle exchange programs in Jerusalem and Ashdod. He is also an Adjunct Lecturer at the Hebrew University School of Social Work and Social Welfare. His areas of interest are creating and improving systems and individual models of rehabilitation for substance use clients, and utilizing technology to improve the quality of social and health services for deprived populations such as substance users. He has several publications on these topics.

Rinat Cohen-Moreno, MPH and licensed paramedic, is the Director of the Branch for Welfare Statistics in the Central Bureau of Statistics, Israel. Her research topics are children at risk and juvenile and adult delinquency. Previously, she worked for several years in a Methadone Maintenance Treatment Program as the Director of methadone distribution. She was a member of the methadone program management and development committee, and led the clinical and research efforts toward extending the HCV testing and treatment coverage at the program.

Dr. Geoffrey C. Williams, Ph.D., MD, is currently the Director of the Healthy Living Center at the University of Rochester. He is also a Professor of medicine, psychiatry, and psychology. He has 20 years of practice experience in academic internal medicine and training as a health psychologist. He has contributed to the development of the self-determination theory model for health behavior change. He has been the recipient of numerous grants including research support from the National Cancer Institute, the National Institute on Drug Abuse, the National Institute of Diabetes and Digestive and Kidney Diseases, Small Business Innovation Research, and the National Institute of Mental Health. He has published over 50 peer-reviewed articles as well as books and numerous book chapters. In addition, he has presented at over 50 major national professional meetings.

Dr. Yehuda Neumark, Ph.D., MPH, is a tenured Senior Lecturer of epidemiology at the Braun School of Public Health and Community Medicine of Hebrew University-Hadassah in Jerusalem, Israel. His research focuses on the epidemiology of alcohol and drug use and misuse in Israel and globally, using his research findings to inform policy whenever possible. He serves as the Director of the Braun School’s International Master of Public Health Program, mentors master and doctoral students, and teaches courses in epidemiology, quantitative research methods in public health, and community-oriented health care. He is becoming increasingly interested in the use of information and communication technologies for health promotion, specifically developing drug and alcohol Internet-based interventions for young adults. If asked about his greatest accomplishment, he would say his children and grandchildren.

Footnotes
This manuscript was previously presented as follows:Society of General Internal Medicine Annual Meeting, Miami, FL, May 2009.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. International Journal on Drug Policy. 2007;18(5):352–358. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Braddock CH, III, Edwards KA, Laidley TL, Levinson W. Informed decision making in out-patient practice: Time to get back to basics. JAMA. 1999;282(24):2313–2320. doi: 10.1001/jama.282.24.2313. [DOI] [PubMed] [Google Scholar]
- Brueck RK, Frick K, Loessl B, Kriston L, Schondelmaier S, Go C, et al. Psychometric properties of the German version of the motivational interviewing treatment integrity code. Journal of Substance Abuse Treatment. 2009;36(1):44–48. doi: 10.1016/j.jsat.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Carels RA, Darby L, Cacciapaglia HM, Konrad K, Coit C, Harper J, et al. Using motivational interviewing as a supplement to obesity treatment: A stepped-care approach. Health Psychology. 2007;26(3):369–374. doi: 10.1037/0278-6133.26.3.369. [DOI] [PubMed] [Google Scholar]
- Castonguay LG, Constantino MJ, Holtforth MG. The working alliance: Where are we and where should we go? Psychotherapy: Theory/Research/Practice/Training. 2006;43(3):271–279. doi: 10.1037/0033-3204.43.3.271. [DOI] [PubMed] [Google Scholar]
- Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, et al. A multicenter randomized controlled trial of motivational interviewing in teens with diabetes. Diabetes Care. 2007;30(6):1390–1395. doi: 10.2337/dc06-2260. [DOI] [PubMed] [Google Scholar]
- Chirkov V, Ryan RM, Youngmee K, Kaplan U. Differentiating autonomy from individualism and independence: A self-determination theory perspective on internalization of cultural orientations and well-being. Journal of Personality and Social Psychology. 2003;84(1):97–110. [PubMed] [Google Scholar]
- Cohen-Moreno R, Schiff M, Levitt S, Bar-Hamburger R, Strauss S, Neumark Y. Knowledge about hepatitis-C among methadone maintenance treatment patients in Israel. Substance Use & Misuse. 2010;45(1–2):58–76. doi: 10.3109/10826080902864894. [DOI] [PubMed] [Google Scholar]
- Crockett B, Gifford SM. “Eyes wide shut”: Narratives of women living with hepatitis C in Australia. Women and Health. 2004;39(4):117–137. doi: 10.1300/J013v39n04_07. [DOI] [PubMed] [Google Scholar]
- Deci EL, Eghrari H, Patrick BC, Leone DR. Facilitating internalization: The self-determination theory perspective. Journal of Personality. 1994;62(1):119–142. doi: 10.1111/j.1467-6494.1994.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: An update. Journal of Viral Hepatitis. 2008;15(1):2–11. doi: 10.1111/j.1365-2893.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clinical Infectious Diseases. 2005;40(5 Suppl):S313–S320. doi: 10.1086/427446. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Law M, MacDonald M, Kaldor JM. Epidemiology of hepatitis C virus infection in Australia. Journal of Clinical Virology. 2003;26(2):171–184. doi: 10.1016/s1386-6532(02)00116-6. [DOI] [PubMed] [Google Scholar]
- Edlin BR, Kresina TF, Raymond DB, Carden MR, Gourevitch MN, Rich JD, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clinical Infectious Diseases. 2005;40(5 Suppl):S276–S285. doi: 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelboym R. Motivational interviewing: Theory and practice. Society and Welfare. 1995;15(2–3):317–326. [Google Scholar]
- Evon D, Simpson K, Esserman D, Verma V, Smith S, Fried M. Barriers to accessing care in patients with chronic Hepatitis C: The impact of depression. Aliment Pharmacol Theraputics. 2010;32(9):1163–1173. doi: 10.1111/j.1365-2036.2010.04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BL, Wright ER. The dimensionality of stigma: A comparison of its impact on the self of persons with HIV/AIDS and cancer. Journal of Health and Social Behavior. 2000;41(1):50–67. [PubMed] [Google Scholar]
- Fischer B, Kalousek K, Rehm J, Powis J, Krajden M, Reimer J. Hepatitis C, illicit drug use and public health: Does Canada really have a viable plan? Canadian Journal of Public Health Revue Canadienne De Sante Publique. 2006;97(6):485–488. doi: 10.1007/BF03405233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DA, Lo Y, Reinus JF, Gourevitch MN, Klein RS. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. Journal of Acquired Immune Deficiency Syndromes (1999) 2004;37(3):1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- Fraenkel L, McGraw S, Wongcharatrawee S, GarciaTsao G. Patients’ experiences related to anti-viral treatment for hepatitis C. Patient Education and Counseling. 2006;62(1):148–155. doi: 10.1016/j.pec.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, et al. Peg interferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Friedman AM, Marchevsky A. No deductions: Enhancing motivation for cooperation in a relapse prevention group. Society and Welfare. 1995;15(2–3):327–336. [Google Scholar]
- George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: A 5-year follow-up of 150 patients. Hepatology. 2009;49(3):729–738. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Genoway KA, Raffa JD, Dhadwal G, Rajan T, Showler G, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug and Alcohol Dependence. 2008;93(1–2):141–147. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Groessl EJ, Weingart KR, Kaplan RM, Clark JA, Gifford AL, Ho SB. Living with hepatitis C: Qualitative interviews with hepatitis C-infected veterans. Journal of General Internal Medicine. 2008;23(12):1959–1965. doi: 10.1007/s11606-008-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallinan R, Byrne A, Agho K, Dore GJ. Referral for chronic hepatitis C treatment from a drug dependency treatment setting. Drug and Alcohol Dependence. 2007;88(1):49–53. doi: 10.1016/j.drugalcdep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Julius RJ, Novitsky MA, Jr, Dubin WR. Medication adherence: A review of the literature and implications for clinical practice. Journal of Psychiatric Practice. 2009;15(1):34– 44. doi: 10.1097/01.pra.0000344917.43780.77. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Goggin K, Nollen N. Adherence to HIV medications: Utility of the theory of self-determination. Cognitive Therapy and Research. 2004;28(5):611–628. [Google Scholar]
- Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: Do they alter their decision with time? Digestive Diseases and Sciences. 2007;52(5):1168–1176. doi: 10.1007/s10620-006-9579-1. [DOI] [PubMed] [Google Scholar]
- Lavanchy D. The global burden of hepatitis C. Liver International. 2009;29(1 Suppl):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the treatment self-regulation questionnaire (TSRQ) across three different health behaviors. Health Education Research. 2007;22(5):691–702. doi: 10.1093/her/cyl148. [DOI] [PubMed] [Google Scholar]
- Litwin AH, Kunins HV, Berg KM, Federman AD, Heavner KK, Gourevitch MN, et al. Hepatitis C management by addiction medicine physicians: Results from a national survey. Journal of Substance Abuse Treatment. 2007;33:99–105. doi: 10.1016/j.jsat.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebstein R, Mahagna R, Maor Y, Kurnik D, Elbaz E, Halkin H, et al. Hepatitis C, B, and human immunodeficiency virus infections in illicit drug users in Israel: Prevalence and risk factors. Israel Medical Association Journal. 2008;10:775–778. [PubMed] [Google Scholar]
- Loguercio C, Di Pierro M, Di Marino MP, Federico A, Disalvo D, Crafa E, et al. Drinking habits of subjects with hepatitis C virus-related chronic liver disease: Prevalence and effect on clinical, virological and pathological aspects. Alcohol and Alcoholism. 2000;35(3):296–301. doi: 10.1093/alcalc/35.3.296. [DOI] [PubMed] [Google Scholar]
- Malnick SD, Sheidvasser V, Basevitz A, Levit S. A model for treating HCV hepatitis in patients receiving methadone maintenance therapy. Gastroenterology. 2009;136(5):A831. [PubMed] [Google Scholar]
- Marcellin P. Hepatitis B and hepatitis C in 2009. Liver International. 2009;29(1 Suppl):1–8. doi: 10.1111/j.1478-3231.2008.01947.x. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Flynn N. Hepatitis C in methadone maintenance patients: Prevalence and public policy implications. Journal of Addictive Diseases. 2001;20(1):19–31. doi: 10.1300/J069v20n01_03. [DOI] [PubMed] [Google Scholar]
- Miller WR. Motivational interviewing with problem drinkers. Behavioural Psychotherapy. 1983;11(2):147–172. [Google Scholar]
- Miller WR, Brown JM. Self-regulation as a conceptual basis for the prevention and treatment of addictive behaviours. In: Heather N, Miller WR, Greeley J, editors. Self-control and the addictive behaviours. Sydney: Maxwell Macmillan; 1991. pp. 3–79. [Google Scholar]
- Miller WR, Mount KA. A small study of training in motivational interviewing: Does one workshop change clinician and client behavior? Behavioural and Cognitive Psychotherapy. 2001;29(4):457–471. [Google Scholar]
- Miller WR National Institute on Drug Abuse. Motivational enhancement therapy with drug abusers. s.l: University of New Mexico Press; 1995. [Google Scholar]
- Miller WR, Rollnick S. Preparing people for change. 2. New York: Guilford Press; 2002. Motivational interviewing. [Google Scholar]
- Miller WR, Villanueva M, Tonigan JS, Cuzmar I. Are special treatments needed for special populations? Alcoholism Treatment Quarterly. 2007;25(4):63–78. [Google Scholar]
- Narasimhan G, Sargios TN, Kalakuntla R, Homel P, Clain DJ, Theise ND, et al. Treatment rates in patients with chronic hepatitis C after liver biopsy. Journal of Viral Hepatitis. 2006;13(11):783–786. doi: 10.1111/j.1365-2893.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- O’Brien S, Day C, Black E, Dolan K. Injecting drug users’ understanding of hepatitis C. Addictive Behaviors. 2008;33:1602–1605. doi: 10.1016/j.addbeh.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes. 2007;46(4):443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Linzy S, Kreek MJ, Adelson M. One-year and cumulative retention as predictors of success in methadone maintenance treatment: A comparison of two clinics in the United States and Israel. Journal of Addictive Diseases. 2008;27(4):11–25. doi: 10.1080/10550880802324382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G, Assor A, Kanat-Maymon Y, Kaplan H. Assessing the experience of autonomy in new cultures and contexts. Motivation and Emotion. 2006;30:365–376. [Google Scholar]
- Rounsaville BJ, Carroll KM, Onksen LS. A stage model of behavioral therapies research: Getting started andmoving on from stage 1. Clinical Psychology Science and Practice. 2001;8:133–142. [Google Scholar]
- Ryan RM, Deci EL. A self-determination theory approach to psychotherapy: The motivational basis for effective change. Canadian Psychology. 2008;49(3):186–193. [Google Scholar]
- Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. Journal of General Internal Medicine. 2010;25(8):803–808. doi: 10.1007/s11606-010-1311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. The Lancet Infectious Diseases. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Latka M, Campbell J, O’Driscoll PT, Golub ET, Kapadia F, et al. Factors associated with interest in initiating treatment for hepatitis C virus (HCV) infection among young HCV-infected injection drug users. Clinical Infectious Diseases. 2005;40(Suppl 5):S304–S312. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SM, Astone-Twerell JM, Munoz-Plaza C, Des Jarlais DC, Gwadz M, Hagan H, et al. Drug treatment program patients’ hepatitis C virus (HCV) education needs and their use of available HCV education services. BMC Health Services Research. 2007;7:39. doi: 10.1186/1472-6963-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffen L, Sheridan S. Improving take-up of hepatitis C services. Nursing Times. 2002;98(43):30–32. [PubMed] [Google Scholar]
- Van den Berg CH, Smit C, Bakker M, Geskus RB, Berkhout B, Jurriaans S, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. European Journal of Epidemiology. 2007;22(3):183–193. doi: 10.1007/s10654-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, Deci EL. Activating patients for smoking cessation through physician autonomy support. Medical Care. 2001;39(8):813–823. doi: 10.1097/00005650-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate glucose control in patients with diabetes. Diabetes Care. 1998;21:1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- Williams GC, Gagné M, Ryan RM, Deci EL. Facilitating autonomous motivation for smoking cessation. Health Psychology. 2002;21(1):40–50. [PubMed] [Google Scholar]
- Williams GC, McGregor H, Sharp D, Kouides RW, Lévesque CS, Ryan RM, et al. A self-determination multiple risk intervention trial to improve smokers’ health. Journal of General Internal Medicine. 2006a;21(12):1288–1294. doi: 10.1111/j.1525-1497.2006.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, et al. Supporting autonomy and competence in a clinical trial. Health Psychology. 2006b;25(1):91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- Zeuzem S. Heterogeneous virologic response rates to interferon-based therapy in patients with chronic hepatitis C: Who responds less well? Annals of Internal Medicine. 2004;140(5):370–381. doi: 10.7326/0003-4819-140-5-200403020-00033. [DOI] [PubMed] [Google Scholar]
- Zickmund SL, Bryce CL, Blasiole JA, Shinkunas L, LaBrecque DR, Arnold RM. Majority of patients with hepatitis C express physical, mental, and social difficulties with antiviral treatment. European Journal of Gastroenterology and Hepatology. 2006;18(4):381–388. doi: 10.1097/00042737-200604000-00011. [DOI] [PubMed] [Google Scholar]
- Zickmund SL, Ho EY, Masuda M, Ippolito L, LaBrecque DR. “They treated me like a leper.” Stigmatization and the quality of life of patients with hepatitis C. Journal of General Internal Medicine. 2003;18(10):835–844. doi: 10.1046/j.1525-1497.2003.20826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]