Abstract
Persistent difficulties in resolving clear lineages in diverging populations of prokaryotes or unicellular eukaryotes (protistan polyphyletic groups) are challenging the classical species concept. Although multiple integrated approaches would render holistic taxonomies, most phylogenetic studies are still based on single-gene or morphological traits. Such methodologies conceal natural lineages, which are considered ‘cryptic’. The concept of species is considered artificial and inadequate to define natural populations. Social organisms display differential behaviors toward kin than to non-related individuals. In ‘social’ microbes, kin discrimination has been used to help resolve crypticity. Aggregative behavior could be explored in a non-social protist to define phylogenetic varieties that are considered ‘cryptic’. Two Entamoeba invadens strains, VK-1:NS and IP-1 are considered close populations of the same ‘species’. This study demonstrates that VK-1:NS and IP-1 trophozoites aggregate only with alike members and discriminate individuals from different strains based on behavioral and chemical signals. Combined morphological, behavioral/chemical and ecological studies could improve Archamoebae phylogenies and define cryptic varieties. Evolutionary processes in which selection acted continuously and cumulatively on ancestors of Entamoeba populations gave rise to behavioral and chemical signals that allowed individuals to discriminate non-population members and gradually, to new lineages; alternative views that claim a ‘Designer’ or ‘Creator’ as responsible for protistan diversity are unfounded.
Keywords: Aggregation, behavior, chemical signals, cryptic species, design creationism
AMOEBAE and amoeboid protists have transitioned from studies based on single morphological traits (pseudopodia; Levine et al. 1980), to single gene (subunit rRNA -SSU rRNA; Adl et al. 2005; Burki and Pawlowski 2006; Pawlowski and Burki 2009; Stensvold et al. 2010, 2011), and to multigene (Bapteste et al. 2002; Pawlowski and Burki 2009) phylogenies at the supertaxon, supergroup, class, genus, or species level. Single gene analyses of metabolic traits (e.g. alcohol dehydrogenase adhe) contribute to conflictive phylogenetic depictions, due to horizontal acquisition (horizontal gene transfer -HGT) of genes from prokaryotes and unicellular eukaryotes (Andersson 2005; Andersson et al. 2006; Bapteste and Boucher 2009; Espinosa et al. 2001, 2009; Paz-y-Miño C. and Espinosa 2010).
In On the Origin of Species (1859), Charles Darwin questioned whether the species concept reflected natural groups, “we shall have to treat species in the same manner as those naturalists treat genera, who admit that genera are merely artificial combinations made for convenience… we shall at least be freed from the vain search for the undiscovered and undiscoverable essence of the term species”. Persistent difficulties in resolving clear lineages have questioned the species concept to describe diverging populations of prokaryotes (Bacteria and Archaea, Pace 2006; Doolittle and Zhaxybayeva 2009) or unicellular eukaryotes (protistan polyphyletic groups, Finlay 2004). Although it is widely accepted that value of multiple integrated approaches would render ‘holistic taxonomies’ (Finlay 2004; Pawlowski and Burki 2009), most phylogenetic studies are still based on single-gene or morphological traits. Such methodologies conceal natural lineages, which are considered ‘cryptic’ (Mallet 2010). Stebbins (1950) defined cryptic species as “… population systems, which were believed to belong to the same species until genetic evidence showed the existence of isolating mechanisms separating them”. In protists, unresolved phylogenies generate heated debates to unravel ‘cryptic’ morphospecies (populations of unicellular eukaryotes with similar physiological properties and mating incompatibilities; Caron et al. 2009; Finlay 2004). Phylogenetic analyses would benefit from the integration of physiological, ecological, and behavioral studies. Most phylogenies of the Entamoeba lineage are based on SSU-rRNA analyses (Stensvold et al. 2010, 2011).
Kin discrimination has been suggested as a behavioral mechanism in social microbes to resolve ‘cryptic’ varieties, given that individuals in a population signal to ‘like’ individuals (Kalla et al. 2011; Pérez-Ponce de León and Nadler 2010; Sáez and Lozano 2005). In single or multicellular organisms behavior toward kin differs from interactions with non-kin (Hamilton 1964; Queller et al. 2003; Wilson 2000). Altruistic behavior increases toward members of the same group, a phenomenon known as ‘the green beard effect’ (Dawkins 1976). Discrimination at the unicellular level has been detected in bacteria (Myxococcus xanthus; Vos and Velicer 2009) and protists (Dictyostelium, Polysphondylium violaceum; Kalla et al. 2011; Li and Purugganan 2011; Mehdiabadi et al. 2006; Ostrowski et al. 2008; Queller et al. 2003). The Entamoeba lineage is an ideal model to combine discrimination in a non-social ameba with morphological, multi-gene and ecological studies in attempting to resolve phylogenetic varieties considered ‘cryptic’.
Here we demonstrate that two strains of Entamoeba invadens (IP-1 and VK-1:NS) aggregate only with members of their own population suggesting they distinguish members of the same strain based on behavioral and chemical signals. Discrimination could help resolve conflictive branching and crypticity among Entamoeba varieties and demonstrate the evolutionary history of this lineage. We discuss how gradual genetic changes combined with selective pressures that gave strain members the ability to discriminate between alike and non-alike-individuals drove the diversification of the Entamoeba lineage and contrast this analysis with proposals invoking ‘common design’ (independent major taxa emergence with no common ancestry; Nelson 1996) and ‘special Entamoeba creation’ (Sherwin 2009), which are unfounded.
MATERIALS AND METHODS
Cells and reagents
All parasite cultures were obtained from Dan Eichinger (NYU School of Medicine) and Graham Clark (London School of Hygiene and Tropical Medicine). Entamoeba invadens strains IP-1 and VK-1:NS were grown at 25 °C under axenic conditions in flat-bottomed 48-well plates containing 1.4 ml of TYI-S-33 (Diamond, 1968) containing 10% ABS (Sigma-Aldrich, St. Louis, MO) modified from Espinosa et al. 2001, 2009. Additional media components were purchased from Fisher Scientific (Agawam, MA); Sigma-Aldrich (St. Louis, MO); Atlanta Biologicals (Atlanta, GA). Growth counts were averaged from three replicate wells and three separate experiments.
Growth conditions and trophozoite growth rates
E. invadens strains were inoculated at 4 × 102 cells / ml in 1.4 ml of media in 48 well plates and incubated at 25 °C. Every 24 h aliquots of cells were harvested by chilling and the cell density was determined using the Cellometer Vision HS RF-150 (Nexcelom BioScience LLC, Lawrence, MA). Trophozoites were subcultured every 72 h by transferring 4 × 102 cells / ml of culture into 1.4 ml of fresh medium and repeating the incubation and cell density determinations as described. Each time point cell density value was determined using triplicate cultures.
Morphological and aggregative measurements
Cell density, cell size, cell spatial distribution, number of aggregated trophozoites, number of aggregated clusters per surface area, and average distance between clusters, were examined using a Zeiss Axiovert 40 CFL fluorescent microscope (10X or 32X; Micro-Tech Optical, New England Inc., Bloomfield, CT). Digital images of each well (1.4 ml) were analyzed to determine interactions among strain and non-strain members. Images were processed with the Image Pro Software (Micro-Tech Optical, New England Inc., Bloomfield, CT).
Fluorescent labeling of E. invadens cells
CellTracker Red and Green CMFD (Invitrogen, Carlsbad, CA) were used to fluorescently label E. invadens VK-1:NS and IP-1 cells. Briefly, 1 × 105 trophozoites were harvested by chilling and centrifuged at 1,361 g for 20 minutes. Trophozoite pellets were resuspended gently in CellTracker Red (1:3 dilution in DMSO) and CellTracker Green CMFD (1:100 dilution in DMSO). Two incubation periods (30 minutes) followed by a 5-min PBS plus formaldehyde fixing period, and a final resuspension in 1.4 ml media were performed following the manufacturer’s protocol. Cells were then incubated at 25 °C for 36 h and analyzed at 12, 14, 18 and 36 h following the dyeing procedure. All experiments were performed in triplicate. To eliminate potential toxicity of both dyes, non-dyed trophozoites were analyzed at the same time points. Table 1 shows the six combined sets. E. invadens-IP-1 and VK-1:NS strains were dyed with both fluorescent tags, alone and together.
Table 1.
Experimental combinations of Entamoeba invadens IP-1 and VK-1:NS labeled with CellTracker Red and / or Green CMFD fluorescent tags (Invitrogen)
| Unlabeled | Labeled (Green or Red) |
|---|---|
| IP-1 / VK-1:NS | IP-1 (Green) / VK-1:NS (Red) VK-1:NS (Green) / IP-1 (Red) |
| IP-1 alone | IP-1 (Green) / IP-1 (Red) |
| VK-1:NS alone | VK-1:NS (Green) / VK-1:NS (Red) |
RESULTS
E. invadens IP-1 and VK-1:NS aggregate with members of their own strain and maintain separation from clusters of non-alike ameba. Quantitative data of individual trophozoites (average length, width, and surface area) show that both strains are morphologically distinguishable when combined traits are examined. As seen in Table 2, IP-1 is larger, wider, and elongated (length 28.77±3.52 µm; width 23.04 ± 1.81 µm) and VK-1:NS is smaller, narrower, and rounded (length 21.20 ± 2.04 µm; width 17.50 ± 1.45 µm). The average distance between IP-1 clusters is smaller (17.10 µm) than between VK-1:NS clusters (69.71 µm) when examined at the three time points (12, 18 and 36 h).
Table 2.
Phenotypic characterization of E. invadens IP-1 and VK-1:NS
| Characteristics | E. invadens IP-1 | E. invadens VK-1:NS |
|---|---|---|
| Average length (µm) | 28.77 ± 3.52 | 21.20 ± 2.04 |
| Average width (µm) | 23.04 ±1.81 | 17.50 ± 1.45 |
| Number of ameba per cluster (µm) | > 20 | > 20 |
| Average distance between clusters (µm) | 17.10 | 69.71 |
| Unlabeled trophozoites | 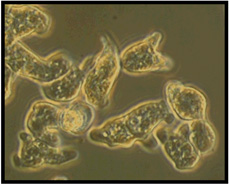 |
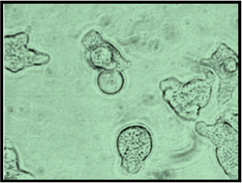 |
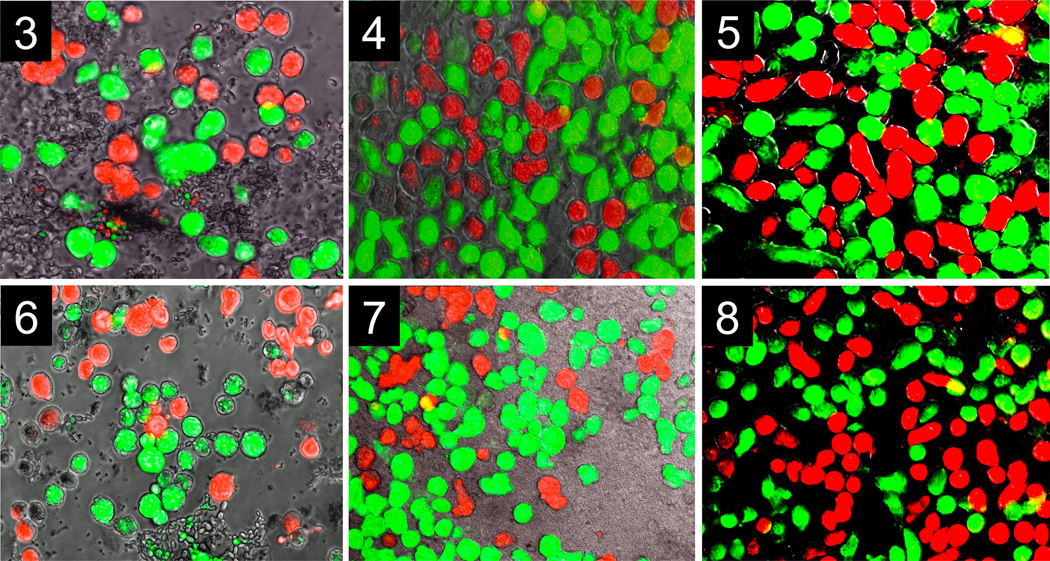
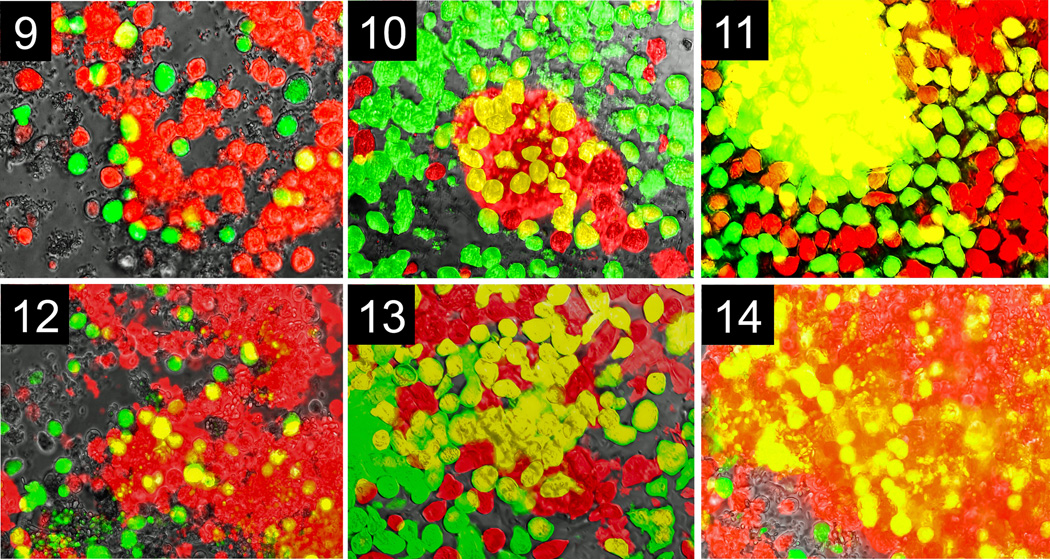
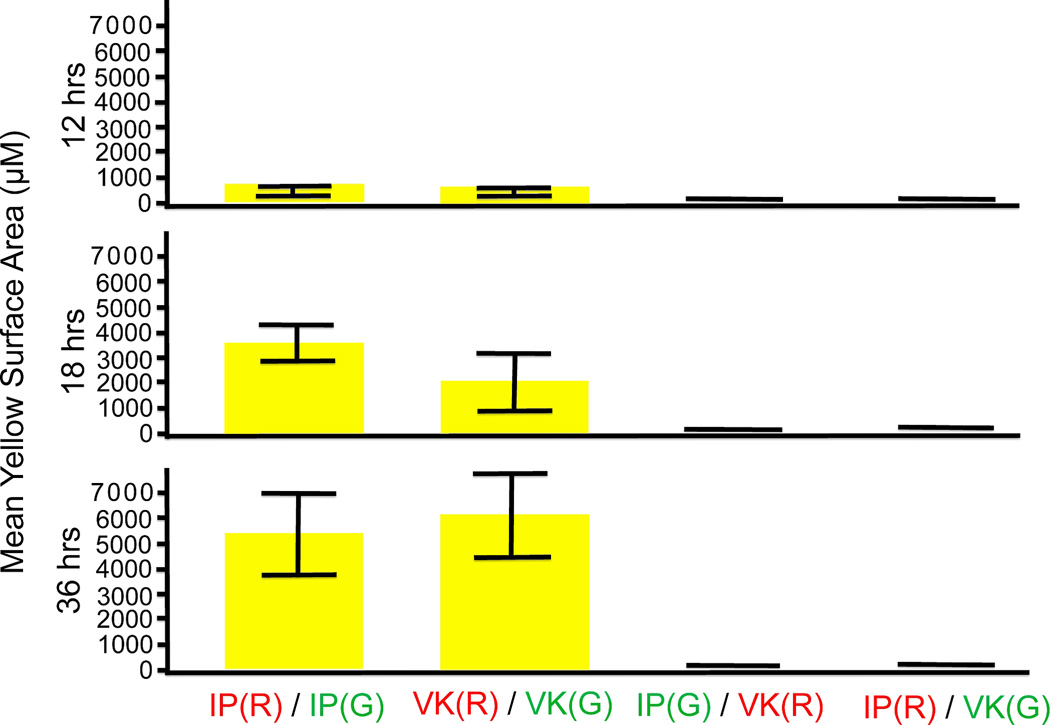
Pair-combinations of IP-1 / VK-1:NS labeled with green / red or, in the reciprocal, red / green dyes (Table 1) were placed together and grown in the same well. IP-1 trophozoites formed distinct and separate color clusters, which expanded in 12, 18, and 36 h without mixing with members of the other strain; a similar pattern of fluorescent single color clusters was observed for VK-1:NS trophozoites. For example, IP-1 aggregated in green clusters; VK-1:NS in red clusters; or IP-1 aggregated in red clusters; VK-1:NS in green clusters (Fig. 3--8). In contrast, when E. invadens VK-1:NS trophozoites were labeled with green and red dye and placed together in the same well, aggregation occurred between all trophozoites, showing strong strain association behavior. Large fluorescent yellow clusters (green + red) increased after 12, 18 and 36 h (Fig. 9--11). Similar behavior was shown by pair combinations of E. invadens IP-1 trophozoites that were labeled with green / red dyes (Fig. 12--14). Histograms showing fluorescent yellow surface area at 12, 18 and 36 h for aggregating strain members (IP-1 / IP-1 or VK-1:NS / VK-1:NS two-color overlapping clusters) are depicted in Fig. 15. IP-1 / VK-1:NS growing together showed little or no mixed aggregation (no yellow overlapping clusters (Fig. 15). There was no detectable toxicity in the trophozoites with either dye for the length of the experiments (36 h, control data not shown).
Fig. 3--8.
Discrimination shown by two strains of Entamoeba invadens, IP-1 and VK-1:NS; Fig. 3--5. Fluorescent micrographs of IP-1, labeled green; VK-1:NS , labeled red, were taken with the same field of view at three different times. 1. Initial aggregates show distinct clusters formed by two strains at 12 h. 2. Intermediate aggregates show distinct clusters of two strains at 18 h. 3. Large aggregates show distinct clusters formed by two strains at 36 h. Fig. 6--8. Fluorescent micrographs of inversely labeled strains, IP-1, labeled red; VK-1:NS , labeled green, were taken with the same field of view at three different times. 4. Initial aggregates show distinct clusters at 12 h. 5. Intermediate aggregates show distinct clusters at 18 h. 6. Large aggregates show distinct clusters formed by two strains at 36 h. The surface area of individual ameba, aggregates, and overlapping areas were determined by Image-pro software (Micro-Tech Optical New England Inc.). Scale bar, 1 mm.
Fig. 9--14.
Aggregative behavior shown by strains of pure Entamoeba invadens cultures with either IP-1 or VK-1:NS cultures. Fluorescent micrographs were taken with the same field of view at three different times. 9--11. E. invadens IP-1 (half of the cells are fluorescently labeled green, and the other half fluorescently labeled red) trophozoites mix equally creating an overlapping fluorescent yellow area that increases with time. 7. Yellow surface area at 12 h. 8. Yellow surface area at 18 h. 9. Yellow surface area at 36 h. 12--14. E. invadens VK-1:NS (half of the cells are fluorescently labeled green, and the other half fluorescently labeled red) trophozoites mix equally creating an overlapping fluorescent yellow area that increases with time. 10. Yellow surface area at 12 h. 11. Yellow surface area at 18 h. 12. Yellow surface area at 36 h. The surface area of individual ameba, aggregates, and overlapping areas were determined by Image-pro software (Micro-Tech Optical New England Inc.). Scale bar, 1 mm.
Fig. 15.
Yellow surface area (µm) histograms that quantitate the aggregative behavior of each combination of Entamoeba invadens IP-1 and/or VK-1:NS cultures (pure and mixed). The increasing yellow surface area is shown at 12, 18, 36 h with error bars. The surface area of individual ameba, aggregates, and overlapping areas were determined by Image-pro software (Micro-Tech Optical New England Inc.). Results were averaged from three replicate wells and three separate experiments (error bars shown).
DISCUSSION
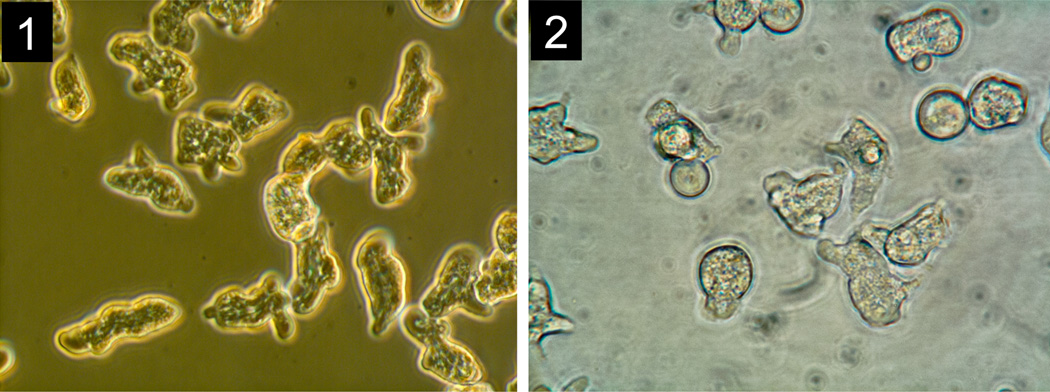
Entamoeba invadens IP-1 and VK-1:NS have been studied as molecular models of encystation (Byers et al. 2005; Mitra et al. 2010), or as part of single-gene phylogenetic studies of the Entamoeba lineage (Stensvold et al. 2010, 2011). Little is known about their morphological, ecological and behavioral properties or their evolutionary history inside their reptilian hosts’ gut. Based on combined measurements of individual trophozoites (average length, width, Table 2) we established clear differences: IP-1 is larger, wider, and elongated (length 28.77±3.52 µm; width 23.04 ± 1.81 µm, Fig. 1) and VK-1:NS is smaller, narrower, and rounded (length 21.20 ± 2.04 µm; width 17.50 ± 1.45 µm, Fig. 2). The average distance between aggregated clusters of IP-1 (17.10 µm) is greater than between aggregated clusters of VK-1:NS (69.71 µm) (Table 2).
Fig. 1, 2.
Phenotypic characterization of Entamoeba invadens, see figures within Table 2.
Our findings provide evidence that non-social protists have evolved discrimination skills previously attributed only to social organisms. Entamoeba invadens IP-1 and VK-1:NS are able to discriminate and interact preferentially with strain members. Distinct green or red clusters of combined sets (e.g. IP-1 red / VK-1:NS green or IP-1 green / VK-1:NS red; Table 1) suggest discrimination and non-aggregative behaviors with non-group members (Fig. 3--8). Fluorescent yellow clusters formed by E. invadens trophozoites increased in size with time if placed with members of the same strain (IP-1 green / IP-1 red; VK-1:NS green / IP-1 red; Fig. 9--14).
The number of studies on cooperative behaviors of social microbes have increased in the last decade: discrimination-dependent resource production (e.g. siderophores; Griffin et al. 2004), quorum sensing (Keller and Surette 2006; Parsek and Greenberg 2005), biofilms (Nadell 2009), aggregative motility (Chaine et al. 2009; Kraemer and Velicer 2011; Vos and Velicer 2009) and formation of fruiting bodies (Kalla et al. 2011; Mehdiabadi et al. 2006; Strassmann and Queller 2011). Most research in chemical signaling in non-social protists has focused in feeding, defense, invasiveness, or reproduction behaviors (e.g marine eukaryotes; dinoflagellates; algae, parasitic amoeba; Brodsky 2009; Paul et al. 2007; Strom et al. 2007, Zaki et al. 2006). Upon sensing the human pro-inflammatory cytokine tumor necrosis factor (TNF), E. histolytica trophozoites migrate directionally and initiate infection (Blazquez et al. 2008; Tavares et al. 2000). This is the first study to show strain association as a trait in non-social ameba, suggesting that discrimination might have evolved as an adaptation not limited to sociality.
In protists, ‘cryptic’ varieties are defined as morphospecies composed of strains with different physiological abilities or mating incompatibilities (Caron et al. 2009). Morphological phylogenies ignore the importance of physiological, ecological, and behavioral traits (Finlay 2004; Pawlowski and Burki 2009). The anaerobic Archamoebae (Entamoebae and pelobionts), were historically placed at the base of the eukaryotic tree, as ‘primitive eukaryotes’ that lacked mitochondria (Embley and Martin 2006). Recent phylogenomic analyses of 100 genes support the grouping of three highly divergent amoebae Dictyostelium, Entamoeba, and Mastigamoeba within the class Conosea (Bapteste et al. 2002). Single gene analyses of metabolic traits (e.g. alcohol dehydrogenase adhe) contribute to conflictive phylogenetic depictions, due to horizontal acquisition (horizontal gene transfer -HGT) of genes from prokaryotes and unicellular eukaryotes (Andersson et al. 2006; Bapteste and Boucher 2009; Espinosa et al. 2001, 2009; Paz-y-Miño C. and Espinosa 2010). If single-celled protists display aggregative behavior only with alike-members, we can use measurable traits to reveal traditionally ‘cryptic’ varieties in the Entamoeba lineage, improve the Archamoebae phylogenies and, possibly, other non-social unicellular eukaryotes.
This study demonstrates that trophozoites aggregate only with members of their strain suggesting they distinguish their closest relatives based on behavioral and chemical signals. Adaptations to different ecological environments (37°C vs. 23°C hosts; intestinal pH, oxygen sensitivity) and horizontal gene exchange could have influenced diversification.
CONCLUSIONS
Here we demonstrate that two strains of Entamoeba invadens (IP-1 and VK-1:NS) aggregate only with members of their own population. Chemical discrimination could help resolve conflictive branching and crypticity among Entamoeba varieties and demonstrate the evolutionary history of this lineage. But the generation of new lineages via classical evolutionary trajectories has been challenged by proponents of ‘common design’ or ‘separate ancestry’ of complex molecular structures (Luskin and Gage 2008) and major taxonomic lineages (Nelson 1996); and by advocates of a ‘creation model’ for Entamoeba parasitic origin (Sherwin 2009). Design creationists claim that ‘common ancestry is merely an assumption that governs interpretation of the data, not an undeniable conclusion’ (Luskin and Gage 2008). The creation model proposes that created Entamoeba “progressed from originally free-living single-celled eukaryotes (neutral or beneficial) toward a pathogenic condition after ‘The Fall’” (Sherwin 2009). Both alternatives hypothesize supernatural causation to life’s essential evolutionary processes. Our case study suggests behavioral traits can be used to address the origin and evolution of the Entamoeba lineage; strengthening the Darwinian perspective. It is possible to envision an evolutionary process in which selection acted continuously and cumulatively on intermediates and ancestors of Entamoeba populations; which evolved chemical and behavioral cues to discriminate non-strain members and gradually diversified in new lineages; sudden emergence of newly ‘designed’ or ‘created’ extant Entamoeba is improbable.
ACKNOWLEDGMENTS
We thank the VI European Congress of Protistology and the International Society of Protistologists for sponsoring the symposium, Using the Diversity of Protists to Educate the Students and the Public About Evolution, and Free University of Berlin for hosting it. A. Espinosa is supported by NIH-NCRR grant # 2 P20RR16457-11 and G. Paz-y-Miño C. by the Center for Teaching Excellence at U. Mass., Dartmouth. Kate Higgins provided technical assistance.
Footnotes
Paper presented at the symposium, Using the Diversity of Protists to Educate the Students and the Public About Evolution, VI European Congress of Protistology---ECOP Meeting, 25--29 July 2011, Free University of Berlin, Germany.
LITERATURE CITED
- Adl SM, Simpson AG, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup Ø, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005;52:399–451. doi: 10.1111/j.1550-7408.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- Andersson JO. Lateral gene transfer in eukaryotes. Cell Mol. Life Sci. 2005;62:1182–1197. doi: 10.1007/s00018-005-4539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JO, Hirt RP, Foster PG, Roger AJ. Evolution of four gene families with patchy phylogenetic distributions: influx of genes into protest genomes. BMC Evol. Biol. 2006;6:27. doi: 10.1186/1471-2148-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapteste E, Boucher Y. Epistemological impacts of horizontal gene transfer on classification in microbiology. In: Gogarten MB, Gogarten JP, Olendzenski L, editors. Horizontal Gene Transfer: Genomes in Flux. New York: Humana Press; 2009. pp. 55–72. [DOI] [PubMed] [Google Scholar]
- Bapteste E, Brinkmann H, Lee JA, Moore DB, Sense CW, Gordon P, Duruflé L, Gaasterland T, Lopez P, Müller M, Philippe H. The analysis of 100 genes supports the grouping of three highly divergent amoebae: Dictyostelium, Entamoeba, and Mastigamoeba. PNAS. 2002;99:1414–1419. doi: 10.1073/pnas.032662799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez S, Guigon G, Weber C, Syan S, Sismeiro O, Coppée JY, Labruyère E, Guillén N. Chemotaxis of Entamoeba histolytica towards the pro-inflammatory cytokine TNF is based on PI3K signalling, cytoskeleton reorganization and the Galactose/N-acetylgalactosamine lectin activity. Cell Microbiol. 2008;10:1676–1686. doi: 10.1111/j.1462-5822.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- Brodsky VY. Direct cell–cell communications and social behavior of cells in mammals, protists, and bacteria. Possible causes of multicellularity. Russian J. Dev. Biol. 2009;40:69–82. [PubMed] [Google Scholar]
- Burki F, Pawlowski J. Monophyly of Rhizaria and multigene phylogeny of unicellular bikonts. Mol. Biol. Evol. 2006;23:1922–1930. doi: 10.1093/molbev/msl055. [DOI] [PubMed] [Google Scholar]
- Byers J, Faigle W, Eichinger D. Colonic short-chain fatty acids inhibit encystation of Entamoeba invadens. Cell. Microbiol. 2005;7:269–279. doi: 10.1111/j.1462-5822.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- Caron DA, Worden AZ, Countway PD, Demir E, Heidelberg KB. Protists are microbes too: a perspective. ISME J. 2009;3:4–12. doi: 10.1038/ismej.2008.101. [DOI] [PubMed] [Google Scholar]
- Chaine A, Schtickzelle N, Pollard T, Huet M, Clobert J. Kin-based recognition and social aggregation in a ciliate. Evolution. 2010;64:1290–1300. doi: 10.1111/j.1558-5646.2009.00902.x. [DOI] [PubMed] [Google Scholar]
- Dawkins R. The Selfish Gene. Oxford: Oxford Univ. Press; 1976. p. 89. [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. 1st ed. London: John Murray; 1859. p. 485. [PMC free article] [PubMed] [Google Scholar]
- Diamond LS. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J. Parasitol. 1968;54:1047–1056. [PubMed] [Google Scholar]
- Doolittle WF, Zhaxybayeva O. On the origin of prokaryotic species. Genome Res. 2009;19:744–756. doi: 10.1101/gr.086645.108. [DOI] [PubMed] [Google Scholar]
- Embley TM, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440:623–630. doi: 10.1038/nature04546. [DOI] [PubMed] [Google Scholar]
- Espinosa A, Perdrizet G, Paz-y-Miño CG, Lanfrachi R, Phay M. Effects of iron depletion on Entamoeba histolytica alcohol dehydrogenase 2 (EhADH2) and trophozoite growth: implications for antiamoebic therapy. J. Antimicrob. Chemother. 2009;63:675–678. doi: 10.1093/jac/dkp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa A, Yan L, Zhang Z, Foster L, Clark D, Li E, Stanley SL. The bifunctional Entamoeba histolytica alcohol dehydrogenase 2 (EhADH2) protein is necessary for amebic growth and survival and requires an intact C-terminal domain for both alcohol dehydrogenase and acetaldehyde dehydrogenase activity. J. Biol. Chem. 2001;276:20136–20143. doi: 10.1074/jbc.M101349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BJ. Protist taxonomy: an ecological perspective. Philos Trans. R. Soc. Lond. B. Biol. Sci. 2004;359:599–610. doi: 10.1098/rstb.2003.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. J. Theoret. Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Kalla SE, Queller DC, Lasagni A, Strassmann JE. Kin discrimination and possible cryptic species in the social amoeba Polysphondylium violaceum. BMC Evol. Biol. 2011;27:11–31. doi: 10.1186/1471-2148-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L, Surette MG. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- Kraemer SA, Velicer GJ. Endemic social diversity within natural kin groups of a cooperative bacterium. Proc. Natl. Acad. Sci. USA. 2011;108:10823–10830. doi: 10.1073/pnas.1100307108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ND, Corliss JO, Cox FEG, Deroux G, Grain J, Honigberg BM, Leedale GF, Loeblich AR, III, Lom J, Lynn D, Merinfeld EG, Page FC, Poljansky G, Sprague V, Vavra J, Wallace FG. A newly revised classification of the Protozoa. J. Protozool. 1980;27:37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Li SI, Purugganan MD. The cooperative amoeba: Dictyostelium as a model for social evolution. Trends Genet. 2011;27:48–54. doi: 10.1016/j.tig.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Luskin C, Gage LP. A reply to Francis Collins’s Darwinian arguments for common ancestry of apes and humans. In: House HW, editor. Intelligent Design 101. Kregel Publications, Grand Rapids; 2008. pp. 215–235. [Google Scholar]
- Mallet J. Why was Darwin’s view of species rejected by twentieth century biologists? Biol. Philos. 2010;25:497–527. [Google Scholar]
- Mehdiabadi N, Jack C, Farnham T, Platt T, Kalla S, Shaulsky G, Queller D, Strassmann J. Social evolution: Kin preference in a social microbe. Nature. 2006;442:881–882. doi: 10.1038/442881a. [DOI] [PubMed] [Google Scholar]
- Mitra BN, Pradelb G, Freverta U, Eichinger D. Compounds of the upper gastrointestinal tract induce rapid and efficient excystation of Entamoeba invadens. Int. J. Parasitol. 2010;40:751–760. doi: 10.1016/j.ijpara.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadell CD. The sociobiology of biofilms. FEMS Microbiol. Rev. 2009;33:206–224. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- Nelson PA. The role of theology in current evolutionary reasoning. Biol. Philos. 1996;11:493–517. [Google Scholar]
- Ostrowski EA, Katoh M, Shaulsky G, Queller DC, Strassmann JE. Kin discrimination increases with genetic distance in a social amoeba. PLoS Biol. 2008;6:e287. doi: 10.1371/journal.pbio.0060287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace NR. Time for a change. Nature. 2006;441 doi: 10.1038/441289a. [DOI] [PubMed] [Google Scholar]
- Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Paul VJ, Arthur KE, Ritson-Williams R, Ross C, Sharp K. Chemical defenses: from compounds to communities. Biol. Bull. 2007;213:226–251. doi: 10.2307/25066642. [DOI] [PubMed] [Google Scholar]
- Pawlowski J, Burki F. Untangling the phylogeny of amoeboid protists. J. Eukaryot. Microbiol. 2009;56:16–25. doi: 10.1111/j.1550-7408.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- Paz-y-Miño CG, Espinosa A. Integrating horizontal gene transfer and common descent to depict evolution and contrast it with ‘common design’. J. Eukaryot. Microbiol. 2010;57:11–18. doi: 10.1111/j.1550-7408.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Ponce de León G, Nadler SA. What we don't recognize can hurt us: a plea for awareness about cryptic species. J. Parasitol. 2010;96:453–464. doi: 10.1645/GE-2260.1. [DOI] [PubMed] [Google Scholar]
- Queller DC, Ponte E, Bozzaro S, Strassmann JE. Single-gene greenbeard effects in the social amoeba Dictyostelium discoideum. Science. 2003;299:105–106. doi: 10.1126/science.1077742. [DOI] [PubMed] [Google Scholar]
- Sáez AG, Lozano E. Body doubles. Nature. 2005;433:111. doi: 10.1038/433111a. [DOI] [PubMed] [Google Scholar]
- Sherwin F. A possible function of Entamoeba histolytica in the creation model. Answers Res. J. 2009;2:117–121. [Google Scholar]
- Stebbins GL. Variation and evolution in plants. New York: Columbia University Press; 1950. p. 193. [Google Scholar]
- Stensvold CR, Lebbad M, Clark CG. Genetic characterisation of uninucleated cyst-producing Entamoeba spp. from ruminants. Int. J. Parasitol. 2010;40:775–778. doi: 10.1016/j.ijpara.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, Lebbad M, Victory EL, Verweij JJ, Tannich E, Alfellani M, Legarraga P, Clark CG. Increased sampling reveals novel lineages of Entamoeba: consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist. 2011;162:525–541. doi: 10.1016/j.protis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Strassmann JE, Queller DC. How social evolution theory impacts our understanding of development in the social amoeba Dictyostelium. Develop. Growth Differ. 2011;53:597–607. doi: 10.1111/j.1440-169X.2011.01272.x. [DOI] [PubMed] [Google Scholar]
- Strom SL, Wolfe GV, Bright KJ. Responses of marine planktonic protists to amino acids: feeding inhibition and swimming behavior in the ciliate Favella sp. Aquatic Microb. Ecol. 2007;47:107–121. [Google Scholar]
- Tavares P, Guillén N, Sansonetti P. Cell polarization and adhesion in a motile pathogenic protozoan: role and fate of the Entamoeba histolytica Gal/GalNAc lectin. Microbes Infect. 2000;2:643–649. doi: 10.1016/s1286-4579(00)00361-0. [DOI] [PubMed] [Google Scholar]
- Velicer GJ, Vos M. Sociobiology of the myxobacteria. Annu. Rev. Microbiol. 2009;63:599–623. doi: 10.1146/annurev.micro.091208.073158. [DOI] [PubMed] [Google Scholar]
- Vos M, Velicer GJ. Social conflict in centimeter-and global-scale populations of the bacterium Myxococcus xanthus. Curr. Biol. 2009;19:1763–1767. doi: 10.1016/j.cub.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EO. Sociobiology: The New Synthesis. Harvard University Press; 2000. pp. 117–118. [Google Scholar]
- Zaki M, Natalie A, Robert H. Entamoeba histolytica cell movement: a central role for self-generated chemokines and chemorepellents. Proc. Natl. Acad. Sci. USA. 2006;103:18751–18756. doi: 10.1073/pnas.0605437103. [DOI] [PMC free article] [PubMed] [Google Scholar]