Abstract
In animal models, hundreds of repetitions of upper extremity (UE) task practice promote neural adaptation and functional gain. Recently, we demonstrated improved UE function following a similar intervention for people after stroke. In this secondary analysis, computerized measures of UE task performance were used to identify movement parameters that changed as function improved. Ten people with chronic post-stroke hemiparesis participated in high-repetition UE task-specific training 3 times per week for 6 weeks. Before and after training, we assessed UE function with the Action Research Arm Test (ARAT), and evaluated motor performance using computerized motion capture during a reach-grasp-transport-release task. Movement parameters included the duration of each movement phase, trunk excursion, peak aperture, aperture path ratio, and peak grip force. Group results showed an improvement in ARAT scores (p = 0.003). Although each individual changed significantly on at least one movement parameter, across the group there were no changes in any movement parameter that reached or approached significance. Changes on the ARAT were not closely related to changes in movement parameters. Since aspects of motor performance that contribute to functional change vary across individuals, an individualized approach to upper extremity motion analysis appears warranted.
Keywords: reach, grasp, kinematic, force
INTRODUCTION
Reduced upper extremity function is a devastating consequence of stroke. Of the nearly 800,000 people who experience stroke each year in the United States, 50% have persistent hemiparesis (Lloyd-Jones et al., 2010; Mayo et al., 1999), and hand function often remains limited, even in those with good overall recovery (Lai et al., 2002). Typically, within the first six months post-stroke, partial functional improvement occurs and is accompanied by compensatory movement strategies that develop either spontaneously or through rehabilitation that focuses on restoring function. Neuroscientific discoveries over the past several decades have shown that the brain undergoes a continual process of reorganization, strongly influenced by behavioral experience, in healthy individuals and particularly in those with recent neural injury (Kleim & Jones, 2008; Nudo et al., 2007). These discoveries have renewed interest in the idea that greater motor recovery may be possible after stroke, and that it may be possible to restore function through return of normal movement patterns instead of through compensatory strategies (Cramer, 2008; Krakauer, 2005; Kwakkel et al., 2004; Levin et al., 2009).
Repetitive training is a powerful behavioral stimulus for driving use-dependent neural adaptation in animals (Butefisch et al., 2000; Kleim et al., 2004; Monfils et al., 2005; Nudo et al., 1996), and in humans (Askim et al., 2009; Jang et al., 2003; Liepert et al., 2000; Schaechter et al., 2002). Rehabilitation protocols that include repetitive task-specific training can produce gains in upper extremity function early after stroke (Harris et al., 2009; Winstein et al., 2004; Wolf et al., 2006), and at later time points as well (Pang et al., 2006; Page et al., 2008; Platz et al., 2001; Taub et al., 2006). Important features of training include acquisition of skills that are salient for the individual, and repetition of the newly learned skills at an adequate intensity (Kleim & Jones, 2008).
Investigators have begun to explore changes in specific movement parameters that may result from task-specific training, and that may contribute to changes in function. For example, in a series of three cases, measures of grasp force and functional task performance both improved after six weeks of distributed repetitive practice (Conti & Schepens, 2009). In studies of constraint-induced movement therapy, four of five participants showed improved grasp force generation in a key-turning task (Alberts et al., 2004), and a group of eight participants showed faster, more coordinated arm movement (Caimmi et al., 2008). In four individuals, positive changes in kinematic variables and measures of muscle activity were reported following task-specific training designed to remedy each person’s key movement impairments (Lum et al., 2009). Several research groups have shown improved shoulder and elbow movement and decreased compensatory trunk movement, after repetitive reaching practice with trunk restraint (Michaelsen et al., 2006; Thielman et al., 2008; Woodbury et al., 2009). These findings support the idea that functional gains after stroke can occur at least partially through recovery of normal movement patterns rather than compensation, and that evaluation of specific movement parameters may provide insights that are useful when selecting and progressing training tasks, and when describing how movement changes after intervention.
In attempts to better understand the movement problems that underlie loss of upper extremity function, numerous motor impairments have been studied in people with post-stroke hemiparesis. These include diminished muscle activation (Canning et al., 2000; McCrea et al., 2005; Wagner et al., 2007), reduced movement speed (Beer et al., 2000; Cirstea et al., 2003; Dewald & Beer, 2001; Lang et al., 2005; Levin et al., 1996; Reisman & Scholz, 2003; Wagner et al., 2007), synergistic movement patterns that constrain multijoint movements proximally and distally (Cirstea et al., 2003; Ellis et al., 2005; Lang & Beebe, 2007; Lang & Schieber, 2004; Li et al., 2003; Schieber et al., 2009), and related compensatory movements of the trunk (Cirstea & Levin, 2000; Levin et al., 2002; Roby-Brami et al., 2003). Moderate correlations have been demonstrated between several of these movement parameters and deficits in upper extremity function (Celik et al., 2010; Depietro et al., 2007; Ellis et al., 2008; Lang et al., 2006a; McCrea et al., 2002). It remains unclear, however, to what extent changes in specific movement parameters underlie the changes in function observed after task-specific training.
Recently, we studied the feasibility of implementing high-repetition doses of upper extremity task-specific training in people post-stroke, and questioned whether the high-repetition protocol would lead to gains in upper extremity function (Birkenmeier et al., 2010). Primary results demonstrated feasibility and functional improvement. The current investigation is a secondary analysis of outcome data collected during that study, in which we measured motor performance of a reach-grasp-transport-release task using computerized motion analysis methods. The purposes of this secondary analysis were to identify movement parameters that improved after training, and to determine whether improvements in upper extremity function were associated with improvements in specific movement parameters. Based on previous descriptions of stroke-related motor impairments and their relationships to function, we hypothesized that functional gains would be associated with decreases in movement time, trunk excursion, and inefficient finger movement, and with increases in thumb-finger separation (aperture) and grip force.
METHODS
Participants
People with hemiparesis due to stroke were recruited from the St. Louis metropolitan area via the Cognitive Rehabilitation Research Group Stroke Registry at Washington University and from local outpatient rehabilitation clinics. Potential participants were included if they had been diagnosed with stroke at least six months prior and had unilateral upper extremity hemiparesis, indicated by a score of 1, 2, or 3 on the Motor Arm item of the National Institutes of Health Stroke Scale (NIHSS). Potential participants were excluded if 1) they had ever been diagnosed with any other neurological or psychiatric condition, 2) they were participating in any other upper extremity stroke intervention (e.g. Botox), 3) they had NIHSS scores indicating insufficient cognitive ability or severe hemineglect (a score of 2 on the Questions item, 1 or 2 on the Commands item, or 2 on the Extinction and Inattention item), or 4) they did not anticipate being able to attend all study related appointments. During the 1-year period of the study, 27 people were screened, 15 were enrolled, and 13 completed the intervention and functional assessments. Ten of the 15 participants were assessed using the motion analysis procedures described in this report. All ten completed the training program, with 97 % attendance. The five enrolled participants who were not assessed using motion analysis included four who began training before motion analysis was added to the study protocol, and one who was unable to complete the initial phase of the assessment task. This study was approved by the Washington University Human Research Protection Office, and all participants provided informed consent before participation.
Intervention
The intervention consisted of supervised massed practice of upper extremity tasks, for three one-hour sessions per week for six weeks (Birkenmeier et al., 2010). During each session, participants were encouraged to perform at least 300 repetitions of task practice (3 tasks per session, ≥100 repetitions each). Each task included four movement components that are essential for most upper extremity functional tasks: reaching for, grasping, moving or manipulating, and releasing an object. In order to identify tasks that were relevant and motivating for each participant, the Canadian Occupational Performance Measure was administered by an occupational therapist during the first baseline assessment session (Dedding et al., 2004; Law et al., 1990). For each participant, three tasks were selected, adjusted for difficulty, and progressed throughout the study, in order to provide a training stimulus that was continually challenging but not overwhelming. Additional detail regarding the selection and progression of training tasks is provided elsewhere (Birkenmeier et al., 2010).
Assessments
The primary outcome measure, used to assess the benefit of the intervention was the Action Research Arm Test (ARAT). This criterion-rated test quantifies the ability to reach, grasp, manipulate, and release a variety of everyday objects. The ARAT consists of 19 items, with each item scaled on a 0–3 point scale (total score = 57). The ARAT is strongly correlated with timed tests of upper extremity function at multiple time points post stroke with absolute r values ranging from 0.87 – 0.95 (Lang & Beebe 2007; Beebe & Lang 2009). It is clinically useful because of its low testing burden and strong psychometric properties (Beebe & Lang 2009; Lang et al., 2006a; Lyle, 1981; Van der Lee et al., 2001; Yozbatiran et al., 2008). The ARAT was administered on the affected side during three baseline assessment sessions one week apart, and at the end of the six-week intervention. For descriptive purposes, spasticity of the elbow flexors was assessed on the affected side during the first baseline session, using the modified Ashworth scale (Bohannon & Smith, 1987). We also measured maximal grip force bilaterally, using a Jamar grip dynamometer and the method described by Fess (1992).
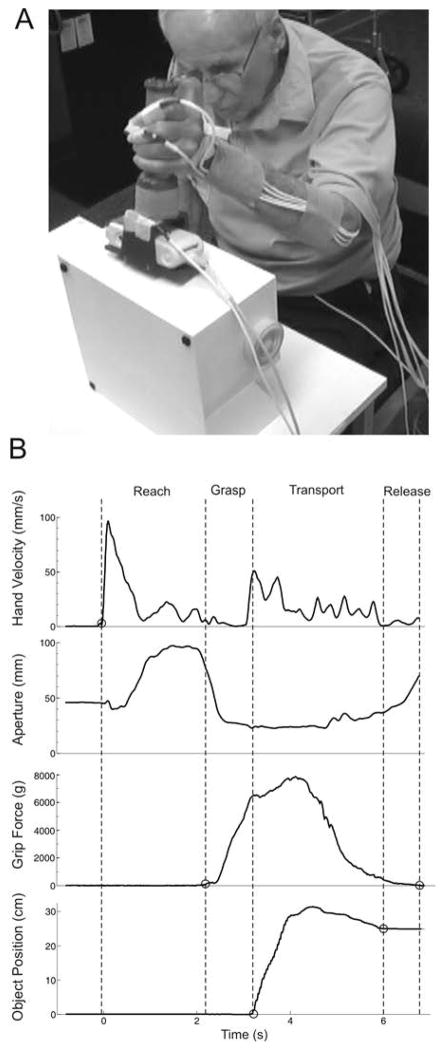
Motor performance of the affected upper extremity was assessed using computerized motion analysis of a reach-grasp-transport-release task, during the last baseline session and at the end of the intervention. All participants performed the same task, which involved reaching for an object on a table, grasping it with a palmar grip, lifting it onto a shelf, and releasing it, with the goal of completing the task as quickly as possible (Figure 1A). None of the participants practiced the assessment task during the intervention. In choosing the assessment task, we tried to accommodate a wide range of motor abilities and to minimize floor and ceiling effects. We considered the chosen task relatively easy, and thought that potential participants who met our study criteria and could participate in the intervention would also be able to complete ten trials of the assessment task. At the same time, we believed that the task would be responsive to change, since improved motor performance could be reflected in measures of movement time, excursion, efficiency, and grip force, each of which has a continuous scale.
Figure 1.
Assessment of motor performance. A) Illustration of the experimental set-up and a participant performing the reach-grasp-transport-release task. B) Example data from one trial. Vertical dashed lines demonstrate division of the task into movement phases. The reach phase began when hand velocity exceeded 5 mm/sec, the grasp phase began when grip force exceeded 5 grams, the transport phase began when the vertical position of the object increased by 3 mm, and the transport phase ended when the vertical position of the object returned to within 3 mm of its final resting position. Duration of the release phase was calculated as the difference in time between the end of transport, and the time when force on the object returned to within 5 grams of its baseline value.
The object to be grasped (seen in Figure 1A) consisted of a custom-fabricated vertical cylinder (3.4 cm diameter, 11.3 cm height) attached to a rectangular base (13.5 cm by 6 cm) that was designed to hold a Tekscan I-scan electronic interface (Tekscan, Inc., South Boston, MA). The cylindrical portion of the object was covered with a Tekscan pressure sensor (I-scan model 5101/3414TI/10, 111.8 × 111.8mm, 1936 sensels, spatial resolution of 15.5 sensels/cm2). Combined weight of the object, sensor and electronics was 420 grams (4.12 N). Pressure data were collected at 100 Hz.
Measurement of grip force is a novel use of pressure sensor technology. This method was chosen instead of a more typical strain gauge system because it does not require that participants place their hand or fingers on specific locations, and instead allows for more natural grasping performance. A disadvantage of the pressure sensor system is that it only measures grip forces (normal forces) and is unable to measure load forces (shear forces). For use in this study, we believed that the advantage of capturing natural movements outweighed the disadvantage of limiting our force analysis to grip (i.e. normal) forces. Psychometric properties of this grip force measurement method have not been reported.
Three dimensional movement of the affected upper extremity was captured at 50 Hz using an electromagnetic tracking system (MotionMonitor, Innovative Sports Training, Chicago, IL). Seven sensors were attached to the trunk and the affected upper extremity, as follows: 1) trunk: midline below the sternal notch, 2) upper arm: proximal to the lateral epicondyle, bisecting the upper arm mass, 3) forearm: midpoint between the radial and ulnar styloids on the dorsum of the forearm, 4) hand: midpoint of the third metacarpal on the dorsum of the hand, and 5 through 7) thumb, index and middle fingers: on the nail of each digit.
Participants were seated in a chair with back support, and a table was placed with its closest edge across the participant’s mid-thighs. Table height was adjusted so the surface was approximately 10 cm above the thighs. For each participant, equal table height for the pre- and post-training assessments was ensured. A 25 cm high shelf was placed on the table, at a distance from the participant equal to 90% of the length of the arm from shoulder to wrist, and the center of the shelf was aligned with the mid-clavicle in the frontal plane. The object to be grasped was placed on the table, near its closest edge, also aligned with the mid-clavicle.
Prior to each trial, the participant was instructed to rest both hands in their lap with thumb and fingers together, wait for the word ‘go’, then use their affected limb to reach and grasp the object with a palmar grip, lift it and place it on the shelf, then release and return their hand to their lap (Figure 1A). They were asked to perform the movement as quickly as possible while still successfully completing the task. Verbal instructions and demonstration were provided. Ten trials were recorded, with approximately ten seconds of rest between trials. We limited each trial to ten seconds, since preliminary testing had shown that healthy adults consistently performed the task in less than two seconds. Onset of data collection was electronically triggered, ensuring synchronization of the Tekscan and MotionMonitor systems. Video was also recorded during each testing session.
Analysis
Pressure data were converted to grams of force, using Tekscan software to multiply recorded pressure by the sensor’s spatial area. After low-pass filtering of kinematic data at 6 Hz using a second-order Butterworth filter, sensor position data were extracted using MotionMonitor software (Innovative Sports Training, Chicago, IL). Video recordings were used to verify whether each movement phase was successfully completed during each trial. Subsequent analysis was then completed using custom software written in MATLAB (The MathWorks, Inc., Natick, MA).
Durations of the reach, grasp, transport, and release phases were determined based on hand velocity, force on the object, and object position, as follows (Figure 1B). The reach phase began when velocity of the hand sensor first exceeded 5 mm/s, and ended when force on the object first exceeded 5 grams. The grasp phase began at the end of the reach, and ended when the vertical position of the object increased by 3 mm from its initial value. The transport phase began at the end of the grasp, and ended when the vertical position of the object was first within 3 mm of its final stable value. Duration of the release phase was calculated as the difference in time between the end of transport, and the time when force on the object returned to within 5 grams of its baseline value. In some cases, force returned to baseline prior to the object reaching a final stable position. In these cases, the calculated duration of the release phase was negative, indicating release of the object before it was placed securely on the shelf. In other cases, the object reached a stable position before force returned to baseline, yielding a positive release phase duration.
Other variables of interest included trunk excursion, peak aperture, aperture path ratio, and peak grip force. Trunk excursion was determined separately for the reach phase and for the transport phase, and was defined as the difference between the maximum and minimum resultant trunk sensor positions. Trunk excursion values close to zero represented normal performance, and higher values indicated compensatory trunk movement. Peak aperture was the maximum three-dimensional distance between sensors on the thumbnail and the index fingernail during the reach phase. Aperture path ratio quantified the smoothness/efficiency of thumb and index finger movement during the reach phase, and was calculated as follows (modified from Lang et al., 2005 and Lang et al., 2006b):
An aperture path ratio equal to one indicates smooth and direct separation of the thumb and index finger to the maximum aperture value, followed by smooth and direct closing onto the object. Higher values indicate abnormal, inefficient opening and closing of the thumb and index fingers, typically seen when participants make multiple attempts to open their hand and then close it on the object. Peak grip force was defined as the maximum force applied to the object during the transport phase.
Variables were calculated separately for each trial. Kolmogorov-Smirnov tests were used to test whether data was normally distributed within and across participants. Since all data met the normality assumption (p > 0.05), parametric statistics were used. Statistically significant pre-post changes for each participant were identified individually using paired t-tests to compare the ten pre-training trials to the ten post-training trials. For analysis of group results, each participant’s performance was represented by the mean value for each variable across the ten trials within each assessment session. Pre-post changes for the group were identified using paired t-tests. Statistica software was used for all statistical analyses (Version 6.1 Statsoft Inc., Tulsa, OK), and the criterion for significance was set at p < 0.05. Given the numerous comparisons required for individual and group analysis of movement parameters, we also noted pre-post differences that were statistically significant using a more stringent Bonferroni-adjusted p value of 0.0005. Effect sizes and estimated sample sizes that would have been needed to detect significant pre-post differences for each movement parameter were derived from change scores (mean change/SD of change) using a paired t test design and assumptions that power = 0.80 and 2-tailed alpha = 0.05. Pearson product moment correlation coefficients were used to examine relationships between changes in UE function (post-training ARAT score minus the mean of the three baseline ARAT scores) and changes in each movement parameter (post-training mean minus pre-training mean). Correlation coefficients were considered low when r < 0.50, moderate when r was between 0.50 and 0.80, and high when r > 0.80.
In order to facilitate interpretation of the movement parameter data, values are reported for a group of twelve healthy controls (6 males, 6 females, 10 right handed, 2 left handed) who performed a similar task in our laboratory. The controls had an average age of 52.4 years (std. dev. 15.7), had no current or prior neurological diagnosis, and had no history of musculoskeletal disorders involving either upper extremity. Using one randomly selected side (7 dominant, 5 non-dominant, 7 right, 5 left), they performed a reach-grasp-lift task that was identical to the task used in the current study, except that instead of placing the object on a shelf and releasing it, they lifted it and held it approximately 10 cm above the table for 5 seconds. As a result, normative data are available for most of the movement parameters included in the current study, but are not available for transport duration, release duration, and trunk excursion during transport. Methods for collecting and analyzing the control data were identical to the procedures used in the current study, including the instruction to perform the task as quickly as possible.
Reliability of upper extremity kinematic measures has been investigated recently in healthy individuals and people with post-stroke hemiparesis. Excellent test-retest reliability has been reported for reach duration (Pearson r > 0.90) in healthy controls reaching at their self-selected speed (Caimmi et al., 2008). In a study of people with post-stroke hemiparesis performing reaching movements, reliability estimates for reach duration ranged from poor to excellent, depending on the speed of movement and the height of the reaching target (Wagner, et al, 2008). In a recent evaluation of a reach-to-grasp task that resembled the task used in the current study, Patterson et al. (In press) reported excellent reliability for reach duration, peak aperture, and trunk excursion (r > 0.75) in a group of people with hemiparesis after stroke. The smallest amount of change that exceeds measurement error and can be considered real change (minimal detectable change, 90% confidence), was estimated to be 280 milliseconds for reach duration, 5 millimeters for peak aperture, and 36 millimeters for trunk excursion. Reliability and minimal detectable change have not been investigated for the other movement parameters used in this study.
Results
Characteristics of the participants are shown in Table 1. For the six females and four males included in this study, time since stroke varied widely from six months to ten years. Five participants had right hemiparesis and five had left hemiparesis. In six participants, the affected side was their dominant side. All except one were right handed.
Table 1.
Characteristics of participants
| Participant | Age (years) | Months post-stroke | Spasticity § | Grip Strength §§ | |
|---|---|---|---|---|---|
| Affected side (kg) | Affected side as % of less affected side | ||||
| R005 | 44 | 6 | 1 | 14 | 47 |
| R007 | 55 | 120 | 3 | 15 | 61 |
| R008 | 28 | 48 | 0 | 11 | 43 |
| R009 | 57 | 18 | 3 | 10 | 25 |
| R010 | 50 | 48 | 0 | 22 | 65 |
| R011 | 65 | 36 | 2 | 6 | 22 |
| R012 | 56 | 57 | 4 | 12 | 25 |
| R013 | 57 | 36 | 1 | 10 | 43 |
| R014 | 90 | 48 | 4 | 15 | 58 |
| R015 | 33 | 22 | 1 | 17 | 34 |
| Mean ± SD | 54 ± 17 | 44 ± 31 | 1.9 ± 1.5 | 13.2 ± 4.4 | 42.3 ± 13.9 |
Elbow flexors were assessed on the affected side using the Modified Ashworth Scale.
Maximum isometric grip strength assessed with a Jamar grip dynamometer
Individual and group results are reported for the ARAT and movement phase durations in Table 2, and for all other movement parameters in Table 3. In Tables 2 and 3, each of the 10 participants (R005 through R015) is represented by two rows, one for the pre-training data (upper row) and one for the post-training data (lower row). Pre- and post-training group means are presented in the bottom rows of tables 2 and 3, along with the number of participants included in each mean.
Table 2.
Changes in ARAT scores and movement phase durations
| ARAT | Reach Duration (msec) | Grasp Duration (msec) | Transport Duration (msec) | Release Duration (msec) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy Controls mean ± 1 SE |
57 ± 0 | 425 ± 17 | 236 ± 14 | not available | not available | |||||
| Participant | mean | n | mean | n | mean | n | mean | n | ||
| R005 | Pre | 38 | 442 | 10 | 314 | 10 | 964 | 10 | 186 | 10 |
| Post | 57 | 548 ‡ | 10 | 263 | 10 | 935 | 10 | −122 ‡ | 10 | |
| R007 | Pre | 20.3 | 1050 | 8 | 365 | 8 | 2196 | 7 | 590 | 7 |
| Post | 28 | 1060 | 10 | 281 ‡ | 10 | 2762 | 10 | 1153 | 10 | |
| R008 | Pre | 43 | 558 | 10 | 327 | 10 | 2630 | 10 | −1647 | 10 |
| Post | 56 | 379 † | 10 | 157 † | 10 | 740 † | 10 | −15 † | 10 | |
| R009 | Pre | 9.3 | 3870 | 10 | 2127 | 9 | 2550 | 2 | 0 | |
| Post | 11 | 2646 | 7 | 1483 | 7 | 0 | 0 | |||
| R010 | Pre | 40 | 593 | 10 | 325 | 10 | 2334 | 10 | −1455 | 10 |
| Post | 53 | 963 † | 10 | 302 | 10 | 1412 ‡ | 10 | −11 † | 10 | |
| R011 | Pre | 26.7 | 1692 | 10 | 447 | 10 | 4011 | 7 | −641 | 7 |
| Post | 31 | 2466 † | 10 | 666 | 10 | 2477 | 7 | 63 | 7 | |
| R012 | Pre | 20 | 2004 | 10 | 1359 | 10 | 2910 | 9 | 909 | 8 |
| Post | 24 | 1794 | 10 | 1754 | 10 | 3764 ‡ | 9 | 400 | 9 | |
| R013 | Pre | 15 | 1491 | 10 | 497 | 10 | 3990 | 1 | −220 | 1 |
| Post | 15 | 1516 | 10 | 827 † | 10 | 0 | 0 | |||
| R014 | Pre | 22 | 2022 | 10 | 1435 | 10 | 3811 | 7 | 0 | |
| Post | 31 | 1367 † | 10 | 681 ‡ | 10 | 1893 ‡ | 10 | 1485 | 10 | |
| R015 | Pre | 20 | 3664 | 10 | 2194 | 5 | 2114 | 5 | −550 | 1 |
| Post | 24 | 1803 ‡ | 9 | 356 ‡ | 9 | 1427 ‡ | 9 | −312 | 9 | |
| Group | Pre | 25 | 1739 | 10 | 939 | 10 | 2751 | 10 | −354 | 8 |
| Post | 33 * | 1454 | 10 | 677 | 10 | 1926 | 8 | 330 | 8 | |
| Effect size (Est. N) | 1.61 (6) | 0.37 (60) | 0.39 (54) | 0.65 (21) | 0.66 (21) | |||||
Post > Pre, p = 0.003
Bold type indicates individual or group pre-post differences that were statistically significant at the p < 0.05 level (‡) or at the p < 0.0005 level (†). Note that some significant changes were in the unexpected direction (e.g. increased reach phase duration for R005, R010, R011). For R005 through R015, n represents the number of trials included in the individual’s mean. Where n < 10, the participant was unable to complete the movement phase during every trial. For Group results, n represents the number of participants for whom data were available. The pre-training ARAT score is the mean of three baseline tests each separated by one week. The post-training ARAT score is from one test administered at the end of the training program. The effect sizes and estimated sample sizes (Est. N) are from post-hoc power analyses for each parameter (see Methods).
Table 3.
Changes in movement parameters
| Reach Phase | Transport Phase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trunk Excursion (mm) | Peak Aperture (mm) | Aperture Path Ratio | Trunk Excursion (mm) | Peak Grip Force (g) | |||||||
| Healthy Controls mean ± 1 SE |
4 ± 2 | 125 ± 3 | 1.13 ± 0.04 | not available | 5659 ± 1629 | ||||||
| Participant | mean | n | mean | n | mean | n | mean | n | mean | n | |
| R005 | Pre | 16 | 10 | 145 | 10 | 1.29 | 10 | 9 | 10 | 3745 | 10 |
| Post | 4 ‡ | 10 | 124 | 10 | 1.11 | 10 | 18 | 10 | 1464 ‡ | 10 | |
| R007 | Pre | 11 | 8 | 82 | 8 | 1.55 | 8 | 20 | 7 | 3075 | 8 |
| Post | 33 ‡ | 10 | 77 | 10 | 1.35 | 10 | 18 | 10 | 3739 | 10 | |
| R008 | Pre | 10 | 10 | 101 | 10 | 3.60 | 10 | 45 | 10 | 6930 | 10 |
| Post | 1 ‡ | 10 | 103 | 10 | 1.08 † | 10 | 5 † | 10 | 3321 ‡ | 10 | |
| R009 | Pre | 31 | 10 | 100 | 10 | 2.24 | 10 | 29 | 2 | 797 | 10 |
| Post | 20 | 7 | 105 ‡ | 7 | 2.02 | 7 | 0 | 610 | 7 | ||
| R010 | Pre | 64 | 10 | 102 | 10 | 1.13 | 10 | 109 | 10 | 2084 | 10 |
| Post | 13 † | 10 | 93 | 10 | 1.40 | 10 | 38 † | 10 | 3620 ‡ | 10 | |
| R011 | Pre | 23 | 10 | 162 | 10 | 1.76 | 10 | 53 | 7 | 4643 | 10 |
| Post | 64 † | 10 | 171 | 10 | 2.09 | 10 | 62 | 7 | 4347 | 10 | |
| R012 | Pre | 105 | 10 | 99 | 10 | 1.39 | 10 | 42 | 9 | 7323 | 10 |
| Post | 124 ‡ | 10 | 105 | 10 | 2.02 ‡ | 10 | 103 | 9 | 7169 | 10 | |
| R013 | Pre | 67 | 10 | 92 | 10 | 1.70 | 10 | 68 | 1 | 1176 | 10 |
| Post | 27 ‡ | 10 | 62 ‡ | 9 | 2.00 | 10 | 0 | 751 † | 10 | ||
| R014 | Pre | 15 | 10 | 119 | 10 | 2.12 | 10 | 37 | 7 | 2091 | 10 |
| Post | 12 | 10 | 121 | 9 | 1.56 ‡ | 10 | 17 ‡ | 10 | 3466 ‡ | 10 | |
| R015 | Pre | 105 | 10 | 45 | 10 | 3.67 | 10 | 82 | 5 | 2566 | 10 |
| Post | 97 | 9 | 59 ‡ | 9 | 3.72 | 9 | 77 | 9 | 1276 | 9 | |
| Group | Pre | 45 | 10 | 105 | 10 | 2.04 | 10 | 49 | 10 | 3443 | 10 |
| Post | 39 | 10 | 102 | 10 | 1.84 | 10 | 42 | 8 | 2976 | 10 | |
| Effect Size (Est. N) | 0.19 (220) | 0.20 (199) | 0.24 (139) | 0.19 (220) | 0.29 (96) | ||||||
Bold type indicates individual or group pre-post differences that were statistically significant at the p < 0.05 level (‡) or at the p < 0.0005 level (†). Note that some significant changes were in the unexpected direction (e.g. increased trunk excursion during reach for R007, R011, R012). For R005 through R015, n represents the number of trials included in the individual’s mean. Where n < 10, the participant was unable to complete the movement phase during every trial. For Group results, n represents the number of participants for whom data were available. The effect sizes and estimated sample sizes (Est. N) are from post-hoc power analyses for each parameter (see Methods).
Pre-training ARAT scores ranged from 9 to 43 (mean 25.4 ± 11.3 SD). Changes on the ARAT ranged from 0 to 19 points. For the group of 10 participants included in this analysis, upper extremity function increased significantly after training, as indicated by an average ARAT score increase of 8 points (p = 0.003). The average improvement of 8 points exceeded the 4-point minimal detectable change for this measure (Lin et al., 2009), and exceeded the 6-point estimate of minimal clinically important difference for people with chronic post-stroke hemiparesis (van der Lee et al., 1999).
In Tables 2 and 3, each participant’s mean pre-training and post-training values are reported for each movement parameter, averaged across all trials for which the movement parameter could be determined. The number of trials included in each mean is also reported. Although 10 trials were attempted during each assessment session, in some cases the participant did not complete all phases of the task, resulting in n < 10 for certain movement parameters. For example, when the reach phase was not completed, no movement parameters could be calculated (e.g. 2 of the 10 pre-training trials for R007), and when the transport phase was not completed, trunk excursion during transport could not be calculated (e.g. 3 of the 10 pre-training trials for R007).
Pre-training data showed impaired motor performance. In all participants, mean values for reach duration, grasp duration, and trunk excursion during the reach phase exceeded mean control values. Aperture path ratio exceeded the control mean for all except one participant (R010). Most participants also showed diminished peak aperture and diminished peak grip force. Exceptions included R005 and R011, whose peak apertures exceeded the control mean, and R008 and R012, whose peak grip force exceeded the control mean.
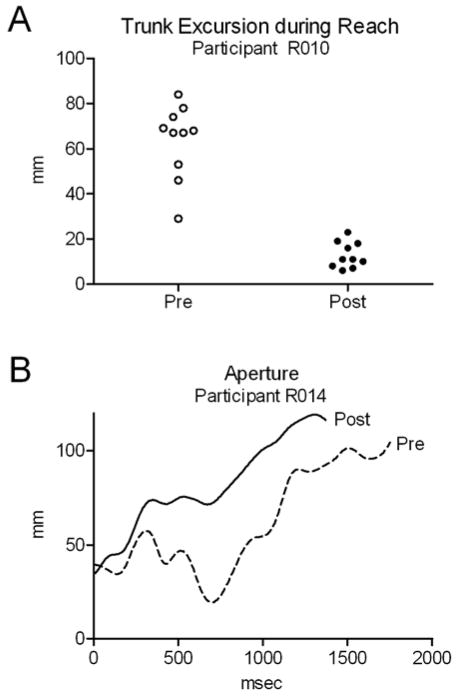
After training, eight of the ten participants showed an improvement in at least one movement parameter, indicated by a change toward the mean value for healthy controls when available, or by a decrease in transport phase duration, a decrease in trunk excursion during transport, or release duration closer to zero. Examples of improvements are illustrated in Figure 2, including a decrease in trunk excursion during the reach phase for R010 and a decrease in aperture path ratio for R014. In six of the ten participants, at least one movement parameter changed in the opposite direction, away from the control mean, possibly representing compensatory movement strategies. Examples include increased reach duration for R005, R010, and R011, and increased trunk excursion during the reach phase for R007, R011, and R012. Of the eight participants who gained at least four points on the ARAT, two showed only improvements in movement parameters (R014, R015), four showed a combination of improvements and compensatory changes (R005, R007, R008, R010), and two showed only compensatory changes (R011, R012).
Figure 2.
Examples of improvements in movement parameters in individual participants. A) After training, R010 showed decreased trunk excursion during the reach phase. Ten pre-training trials and ten post-training trials are shown. B) After training, R014 showed improved efficiency of finger movement, seen as a smoother aperture trace and quantified by a decrease in the aperture path ratio (see Methods). Reach duration also decreased. One representative trial is shown for each time point.
In some cases, changes in movement parameters were consistent with changes in upper extremity function. For example, participant R014 improved by 9 points on the ARAT, with faster completion of the reach, grasp, and transport phases, lower aperture path ratios during the reach, increased peak grip force, and decreased trunk excursion during the transport phase. R009 showed little change on the ARAT, and also showed no advantageous changes in movement parameters other than a 5 mm increase in peak aperture. In participant R015, however, large improvements in the reach, grasp, and transport phase durations occurred despite a modest functional gain. The participant with the largest functional improvement (R005) showed small improvements in trunk excursion during reaching and in the timing of object release. Reach duration, however, increased slightly and grip force was further diminished after training. These examples suggest that functional gains are not necessarily reflected in movement parameter changes, and vice versa.
Despite the improvement in upper extremity function, group results revealed no significant changes in any of the movement parameters (p > 0.20). Highly variable performance across participants kept the mean pre-post differences from reaching statistical significance. Effect sizes were calculated as the mean change score divided by the standard deviation of change scores (bottom row of Tables 2 and 3). While the effect size was very large for the ARAT, effect sizes for the movement parameters were small to moderate. Accordingly, much larger sample sizes would be required in order to detect statistically significant changes in the movement parameters.
Across individuals, no consistent pattern emerged linking specific movement parameters to changes in function. Changes in ARAT scores and ARAT subscores were not highly correlated with changes in movement parameters (Table 4). The only statistically significant correlation was between the grasp subscale and the aperture path ratio (r = −0.67, p < 0.05).
Table 4.
Correlations between changes on the ARAT and changes in movement parameters
| Total Score | Grip Subscale | Grasp Subscale | Pinch Subscale | Gross Movement Subscale | |
|---|---|---|---|---|---|
| Reach Duration | 0.32 | 0.22 | 0.12 | 0.43 | 0.31 |
| Grasp Duration | 0.08 | −0.12 | −0.14 | 0.30 | 0.27 |
| Transport Duration | −0.11 | −0.16 | −0.37 | 0.03 | 0.15 |
| Release Duration | 0.19 | 0.21 | 0.31 | 0.14 | −0.05 |
| Trunk Excursion during Reach | −0.19 | −0.25 | −0.06 | −0.10 | −0.29 |
| Peak Aperture | −0.10 | <0.01 | 0.10 | −0.17 | −0.35 |
| Aperture Path Ratio | −0.41 | −0.17 | −0.67 * | −0.36 | −0.28 |
| Trunk Excursion during Transport | −0.45 | −0.60 | −0.46 | −0.24 | −0.16 |
| Peak Grip Force | −0.29 | 0.05 | −0.39 | −0.36 | −0.42 |
The only correlation that reached statistical significance was between the Grasp Subscale and the Aperture Path Ratio. For transport duration, trunk excursion during transport, and release duration, n = 8 and p < 0.05 when r > 0.62. For all other movement parameters, n = 10 and p < 0.05 when r > 0.55 (one-tailed).
Discussion
It is logical to think that improvements in upper extremity function after task-specific training would be produced by changes in certain measurable aspects of motor performance. Our data support this idea at the level of the individual participant, but not at the group level. Each participant’s motor performance changed as they achieved greater function, and such changes were identified using a set of movement parameters that quantified different aspects of motor performance. Numerous significant changes were observed within individuals, including improvements toward more normal motor performance, as well as changes in the opposite direction, possibly representing compensation. Several participants demonstrated improvement in some movement parameters and compensatory changes in others.
The appreciation of change, however, was lost in the group analysis. Reasons for the lack of significant group findings include the low effect sizes for most movement parameters, and the small number of participants. Post-hoc power analyses showed that effect sizes were much lower for all movement parameters than for the ARAT score, and that a large sample size would be required for most of the observed movement parameter effects to reach statistical significance. Exceptions include the transport and release phase duration effects, which would have reached significance with a sample size of 21. Since effect size is diminished by high variability between-participants, it is likely that the heterogeneity of participants in this study contributed to the lack of significant group findings. Severity of motor deficits, in particular, is known to be strongly negatively correlated with motor recovery after stroke, and likely limited the effect sizes observed in this study. Additional factors may have also contributed, including other participant characteristics such as time since stroke and/or lesion location, measurement error, and individual differences in terms of which movement parameters changed and in which direction. Statistical power in this study was not sufficient to conclude that any movement parameters fail to change with intervention or with functional improvement. Our findings do show, however, that changes in specific movement parameters may not be large enough and consistent enough to show change across a small group, even when functional gains are significant across the group.
An important finding in this study is the lack of strong relationships between upper extremity functional gains and changes in specific movement parameters. Correlation analysis illustrates that none of the movement parameters included in this study is a suitable substitute for the measurement of upper extremity function as an outcome of intervention. Rather, motion analysis is a useful tool for studying how function improves, through restoration of normal movement patterns, development of compensatory strategies, or through a combination of the two. As discussed by Lum et al. (2009), principle components analysis, confirmatory factor analysis, and structural equation modeling hold promise as alternative methods to analyze motor performance, but their application to upper extremity rehabilitation studies is currently limited because of the large sample sizes required and the need for further theoretical understanding about upper extremity kinematic analysis.
The lack of strong relationships between functional gains and changes in movement parameters suggest that, to some extent, the two assessments measure different constructs. This highlights the importance of matching assessment tools to the purpose of research studies. For example, in clinical trials where the goal is to assess effectiveness of intervention aimed at improving upper extremity function, we suggest that measures of function should serve as the primary outcome. Motion analysis is clearly useful, however, in studies that seek to distinguish between restoration of normal movement and development of compensatory movement strategies, and in studies of intervention aimed at improving specific movement problems. Given the numerous changes within individuals and the lack of significant group changes in this study, we further suggest that an individualized approach to upper extremity motion analysis may be optimal in studies that explore changes in motor performance. For example, baseline motion analysis could be used to identify each person’s most limiting movement problems and then to develop an individualized task-specific training program to address those specific deficits. Post-training motion analysis could then be used to evaluate outcomes. Group analysis is clearly useful when all group members share a common movement problem that is the target of intervention.
Several limitations should be considered when interpreting our data. First, this study included a small, heterogeneous sample of people with hemiparesis that varied in terms of severity and time since stroke. Further, the intervention was individualized and was aimed at improving function rather than improving specific movement parameters. While more stringent recruiting criteria and a more focused intervention may have yielded more significant group results, our study closely paralleled the circumstances encountered in clinical settings and in many other studies of upper extremity rehabilitation. Given the small sample size in this study, we were unable to explore the effects of participant characteristics on responsiveness to intervention. Larger studies are needed to investigate whether initial movement problems, lesion location, and time since stroke affect the magnitude or type of changes seen in movement parameters after training.
For certain variables, interpretation of our results is limited by a lack of control data and reliability estimates. It is not clear how long the transport and release phases last in healthy individuals, and the amount of trunk excursion that typically occurs during the transport phase is unknown. Nevertheless, it is reasonable to assume that in people with hemiparesis, a faster transport phase with less trunk excursion represents better upper extremity performance, so the desirable direction of change is fairly clear. Similarly, release durations that approach zero can be considered advantageous, indicating that release of grip force closely coincides with placement of the object securely on the shelf. Although the minimal detectable change is unknown for several of the movement parameters we studied, statistically significant pre-post changes in those variables were quite large, exceeding a 50% change in 17 of 24 instances, and exceeding a 20% change in all instances.
In summary, our results suggest that changes in motor performance after training vary across individuals, and that group analysis of movement parameters can obscure significant changes within individuals, particularly in small samples. After high-repetition task specific training, upper extremity function improved, and each participant changed significantly on at least one variable that quantified timing, movement or grip force. None of the movement parameters, however, changed significantly across the group, and improvements in upper extremity function were not closely related to changes in any of the movement parameters. Since functional assessments and measures of motor performance can produce different results, outcome measures used in research studies should be carefully selected depending on the purpose of the study. Our findings further suggest that an individualized approach to upper extremity motion analysis may be more informative than group designs when exploring changes in motor performance.
Acknowledgments
This work was supported in part by HealthSouth Corporation, the Missouri Physical Therapy Association, the Foundation for Physical Therapy (SLD), NIH K01HD047669 (CEL), NIH R01HD055964 (CEL), and NIH T32HD007434 (SLD).
Contributor Information
Stacey L. DeJong, Email: sldejong@wustl.edu.
Rebecca L. Birkenmeier, Email: birkenmeierr@wustl.edu.
Catherine E. Lang, Email: langc@wustl.edu.
References
- Alberts JL, Butler AJ, Wolf SL. The effects of constraint-induced therapy on precision grip: a preliminary study. Neurorehabilitation and Neural Repair. 2004;18:250–258. doi: 10.1177/1545968304271370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askim T, Indredavik B, Vangberg T, Haberg A. Motor network changes associated with successful motor skill relearning after acute ischemic stroke: a longitudinal functional magnetic resonance imaging study. Neurorehabilitation and Neural Repair. 2009;23:295–304. doi: 10.1177/1545968308322840. [DOI] [PubMed] [Google Scholar]
- Beebe JA, Lang CE. Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. Journal of Neurologic Physical Therapy. 2009;33:96–103. doi: 10.1097/NPT.0b013e3181a33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Experimental Brain Research. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabilitation and Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, et al. Mechanisms of use-dependent plasticity in the human motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimmi M, Carda S, Giovanzana C, Maini ES, Sabatini AM, Smania N, et al. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabilitation and Neural Repair. 2008;22:31–39. doi: 10.1177/1545968307302923. [DOI] [PubMed] [Google Scholar]
- Canning CG, Ada L, O’Dwyer NJ. Abnormal muscle activation characteristics associated with loss of dexterity after stroke. Journal of the Neurological Sciences. 2000;176:45–56. doi: 10.1016/s0022-510x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- Celik O, O’Malley MK, Boake C, Levin HS, Yozbatiran N, Reistetter TA. Normalized movement quality measures for therapeutic robots strongly correlate with clinical motor impairment measures. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2010;18:433–444. doi: 10.1109/TNSRE.2010.2047600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Cirstea MC, Mitnitski AB, Feldman AG, Levin MF. Interjoint coordination dynamics during reaching in stroke. Experimental Brain Research. 2003;151:289–300. doi: 10.1007/s00221-003-1438-0. [DOI] [PubMed] [Google Scholar]
- Conti GE, Schepens SL. Changes in hemiplegic grasp following distributed repetitive intervention: a case series. Occupational Therapy International. 2009;16:204–217. doi: 10.1002/oti.276. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Annals of Neurology. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- Dedding C, Cardol M, Eyssen IC, Dekker J, Beelen A. Validity of the Canadian Occupational Performance Measure: a client-centred outcome measurement. Clinical Rehabilitation. 2004;18:660–667. doi: 10.1191/0269215504cr746oa. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle and Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dipietro L, Krebs HI, Fasoli SE, Volpe BT, Stein J, Bever C, et al. Changing motor synergies in chronic stroke. Journal of Neurophysiology. 2007;98:757–768. doi: 10.1152/jn.01295.2006. [DOI] [PubMed] [Google Scholar]
- Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JP. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle and Nerve. 2005;32:170–178. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MD, Sukal T, DeMott T, Dewald JP. Augmenting clinical evaluation of hemiparetic arm movement with a laboratory-based quantitative measurement of kinematics as a function of limb loading. Neurorehabilitation and Neural Repair. 2008;22:321–329. doi: 10.1177/1545968307313509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fess EE. Grip strength. In: Casanova JS, editor. Clinical assessment recommendations. Vol. 2. Chicago: American Society of Hand Therapists; 1992. pp. 41–45. [Google Scholar]
- Harris JE, Eng JJ, Miller WC, Dawson AS. A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi-site randomized controlled trial. Stroke. 2009;40:2123–2128. doi: 10.1161/STROKEAHA.108.544585. [DOI] [PubMed] [Google Scholar]
- Jang SH, Kim YH, Cho SH, Lee JH, Park JW, Kwon YH. Cortical reorganization induced by task-oriented training in chronic hemiplegic stroke patients. Neuroreport. 2003;14:137–141. doi: 10.1097/00001756-200301200-00025. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. Journal of Neuroscience. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research. 2008;51:S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Arm function after stroke: from physiology to recovery. Seminars in Neurology. 2005;25:384–395. doi: 10.1055/s-2005-923533. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restorative Neurology and Neuroscience. 2004;22:281–299. [PubMed] [Google Scholar]
- Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke. 2002;33:1840–1844. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabilitation and Neural Repair. 2007;21:279–291. doi: 10.1177/1545968306296964. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. Journal of Neurophysiology. 2004;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Bastian AJ, Hu Q, Edwards DF, Sahrmann SA, et al. Deficits in grasp versus reach during acute hemiparesis. Experimental Brain Research. 2005;166:126–136. doi: 10.1007/s00221-005-2350-6. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Archives of Physical Medicine and Rehabilitation. 2006a;87:1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabilitation and Neural Repair. 2006b;20:444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Canadian Journal of Occupational Therapy. 1990;57:82–87. doi: 10.1177/000841749005700207. [DOI] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119:281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabilitation and Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- Levin MF, Michaelsen SM, Cirstea CM, Roby-Brami A. Use of the trunk for reaching targets placed within and beyond the reach in adult hemiparesis. Experimental Brain Research. 2002;143:171–180. doi: 10.1007/s00221-001-0976-6. [DOI] [PubMed] [Google Scholar]
- Li S, Latash ML, Yue GH, Siemionow V, Sahgal V. The effects of stroke and age on finger interaction in multi-finger force production tasks. Clinical Neurophysiology. 2003;114:1646–1655. doi: 10.1016/s1388-2457(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Liepert J, Graef S, Uhde I, Leidner O, Weiller C. Training-induced changes of motor cortex representations in stroke patients. Acta Neurologica Scandinavica. 2000;101:321–326. doi: 10.1034/j.1600-0404.2000.90337a.x. [DOI] [PubMed] [Google Scholar]
- Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, Hsieh CL. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Physical Therapy. 2009;89:840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: Potential differential effects on amount of real-world limb use. Topics in Stroke Rehabilitation. 2009;16:237–253. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- Mayo NE, Wood-Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, et al. Disablement following stroke. Disability and Rehabilitation. 1999;21:258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ, Hodgson AJ. Biomechanics of reaching: clinical implications for individuals with acquired brain injury. Disability and Rehabilitation. 2002;24:534–541. doi: 10.1080/09638280110115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ, Hodgson AJ. Saturated muscle activation contributes to compensatory reaching strategies after stroke. Journal of Neurophysiology. 2005;94:2999–3008. doi: 10.1152/jn.00732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen SM, Dannenbaum R, Levin MF. Task-specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke. 2006;37:186–192. doi: 10.1161/01.STR.0000196940.20446.c9. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SJ, Levine P, Leonard A, Szaflarski JP, Kissela BM. Modified constraint-induced therapy in chronic stroke: results of a single-blinded randomized controlled trial. Physical Therapy. 2008;88:333–340. doi: 10.2522/ptj.20060029. [DOI] [PubMed] [Google Scholar]
- Pang MY, Harris JE, Eng JJ. A community-based upper-extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2006;87:1–9. doi: 10.1016/j.apmr.2005.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TS, Bishop MD, McGuirk TE, Sethi A, Richards LG. Reliability of upper extremity kinematics while performing different tasks in individuals post stroke. Journal of Motor Behavior. doi: 10.1080/00222895.2010.548422. In Press. [DOI] [PubMed] [Google Scholar]
- Platz T, Winter T, Muller N, Pinkowski C, Eickhof C, Mauritz KH. Arm ability training for stroke and traumatic brain injury patients with mild arm paresis: a single-blind, randomized, controlled trial. Archives of Physical Medicine and Rehabilitation. 2001;82:961–968. doi: 10.1053/apmr.2001.23982. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain. 2003;126:2510–2527. doi: 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Feydy A, Combeaud M, Biryukova EV, Bussel B, Levin MF. Motor compensation and recovery for reaching in stroke patients. Acta Neurologica Scandinavica. 2003;107:369–381. doi: 10.1034/j.1600-0404.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Kraft E, Hilliard TS, Dijkhuizen RM, Benner T, Finklestein SP, et al. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabilitation and Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Lang CE, Reilly KT, McNulty P, Sirigu A. Selective activation of human finger muscles after stroke or amputation. Advances in Experimental Medicine and Biology. 2009;629:559–575. doi: 10.1007/978-0-387-77064-2_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- Thielman G, Kaminski T, Gentile AM. Rehabilitation of reaching after stroke: comparing 2 training protocols utilizing trunk restraint. Neurorehabilitation and Neural Repair. 2008;22:697–705. doi: 10.1177/1545968308315998. [DOI] [PubMed] [Google Scholar]
- Van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Archives of Physical Medicine and Rehabilitation. 2001;82:14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- Wagner JM, Dromerick AW, Sahrmann SA, Lang CE. Upper extremity muscle activation during recovery of reaching in subjects with post-stroke hemiparesis. Clinical Neurophysiology. 2007;118:164–176. doi: 10.1016/j.clinph.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Physical Therapy. 2008;88:652–663. doi: 10.2522/ptj.20070255. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Archives of Physical Medicine and Rehabilitation. 2004;85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Journal of the American Medical Association. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Woodbury ML, Howland DR, McGuirk TE, Davis SB, Senesac CR, Kautz S, et al. Effects of trunk restraint combined with intensive task practice on poststroke upper extremity reach and function: a pilot study. Neurorehabilitation and Neural Repair. 2009;23:78–91. doi: 10.1177/1545968308318836. [DOI] [PubMed] [Google Scholar]
- Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabilitation and Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]