Abstract
Objective
The onset of neurological signs in experimental autoimmune encephalomyelitis (EAE) is tightly associated with infiltration and reactivation of T cells in the CNS. The anatomical localization of the initial T cell-APC interactions leading to reactivation of T cells in the CNS is, however, still unclear. We hypothesized that activated CD4+ T cells gain direct access to the subarachnoid space and become reactivated upon encounter with cognate antigen in this compartment.
Methods
C57Bl/6 mice were immunized with MOG35-55 and interactions between CD4+ T cells and MHC class II+ APCs in the subarachnoid space were investigated using flow cytometry, confocal microscopy of leptomeningeal whole-mount preparations, time-lapse microscopy of leptomeningeal explants, and in vitro proliferation assays.
Results
CD4+ T cells, polarized to produce Th1/Th17 cytokines, accumulated in the subarachnoid space early during the course of EAE, before CD4+ T cells were detected in the spinal cord parenchyma. At this time point, leptomeningeal, but not parenchymal CD4+ T cells incorporated BrdU, indicating local proliferation of CD4+ T cells in the subarachnoid space. Time lapse microscopy indicated that these CD4+ T cells actively scanned the tissue and interacted with local MHC class II positive APCs, resulting in long-lasting interactions between CD4+ T cells and MHC class II positive APCs, suggestive of immunological synapses.
Interpretation
These results support the concept that immune surveillance of the CNS involves the subarachnoid space and indicate that the leptomeninges play an important role in EAE initiation.
INTRODUCTION
It has long been noted that the inflammatory process during experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), occurs earlier in the subarachnoid space than in the brain parenchyma, suggesting an important role for this compartment in the initiation and development of central nervous system (CNS) inflammation1,2. More recent data have suggested that the initial events underlying EAE consist of a two-step process: (i) priming of myelin-reactive CD4 cells in the periphery; and (ii) reactivation of primed CD4+ T cells in the CNS3. Reactivation of primed CD4+ T cells in the CNS requires that interactions take place between CD4+ T cells and antigen presenting cells (APCs) locally in the CNS during the initiation of an inflammatory response. Indeed, encephalitogenic T cells accumulate within the CNS, recognize target antigens and cause disease even in the absence of a peripheral lymphoreticular system4. Furthermore, the difference between highly and weakly pathogenic myelin-specific T cells was not related to their ability to enter the CNS, but to the degree these cells are reactivated after entry into the CNS5.
MHC class II+ APCs located in the CNS, either in the form of radio-resistant parenchymal microglia or radio-sensitive dendritic cells/perivascular macrophages, mediate this local reactivation of primed myelin-reactive T cells resulting in CNS inflammation and EAE development4,6–8. However, virtually nothing is known about the anatomical localization of initial T cell-APC interactions leading to reactivation of T cells in the CNS. The brain and spinal cord are surrounded by the subarachnoid space consisting of the leptomeninges (the arachnoid and the pial membranes) and the compartment between them containing cerebrospinal fluid (CSF), fibrous trabeculae, blood vessels, and APCs. During inflammation, T cells enter the CNS in large numbers across the blood-brain barrier (BBB) via parenchymal post-capillary venules. The absence of adhesion molecule expression on resting brain parenchymal endothelium renders it questionable to which extent T cells utilize this route during immune surveillance of the non-inflamed CNS. Instead, T cells may enter the CSF across the choroid plexus or through postcapillary venules located in the subarachnoid space9–12.
In the current study, we examined the hypothesis that activated CD4+ T cells are reactivated within the subarachnoid space upon encounter with cognate antigens, early during the course of EAE. We have previously suggested that activated memory T cells enter CSF from the systemic circulation and monitor the subarachnoid space as part of immune surveillance of the healthy CNS9,13. This proposal was supported by studies in mice that are unable to reactivate T cells in the CNS parenchyma, such as mice lacking the costimulatory molecules CD28 or B7-1/B7-2, where there is a preferential accumulation of inflammatory cells in the meningeal compartment, with little or no parenchymal inflammation14,15. To address our hypothesis in the current study, we monitored T cell/APC interactions in the subarachnoid space early during the course of EAE as follows: (i) cells from the leptomeninges were characterized using flow cytometry; (ii) APCs were isolated from meningeal cell suspensions and used for in vitro studies; (iii) whole-mount preparations of leptomeninges were analyzed using confocal microscopy; and (iv) leptomeningeal explants were embedded in collagen matrix directly ex vivo and interactions between CD4+ cells and APCs were visualized using time-lapse confocal microscopy. We found that interactions between CD4+ T cells and MHC class II+ APCs occurred in the subarachnoid space before neurobehavioral onset of EAE, supporting the notion that immune surveillance of the CNS involves the meningeal/CSF compartment and suggesting that the leptomeninges play an important role in EAE initiation.
Materials and Methods
Animals and EAE induction
Mice were kept in a specific pathogen-free environment at Harvard Institutes of Medicine. All experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School. Naive C57Bl/6 females (Jackson Laboratories, Inc.) were used between 6–12 weeks of age. EAE was induced by injecting the mice s.c. in the flanks with an emulsion containing 150 μg of MOG35-55 peptide (Quality Controlled Biochemicals) and 200 μg M. Tuberculosis in incomplete Freund’s adjuvant. In addition, 200 ng pertussis toxin (PT) was injected i.p. on days 0 and 2 post immunization (pi). Control mice received complete Freund’s adjuvant (CFA) and PT without MOG35-55. To study antigen-specific responses, 5×106 MOG TCR tg CD4 cells were transferred i.v. to naive C57Bl/6 females immediately before immunization. MOG TCR tg CD4+ T cells were isolated from spleen and lymph nodes of naive 2D2 mice (kindly provided by VK Kuchroo, Brigham and Women’s Hospital, Boston16) using MACS separation.
Antibodies
The following mAbs were used: CD4 (clone RM4-5), CD11a (M17/4), CD11b (M1/70), CD11c (HL3), CD25 (PC61), CD69 (H1.2F3), CD86 (GL1), TCR Vα3.2 (RR3-16), TCR Vβ11 (RR3-15), I-Ab (KH74), IFN-γ (XMG1.2), IL-10 (JES5-16E3), and TNF-α (MP6-XT22) from BD Biosciences; CD4 Alexa 488 (RM4-5), BrdU (PRB-1), Q-dot 705 labeled goat anti-mouse IgG (H+L) F(ab′)2, and Alexa Fluor 488, 594 or 680 labeled goat anti-mouse, anti-rabbit or anti-rat IgG (H+L) from Invitrogen; IL-17 (TC11-18H10.1) from BioLegend; DEC-205 (NLDC-145) from AbD Serotec.
Preparation of mononuclear cells from the leptomeninges and spinal cord parenchyma for flow cytometry and in vitro cultures
Mice were perfused with 30 ml cold PBS and the leptomeninges were carefully dissected from the CNS using a dissection microscope. For flow cytometry, meninges were incubated in HBSS containing 2 mM EDTA and 5 mM HEPES for 1 hour at 4°C to break cell-cell interactions. Remaining meningeal tissue was mechanically dissociated by trituration and passage through a 70 μm cell strainer. Spinal cords were flushed out of the spinal column by hydrostatic pressure to remove the meninges, cut into pieces, and incubated in HBSS as described above. After the incubation, the spinal cord suspension was passed through a 70 μm cell strainer and centrifugated through 37% Percoll (Pharmacia) to remove myelin debris. Samples intended for in vitro assays were digested with collagenase type IV (1 mg/ml, Roche Diagnostics) for 20 min at 37°C. Due to technical reasons, it was not possible to isolate both meninges and parenchyma from the spinal cord of the same animal. Since our initial experiments demonstrated that while parenchymal T cell infiltration is limited to the spinal cord, meningeal T cell infiltration was detected throughout the CNS, we used leptomeninges isolated from the forebrain, cerebellum and brain stem for experiments comparing cell infiltration in the two compartments.
Staining for flow cytometry
Mononuclear cells were blocked with 20% normal rat serum and 10 μg/ml anti-mouse CD16/CD32 in PBS with 5% FCS and 0.05% sodium azide for 15 min at 4°C. Cells were stained with directly conjugated mAbs for 40 min at 4°C, followed by fixation with 1% PFA for 5 min at RT.
For intracellular cytokine staining, cells were stimulated in full culture medium containing 50 ng/ml PMA, 1 μg/ml ionomycin and monensin (GolgiStop, 0.65 μl/ml) for 4 hours at 37°C. After staining of surface markers, cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences), and incubated with anti-cytokine antibodies for 20 min at RT. Samples were acquired on a FACS Calibur or an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo version 8.4.3 (Tree Star, Inc.).
BrdU incorporation
Immunized mice received BrdU (120 mg/kg bodyweight, BD Biosciences) two hours before harvest on day 10 and 18 p.i. This interval between BrdU injection and harvest was chosen to minimize cell trafficking between the periphery and the CNS, as well as to provide ample time for BrdU to evenly penetrate the CNS parenchyma17. CNS mononuclear cells were prepared as described and BrdU containing cells identified using BrdU Flow Kit (BD Biosciences) according to the manufacturer’s instructions. Whole leptomeninges were fixed with 1% PFA for 15 min at RT and incubated in 2M HCl for 2 hours to expose BrdU. The samples were blocked with IHC buffer (PBS with 8% horse serum, 3 g bovine serum albumin and 0.3% Triton-X), stained with Alexa Fluor 595-labeled anti-BrdU and Alexa Fluor 488-labeled anti-CD4 for 40 min at 4°C, mounted on microscope slides and examined using a Zeiss LSM 510 Laser Scanning confocal microscope using LSM 3D analysis software (Zeiss).
In vitro proliferation assays
Meningeal and spinal cord CD11b+/CD11c− and CD11c+ APCs were isolated using directly conjugated antibodies and FACS (FACS Aria, BD Biosciences). The purity of the isolation was >97%. CD4+ MOG TCR tg cells were isolated from naive 2D2 mice using MACS beads and labeled with 5 μM CFSE in PBS containing 5% FCS for 5 min at RT. CD11b+/CD11c− or CD11c+ APCs were co-cultured with 5×104 CD4 cells at a ratio of 1:1–1:100 for 96 hours at 37°C in the presence of 5 μg/ml MOG35-55 peptide, stained with 7-AAD, Annexin-V and cell surface markers, and acquired using an LSR II flow cytometer. CFSE intensity was determined in viable (7-AAD−/Annexin V−) CD4+/Vα3.2+/Vβ11+ cells.
Whole-mount leptomeningeal staining
Whole leptomeninges were fixed with 1% PFA for 15 min at RT and blocked with IHC buffer for a minimum of one hour at 4°C. The samples were stained with unconjugated mAbs in 1.5 ml Eppendorf tubes for 1 hour at 4°C, washed, stained with species-specific fluorochrome-labeled (Alexa Fluor 488, 594, or 705) secondary antibodies for 1 hour at 4°C, mounted on microscope slides and examined using a Zeiss LSM 510 Laser Scanning confocal microscope and LSM 3D analysis software (Zeiss). To visualize blood vessels, 100 μl of tetramethylrhodamin-labeled dextran (10 mg/ml; MW 2×106 daltons) was injected i.v. to the mice two minutes before harvesting the animals and the leptomeninges dissected without preceding perfusion.
Leptomeningeal explants and time-lapse microscopy
Leptomeninges were briefly stained with anti-MHC class II followed by Alexa Fluor 594 labeled goat anti-mouse IgG and Alexa Fluor 488 anti-CD4 for 5 min at 4°C in PBS containing 10% FCS. Kinetic experiments demonstrated that treatment of splenocytes with anti-CD4 for 5 min did not change their migratory behavior during the initial four hour period after the staining (which is comparable to the time period when imaging was performed; data not shown). The leptomeningeal explants were attached to poly-D-lysine coated glass bottom culture dishes (MatTek Corp.), covered with a thin layer of growth factor reduced matrigel matrix (BD Biosciences) and gelled at for 15 min 37°C to minimize tissue movements during recording, before being submersed in full culture medium as previously described18–20. The samples were maintained at physiological cell culture conditions during imaging using an environmental controller system (Zeiss). Time-lapse microscopy and computer-assisted single cell tracking was used to analyze the migration of cells within the meninges. Images were recorded with a Zeiss LSM 510 Laser Scanning confocal microscope as vertical Z-stacks over time (every 1–6 min for a total of up to 90 min). Sequences of image stacks were transformed into volume-rendered three-dimensional movies using LSM 3D analysis software (Zeiss). Velocities were determined using frame-by-frame measurements of migrated distance by period of time scanned using computer-assisted single cell tracking (LSM 510 software, Zeiss). Preliminary experiments demonstrated intact cell motility during scanning times for up to four hours. The parameters used for the laser scanning do not cause photodamage and this technique allows for following normal T cell physiology as previously shown21. Explants from 3–4 mice were examined at each time point.
Results
Infiltration of CD4+ T cells in the subarachnoid space occurs early during EAE
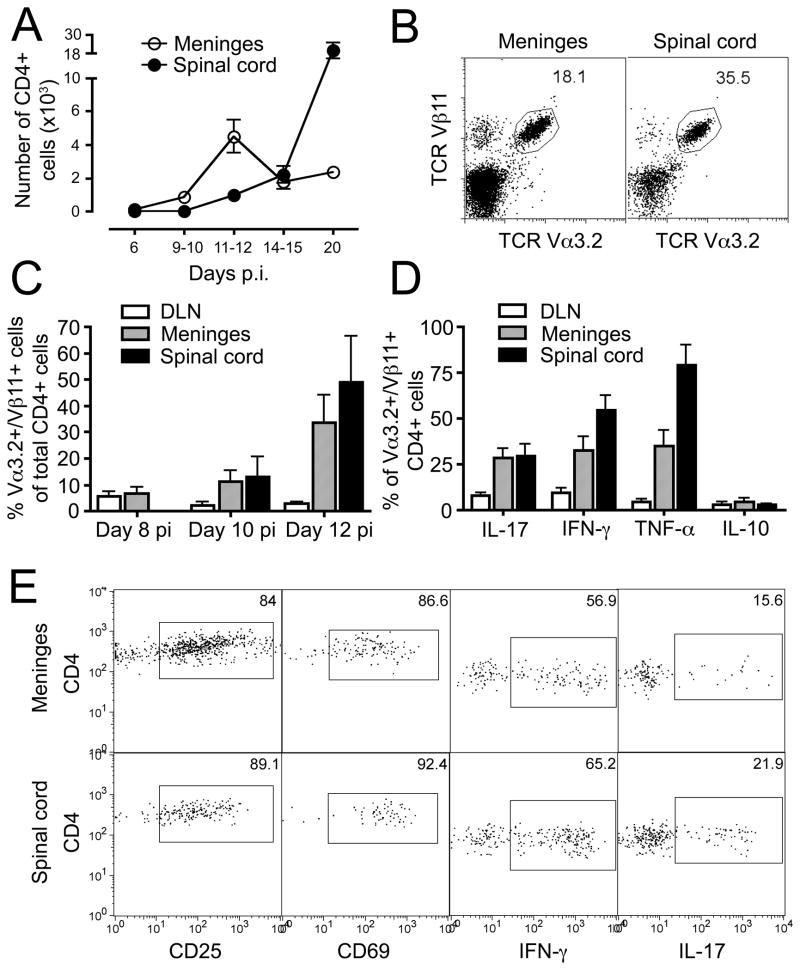
To study the role of the subarachnoid space in initiating CNS inflammation, we carefully dissected leptomeninges from spinal cord parenchyma of C57Bl/6 mice immunized with MOG35-55 and prepared single-cell suspensions of inflammatory cells for analysis by flow cytometry. On day 6 pi, well before the onset of EAE signs, few CD4+ cells were present in the leptomeninges or in paired spinal cord samples (Fig 1A; Table 1). The number of CD4+ cells increased significantly in the leptomeninges on day 9–10 pi, immediately before the mice developed neurobehavioral signs of EAE, while the number of CD4+ cells in the spinal cord remained unchanged. The number of CD4+ cells in the leptomeninges peaked around the onset of clinical signs of EAE (at day 11–12 pi), which coincided with the emergence of CD4+ cells in the spinal cord parenchyma. At this time point, the number of CD4+ T cells was still significantly lower in the spinal cord compared to the leptomeninges (p<0.005). As leptomeningeal CD4+ T cell numbers started to decline around the peak of EAE (day 14–15 pi), the number of CD4+ cells in the spinal cord parenchyma continued to increase. Consequently, there was a switch in the main localization of the CD4+ T cell accumulation from the leptomeninges to the spinal cord parenchyma as EAE developed: on day 11–12 pi, all mice evaluated had more CD4+ T cells in the leptomeninges compared to the spinal cord (8/8, p<0.005), while all mice had more CD4+ T cells in the spinal cord on day 20 pi (7/7, p<0.02). The number of CD4+ T cells in the meninges or spinal cord from control animals immunized with complete Freund’s adjuvant (CFA) and pertussis toxin (PT) without MOG35-55 was consistently less than 150 cells (data not shown).
Figure 1. Characterization of meningeal CD4+ T cells.
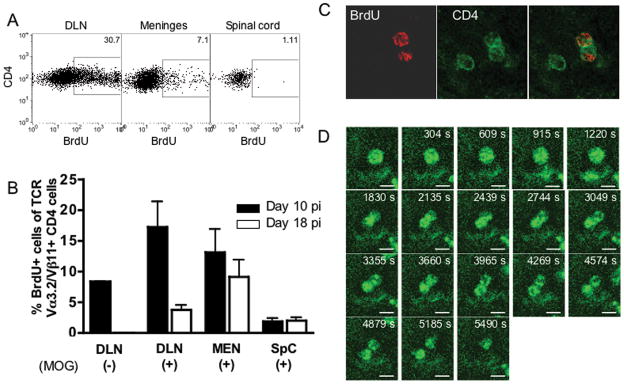
(A) Numbers of CD4+ T cells isolated from the leptomeninges and spinal cord at different time points after immunization with MOG35-55. Clinical signs of EAE appeared on day 11–12 pi. Results are presented as total numbers of cells in each compartment/mouse and show mean±SEM for data from four independent experiments. (B–C) 5×106 MOG TCR tg cells from naive 2D2 mice16 were transferred into naive C57Bl/6 recipients, which were subsequently immunized with MOG35-55. MOG-specific CD4+ T cells were traced by their homogenous use of TCR Vα3.2/Vβ11 chains. Flow plots in B show characteristic TCR usage on day 12 pi (gated on CD4+ cells). (D–E) Cells from draining LN (DLN), meninges and spinal cord were stimulated with PMA and ionomycin for 4 hours at 37°C and intracellular cytokine expression determined by flow cytometry. The expression of CD25 and CD69 was analyzed in unstimulated cells. Figures show expression in CD4+ MOG TCR tg donor cells during clinical EAE (days 11–15 pi).
Table 1.
Number of CD4+ T cells present in the leptomeninges or spinal cord of C57Bl/6 mice after immunization with MOG35-55.
| Days pi | Meninges | Spinal cord | ||
|---|---|---|---|---|
| 6 | 91±69 | 15±14 | ||
| 9–10 | 860±840 | p<0.05 | 32±16 | n.s. |
| 11–12 | 4,500±2,780 | p<0.007 | 970±520 | p<0.005 |
| 14–15 | 1,770±1,150 | n.s. | 2,190±1,330 | p<0.05 |
| 20 | 2,340±760 | p<0.0071 | 19,400±13,900 | p<0.007 |
p-values refer to the comparison between one time point and the preceding time point (except in 1, which refers to the comparison with day 11–12 pi).
These results suggested that antigen-specific CD4+ T cells might be reactivated and expanded in the subarachnoid space at early time points during EAE. To address more directly the accumulation of antigen-specific CD4+ T cells within the subarachnoid space, we took advantage of MOG TCR tg (2D2) mice, which bear a transgenic TCR specific for MOG35–55, presented by H2b class II MHC16. By transferring 5×106 CD4+ 2D2 cells to naive C57Bl/6 recipients before immunization with MOG35-55, we were able to track the 2D2 MOG-specific cells based on their homogenous use of TCR Vα3.2/Vβ11 chains (Fig 1B). At the earliest time point analyzed (day 8 pi), frequencies of Vα3.2+/Vβ11+/CD4+ cells were comparable in the meninges (6.6±3.3%; mean±SD) and draining lymph nodes (5.5±2.7%), whereas numbers of CD4+ cells were too low to allow further analysis in the spinal cord parenchyma (Fig 1C). The frequencies of Vα3.2+/Vβ11+/CD4+ cells started to increase at day 10 pi both in the meninges and the spinal cord parenchyma, with up to 50% of all infiltrating CD4+ T cells bearing the MOG35-55 specific Vα3.2+/Vβ11+ TCR on day 12 pi (meninges: 33.5±15.0%; spinal cord parenchyma: 49.1±24.8%).
The phenotype of Vα3.2+/Vβ11+/CD4+ cells isolated from the subarachnoid space and the spinal cord parenchyma were strikingly similar and consisted predominantly of CD44hi/CD62Llow/CCR7low effector/memory cells (data not shown). As expected, the majority of these cells were recently activated Th1/Th17 cells expressing high levels of CD25, CD69, IFN-γ, IL-17 and TNF-α (Fig 1D–E).
CD4+ T cell infiltration in the subarachnoid space starts from the vessels
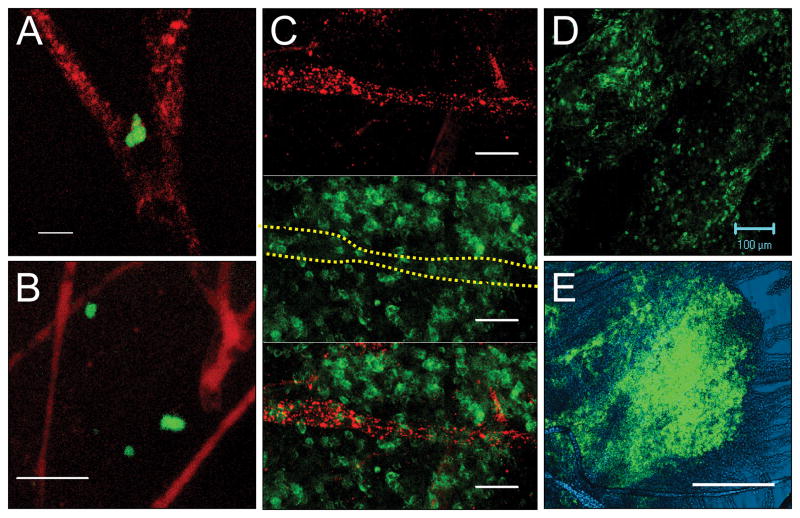
Having demonstrated that early infiltration of CD4+ cells occurs in the meningeal compartment, we addressed the anatomical distribution of these cells. We previously provided evidence that CD4+ T cells can enter this compartment via meningeal venules13. To visualize blood vessels in the subarachnoid space, we injected mice with tetramethylrhodamin-labeled dextran immediately before harvest and prepared whole-mount sections of the leptomeninges. Few CD4+ cells were observed in the subarachnoid space of naive mice. These cells were primarily located adjacent to blood vessels, with sporadic cells further away from the vasculature (data not shown). In mice harvested on day 9–10 (before onset of EAE) the number of CD4+ cells had increased in the leptomeninges. While CD4+ cells were still observed next to vessels, cells were also widely distributed throughout the subarachnoid space (Fig 2A–B). During the peak of EAE, large numbers of CD4 cells were observed throughout the leptomeninges (Fig 2C–D). The distribution of CD4+ T cells was not uniform, but rather formed large aggregates of cells (Fig 2E).
Figure 2. CD4+ T cells are present in the extravascular compartment of the subarachnoid space.
Tetramethylrhodamin-labeled dextran (red) was injected i.v. two minutes before harvest to visualize blood vessels. Whole-mount preparations of leptomeninges were obtained, stained for CD4 (green) and scanned using a confocal laser-scanning microscope. (A–B) At day 9–10 pi (before onset of EAE signs), CD4+ T cells were detected in close contact with blood vessels as well as further into the surrounding space. (C–D) During overt EAE, the leptomeninges contained large numbers of CD4+ T cells without any association with blood vessels. Dashed yellow line in middle panel of C indicates location of blood vessel. (E) Differential interference contrast (DIC) microscopy using a low power objective demonstrated large conglomerates of CD4+ T cells in the leptomeninges during peak of EAE. Scale bars: A=10 μm, B–C=50 μm, D=100 μm, E=200 μm.
Mature dendritic cells are present in the leptomeninges and have the capacity to present antigen to naive CD4+ T cells
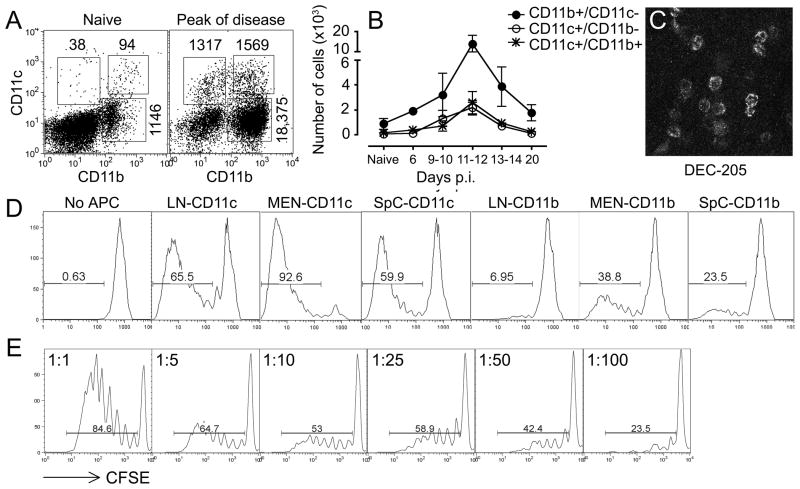
Next, we studied the presence of APCs in the leptomeninges. A substantial number of CD11b+/CD11c− monocytes/macrophages (873±629 cells/animal) could be isolated from the meninges of naive, non-immunized mice (Fig 3A). In addition, naive mice had small numbers of CD11c+/CD11b+ (132±35) and CD11c+/CD11b− (54±25) DCs in the meninges. During the course of EAE, the numbers of all APC populations (CD11b+/CD11c−, CD11c+/CD11b+ and CD11c+/CD11b−) followed similar kinetics of accumulation within the leptomeninges as observed for CD4+ T cells: APC numbers started to increase by day 9–10 pi, and reached their highest levels on day 11–12 pi, concomitant with the first signs of EAE (Fig 3A–B). CD11c+ DCs isolated from the leptomeninges of both naive mice and mice with signs of EAE expressed high levels of MHC class II (44.6±10.7%; mean±SD) and CD86 (33.9±11.3%), consistent with a mature phenotype and an ability to present antigens to T cells. Immunofluorescent staining of whole-mount preparations verified the presence of CD11b+ monocytes/macrophages (data not shown) and DEC-205+ DCs (Fig 3C) in the leptomeninges.
Figure 3. The meningeal compartment contains CD11c+ DCs with the capacity to induce proliferation of naive CD4+ T cells in vitro.
(A–B) Numbers of CD11b+ and CD11c+ cells isolated from the meninges at different time points after immunization with MOG35-55. Neurobehavioral signs of EAE appeared on day 11–12 pi. Results are presented as total cells in each compartment/mouse and show mean±SEM for data from 3–9 mice. (C) Whole-mount preparation of leptomeninges from one mouse with early EAE (day 12 pi) stained for DEC-205. (D) CD11b+/CD11c− and CD11c+ cells from LN, leptomeninges (MEN) and spinal cords (SpC) of immunized mice harvested before onset of EAE (day 10–11 pi) were isolated using FACS and co-cultured with naive CFSE-labelled MOG TCR tg CD4 cells (at a ratio of 1:1) in the presence of 5 μg/ml MOG35-55 for 96 hours in vitro. (E) CD11c+ DC were co-cultured in vitro with naive CSFE-labelled MOG TCR tg CD4+ cells at decreasing APC:T cell ratios (1:1–1:100).
To define the functional capacity of leptomeningeal APCs for antigen presentation to CD4+ T cells, we sorted CD11b+/CD11c− and CD11c+ cells from the CNS of immunized mice before the onset of EAE (day 10–11 pi) and co-cultured the cells with naive 2D2 CD4+ T cells (at a ratio of 1:1) in the presence of MOG35-55. Meningeal CD11c+ DCs and CD11b+/CD11c-monocytes/macrophages induced vigorous proliferation of naive antigen-specific CD4+ T cells, comparable to or exceeding the proliferation induced by comparable APC populations in draining LN or spinal cord (Fig 3D). Serial dilutions demonstrated that CD11c+ DCs isolated from the meninges stimulated CD4+ T cells at CD11c:CD4 ratios as low as 1:50–1:100 (Fig 3E).
CD4+ T cells interact with MHC class II+ APCs in the subarachnoid space
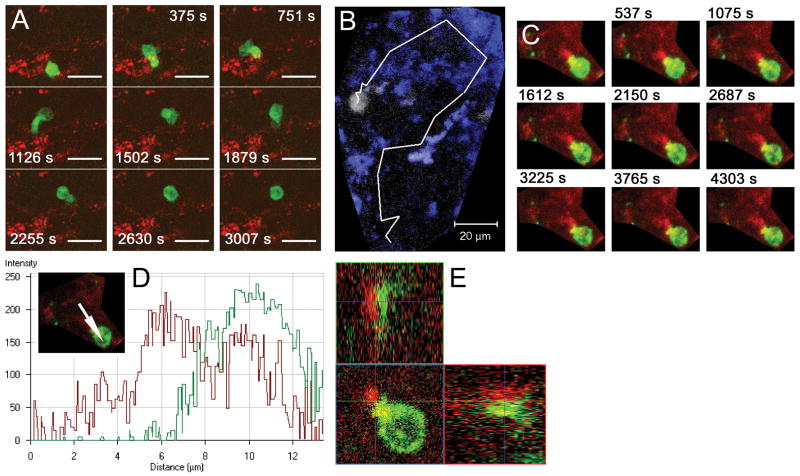
Our data suggested that CD4+ T cells interact with APCs in the subarachnoid space early during the development of EAE. We prepared leptomeningeal explants and performed two-color time-lapse imaging using confocal immunofluorescence microscopy to study CD4+ T cell behavior and MHC class II interactions in the subarachnoid space directly ex vivo. In naive mice, only a few CD4+ T cells were identified. These cells were viable and displayed an activated phenotype with ruffled cell membranes and continuous reorganization of CD4 clusters on the surface, as well as process extension and retraction. One cell (of eight) was migrating in a scanning motion, characterized by high motility, sharp turn angles and a large pseudopod preceding the main cell body, surveying the microenvironment and forming serial brief interactions with MHC class II+ cells (Fig 4A, Supplementary movie 1).
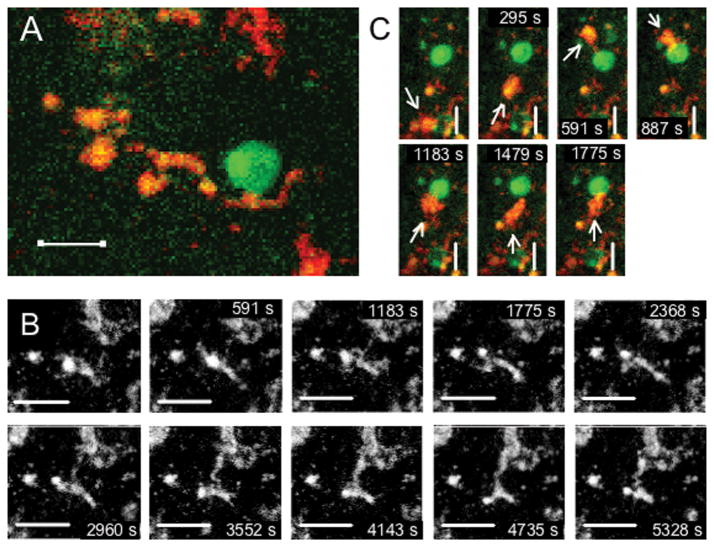
Figure 4. CD4+ T cells interacting with MHC class II+ APCs in the subarachnoid space.
Leptomeningeal explants were prepared from naive mice (A) or mice immunized with MOG35-55 harvested before onset of neurobehavioral signs of EAE (B–E) and imaged using confocal time-lapse microscopy directly ex vivo. Figures show merged z-stacks evenly spaced in time. (A) A highly motileCD4+ cell (green) migrating in a scanning fashion and forming serial brief interactions with multiple MHC class II+ APCs (red). (B) CD4+ cell (white) from mouse harvested immediately before onset of EAE migrating with higher instant velocity than cell from naive mouse (A), but retaining a scanning migration pattern and interacting with multiple MHC class II+ cells (blue). Image shows CD4+ cell trajectory outlined in white. (C) Stable interaction between CD4+ cell (green) and MHC class II+ APC (red) lasting throughout the scanning time of 70 min. (D) Pixel intensity histogram (for path indicated with arrow in insert) and (E) orthogonal 3D display of interaction in panel C showing intimate contact between the two cells. Scale bars: A–B=20 μm; C=10 μm. Time-lapse animation of cells in A–C are available in Supplementary Movies 1 and 2.
Dramatically more CD4+ T cells were present in the meninges of immunized mice harvested either on day 10–11 pi, immediately before onset of EAE signs, or during overt EAE. Most of these cells had a phenotype resembling cells from naive mice, i.e. ruffled cell membranes and continuous reorganization of CD4 clusters on their surface. CD4+ T cells were often situated in close proximity to MHC class II+ APCs and were involved in two distinct types of interactions: (i) serial brief interactions with multiple MHC class II+ cells as described for CD4+ T cells isolated from naive mice, and (ii) stable, long-lasting interactions between a highly polarized CD4+ T cell and an APC often lasting for more than an hour (Fig 4B–E, Supplementary movie 2). MHC class II+ cells possessed numerous processes and were highly motile (Fig 5A–B, Supplementary movie 3). Their behavior was consistent with continuous sampling of the environment by extending and retracting processes. While CD4+ T cells displayed higher velocities and covered larger surface areas in their search for APCs, MHC class II+ cells were also noted to identify and approach a more stationary CD4+ T cell initiating contact between the two cells (Fig 5C).
Figure 5. Behavior of MHC class II+ cells in the subarachnoid space.
Leptomeningeal explants were prepared from mice harvested on day 10–11 pi (before onset of EAE signs) and imaged using confocal time-lapse microscopy directly ex vivo. Figures show merged z-stacks evenly spaced in time. (A) CD4+ cell (green) localized in close contact with an MHC class II+ APC (red) exhibiting a branched process-bearing DC-like morphology. (B) Representative movie of highly motile MHC class II+ APC continuously extending and retracting processes and pseudopods. (C) Migrating MHC class II+ APC (red, marked with arrow) identifying and forming interaction with CD4+ cell (green). Scale bars: 10 μm. Time-lapse animations are available in Supplementary Movie 3.
The migratory behavior of leptomeningeal CD4+ T cells changes during the course of EAE
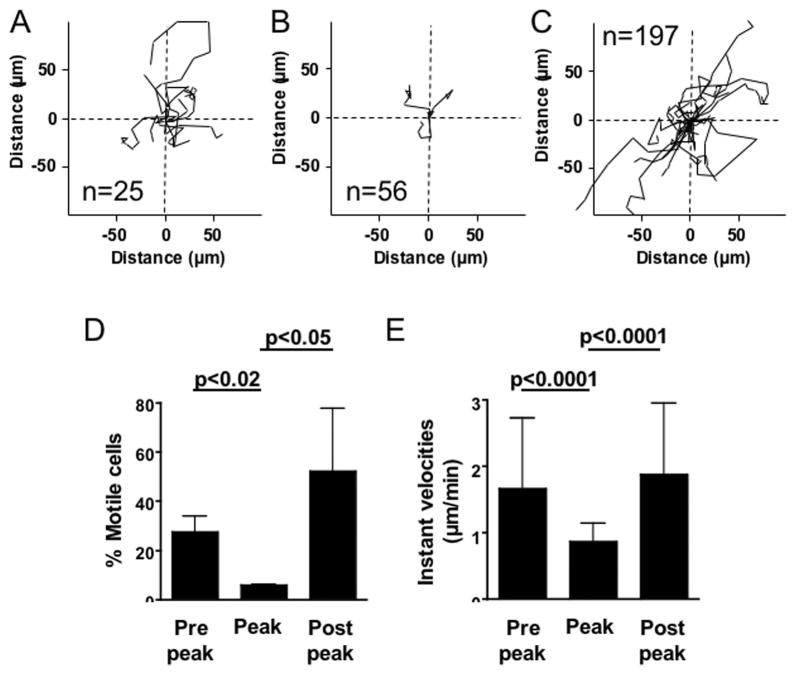
Interestingly, the migratory behavior of CD4+ T cells changed as EAE developed. On day 10–11 pi immediately before onset of EAE signs, 27.5±6.4% of all CD4+ T cells in leptomeningeal explants were highly motile. Individual CD4+ T cells moved in a scanning pattern with frequent sharp turns, touching multiple MHC class II+ cells. The vast majority of CD4-MHC class II interactions were brief before the CD4+ T cells detached and migrated to another APC, but more long-lasting interactions were also observed. The average two-dimensional velocity of migratory CD4+ T cells in the meninges at day 10–11 pi was 1.66±1.06 μm/min (Fig 6A, D, E, Supplementary movie 4). The behavior of CD4+ T cells during peak of EAE was distinctly different. The majority (94.2±0.4%) of all analyzed CD4+ T cells were stationary and involved in long-lasting interactions with MHC class II+ cells. These interactions often lasted throughout the scanning time of up to 90 min. Both the number of cells that were migrating and their instant velocities (0.86±0.28 μm/min) were significantly lower compared to day 10–11 pi (number of cells: p<0.02; velocities: p<0.0001; Fig 6B, D–E, Supplementary movie 4). The period immediately after the peak, when clinical signs of EAE started to regress (day 17–18 pi), had the highest frequency of highly motile CD4+ T cells (52.2±25.5%, p<0.05 compared to peak of EAE) and CD4+ T cells were again migrating and moving with high instant velocities (1.87±1.08 μm/min; p<0.0001 compared to peak of EAE; Fig 6C–E). While many CD4+ T cells moved with frequent steep turns, following a path touching multiple MHC class II+ cells consistent with a scanning behavior, other cells were rapidly migrating through the subarachnoid space without interacting with other cells (Supplementary movie 4).
Figure 6. T cell motility in the leptomeninges during the development of EAE.
Leptomeningeal explants were prepared from MOG35-55 immunized mice harvested at various time points after immunization and imaged using confocal time-lapse microscopy directly ex vivo. The trajectories of individual CD4+ cells were calculated during a 1-hour time period and overlaid in two-dimensional plots, with each cell’s track originating at zero. The figures show trajectories for all migratory cells in each explant with the total number of CD4+ cells indicated. Multiple highly motile cells were identified in mice harvested before onset of clinical EAE on day 10–11 pi (A) and during the period immediately after peak of signs on day 17–18 pi (C), while few cells displayed a migratory behavior during peak of EAE on day 14–15 (B). (D) Percent motile CD4+ cells and (E) Instant velocities (μm/min) of migratory CD4+ cells (stationary cells excluded from analysis) in leptomeningeal explants harvested at different time points after immunization.
CD4+ MOG-specific 2D2 cells proliferate in the subarachnoid space
The presence of CD4+ T cells interacting with local APCs in the subarachnoid space before onset of clinical signs of EAE indicates a specific physiological role of these cells. We investigated whether MOG-specific CD4+ T cells proliferate in the subarachnoid space by injecting BrdU ip (120 mg/kg bodyweight) on day 10 pi and harvesting the CNS two hours after injection. Roughly 15% (16.4±10.5%; mean±SD; n=6) of all Vα3.2+/Vβ11+/CD4+ isolated from the leptomeninges had incorporated BrdU during this 2-hour time period, while only sporadic cells had divided in the spinal cord parenchyma (1.8±1.3%; p<0.03 compared to meninges; Fig 7A–C). Since very few CD4+ T cells were present in the spinal cord at this time point, we repeated the experiment on day 18 pi. Slightly lower numbers of Vα3.2+/Vβ11+/CD4+ cells were incorporating BrdU in the leptomeninges on day 18 pi compared to day 10 pi, but there was still evidence of active proliferation in this compartment (9.2±6.7%; p=0.2; n=6). In contrast, the frequency of dividing Vα3.2+/Vβ11+/CD4+ cells in the spinal cord parenchyma was still low on day 18 pi (2.0±1.5%; p<0.05 compared to meninges). While it is technically difficult to discriminate between a dividing CD4+ T cell and two CD4+ T cells detaching after having interacted with each other, we observed at least one large CD4+ T cell going through cytokinesis during a one-hour scanning time (Fig 7D).
Figure 7. CD4+ cells in the subarachnoid space proliferate early during the course of EAE.
5×106 MOG TCR tg CD4+ cells isolated from naive 2D2 mice were transferred iv into C57Bl/6 mice and the recipients immunized with MOG35-55. BrdU (120 mg/kg bodyweight) was injected ip two hours before harvest at day 10 and day 18 pi and percentages of BrdU-containing MOG TCR tg Vα3.2+/Vβ11+/CD4+ cells were determined in draining lymph nodes (DLN), leptomeninges (MEN) and spinal cord (SpC) were determined using flow cytometry (A–B). The amount of antigen-non-specific proliferation was analyzed in MOG TCR tg CD4 cells in DLN from animals immunized without MOG35-55 (MOG−). It was not possible to assess proliferation of MOG TCR tg CD4+ cells in the CNS of mice immunized without MOG35-55 due to low numbers of cells present. (C) Two-color immunofluorescence staining of leptomeningeal whole-mount preparations obtained on day 10 pi verifying uptake of BrdU (red) in CD4 cells (green). (D) Leptomeningeal explant prepared from a mouse during the early recovery phase of EAE imaged using confocal time-lapse microscopy directly ex vivo. Images show merged z-stacks demonstrating an initially large CD4+ cell (green) going through cytokinesis during 60 min scanning. Scale bar=5 μm.
Discussion
In the current report, we studied the early phases of EAE, and used flow cytometry to show that CD4+ T cells accumulated and proliferated in the subarachnoid space before CD4+ T cells were detected in the spinal cord parenchyma. These CD4+ T cells appeared to scan the subarachnoid space by interacting serially with local MHC class II positive APCs. In some cases, long-lasting contacts between CD4+ T cells and MHC class II positive APCs were established. In the context of previous reports, we interpret these findings to support our hypothesis that immune surveillance and initial immune activation occur in the subarachnoid space.
Even though CNS historically has been characterized as an immunologically privileged site, it is well documented that intravenously injected T cell blasts enter the perivascular spaces of the spinal cord22. These cells localize in parenchymal perivascular spaces and are competent to interact with antigen presented by MHC class II+ perivascular macrophages8,23. The efficiency of T cell extravasation to the non-inflamed brain is, however, negligible compared to the frequency of cells entering the spleen or lung under similar conditions24. More recent studies with intravital microscopy confirmed that CD4+ T cells do not interact with cerebrovascular endothelium unless the cerebral vasculature is activated by LPS or TNF-α25 and that resting human brain vasculature does not express the trafficking determinants required for T cell adhesion and extravasation13. Therefore, it is unlikely that CNS immune surveillance relies on T cell-APC contacts in the perivascular spaces of the parenchyma. Instead, evidence favors physiological T cell trafficking to the subarachnoid space during immune surveillance, either directly through meningeal postcapillary venules or across the choroid plexus9,12. Fluorescently labeled splenocytes can, independent of antigen-specificity, be detected two hours after transfer in the meninges and choroid plexus26. In healthy humans, the vast majority of the cells in the CSF are CD4+/CD45RO+/CD27+/CD69+, recently activated central memory T cells, expressing high levels of CCR7 and L-selectin13, a phenotype consistent with immune surveillance rather than immediate effector functions27. In the present study, low numbers of CD4+ T cells were detected in the leptomeninges of naive mice. These cells were frequently located adjacent to meningeal blood vessels, but were also observed migrating between MHC class II+ APCs in an apparent scanning behavior with brief cell-cell interactions before detaching and migrating to another APC.
The anatomical and histological structure of the subarachnoid space renders this compartment highly suitable for CNS immune surveillance. There is a multitude of APCs in the CSF compartment, including ventricular epiplexus cells; meningeal, choroid plexus, and perivascular macrophages, all of which are competent for local restimulation of previously activated memory T cells28. Since the total CSF volume is turned over approximately three times a day29, this path of circulation results in exposure of subarachnoid space T cells to a large number of APCs, optimizing the chances for finding their cognate ligands. In addition, interstitial fluid from CNS white and gray matter drains into the CSF30, which is accordingly a partial functional equivalent of the lymph for the CNS31. Indeed, about half of the CSF is absorbed via the cribrifom plate into the lymphatics of the nasal mucosa, after circulating from its site of secretion in the choroid plexus, through the subarachnoid space. Because soluble proteins efficiently drain from CNS interstitial fluid into the CSF, it is not surprising that CNS derived proteins are present in the CSF from patients with different neurological disorders, including multiple sclerosis (MS), Alzheimer’s disease and Creutzfeldt-Jacob’s disease32,33. It is still unknown to which extent CNS antigens are presented by APCs in the subarachnoid space of healthy individuals, but it is plausible that CNS proteins liberated during normal cellular turnover are cleared from the interstitial fluid to the CSF. It is also possible that various challenges to the CNS, such as minor trauma or asymptomatic/latent infections may trigger release of brain antigens34. We attempted to address if meningeal APCs were able to stimulate naive CD4 cells in vitro without exogeneous antigen, but the low number of APCs obtained from the meninges made this analysis technically challenging and the levels of endogeneous antigen presentation were variable and close to the detection level in our experiments.
Consistent with the classical reports by Waksman1 and Lassman2, we observed that CD4+ T cells accumulated in the leptomeninges before onset of clinical signs of EAE, while infiltration of CD4+ T cells to the spinal cord parenchyma occurred two days later and coincided with the onset of clinical signs of disease. A striking observation was the intense activity of CD4+ T cells and MHC class II+ APCs in the subarachnoid space during the few days preceding CD4+ T cell infiltration into the spinal cord parenchyma. At this stage, CD4+ T cells were highly motile and engaged in multiple, predominantly brief interactions with APCs. This pattern of behavior changed dramatically during overt EAE when CD4+ T cells became involved in long-lasting, stable interactions with MHC class II+ APCs, often lasting throughout the scanning time of 60–90 minutes. We did not address if the observed CD4-APC interactions were antigen-specific, but the long contact time argues in favor for the formation of functional immunologic synapses35,36. The formation of mature synapses between naive T cells and APCs in vitro requires about 30 min and is preceded by TCR signaling37. When calculating in vivo contact times between T cells and APCs in lymph nodes, it was demonstrated that T cells form prolonged contacts with APCs even in the absence of antigen, but that 50% of these contacts lasted <10 min, while >50% of contacts in the presence of antigen lasted >60 min38.
Interestingly, during the period immediately following the peak of clinical EAE, CD4+ T cells reverted to behavior characterized by high instant velocities and multiple, brief APC interactions. The high motility of CD4 cells at this stage may represent an inability to find an activating signal or be the result of reactivation and a search for an exit from the subarachnoid space. Taken together, the behavior of CD4+ T cells in the subarachnoid space was remarkably similar to primary T cell responses in peripheral lymph nodes, where naive T cells interact with antigen presenting DCs in three sequential stages: (i) during the first 8 hours, T cells migrate rapidly and are involved in multiple short encounters with numerous DCs; (ii) during the subsequent 16 hours, stable conjugates of T cells and DCs lasting for more than an hour were formed; and (iii) one day after homing into the LN, T cells dissociated from DCs, migrated rapidly and proliferated vigorously38.
Taken in the context of our prior results (reviewed in9), the findings in the present study suggest that CD4+ T cells enter the CSF compartment and scan the subarachnoid space as part of normal immune surveillance. Upon meeting cognate ligands presented by local APCs, previously experienced T cells activated by antigen in the periphery39 become involved in long-lasting interactions resulting in the formation of immunological synapses and T cell reactivation, followed by local production of inflammatory mediators including TNF-α, IFN-γ, and IL-17. Subpial gray matter lesions adjoining the subarachnoid space contain relatively low numbers of T cells40 and the role(s) of meningeal lymphocytes in generating the cortical pathology of MS remain undefined. One possibility is that soluble factors produced in the subarachnoid space diffuse through the pial membrane resulting in microglia activation, as soluble proteins injected into the subarachnoid space are readily detected on the pial surface and within subpial cortical gray matter in adult rat brain41. Indeed, in tissue sections from patients with long-standing MS, one observes an interdependence between meningeal T cell infiltration and levels of cortical activation of microglia, suggesting that microglia activation is, at least in part, driven by the meningeal inflammatory response42. Similarly, it is not uncommon to detect myelin loss in cerebral grey matter adjacent to meningeal infiltrates43.
Despite this progress, it has remained enigmatic how antigen recognition in the subarachnoid space could be coupled to inflammation deep within the CNS parenchyma. It has been proposed44 that activation of subpial microglia results in neuronal injury in white matter tracts adjoining the inflamed meninges. Secondary to this first wave of inflammation and tissue injury, Wallerian degeneration leads to distal microglial activation, upregulation of adhesion molecules such as ICAM-1 on parenchymal vasculature and large-scale perivascular accumulation of leukocytes within CNS white matter distant from the initial site of antigen recognition in the meninges. Another, not mutually exclusive possibility is that productive T cell-APC interactions in the subarachnoid space bring about cytokine elaboration, which activates vascular elements to become sites of leukocyte infiltration.
Our current data support and extend this pathogenic scheme by quantifying the T cell infiltrate in the subarachnoid space and documenting lymphocyte re-activation by resident APCs. It may be noteworthy that effective treatments for MS such as IFN-β45 and natalizumab46, reduce CSF cell counts. In this regard, our current findings may carry implications for initiating MS relapses, as well as events that set the stage for neolymphoid meningeal aggregates, which have been implicated in the progressive phases of MS47–49.
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Multiple Sclerosis Society (RG3666, RG2988 to SJK) and the National Institutes of Health (AI058680 and AI043496 to SJK). PK is a recipient of an advanced postdoctoral fellowship from the National Multiple Sclerosis Society.
Footnotes
The authors have no conflicting financial interests to declare.
References
- 1.Waksman BH, Adams RD. A histologic study of the early lesion in experimental allergic encephalomyelitis in the guinea pig and rabbit. Am J Pathol. 1962;41:135–162. [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H, Wisniewski HM. Chronic relapsing EAE. Time course of neurological symptoms and pathology. Acta Neuropathol (Berl) 1978;43:35–42. doi: 10.1007/BF00684996. [DOI] [PubMed] [Google Scholar]
- 3.Platten M, Steinman L. Multiple sclerosis: trapped in deadly glue. Nat Med. 2005;11:252–253. doi: 10.1038/nm0305-252. [DOI] [PubMed] [Google Scholar]
- 4.Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami N, Lassmann S, Li Z, et al. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J Exp Med. 2004;199:185–197. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heppner FL, Greter M, Marino D, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 7.McMahon EJ, Bailey SL, Castenada CV, et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 8.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 9.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 10.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 12.Schulz M, Engelhardt B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 2005;2:8. doi: 10.1186/1743-8454-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivisakk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang TT, Sobel RA, Wei T, et al. Recovery from EAE is associated with decreased survival of encephalitogenic T cells in the CNS of B7-1/B7-2-deficient mice. Eur J Immunol. 2003;33:2022–2032. doi: 10.1002/eji.200323180. [DOI] [PubMed] [Google Scholar]
- 15.Chitnis T, Najafian N, Abdallah KA, et al. CD28-independent induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;107:575–583. doi: 10.1172/JCI11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettelli E, Pagany M, Weiner HL, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. The Journal of experimental medicine. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci U S A. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasaki A, Yamanaka N, Kubo M, et al. Three-dimensional two-layer collagen matrix gel culture model for evaluating complex biological functions of monocyte-derived dendritic cells. J Immunol Methods. 2004;287:79–90. doi: 10.1016/j.jim.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Gunzer M, Schafer A, Borgmann S, et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 20.Huang NN, Han SB, Hwang IY, Kehrl JH. B cells productively engage soluble antigen-pulsed dendritic cells: visualization of live-cell dynamics of B cell-dendritic cell interactions. J Immunol. 2005;175:7125–7134. doi: 10.4049/jimmunol.175.11.7125. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature immunology. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 22.Hickey WF. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1:97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 23.Cross AH, Cannella B, Brosnan CF, Raine CS. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990;63:162–170. [PubMed] [Google Scholar]
- 24.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 25.Piccio L, Rossi B, Scarpini E, et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- 26.Carrithers MD, Visintin I, Kang SJ, Janeway CA., Jr Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123 (Pt 6):1092–1101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- 27.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 28.McMenamin PG, Wealthall RJ, Deverall M, et al. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 2003;313:259–269. doi: 10.1007/s00441-003-0779-0. [DOI] [PubMed] [Google Scholar]
- 29.Fishman R. Cerebrospinal fluid in diseases of the nervous system. 2. Philadelphia: WB Saunders; 1992. p. 431. [Google Scholar]
- 30.Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13:507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 31.Weller RO, Engelhardt B, Phillips MJ. Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol. 1996;6:275–288. doi: 10.1111/j.1750-3639.1996.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Shoji M, Harigaya Y, et al. Amyloid beta protein levels in cerebrospinal fluid are elevated in early-onset Alzheimer’s disease. Ann Neurol. 1994;36:903–911. doi: 10.1002/ana.410360616. [DOI] [PubMed] [Google Scholar]
- 33.Hsich G, Kenney K, Gibbs CJ, et al. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Engl J Med. 1996;335:924–930. doi: 10.1056/NEJM199609263351303. [DOI] [PubMed] [Google Scholar]
- 34.Popovich PG, Stuckman S, Gienapp IE, Whitacre CC. Alterations in immune cell phenotype and function after experimental spinal cord injury. J Neurotrauma. 2001;18:957–966. doi: 10.1089/089771501750451866. [DOI] [PubMed] [Google Scholar]
- 35.Delon J, Stoll S, Germain RN. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol Rev. 2002;189:51–63. doi: 10.1034/j.1600-065x.2002.18906.x. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami N, Nagerl UV, Odoardi F, et al. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. The Journal of experimental medicine. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KH, Holdorf AD, Dustin ML, et al. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 38.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 39.McCoy L, Tsunoda I, Fujinami RS. Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity. 2006;39:9–19. doi: 10.1080/08916930500484799. [DOI] [PubMed] [Google Scholar]
- 40.Peterson JW, Bo L, Mork S, et al. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 41.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 42.Dal Bianco A, Bradl M, Frischer J, et al. Multiple sclerosis and Alzheimer’s disease. Ann Neurol. 2007 doi: 10.1002/ana.21240. [DOI] [PubMed] [Google Scholar]
- 43.Serafini B, Rosicarelli B, Franciotta D, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. The Journal of experimental medicine. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- 45.Rudick RA, Cookfair DL, Simonian NA, et al. Cerebrospinal fluid abnormalities in a phase III trial of Avonex (IFNbeta-1a) for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group. J Neuroimmunol. 1999;93:8–14. doi: 10.1016/s0165-5728(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 46.Stuve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 47.Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 48.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 49.Serafini B, Rosicarelli B, Magliozzi R, et al. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.