Abstract
Introduction
Ultrasound (US)-enhanced thrombolytic treatment protocols currently in clinical trials for stroke applications involve systemic administration of tissue plasminogen activator (tPA; Alteplase), which carries a risk of adverse bleeding events. The present study aimed to compare the thrombolytic efficacy of a tPA-loaded echogenic liposome (ELIP) formulation with insonification protocols causing rapid fragmentation or acoustically-driven diffusion.
Materials and Methods
Thrombi were induced in the abdominal aortas of male New Zealand white rabbits (2–3 kg) using thrombin and a sclerosing agent (sodium ricinoleate) after aortic denudation with a balloon catheter. Thrombolytic and cavitation nucleation agents (200 μg of tPA alone, tPA mixed with 50 μg of a microbubble contrast agent, or tPA-loaded ELIP) were bolus-injected proximal to the clot through a catheter introduced into the abdominal aorta from the carotid artery. Clots were exposed to transabdominal color Doppler US (6 MHz) for 30 minutes at a low mechanical index (MI = 0.2) to induce sustained bubble activity (acoustically-driven diffusion), or for 2 minutes at an MI of 0.4 to cause ELIP fragmentation. Degree of recanalization was determined by Doppler flow measurements distal to the clots.
Results
All treatments showed thrombolysis, but tPA-loaded ELIP was the most efficacious regimen. Both US treatment strategies enhanced thrombolytic activity over control conditions.
Conclusions
The thrombolytic efficacy of tPA-loaded ELIP is comparable to other clinically described effective treatment protocols, while offering the advantages of US monitoring and enhanced thrombolysis from a site-specific delivery agent.
Keywords: thrombolysis, ultrasound, plasminogen activators, thrombus
The abnormal formation of clots accounts for most of the morbidity and mortality associated with cardiovascular diseases [1, 2]. The introduction of recombinant tissue plasminogen activator (tPA, Alteplase) as a clinical thrombolytic agent about 20 years ago constituted a breakthrough in the treatment of myocardial infarction and ischemic stroke caused by clots in the coronary and cerebral arteries [3, 4]. Hemorrhagic side effects and the difficulty of treating clots in the cerebral arteries, possibly because of plasminogen depletion in the local environment, have limited the indications of using free tPA in acute ischemic stroke treatment [5–7]. Recently, ultrasound has been found to improve the effectiveness of tPA for this indication, but hemorrhagic side effects, which can be fatal, continue to limit optimization of the protocols [8–11].
We have developed intrinsically echogenic liposomes (ELIP) that can serve not only as an ultrasound contrast agent, but also as a vehicle for ultrasound-triggered controlled drug release [12, 13]. The thrombolytic tPA possesses two fibrin-binding sites that are exposed after loading into ELIP (located both on the surface of the lipid bilayer and on the interior of the liposomes), so that the formulation is “self-targeting” to thrombi [14]. Targeted liposomal drug delivery is a proposed method for extending the therapeutic window by increasing tissue-specific efficacy while decreasing systemic toxicity [13, 15]. We have succeeded in loading tPA both into and onto ELIP, creating an ultrasound-controlled thrombolytic drug-delivery nanotechnology [16].
Previous studies have verified the fibrin-targeting properties of tPA-loaded ELIP (TELIP) in vitro [15]. TELIP thrombolytic activity, which is comparable to that of free tPA, was enhanced by both continuous wave and clinical Doppler ultrasound [16–19]. A preliminary in vivo study using an acute rabbit aorta clot model demonstrated Doppler ultrasound-enhanced thrombolysis by TELIP within a 15-minute period [19]. These observations have led us to hypothesize that TELIP with ultrasound will confer thrombolytic efficacy similar (or superior) to that of other clinically used or proposed tPA treatment modalities for ischemic strokes. The purpose of the present study was to test this hypothesis in terms of in vivo thrombolytic efficacy by comparing TELIP with other thrombolytic protocols being used in preclinical or clinical investigations. These other ultrasound-assisted thrombolytic protocols include the use of free tPA alone [20], and free tPA co-administered with microbubbles, such as the clinical perflutren lipid microbubble preparation, Definity®, an ultrasound contrast agent used for cavity opacification [21–25].
Materials and Methods
Preparation of tPA-Loaded ELIP (TELIP)
ELIP were prepared by the sonication-lyophilization-rehydration method as described previously [26]. The liposomal composition used was DPPC:DOPC:DPPG:CH in a 46:24:24:6 molar ratio (DPPC = dipalmitoylphosphatidylcholine; DOPC = dioleoylphosphatidylcholine; DPPG = dipalmitoylphosphatidylglycerol; CH = cholesterol). The component lipids were dissolved in chloroform and the solvent was allowed to evaporate completely. The resulting lipid film was placed under vacuum for full removal of the solvent and then hydrated with a 1 mg/ml tPA (Activase®, Genentech Inc., South San Francisco, CA) solution in 0.1 M phosphate-buffered saline (PBS; 0.08 mg tPA/mg lipid).
The suspension was sonicated for 5 minutes, frozen at −80° C for 2 hours and thawed. In order to separate free from liposome-associated tPA, the suspension was centrifuged in an Eppendorf microfuge (Model 5415D) at 13,200 rpm. The pellet was resuspended in 0.32M D-mannitol. Aliquots (0.5 ml) were frozen at −80° C and lyophilized for 48 hours. When reconstituted with water, the lipid concentration was 10 mg/ml. Zetasizer (Malvern Instruments, Inc., Westborough, MA) dynamic light scattering particle size analysis of TELIP, both in PBS and in bovine serum albumin (BSA), revealed a discrete homogeneous number average peak around 600 nm. Prior studies indicated a 50% encapsulation efficiency of tPA (based on tPA enzyme activity) in the TELIP formulation [16]. Thus, each aliquot contained 200 μg tPA associated with ELIP, which was injected without further dilution. Measurement of immunoreactive tPA in detergent-solubilized TELIP, using a quantitative sandwich immunosorbent assay enzyme-linked (ELISA) [14], yielded a value of 156 ± 49 μg tPA per aliquot.
To determine TELIP ultrastructure and the effect of Tween-20 treatment on it, a TELIP aliquot (5 mg lipid) was freshly reconstituted with nanopure water and diluted in PBS to a concentration of 1.0 mg lipid/ml. Alternatively, an equal volume of 1% Tween-20/PBS was mixed with a 2.0 mg TELIP/PBS suspension. Samples were subjected to a negative staining procedure with 1% uranyl acetate on 300 mesh formvar carbon grids (EMS, Hatfield, PA). Stained samples were examined with a JEOL 1200 TEM at 60kv. Images were captured using a Gatan BioScan 792 CCD camera.
Evaluation of Thrombolytic Formulations with the Rabbit Aorta Thrombus Model
Male New Zealand white rabbits (2–3 kg) were anesthetized with a subcutaneous injection of ketamine HCl (40 mg/kg) and xylazine HCl (7.5 mg/kg) and surgically prepared according to a protocol approved by the University of Texas Health Science Center-Houston institutional animal review board, in compliance with the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. The rabbits were intubated, ventilated and maintained on a low level of isoflurane (2%) with oxygen during the procedure. Small cutdown incisions were made in the neck and the right thigh to place intra-arterial sheaths into the right carotid and femoral arteries. A Fogarty embolectomy catheter (2–4 French) was inserted through the femoral sheath into the abdominal aorta. Balloon inflation was performed until gentle resistance was felt. The catheter was moved distally 5–8 cm to denude the endothelium. With the balloon inflated, a 5% sodium ricinoleate solution, combined with 1,000 U of thrombin (MP Biomedicals, LLC, Solon, OH), was administered through the balloon catheter into the abdominal aorta immediately following denudation to produce a thrombus in the abdominal aorta. A catheter was also introduced through the carotid intra-arterial sheath and advanced under ultrasonic or fluoroscopic guidance to the abdominal aorta proximal to the aortic clot.
After 10 minutes (during which time, thrombus formation had occurred), the rabbit received treatment delivered intra-arterially, by injection into the carotid arterial sheath. Treatments (n = 5 per group) consisted of 0.02M PBS, pH 7.4 (control), 200 μg free tPA, 200 μg free tPA mixed with 50 μl Definity, or TELIP (200μg tPA/5 mg lipid), all in a 0.5-ml volume. Rabbits were also randomized to receive ultrasound (US) exposure, delivered transabdominally over the thrombus with a Philips HDI 5000 ultrasound system using an L12–5 linear array probe. The probe was coupled to shaved skin with Aquasonic 100 ultrasound transmission gel (Parker Laboratories, Inc., Fairfield, NJ).
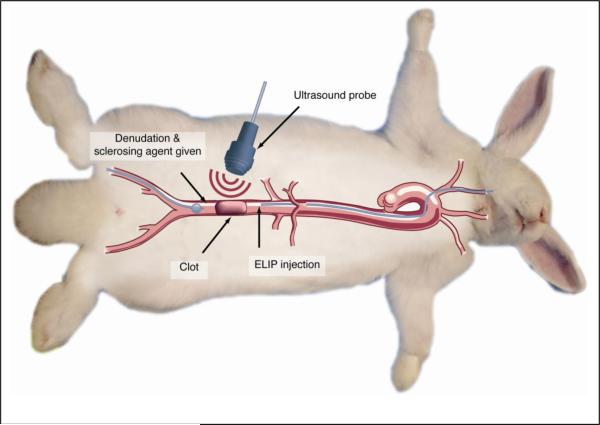
Two completely different insonification protocols were used. The first consisted of 6.0 MHz duplex (color) Doppler imaging pulses (pulse duration = 3.33 μs, pulse repetition frequency = 5 kHz) with a mechanical index (MI) of 0.4 (peak rarefactional pressure, Pr, = 1.51 MPa) for 2 minutes (high MI protocol). The second used similar 6.0 MHz duplex (color) Doppler at an MI of 0.2 (Pr = 0.75 MPa) intermittently (60–90 seconds at a time) for 30 minutes (low MI protocol). The former (MI = 0.4 continuously) ultrasound condition has been shown to produce rapid fragmentation of ELIP with release of encapsulated tPA, while the latter (MI = 0.2 intermittently) conditions have been shown to produce acoustically-driven diffusion from ELIP with concomitant loss of echogenicity [27]. The experimental surgical procedure is summarized pictorially in Figure 1.
Figure 1.
Diagrammatic representation of procedures comprising the rabbit aorta thrombus model. Sequentially, these procedures include 1) denudation of the abdominal aorta, using an embolectomy catheter introduced into the right femoral artery; 2) measurement of baseline abdominal aortic flow by spectral Doppler echocardiography; 3) induction of a thrombus by administration of 5% sodium ricinoleate and thrombin; 4) measurement of post-thrombotic flow distal to the thrombus; 5) introduction of thrombolytic preparation proximal to the thrombus, through a catheter introduced into the right carotid artery; 6) in rabbits receiving ultrasound treatment, transabdominal administration of color Doppler ultrasound for 2 minutes or intermittently for 30 minutes; and 7) spectral Doppler flow measurements every 2 minutes for 30 minutes.
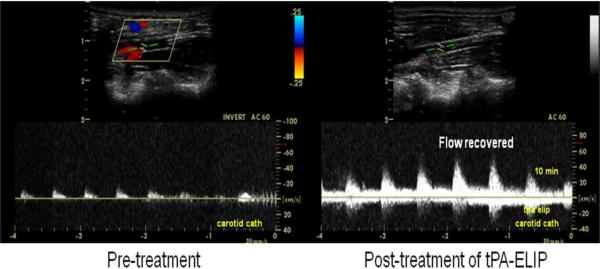
Blood flow through the abdominal aorta was monitored before and after thrombus formation and following treatment by pulsed spectral Doppler ultrasound imaging with the same ultrasound system. We utilized the pulsed spectral Doppler default clinical setting of the Philips HDI 5000 ultrasound system for all the in vivo blood flow velocity measurements, i.e., frequency 6 MHz, gate size 1.5 mm, pulse repetition frequency 5 kHz. The gate was placed in the center of the vessel and aligned at an angle ≤ 60° parallel to blood flow to measure the maximal velocity of blood flow. (Fig. 2). Degree of recanalization was determined by flow measurements distal to the clots relative to baseline measurements at the same location prior to clot formation. Acoustic enhancement of thrombi after TELIP binding to fibrin was assessed by digitally recording B-mode ultrasound images at 2-minute intervals following TELIP injection.
Figure 2.
Spectral Doppler sonographic flow measurements. a: after thrombus formation; b: recanalization following treatment.
In Vitro Simulation Experiment
To confirm that high-MI color Doppler ultrasound at an acoustic output with an MI of 0.4 causes fragmentation of TELIP, releasing free tPA into the medium, individual vials of TELIP were each reconstituted with 0.5 ml of nanopure (MilliQ, 18 MΩ, 96% O2 saturated) water. The suspensions were divided into two equal aliquots, each of which was brought to 50 ml with PBS and placed into a latex condom sealed with a dialysis clamp. Condoms were submerged in a water-filled chamber, the bottom of which was covered with a rho-C rubber acoustic absorber (Precision Acoustics, Inc., Dorchester, Dorset, UK) to render it anechoic, and subjected to ultrasound exposure as in the in vivo protocol.
The suspensions were removed from the condoms and 0.5 ml aliquots were centrifuged at 14,100 × g for 20 minutes at room temperature. Based on optical absorbance at 440 nm, which has been established as a measure of intact liposomal concentration within the submicron size range[28], centrifugal pellets contained 97.4 ± 2.1% (SD, n = 6) of total ELIP. The phospholipid content of centrifugal fractions was also measured by the colorimetric Stewart assay [29], which indicated that 96.2% of total liposomal phospholipid was recovered in the pellet. Aliquots of the liposomal suspension and the resuspended pellet in 0.5% Tween-20 (total tPA and total pellet tPA), as well as the supernate and the intact resuspended pellet were assayed for immunoreactive tPA with a sandwich ELISA as previously described [14]. Immunoreactive tPA in intact pellet suspensions was considered to be tightly associated with ELIP, while having epitopes exposed on the liposomal surface.
Statistics
For the in vivo study, four variables were assessed to compare the efficacy of the treatment groups:
-
1)percent recanalization at each measurement time point, defined as
where peak occluded flow velocity is measured after clot formation; -
2)maximum percent recanalization within the 30 minute treatment period
-
3)
rate of total recanalization (number of animals in each group achieving total recanalization [defined as ≥ 95% recanalization] within 30 minutes/total number of animals in group); and 4) time to recanalization for those animals that achieved total recanalization within the 30 minute period.
Parametric data were compared by analysis of variance (ANOVA), Student's t-test, paired t-test, or Fisher's Exact Test. Nonparametric data were analyzed by the ANOVA on Ranks or the Wilcoxon signed rank test. All analyses were performed using SigmaStat 3.5 (Systat Software, Inc., Chicago, IL). A p<0.05 was considered significant.
Results
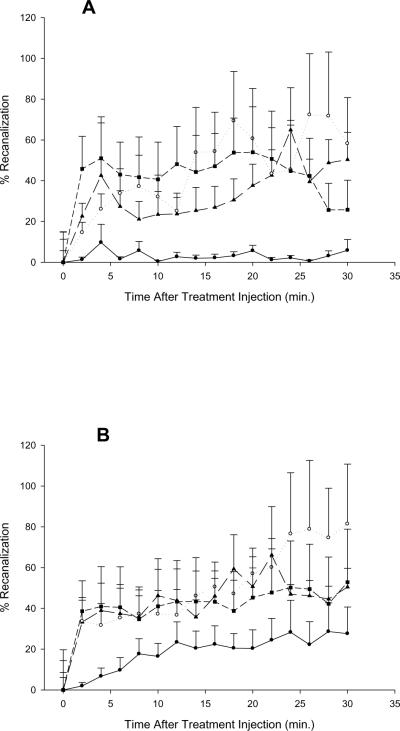
Doppler measurement of aortic blood flow distal to the clots demonstrated a 68 ± 19% reduction after clot formation, compared to baseline. For all tPA treatment groups, there was significant (p < 0.001) percent recanalization at each measurement time point, regardless of US treatment used. There was significant percent recanalization for TELIP vs. free tPA for the No US and 0.2 MI US treatment groups (p < 0.05), but was not significant for the 0.4 MI US treatment. There was no difference in percent recanalization rates between TELIP and tPA/Definity at low or high MI. The enhancement of recanalization by thrombolytic treatments was not significantly different (p > 0.05) for the two ultrasound insonification protocols. However, the thrombolytic effects of color Doppler ultrasound alone at an MI of 0.4, previously shown to cause rapid fragmentation of ELIP [27], relative to acoustically-driven diffusion alone at an MI of 0.2, was evident in the controls (p < 0.05 vs. 0.2 MI ultrasound treatment; Fig. 3).
Figure 3.
Spectral Doppler flow measurements (expressed as % recanalization; see Methods text) distal to clots at 2-minute intervals following treatment administration. Each point is the mean of 2–5 determinations (due to technical inconsistencies in obtaining measurements for all time points); bars represent standard errors (S.E.; also calculated for the 0-time point). Filled circles are controls (0.02M phosphate-buffered saline, pH 7.4; PBS); filled triangles are free tissue plasminogen activator (tPA); filled squares are tPA mixed with Definity; open circles are tPA-loaded echogenic liposomes (TELIP). A, 0.2 MI (mechanical index) ultrasound applied for 30 minutes after treatment initiation; B, 0.4 MI ultrasound applied for 2 minutes after treatment initiation.
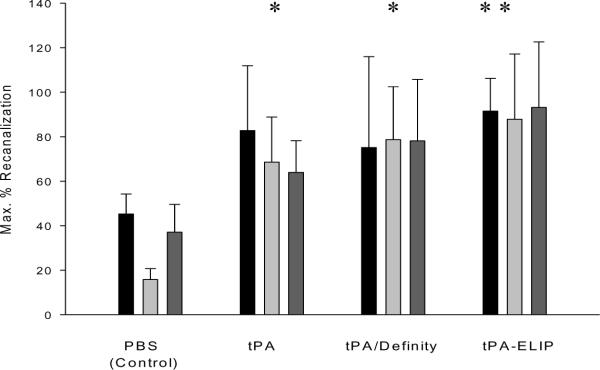
Maximum percent recanalization achieved within a 30-minute period was appreciably higher than controls for all tPA treatment groups (Fig. 4). In all ultrasound treatment groups, TELIP appeared to be more efficacious than other thrombolytic treatments, although the differences were not statistically significant. Two-way ANOVA indicated that there were significant differences between thrombolytic treatment groups with regards to maximum percent recanalization (p = 0.019), but not between ultrasound treatment groups (p > 0.05).
Figure 4.
Spectral Doppler flow measurements, expressed as maximum % recanalization achieved within a 30-minute period following treatment administration. Bar heights are the means of 4–5 animals; error bars = S.E. Asterisks denote significant difference (p < 0.05) from corresponding control groups. Black bars are no ultrasound; light gray bars are 0.2 MI ultrasound; dark gray bars are 0.4 MI ultrasound.
Total recanalization (defined as ≥ 95% recanalization) was not observed in any of the control animals, but was achieved at least once in all treatment groups (Table 1). When compared to all controls (0/15), significant recanalization rates were achieved in rabbits receiving tPA mixed with Definity without ultrasound (2/4, p = 0.035) and with 0.4 MI ultrasound (3/5, p = 0.009), as well as in rabbits receiving TELIP without ultrasound and with 0.2 MI ultrasound (both 3/5). For those animals that achieved total recanalization (where n ≥ 3/group), the mean time to recanalization was approximately 12 minutes for TELIP without ultrasound and tPA plus Definity with 0.4 MI ultrasound. The mean time to recanalization for TELIP with 0.2 MI ultrasound was longer (19.3 minutes, Table 1), but was not significantly different from the groups achieving recanalization in 12 minutes. When all the recanalized animals were considered according to ultrasound or thrombolytic treatment, the mean time to recanalization was not significantly different for the 3 US treatment groups, as determined by one-way ANOVA. The mean recanalization time for all thrombolytic treatment groups were also not significant (p > 0.05).
Table 1.
Total Recanalization Rates for Rabbit Treatment Groups.
| Ultrasound Treatment | Thrombolytic Treatment | Total Recan./ Total Number Animals | Average Time to Recan. (min.) |
|---|---|---|---|
| None | None (Control) | 0/5 | |
| tPA | 2/5 | 8.0 | |
| tPA/Definity | 2/4* | 6.0 | |
| TELIP | 3/5* | 12.0 | |
|
| |||
| Low (0.2) MI | None (Control) | 0/5 | |
| tPA | 1/5 | 4.0 | |
| tPA/Definity | 2/5 | 21.0 | |
| TELIP | 3/5* | 19.3 | |
|
| |||
| High (0.4) MI | None (Control) | 0/5 | |
| tPA | 1/5 | 10.0 | |
| tPA/Definity | 3/5* | 12.7 | |
| TELIP | 1/5 | 20.0 | |
Higher than total controls (0/15) by Fisher's Exact Test (p < 0.05)
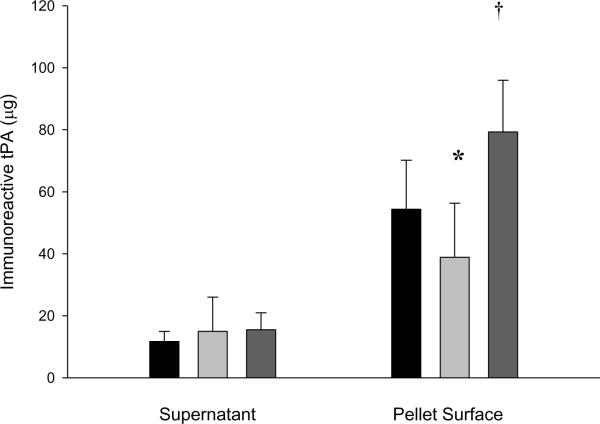
B-mode imaging of thrombi after TELIP injection, during which intraluminal opacification was observed, demonstrated discrete punctate highlighting of the thrombus (Fig. 5) that was apparent almost immediately after injection and which persisted for 8 minutes in vivo. In vitro color Doppler ultrasound treatments of dilute TELIP suspensions released insignificant amounts of free tPA into the medium, but caused significant changes in the amount of exposed ELIP-associated tPA. The low (0.2) MI US decreased the exposed tPA, while the high (0.4) MI US increased the amount of tPA exposed, yet tightly associated with the TELIP (Fig. 6).
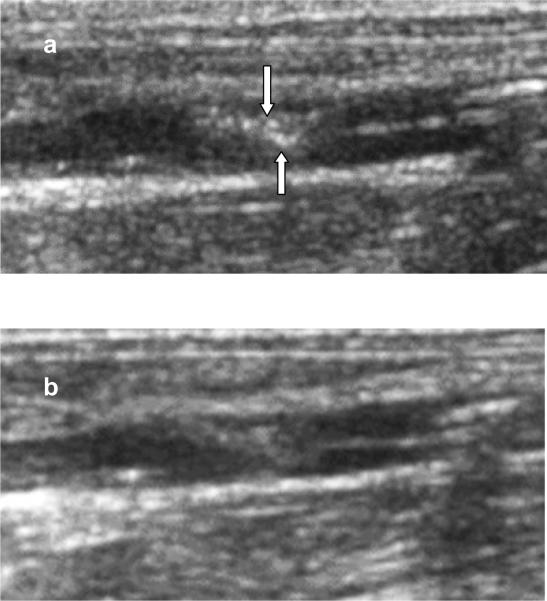
Figure 5.
B-mode ultrasound images of an induced thrombus in a rabbit abdominal aorta; a: 4 minutes after local administration of TELIP; b: 30 minutes after TELIP administration. Arrows indicate areas of highlighting.
Figure 6.
Effect of ultrasound on tPA release from TELIP. Immunoreactive tPA levels of TELIP fractions after treatment with color Doppler ultrasound in vitro. Values are mean ± S.D. (n = 3−8). Black bars are no ultrasound; light gray bars are 0.2 MI ultrasound; dark gray bars are 0.4 MI ultrasound. The star denotes a significantly decreased mean level (p < 0.05) and the dagger denotes an increased mean level (p < 0.005) of immunoreactive tPA exposed, but tightly bound to the liposomal surface, relative to the non-sonified control value.
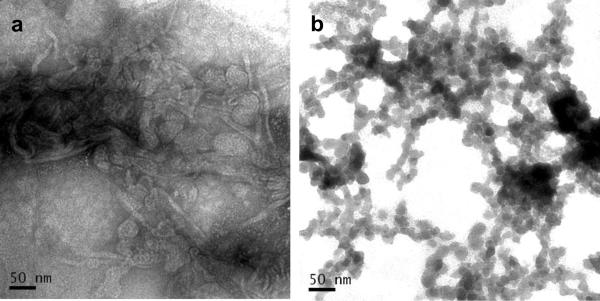
Negative staining transmission electron micrographs revealed that the TELIP production process produces aggregates of multilamellar echogenic liposomes, possibly held together by insoluble protein, which would be expected to be electron-dense (Fig. 7a). Detergent (0.5% Tween-20) treatment completely destroyed the liposomal structure, creating micellar networks surrounding electron-dense proteinaceous regions (7b).
Figure 7.
Negative staining transmission electron micrographs of TELIP samples. 250,000X magnification. a. Intact TELIP, 1 mg lipid/ml PBS; b. TELIP in 0.5% Tween-20/PBS, same concentration.
Discussion
Because of relatively low rates of complete recanalization and increased rates of symptomatic intracerebral hemorrhage, which have been reported to be as high as 15.7%, only about 2% of acute ischemic stroke patients in the United States receive intravenous tPA treatment [30–33]. The use of intracranial Doppler ultrasound treatment in patients with acute ischemic stroke as an adjunct to intravenous tPA has been shown to cause a significant increase in complete recanalization or dramatic clinical recovery (49% vs. 30% tPA only, p = 0.03) within two hours of tPA administration in acute ischemic stroke patients in the CLOTBUST study [20]. Persistence of recanalization enhancement, in the form of favorable neurological outcome three months after treatment, however, was not significant (42 vs. 29%, p = 0.20).
We have previously demonstrated color Doppler ultrasound enhancement of thrombolysis using TELIP, both in vitro and in vivo [17, 19, 34]. In a preliminary study using the same acute rabbit aorta thrombosis model employed presently, we showed achievement of complete recanalization of the aorta 15 minutes after TELIP administration in conjunction with 5.7 MHz pulsed Doppler ultrasound (vascular 8L linear probe, Vivid i Cardiovascular Ultrasound System, GE Healthcare, Waukesha, WI; MI = 0.43), significantly better than TELIP alone [19]. That study demonstrated that empty ELIP alone in the presence of ultrasound exhibited no thrombolytic effect relative to untreated controls. Therefore, an ELIP control group was not included in this study. Furthermore, in preliminary in vitro studies, free tPA plus empty ELIP did not exhibit greater ability to cause clot mass loss than free tPA alone and, therefore, this treatment group was also not included in these in vivo studies.
The purpose of the present study was to determine whether TELIP would exhibit a thrombolytic effect comparable to other ultrasound-assisted thrombolytic treatment protocols in a pre-clinical efficacy trial. The findings reported here indicate that 1) encapsulated tPA has similar efficacy to free tPA for thrombus dissolution in vivo; 2) while recanalization rates tend to be relatively variable in the absence of ultrasound treatment, there is a clear contribution of cavitation effects at MI = 0.4 to clot dissolution that is not provided by acoustically-driven diffusion at MI = 0.2; 3) since all three of the thrombolytic therapeutic protocols evaluated showed similar efficacy using both insonification strategies, the net ultrasound enhancement of thrombolytic therapy appears to be greater for acoustically-driven diffusion alone at the lower peak rarefactional pressures than when cavitation effects occur at the higher MI.
Although the ultrasound insonification groups did not show a difference in lytic effect versus the non-ultrasound group, the data with ultrasound insonification shows much less variability. In these data, the intermittent, low MI groups (acoustically-driven diffusion) showed no recanalization, whereas the continuous, high MI groups (producing rapid fragmentation of TELIP) showed recanalization in the controls compatible with previously demonstrated cavitation radiation forces of the ultrasound in the thrombus [9]. The tPA groups showed added recanalization over controls in both groups. These data do emphasize the added value of ultrasound in improving tPA efficacy for thrombus dissolution. It remains to be determined which type of ultrasound is of most use in tPA delivery and efficacy (acoustically-driven diffusion vs. rapid fragmentation).
With regard to factors affecting encapsulated tPA delivery, we have previously shown that only 12.5–15% of the ELIP-associated tPA was fully encapsulated, while 67.7% was loosely or tightly liposome-associated, with both fibrin-binding and catalytic sites exposed [16, 35]. In vitro simulation experiments demonstrated that 0.4 MI color Doppler ultrasound caused rearrangement of the tPA associated with the liposomal surface, resulting in greater exposure of the tPA molecule that may explain previously observed ultrasound potentiation of the TELIP thrombolytic efficacy. Negative staining TEM images obtained in this study are consistent with this interpretation. In previous studies utilizing 5.7–6.0 MHz transducers (under conditions producing rapid fragmentation) that showed ultrasound enhancement of TELIP fibrinolytic activity [36], it is possible that the ultrasound potentiated the catalytic activity of exposed ELIP-associated tPA, rather than caused release of the enzyme into the medium.
The likelihood that ELIP-associated tPA is fully functional raises the possibility that the apparent increased efficiency of TELIP thrombolytic activity is due to the multiple tPA molecules per liposome (or liposomal aggregate), resulting in a higher fibrin-binding avidity and catalytic turnover rate than can be achieved with free tPA, which may be lost with superficial fibrinolysis, while more substantial clot disintegration may be necessary to displace the liposomes. This hypothesis was supported by B-mode ultrasound monitoring of clot highlighting after TELIP injection. Since previous in vivo studies have shown that fibrin-targeted ELIP retain clot-localized echogenicity for at least 15 minutes [37], loss of highlighting after 8 minutes suggests that the liposomes are being displaced from the disintegrating clot surface.
Future studies should focus on optimization and translational development of the TELIP therapeutic protocol to provide increased efficacy and reduced hemorrhagic effects, especially when combined with ultrasound potentiation. Protocols that could be investigated may include a repeat TELIP dosage if complete recanalization is not observed within 10 minutes or longer periods of 0.4 or higher MI color Doppler US to increase clot permeability. These optimization strategies may afford a reproducibly higher incidence of arterial recanalization, which in turn is likely to increase the use of thrombolytic therapy for acute ischemic strokes, having a positive impact on ischemic stroke mortality and morbidity.
Recombinant tPA (Alteplase) is a good example of the disadvantages presented by systemic administration of a potentially toxic drug that could be ameliorated by a liposomal formulation of the same agent. Molecular targeting of the liposomal formulation provides the added benefit of being able to lower the administered drug dose appreciably. In this case, a lower incidence of hemorrhagic sequelae after TELIP treatment, relative to other thrombolytic protocols, which would be expected for a liposomal drug formulation retaining the fibrin-targeting characteristics of tPA, may provide a more favorable modality for the treatment of acute ischemic stroke.
Supplementary Material
Acknowledgements
We wish to thank the staff of the UTHSC-H Center for Laboratory Animal Medicine and Care for expert assistance in performing the experimental rabbit protocols. We also wish to thank Steven Kolodziej of the UTHSC-H pathology department for preparing negative staining TEM images of the TELIP.
Sources of Funding This work was supported, in part, by a grant from the Texas Ignition Fund and by NIH grant numbers 2R01 HL074002, 2R01 NS047603 and 3R01 HL059586.
Abbreviations
- US
ultrasound
- tPA
tissue plasminogen activator
- ELIP
echogenic liposomes
- MI
mechanical index
- TELIP
tPA-loaded ELIP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement There are no potential conflicts to report.
References
- 1.Ambrose JA, Weinrauch M. Thrombosis in ischemic heart disease. Arch Intern Med. 1996;156(13):1382–94. [PubMed] [Google Scholar]
- 2.van der Worp HB, van Gijn J. Clinical practice. Acute ischemic stroke. N Engl J Med. 2007;357(6):572–9. doi: 10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]
- 3.Dundar Y, Hill R, Dickson R, Walley T. Comparative efficacy of thrombolytics in acute myocardial infarction: a systematic review. QJM. 2003;96(2):103–13. doi: 10.1093/qjmed/hcg016. [DOI] [PubMed] [Google Scholar]
- 4.Collen D, Lijnen HR. Tissue-type plasminogen activator: a historical perspective and personal account. J Thromb Haemost. 2004;2(4):541–6. doi: 10.1111/j.1538-7933.2004.00645.x. [DOI] [PubMed] [Google Scholar]
- 5.Onundarson PT, Francis CW, Marder VJ. Depletion of plasminogen in vitro or during thrombolytic therapy limits fibrinolytic potential. J Lab Clin Med. 1992;120(1):120–8. [PubMed] [Google Scholar]
- 6.Sakharov DV, Rijken DC. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92(7):1883–90. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- 7.Smalling RW. Molecular biology of plasminogen activators: what are the clinical implications of drug design? Am J Cardiol. 1996;78(12A):2–7. doi: 10.1016/s0002-9149(96)00736-9. [DOI] [PubMed] [Google Scholar]
- 8.Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36(7):1441–6. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 9.Datta S, Coussios CC, McAdory LE, et al. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol. 2006;32(8):1257–67. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland CK, Vaidya SS, Datta S, Coussios CC, Shaw GJ. Ultrasound-enhanced tissue plasminogen activator thrombolysis in an in vitro porcine clot model. Thromb Res. 2008;121(5):663–73. doi: 10.1016/j.thromres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier JM, Holland CK, Pancioli AM, Lindsell CJ, Shaw GJ. Effect of low frequency ultrasound on combined rt-PA and eptifibatide thrombolysis in human clots. Thromb Res. 2009;123(3):528–36. doi: 10.1016/j.thromres.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Hamilton AJ, Tiukinhoy SD, et al. Liposomes as ultrasound imaging contrast agents and as ultrasound-sensitive drug delivery agents. Cell Mol Biol Lett. 2002;7(2):233–5. [PubMed] [Google Scholar]
- 13.Huang SL. Liposomes in ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60(10):1167–76. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Klegerman ME, Zou Y, McPherson DD. Fibrin targeting of echogenic liposomes with inactivated tissue plasminogen activator. J Liposome Res. 2008;18(2):95–112. doi: 10.1080/08982100802118482. [DOI] [PubMed] [Google Scholar]
- 15.Tiukinhoy-Laing SD, Buchanan K, Parikh D, et al. Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. J Drug Target. 2007;15(2):109–14. doi: 10.1080/10611860601140673. [DOI] [PubMed] [Google Scholar]
- 16.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119(6):777–84. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moody M, Laing ST, Huang S, et al. Doppler ultrasound enhances the thrombolytic activity of tissue-plasminogen activator-loaded echogenic liposomes. Circulation. 2007;116(16):II–646. [Google Scholar]
- 18.Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res. 2009;124(3):306–10. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laing ST, Moody M, Smulevitz B, et al. Ultrasound-enhanced thrombolytic effect of tissue plasminogen activator-loaded echogenic liposomes in an in vivo rabbit aorta thrombus model--brief report. Arterioscler Thromb Vasc Biol. 2011;31(6):1357–9. doi: 10.1161/ATVBAHA.111.225938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170–8. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 21.Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol. 2011;46(3):202–7. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culp WC, Porter TR, Lowery J, Xie F, Roberson PK, Marky L. Intracranial clot lysis with intravenous microbubbles and transcranial ultrasound in swine. Stroke. 2004;35(10):2407–11. doi: 10.1161/01.STR.0000140890.86779.79. [DOI] [PubMed] [Google Scholar]
- 23.Ribo M, Molina CA, Alvarez B, Rubiera M, Alvarez-Sabin J, Matas M. Intra-arterial administration of microbubbles and continuous 2-MHz ultrasound insonation to enhance intra-arterial thrombolysis. J Neuroimaging. 2010;20(3):224–7. doi: 10.1111/j.1552-6569.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- 24.Xie F, Tsutsui JM, Lof J, et al. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;31(7):979–85. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Culp WC, Erdem E, Roberson PK, Husain MM. Microbubble potentiated ultrasound as a method of stroke therapy in a pig model: preliminary findings. J Vasc Interv Radiol. 2003;14(11):1433–6. doi: 10.1097/01.rvi.0000096767.47047.fa. [DOI] [PubMed] [Google Scholar]
- 26.Huang SL, Hamilton AJ, Nagaraj A, et al. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci. 2001;90(12):1917–26. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- 27.Smith DA, Porter TM, Martinez J, et al. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol. 2007;33(5):797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Pozharski EV, McWilliams L, MacDonald RC. Relationship between turbidity of lipid vesicle suspensions and particle size. Anal Biochem. 2001;291(1):158–62. doi: 10.1006/abio.2001.5012. [DOI] [PubMed] [Google Scholar]
- 29.Zuidam NJ, de Vrueh R, Crommelin DJA. Characterization of liposomes. In: Torchilin VP, Weissig V, editors. Liposomes. 2nd ed. Oxford University Press; New York: 2003. pp. 34–35. [Google Scholar]
- 30.Graham GD. Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke. 2003;34(12):2847–50. doi: 10.1161/01.STR.0000101752.23813.C3. [DOI] [PubMed] [Google Scholar]
- 31.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004;61(3):346–50. doi: 10.1001/archneur.61.3.346. [DOI] [PubMed] [Google Scholar]
- 32.Liang BA, Lew R, Zivin JA. Review of tissue plasminogen activator, ischemic stroke, and potential legal issues. Arch Neurol. 2008;65(11):1429–33. doi: 10.1001/archneur.65.11.1429. [DOI] [PubMed] [Google Scholar]
- 33.Zaidat OO, Wolfe T, Hussain SI, et al. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke. 2008;39(8):2392–5. doi: 10.1161/STROKEAHA.107.510966. [DOI] [PubMed] [Google Scholar]
- 34.Laing ST, Moody MR, Smulevitz B, et al. Doppler ultrasound enhances the thrombolytic activity of tissue plasminogen activator-loaded echogenic liposomes in vivo. Circulation. 2008;118(18):S643. [Google Scholar]
- 35.Klegerman ME, Parikh D, Huang S, Tiukinhoy-Laing S, McPherson DD. Ability of human tissue plasminogen activator to act as a targeting agent in tPA-loaded echogenic liposomes. AAPS J. 2006;8(S2) Abstr T2111. [Google Scholar]
- 36.Smith DA, Vaidya SS, Kopechek JA, et al. Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol. 2010;36(1):145–57. doi: 10.1016/j.ultrasmedbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demos SM, Alkan-Onyuksel H, Kane BJ, et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol. 1999;33(3):867–75. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.