Abstract
The silk-elastinlike protein polymer, SELP 815K, and poloxomer 407, a commercially available synthetic copolymer, were evaluated to compare their relative performance in matrix mediated viral gene delivery. Using a xenogenic mouse tumor model of human head and neck squamous cell carcinoma, the efficacy of viral gene-directed enzyme prodrug therapy with these polymers was characterized by viral gene expression in the tumor tissue, tumor size reduction, and survivability with treatment. Viral injection in SELP 815K produced a greater level and more prolonged extent of gene expression in the tumor, a statistically greater tumor size reduction, a longer time until tumor rebound, and a significantly increased survivability, as compared to injection of virus alone or in poloxamer 407. Safety of treatment with these polymers was evaluated in a non-tumor bearing immunocompetent mouse model. Compared to virus injected alone or in poloxamer 407, virus injected in SELP 815K had fewer and less severe indications of toxicity related to treatment as assessed by blood analysis, body weight, and histopathology of distant organs and the injection sites. Similar to virus alone or in poloxamer 407, virus injected in SELP 815K elicited a mild injection site inflammatory response characterized primarily by a mononuclear leukocyte infiltrate and the formation of granulation tissue. Virus injected in SELP 815K resulted in fewer animals with elevated white blood cell counts and a less pronounced local toxicity reaction than was observed with virus in poloxamer 407. In contrast to virus injected alone or in poloxamer 407, which were not retained in the injection site tissues beyond week 1, SELP 815K was retained at the injection sites and by the end of the study (week 12), displayed limited absorption, and mild encapsulation. These results demonstrate the benefits of SELP 815K for matrix-mediated gene delivery over the injection of free virus and the injection of virus in Poloxamer 407. Virus in SELP 815K had greater efficacy of tumor suppression, promoted greater levels and greater duration of viral gene expression, and displayed reduced levels of injection site toxicity. Combining these performance and safety benefits with the degree of control with which they can be designed, synthesized and formulated, SELPs continue to show promise for their application in viral gene delivery.
Keywords: Protein polymers, Silk-elastinlike polymers, gene delivery, poloxomers, head and neck cancer
1.Introduction
The possibility of correcting the genetic cause of disease has drawn researchers to study gene therapy as a treatment method for a variety of deleterious conditions, including cancer.(Friedmann and Roblin, 1972) With all of its promise, gene therapy is confronted with a number of obstacles to its widespread clinical use. A major challenge is the safe and effective delivery of the therapeutic gene to the targeted cells while avoiding potentially harmful effects to non-targeted cells and organs.(Verma and Weitzman, 2005) Methods for accomplishing this involve the use of viruses as gene carriers (Kay et al., 2001) or non-viral gene carriers such as cationic polymers or liposomes.(Li and Huang, 2006)
While viral gene carriers exploit the highly evolved transfection efficiencies of viruses, they also suffer from the host immune system's ability to combat the infectious threat.(Muruve, 2004) Several approaches to minimize the systemic effects of viral carriers have resulted in limited success. One approach, the surface chemical modification of the viral carrier to suppress its activation of the immune system, has unfortunately resulted in a reduction in transfection efficiency. (Wortmann et al., 2007) A second approach, administering the viral carrier in a localized manner to avoid systemic exposure and off target transfection, has yielded less than optimal results due to the propensity of the viral carrier to disseminate from a local injection site when unconstrained.(Gustafson and Ghandehari, 2010) A combination of the two approaches has led to a promising solution, i.e., matrix mediated viral carrier delivery. The viral carrier is injected in a biomaterial that limits its exposure to the immune system and limits its dissemination from the injection site. Furthermore, the specific physical and chemical characteristics of the biomaterial can be used to control the release of the viral carrier to the surrounding tissues in a localized and sustained manner.(Gu et al., 2004)
Among the numerous biomaterials investigated for matrix-mediated gene delivery, genetically engineered, silk-elastinlike protein polymers (SELPs) have demonstrated important potential advantages. SELPs are block copolymers that consist of repeating amino acid sequence blocks modeled from silkworm silk fibroin (GAGAGS) and mammalian elastin (GVGVP).(Cappello et al., 1990) Depending on their specific sequence and composition, which can be precisely controlled, SELPs are soluble in aqueous solution at room temperature, can be mixed with viral gene carriers, and injected intratumorally where they form insoluble hydrogels for localized delivery. These polymers are produced using recombinant DNA technology, which allows for a high degree of control over both the sequence and length of the polymers formed. This class of polymers has undergone extensive characterization and analysis for their physicochemical properties and release characteristics cited elsewhere. (Cresce et al., 2008; Dandu et al., 2009; Dandu et al., 2008; Dandu, 2007; Dinerman et al., 2010; Dinerman et al., 2002a, b) Our investigations of the structure-function relationship of SELPs enabled us to design SELP 815K (Figure 1) to provide prolonged release of adenoviral vectors in a head and neck tumor model.(Greish et al., 2010; Gustafson et al., 2009)
Figure 1.
Structure of investigated polymers: (A) silk, elastin, and lysine substituted elastin blocks in addition to SELP-815K in single amino acid code, (B) chemical structure of poloxamer 407.
However, SELPs present several challenges as biomaterials. SELPs have no prior history of FDA approved medical use, their biosynthesis is complex, and their manufacture can be costly. The question arises as to whether a readily available and FDA approved copolymer with appropriate injectability such as a Poloxomer can provide comparable performance to SELPs in intratumoral controlled release of adenoviruses. Poloxamers are tri-block copolymers that consist of repeats of poly(oxyethylene) and poly(oxypropylene). These polymers have been studied and largely characterized in the controlled release and pharmaceutical industries. (Dumortier et al., 2006) Previous studies have shown that matrix-mediated delivery by these copolymers prolongs and localizes viral gene expression in vivo. In particular Wang et. al. studied this class of polymers with respect to localized adenoviral administration finding poloxamer 407 at a concentration of 21 wt. % to be the most effective formulation. (Wang et al., 2005) The current studies were designed to compare directly the in-vivo safety and efficacy of these two biomaterials for matrix-mediated viral gene delivery.
2.Materials and Methods
2.1 Materials
SELP 815K was synthesized and characterized as previously described (structure in Figure 1A).(Dandu et al., 2009) Poloxamer 407 (Figure 1B) was obtained from Sigma-Aldrich (St. Louis, MO) under the trade name Pluronic F-127. Replication deficient human adenovirus, containing E1 and E3 deletions, and the genes for thymidine kinase and firefly luciferase (Ad.Luc.HSVtk) were obtained from Vector Biolabs (Philadelphia, PA). The JHU-022 human oral cancer cell line was a gift from Prof. David Sidransky of Johns Hopkins University. Luciferin was obtained from Gold Biotech (St. Louis, MO), and bioluminesence was measured using a Xenogen IVIS100 bioluminescence imaging system from Caliper Life Sciences (Hopkinton, MA). Roswell Park Memorial Institute Advanced 1640 medium (RPMI 1640 Advanced) and 100 mM L-glutamine were obtained from Invitrogen (Carlsbad, CA). Fetal Bovine Serum was purchased from HyClone (Logan, UT). Sodium heparin was obtained from APP Pharmaceuticals (Schaumburg, IL). The prodrug Ganciclovir (GCV) was purchased from Sigma-Aldrich (St.Louis, MO) Athymic nu/nu mice and CD-1 mice (both female, 6 weeks old) were obtained from Charles River Laboratories (Wilmington, MA ). Blood analysis was conducted using a HESKA Complete Blood Count Analyzer and HESKA DriCHEM 4000, HESKA (Loveland, CO).
2.2 Study Designs
2.2.1 Squamous Cell Carcinoma Model
JHU-022 was thawed from frozen stocks of passage number less than 26 and expanded in culture for two weeks in RPMI 1640 Advanced medium from one T-75 culture flask to approximately 12 T-500 triple flasks. Cells were harvested from flasks by treatment with Tryple Express trypsin-like enzyme. Tryple Express was removed by centrifugation of the cells at 1000xg for 5 minutes and aspiration of the medium. Cells were resuspended in 15 mL of cold, sterile 0.9% saline to a concentration of 12.5 million per mL. Tumors were induced in female athymic nu/nu mice by injecting 2.5 million cells (200μL) into the right flank of each animal and were allowed to grow for 10 days until they were 5mm to 7mm in largest dimension.
Following tumor induction, 36 mice were randomly assigned to four treatment groups (9 animals per group): Control (saline injection), Virus Only injection, Poloxamer 407 + Virus, and SELP 815K + Virus. Animals of each group except control were injected with 5×108 plaque forming units (pfu) of Ad.Luc.HSVtk suspended in a consistent volume of 25μL. Control animals were injected with 25μL of 0.9% saline. 25μL injection volumes were determined optimal from past laboratory experience for xenograft, head and neck mouse tumor models. (Greish et al., 2009; Greish et al., 2010). The SELP 815K + Virus injection solution was prepared by thawing 12 wt% stock polymer solution stored in -80° C, mixing with the virus stock solution, and diluting with 0.9% saline to a final polymer concentration of 4% (w/v). The Poloxamer 407 + Virus injection solution was prepared by dissolving 0.21g dry poloxamer 407 to a final volume of 1.0 mL with fluid comprised of 0.9% saline and virus stock solution to yield a final poloxamer 407 concentration of 21% (w/v). This concentration was chosen appropriate for release of viral carriers as determined by previous work. (Wang et al., 2005) Solvation of dry poloxamer 407 was achieved with repeated steps of ice chilling and vortexing. Virus or control injections were administered to each animal after solid tumor formation. The time of virus injection was designated as Day 0 of the study.
Ganciclovir injection solution was prepared by dissolving the drug in 0.9% saline to a concentration of 1mg/mL. The drug was administered daily at a dose of 25mg/kg for the first 28 days of the study (the treatment period) by intraperitoneal injection. The duration of the treatment period was previously determined from pilot study data demonstrating that intratumoral expression of the therapeutic enzyme diminished to subtherapeutic levels after this time period. Tumor size was measured using calipers biweekly for 50 days. All measurements were by the same investigator. Body weight was measured daily. Animals were observed daily for mortality and signs of tumor or treatment morbidity. There was no premature mortality in the study. By protocol, any animal that displayed tumor volume >2000 mm3, necrosis of tumor site, or greater than 10% weight loss was euthanized. Euthanasia was performed by CO2 asphyxiation. Final tumor size and body weight were recorded prior to euthanasia. The study protocol was approved and the study conducted under the authority of the Institutional Animal Care and Use Committee of the University of Utah.
2.2.2 Intratumoral Bioluminesence Expression
Cell culture, tumor induction, material preparation and administration, and treatment were performed as described in section 2.2.1. Bioluminesence was generated in vivo by intraperitoneal injection of 200μL luciferin at a concentration of 15mg/mL in 0.9% saline. Imaging was performed 30 minutes after administration of luciferin to each mouse using a 30 second exposure time in a Xenogen IVIS 100 Imager. Images were analyzed using Igor Pro software from Caliper Life Sciences. Bioluminesence images were taken biweekly for 21 days. Animal body weight was monitored daily. Total photon count density for each animal tumor was determined using Igor Pro image analysis software provided by Caliper Life Sciences working in conjunction with the IVIS 100 imaging system. This software is capable of yielding photon count numbers for any region of interest in images taken with the IVIS 100 imaging system. The region of interest was defined in this study as the area approximately the size of the tumor that encompassed all photon emissions from the tumor. Individual total photon counts for all animals in each group were averaged to yield the average total photon count for each study group. The study protocol was approved and the study conducted under the authority of the Institutional Animal Care and Use Committee of the University of Utah.
2.2.3 Safety Study
160 female CD-1 mice were randomly assigned to four treatment groups (40 animals per group): Control (saline), Virus Only injection, Poloxamer 407 + Virus, and SELP 815K + Virus. Within each treatment group, animals were assigned to four sacrifice time points (10 per time point): 1 week, 2 weeks, 4 weeks, and 12 weeks. Animals of each group except control were injected with 3.79×109 plaque forming units (pfu) of Ad.Luc.HSVtk suspended in each polymer or 0.9% saline. Material preparations for injections and treatment were performed in the same manner as described in section 2.2.1 except that the injections on Day 1 were administered subcutaneously and the volumes were increased to 50uL for each mouse in order to accommodate the increased viral dose.
Each mouse was weighed and inspected daily during the treatment phase of the study (through Day 28), then weekly for the remainder of 12 weeks. At the conclusion of each time point, designated mice were euthanized by CO2 asphyxiation and blood was immediately collected using 1.0 cc tuberculin syringes and 25G needles that had been previously flushed with 1000 IU/mL of sodium heparin to prevent coagulation. The heart, liver, lung, spleen, kidney, and the injection site of each animal were harvested and stored in 10% formalin for histological analysis. The study protocol was approved and the study was conducted under the authority of the Institutional Animal Care and Use Committee of the University of Utah.
2.3 Analysis
2.3.1 Blood analysis
For complete blood counts, 20uL samples of heparinized, fresh, whole blood were drawn from each animal and analyzed within 1 hour of collection. Blood counts were performed using a Heska CBC Diff blood analyzer. Blood count parameters measured were total white blood cell count, lymphocyte count, monocyte count, granulocyte count, hematocrit, red blood cell count, mean corpuscular volume, red blood cell distribution width, hemoglobin concentration, mean corpuscular hemoglobin concentration, mean corpuscular hemoglobin, platelet count, and mean platelet volume. For blood chemistry analysis, blood samples were centrifuged in heparinized vials at 10,000 RPM for 2 minutes and the plasma was analyzed for blood urea nitrogen, creatinine, total protein, albumin, total bilirubin, alanine aminotransferase, and aspartate aminotransferase. Blood chemistry was performed using a Heska Dri Chem 4000 blood analyzer. Normal ranges were used as defined from the blood analyzer manufacturer.
2.3.2 Histology
Animal tissue samples and organs were stored in 10% formalin for a minimum of 24 hours to achieve fixation. Organs were further sectioned into slices approximately 2mm thick and embedded in paraffin. Subcutaneous injection sites including the surrounding skin and the underlying muscle were embedded in block. Embedded tissues were sectioned by microtome to 5μm thick sections, mounted on glass slides, and stained with hematoxylin and eosin. Tissue and histological slide preparation was conducted by ARUP Laboratories (Salt Lake City, Utah). Slides were examined microscopically.
2.3.3 Statistical analysis
Student's t-test was used to compute statistical significance between the treatment and control groups within studies. Mantel-Cox test was used for analysis of survival curves. A value of p ≤ 0.05 was considered statistically significant and p ≤ 0.01 was considered highly statistically significant.
3.Results
3.1 Efficacy in Squamous Cell Carcinoma Model
The tumor model of head and neck squamous cell carcinoma (HNSCC) in mice was used to compare the performance of SELP 815K and poloxamer 407 as matrices for viral gene delivery. This model was chosen for its ease of access to a tumor for local injection and treatment assessment, and because of the presence of the Coxsackievirus and adenovirus receptor (CAR) making them sensitive to adenoviral transfection. Immune compromised athymic nu/nu mice were subcutaneously inoculated with the JHU-022 human HNSCC cell line isolated from a solid tumor of oral origin at Johns Hopkins University in the laboratory of Prof. David Sidransky.(Liggett et al., 1996) Induced subcutaneously, these tumors grow rapidly to assess treatment benefits in a relatively short time period while avoiding the much greater mortality and morbidity of orthotopic HNSCC tumors, for example, induced in the oral cavity.
Gene directed enzyme prodrug therapy (GDEPT) was the treatment modality used in this study. GDEPT relies on delivery of a gene for a therapeutic enzyme to targeted cells. The encoded enzyme converts a non-toxic, systemically administered prodrug into a cytotoxic metabolite that causes cell death only in transfected cells.(Denny, 2003) In this case, adenovirus containing the genes for thymidine kinase and luciferase was injected intratumorally at Day 0 followed by daily systemic dosing of the nontoxic prodrug ganciclovir for the next 28 days. Transfected cells expressing thymidine kinase convert ganciclovir to ganciclovir phosphate, a cytotoxic metabolite. A limitation of this approach is the rapid dissemination of adenovirus from the site of injection, thus reducing the amount of local transfection and causing undesired systemic side effects. A matrix-mediated approach is designed to control the release rate of virus over several weeks, limiting dissemination of virus from the injection site and increasing transfection in targeted local tissues over a longer period of time. While SELP hydrogels prolong viral release, it has not been proven if and to what extent they increase viral stability in vivo. The increased duration of viral protein expression in tumor cells released from SELP hydrogels compared to free virus suggests that they also increase viral bioactivity in vivo possibly through protein-protein interactions or physical sequestration. (Greish et al., 2009; Greish et al., 2010)
We used this tumor model and treatment strategy to compare the performance of the polymers SELP 815K and Poloxamer 407 in matrix-mediated viral gene delivery. On Day 1 of the study, mice with a tumor between 5–7 mm in longest diameter were injected intratumorally with 5×108 plaque forming units (pfu) of Ad.Luc.HSVtk. The virus was either injected alone (in 0.9% saline) or in SELP 815K (4% w/v concentration chosen based on our previous work (Gustafson et al., 2009) or poloxamer 407 (21% w/v concentration chosen based on published work). (Wang et al., 2005) Control mice were injected with 0.9% saline. Ganciclovir was administered daily thereafter for 28 days to all groups including control. Animals were observed for 22 additional days post-treatment for a total study duration of 50 days (Figure 2A). As a standalone therapy, this treatment protocol is not intended to achieve tumor elimination in mice. The tumor size reduction followed by tumor rebound expected with this protocol provides a baseline for assessing the effectiveness of treatment options and variables.
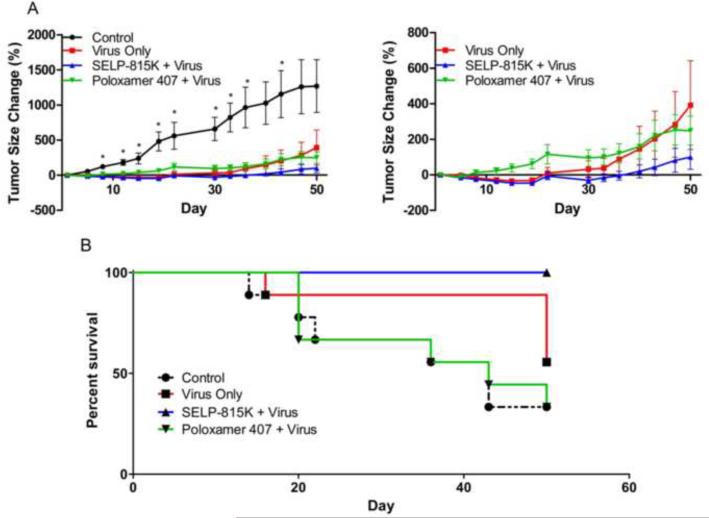
Figure 2.
Effect of matrix mediated gene directed enzyme prodrug therapy on induced JHU-022 squamous cell carcinoma tumors in athymic nu/nu mice, (A) Left panel: tumor size measured with calipers biweekly and normalized to day 0; Right panel: larger view of treatment groups alone. (B) Survivability observed as number of animals not having: tumor volume exceed 2000mm3, unacceptable necrosis, or weight loss of >10%, *SELP 815K + Virus statistically significant from Poloxamer 407 21% + Virus
All virus treatment groups achieved greater reduction in mean tumor size than control, indicating that the virus was therapeutically active and that injection of the virus in SELP 815K or poloxamer 407 did not abolish its therapeutic activity. Throughout the study, the SELP 815K + Virus group had the least mean tumor size of all treatment groups. The Poloxamer 407 + Virus group had the greatest mean tumor size of all treatment groups at all time points except Days 47 and 50, when the Virus Only group was greater. However, at these last time points a greater number of animals in the Poloxamer 407 + Virus group were eliminated by sacrifice due to excessive tumor morbidity. The differences in mean tumor size of the SELP 815K + Virus and the Poloxamer 407 + Virus groups were statistically significant from Day 8 to Day 36 and at Day 43. Neither polymer treatment group achieved statistical significance from the Virus Only group.
Tumor rebound, defined as the time after treatment that the mean tumor size of a group regained its Day 0 average volume, demonstrated a similar difference between treatment groups. The first group to rebound was Poloxamer 407 + Virus at Day 5. Subsequent to this, the Virus Only group rebounded at Day 19 followed by the SELP 815K + Virus group at Day 36.
The second outcome assessment of the efficacy study was survivability. Survival endpoint was defined by protocol as unscheduled mortality or sacrifice due to a tumor burden >2000mm3, an unacceptable level of tumor necrosis, or an unacceptable level of weight loss (>10%). The SELP 815K + Virus group did not have any unscheduled animal deaths for the duration of the study (100% survivability) while control and other treatment groups had multiple unscheduled sacrifices (Figure 2B). The Poloxamer 407 + Virus group and the Virus Only group had a final survivability of 33% and 56%, respectively. Statistical analysis using the Mantel-Cox test revealed that the SELP 815K + Virus treatment group had a highly statistically significant difference in survivability compared to Poloxamer 407 + Virus (p=0.0051) and was also statistically significant over Virus Only injection (p=0.038).
3.2 Intratumoral Expression
To compare the release profiles of the matrix-mediated delivery systems in vivo, the intratumoral expression of viruses was analyzed as a function of bioluminescence produced by luciferase expression caused by transfection of released adenoviruses. This study was performed in the same mouse tumor model as the efficacy study using the same viral injection compositions and treatments (Section 3.1). At early time points, the Virus Only and SELP 815K + Virus treatment groups had indistinguishable average expression levels, however the Poloxamer 407 + Virus group had a statistically significantly lower expression than the SELP 815K + Virus group at Day 4 (Figure 3). As the study progressed to Day 21, SELP 815K + Virus group maintained a higher average total expression level than the virus alone and the Poloxamer 407 + Virus groups. The Poloxamer 407 + Virus group did have higher tumor expression over virus alone from Day 14 to Day 21. While bioluminescence is semi-quantitative, this data was compared to quantitative Beta-galactosidase expression from a separate adenoviral carrier in a separate study over similar time points and the trends were found to be similar (data not shown).
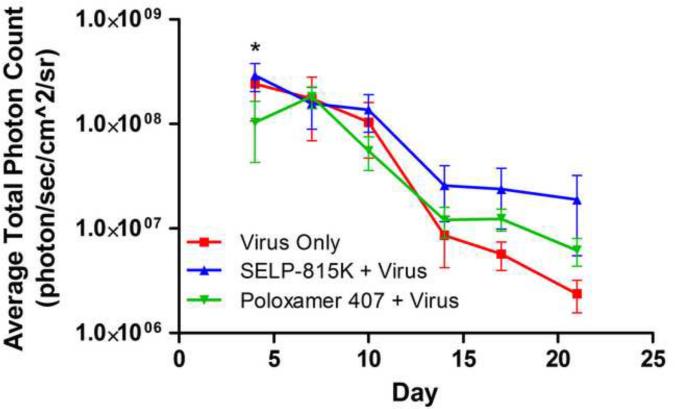
Figure 3.
Measured intratumoral bioluminescence from matrix delivered luciferase encoding adenovirus to JHU-022 squamous cell carcinoma tumors in athymic nu/nu mice, bioluminescence measured biweekly and reported as average total photon count emitted from tumors during recording period. *SELP 815K statistically significant from Poloxamer 407
3.3 Safety
An important parameter for a matrix-mediated gene delivery approach is its safety. The possible effects on safety of SELP 815K and Poloxamer 407 viral injection on GDEPT treatment were evaluated. In this study, immunologically competent, non-tumor bearing mice were used in order to include possible host-related immunological effects. Treatments and control were administered as in the efficacy and expression studies, except that a greater dosage of adenovirus was injected subcutaneously (3.79×109 pfu of Ad.Luc.HSVtk per mouse) in a greater injection volume (50μL) to accommodate the larger viral dose. Animals were administered ganciclovir for 28 days, followed by a post-treatment observation period through the 12-week endpoint of the study.
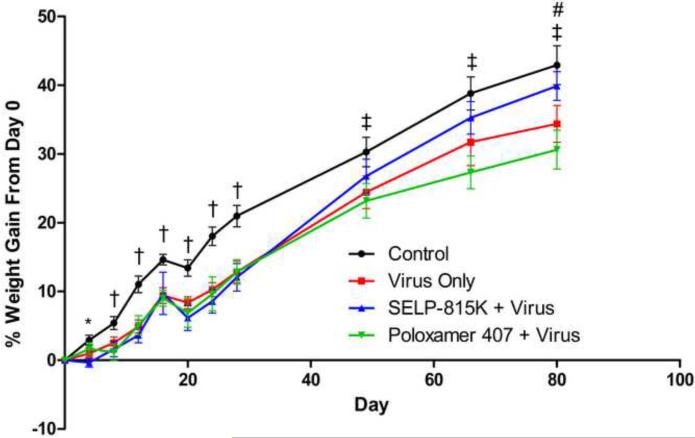
Animal body weight was used as an indicator of overall animal health. As observed in Figure 4 no treatment group had a net mean body weight loss for the duration of the treatment. There were however, statistically significant differences in mean body weight between all treatment groups compared to control from day 7 to day 28 (during the treatment period with ganciclovir). Immediately after Day 28, the mean body weight of the SELP 815K + Virus treatment group recovered to non-significance compared to control. In contrast, the mean body weight of the Poloxamer 407 + Virus group remained statistically significantly less than control for the remainder of the study. The Virus Only group had a statistically significant difference in mean body weight from control for approximately half of the post-treatment observation period.
Figure 4.
Effect of matrix mediated controlled release of therapeutic adenovirus to immune competent, non-tumor bearing CD-1 mice on animal weight as measured daily and normalized for percent weight change from Day 0. †All treatment groups statistically significant from control. ‡Poloxamer 407 statistically significant from control *SELP 815K statistically significant from control #Virus statistically significant from control
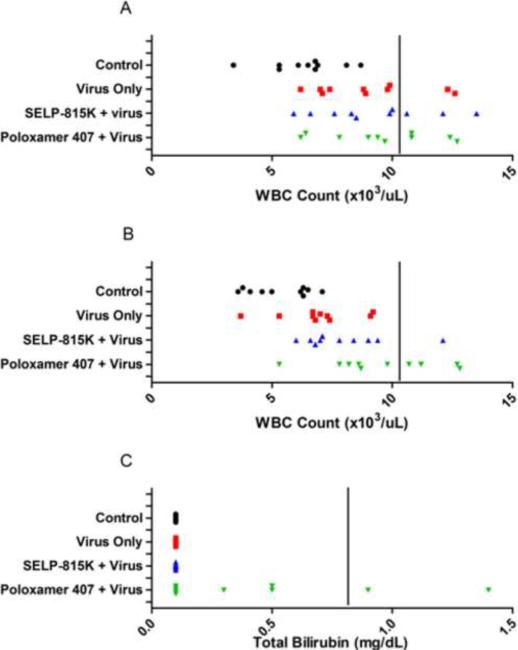
At the 1, 2, 4, and 12 week time points, 10 pre-designated animals per group were euthanized and blood analysis was performed. All parameters measured in complete blood count and blood chemistry analyses (Section 2.3.1) yielded values similar to the control group except for white blood cell counts and total bilirubin. Individual values for total white blood cell counts at weeks 1 and 2 and total bilirubin at week 2 are shown in Figure 5. At week 1, an elevation in total white blood cell counts above the normal range was observed in 20%, 30%, and 40% of animals in the Virus Only, SELP 815K + Virus, and Poloxamer 407 + Virus treatment groups, respectively. At week 2, the percent of animals above the normal WBC range remained at 40% for the Poloxamer 407 + Virus group while it fell to 10% and none in the SELP 815K + Virus and Virus Only groups, respectively. At time points thereafter, WBC counts for all groups were within the normal range. The percent of animals with total bilirubin values outside the normal range was 20% in the Poloxamer 407 + Virus group and none in the SELP 815K + Virus and Virus Only groups. No other abnormal blood count or blood chemistry values were obtained in the study.
Figure 5.
Effect of matrix mediated controlled release of therapeutic adenovirus to immune competent, non-tumor bearing CD-1 mice on blood parameters as measured at necropsy, (A) week 1 total white blood cell count (B) week 2 total white blood cell count (C) week 2 total bilirubin. Normal ranges defined from blood analyzer manufacturer for mice bounded by solid black line. Each point represents individual animal.
Microscopic examination of histological tissue sections obtained from the animals of all treatment groups and at all sacrifice time points showed no abnormalities or signs of pathology for liver, kidney, spleen, heart, or lungs.
3.4 Injection Site Histological Evaluation
Injection site tissues of animals in the safety study were prepared histologically and examined microscopically after staining with hematoxylin and eosin. Photomicrographs of representative injection site tissues at Weeks 1, 2, 4, and 12 after injection are presented in Figure 6. The injection sites of animals administered virus in all groups displayed a subcutaneous lesion characterized by a granulomatous cellular infiltration. For animals of the SELP 815K + Virus group, the lesions were localized to an amorphous mass presumed to be the SELP 815K polymer hydrogel. In the Virus Only and Poloxamer 407 + Virus groups, the lesions were more diffuse with multiple lesion locations. At week 1, the cellular infiltrate of injection sites of all virus injection groups was composed primarily of mononuclear leukocytes including neutrophils, monocytes, macrophages, and lymphocytes. At later time points, fibroblasts and occasional foreign body giant cells were observed.
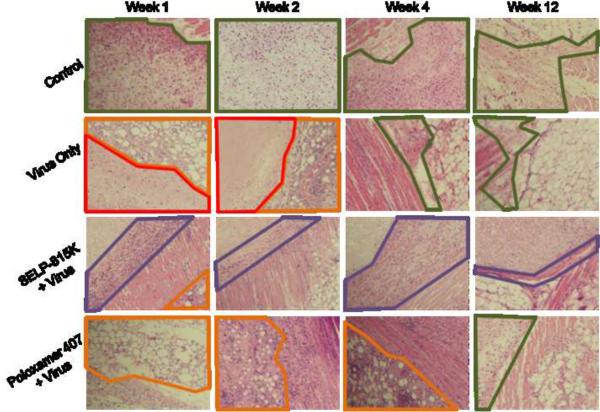
Figure 6.
Effect of matrix mediated controlled release of adenovirus on injection site structure visualized via H&E stained injection sites from weeks 1,2,4, and 12 (left to right) and groups Control, Virus Only, SELP 815K + Virus, and Poloxamer 407 + Virus (top to bottom). Normal fibrosis (green outline) due to injection is seen in control animals while necrotic tracts (red outline) and adipocytic necrosis (orange outline) can be seen in Virus Only weeks 1 and 2. Adipocytic necrosis is also seen in weeks 1, 2, and 4 in Poloxamer 407 + Virus. Progressive encapsulation (purple outline) is seen in SELP 815K + Virus panels.
A notable difference between the treatment groups was the presence of adjacent tissue necrosis observed in the Virus Only and Poloxamer 407 + Virus groups, which was absent in the SELP 815K + Virus group. At week 1 and 2, tissue necrosis characterized by acellular prefibrotic tracts within the injection site was noted in the Virus Only injection group. These were not observed in the Poloxamer 407 + Virus or SELP 815K + Virus groups. Primarily at the early time points (Weeks 1 to 4), cellular necrosis of adipocytes was observed in the Virus Only and Poloxamer 407 + Virus group. Additionally, evidence of neural degeneration was observed in the Poloxamer 407 + Virus group. This was generally absent in the SELP 815K + Virus group. By week 12 the signs of injection were largely absent in all groups except the SELP 815K + Virus group in which there was a clear non-degraded, mildly encapsulated gel mass present.
4. Discussion
Consistent with previous work, our results demonstrate that gene-directed enzyme prodrug therapy (GDEPT) using an adenoviral vector is effective in reducing tumor size and increasing survivability in a mouse tumor model of HNSCC. Moreover, the efficacy and the safety of the treatment are improved using SELP 815K for matrix-mediated localized delivery of the virus. The current studies compare SELP 815K, a silk-elastinlike protein copolymer, with poloxamer 407, a commercially available synthetic copolymer, in their effectiveness and safety of administration of GDEPT.
The results of the efficacy study indicated that GDEPT was more effective when virus was administered in SELP 815K. The SELP 815K + Virus group had greater tumor size reduction and longer time until tumor rebound than the Poloxamer 407 + Virus and the Virus Only groups (Figure 2A). Survivability of the SELP 815K + Virus group was greater than for the Poloxamer 407 + Virus and the Virus Only groups (Figure 2B).
We hypothesize that the increased effectiveness of GDEPT when administered in SELP 815K is linked to increased levels and/or greater duration of expression of the viral genes. Indeed, the intratumoral expression analysis revealed that administration of virus in SELP 815K produced early high-level viral gene expression comparable to Virus Only and maintained the highest levels of the three groups after Day 14, when Virus Only levels were considerably reduced. Administration of virus in poloxamer 407, by contrast, produced the lowest early expression of the three groups, but the lower level was somewhat maintained after Day 14, greater than the Virus Only level over this period.
The greater intratumoral expression of viral genes in the SELP 815K + Virus and the Virus Only groups correlated with the observed greater early reduction in tumor size compared to the Poloxamer 407 + Virus group. Additionally, the higher-level expression of viral genes in the SELP 815K + Virus group through Day 21 correlated with the longest tumor rebound time (Day 36) as compared to Day 19 for the Virus Only and Day 5 for the Poloxamer + Virus groups. These correlations are consistent with the presumption that high initial expression of the therapeutic gene is necessary for initial tumor reduction. When high initial expression was not present, as in the Poloxamer 407 + Virus group, the effectiveness of treatment decreased. Secondly, prolonged expression of the therapeutic gene prevented or delayed tumor rebound or recurrence. Even though the expression of the Poloxamer 407 + Virus group was sustained from Day 14 to Day 21 at a level greater than the Virus Only group, it apparently was not at a sufficient level to delay tumor rebound.
A possible explanation could be the inherent release characteristics of a poloxamer 407 gel. Previous observation by others has revealed that the prevalent mechanism of small molecular weight drug release from poloxamer gels is dissolution of the matrix, not diffusion through the matrix.(Dumortier et al., 2006) The large size of adenovirus would likely entrap viral particles in the poloxamer gel limiting initial release at early time points, preventing high transfection and expression levels. Indeed, histological examination of the Poloxamer 407 + Virus injection sites revealed an absence of injected material after week 1, consistent with the rapid loss of the matrix.
Our data suggests that there may be continuing efficacy beyond Day 28 in the SELP 815K + Virus group when ganciclovir administration was discontinued. This could be due to the continued presence of the active metabolic product of ganciclovir, which has a half life of 12–18 hours in cells.(Tomicic et al., 2001) Alternatively, the effect could simply be an accounting lag in the determination of tumor rebound. Tumors that had responded to treatment by reduction in size relative to Day 0 would require several days after the end of treatment efficacy to regain their pre-treatment size, thus delaying their designation as rebounded tumors. Further, cellular tumor death can result in the release of intracellular components increasing the measured physical size of the tumor due to an inflammatory response. Such inflammatory mass was noted in our previous work and can require days to weeks to subside, which also may explain the extended apparent therapeutic effect beyond the temporal administration of ganciclovir. (Greish et al., 2009; Greish et al., 2010)
Our safety study demonstrated no significant adverse effects related to the injection of virus in SELP 815K or Poloxamer 407. Even at a viral dose nearly 8 times that used in the efficacy and intratumoral expression studies, no treatment group showed signs of systemic toxicity indicated by significant loss of weight (Figure 4). This is a promising result as systemic immune reactions manifested as body weight loss or reduced weight gain and significantly elevated white blood cell populations to adenoviral administration were observed previously in our studies using non-matrix mediated administration. (Gustafson et al., 2010) Histological examination of heart, liver, lung, spleen, and kidney did not reveal any signs of pathology or abnormal anatomy. At early time points, elevated total white blood cell counts were observed as expected in response to adenoviral administration. Virus injected in SELP 815K did reduce the number of animals with an elevated total white blood cell count in comparison with the Poloxamer 407 + Virus group at Week 2. Elevated total bilirubin levels at Week 2 were observed in the Poloxamer 407 + Virus group. However, the absence of elevated ALT and AST liver enzyme levels indicated that significant liver toxicity was unlikely.
Examination of injection site histology revealed signs of localized inflammation and mild toxicity. These observations were most pronounced at early time points and in animals injected with Virus Only. The effects could be due simply from the injection of a large titer of free adenovirus. Gross tissue necrosis was only observed in the Virus Only group, lending support to this hypothesis. Adipocytic necrosis was observed more broadly in both the Virus Only and Poloxamer 407 + Virus injection sites. This observation also persisted longer in the Poloxamer 407 + Virus group. While the effect was likely virus related, it also could have been exacerbated by poloxamer 407, which has been reported to have an adjuvant effect when administered with an immunological agent. (Westerink et al., 2001) In fact, SELP 815K + Virus injection, which produced high early viral gene expression and tumor growth suppression, had a substantially reduced incidence of adipocytic necrosis indicating that the effect is not obligatorily associated with high early viral release.
SELP 815K hydrogel material was observed in the SELP 815K + Virus injection sites throughout the 12-week study. In contrast, Virus Only and Poloxamer 407 + Virus had no observable material present in the injection site tissues beyond 1 week. Over time, a fibrotic layer formed around the remaining gel mass eventually maturing to a compact, likely collagenous layer by week 12. Encapsulation appeared to occur after viral release and therapeutic activity was completed at Day 28 (the treatment period) of the efficacy study, and therefore had no apparent effect on viral release from SELP 815K. Beyond this point any effects on viral release of encapsulation would not be observed.
Together our results confirm the utility of SELP 815K for matrix-mediated gene delivery. Compared to poloxamer 407, a commercially available synthetic copolymer, SELP 815K had greater efficacy in tumor suppression, promoted greater levels and greater duration of viral gene expression, and displayed reduced levels of injection site toxicity. Additionally, SELP 815K displayed a comparable or better safety profile than poloxamer 407. With immunoreactivity among the chief concerns over the therapeutic administration of adenoviruses, it is noteworthy that SELP 815K + Virus injection resulted in less animals with elevated white blood cell counts and an absence of local toxicity reaction than was observed with poloxamer 407. SELP 815K did, however, display encapsulation after injection in the subcutaneous tissue, likely due to its longer-term in-vivo durability. Combining performance and safety with the degree of control with which they can be designed, synthesized and formulated, SELPs continue to show promise for their application in localized gene delivery.
5.Conclusion
SELP 815K mediated viral GDEPT for treatment of HNSCC in a mouse tumor model showed the greatest average tumor size reduction, longest time to tumor rebound, and greatest survivability compared to Virus Only and virus administered in poloxamer 407. Its highest initial intratumoral expression of viral genes coupled with the increased duration of expression proved SELP 815K superior to poloxamer 407 as a viral delivery matrix in this model. A safety assessment of local and systemic effects also demonstrated SELP 815K comparable or superior to poloxamer 407 in moderating or avoiding possible immunological and local toxicity effects of adenoviral administration. Consistent with previous reports, our findings indicate that Poloxamer 407 may exacerbate some of these effects. Although it did not affect its therapeutic efficacy, SELP 815K injection did result in an encapsulated hydrogel mass in the tissues.
6. Acknowledgements
Financial support for this work was provided by National Institutes of Health grant (R01-CA107621), the Utah Science Technology and Research (USTAR) Initiative, and a University of Utah Nanotechnology Training Program Predoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnology Progress. 1990;6:198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- Cresce AW, Dandu R, Burger A, Cappello J, Ghandehari H. Characterization and real-time imaging of gene expression of adenovirus embedded silk-elastinlike protein polymer hydrogels. Mol Pharm. 2008;5:891–897. doi: 10.1021/mp800054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandu R, Cresce AV, Briber R, Dowell P, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties. Polymer. 2009;50:366–374. [Google Scholar]
- Dandu R, Ghandehari H, Cappello J. Characterization of Structurally Related Adenovirus-laden Silk-elastinlike Hydrogels. Journal of Bioactive and Compatible Polymers. 2008;25:441–454. [Google Scholar]
- Dandu R.a.H.G. Delivery of bioactive agents from recombinant polymers. Progress in Polymer Science. 2007;32:1008–1030. [Google Scholar]
- Denny WA. Prodrugs for gene-directed enzyme-prodrug therapy (suicide gene therapy) Journal of Biomedicine and Biotechnology. 2003;3:48–70. doi: 10.1155/S1110724303209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinerman AA, Cappello J, El-Sayed M, Hoag SW, Ghandehari H. Influence of solute charge and hydrophobicity on partitioning and diffusion in a genetically engineered silk-elastin-like protein polymer hydrogel. Macromol Biosci. 2010;10:1235–1247. doi: 10.1002/mabi.201000061. [DOI] [PubMed] [Google Scholar]
- Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Solute diffusion in genetically engineered silk-elastinlike protein polymer hydrogels. Journal of Controlled Release. 2002a;82:277–287. doi: 10.1016/s0168-3659(02)00134-7. [DOI] [PubMed] [Google Scholar]
- Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Swelling behavior of a genetically engineered silk-elastinlike protein polymer hydrogel. Biomaterials. 2002b;23:4203–4210. doi: 10.1016/s0142-9612(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23:2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972;175:949–955. doi: 10.1126/science.175.4025.949. [DOI] [PubMed] [Google Scholar]
- Greish K, Araki K, Li D, O'Malley BW, Jr., Dandu R, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels for localized adenoviral gene therapy of head and neck tumors. Biomacromolecules. 2009;10:2183–2188. doi: 10.1021/bm900356j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greish K, Frandsen J, Scharff S, Gustafson J, Cappello J, Li D, O'Malley BW, Jr., Ghandehari H. Silk-elastinlike protein polymers improve the efficacy of adenovirus thymidine kinase enzyme prodrug therapy of head and neck tumors. J Gene Med. 2010;12:572–579. doi: 10.1002/jgm.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D, Nguyen T, Gonzalez AM, Printz MA, Pierce GF, Sosnowski BA, Phillips ML, Chandler LA. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Molecular Therapy. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: from molecular definition to controlled gene expression. Journal of Controlled Release. 2009;140:256–261. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson JA, Ghandehari H. Silk-elastinlike protein polymers for matrix-mediated cancer gene therapy. Adv Drug Deliv Rev. 2010;62:1509–1523. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Gustafson JA, Price RA, Greish K, Cappello J, Ghandehari H. Silk-elastin-like hydrogel improves the safety of adenovirus-mediated gene-directed enzyme-prodrug therapy. Mol Pharm. 2010;7:1050–1056. doi: 10.1021/mp100161u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nature medicine. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Li S, Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther. 2006;13:1313–1319. doi: 10.1038/sj.gt.3302838. [DOI] [PubMed] [Google Scholar]
- Liggett WH, Jr., Sewell DA, Rocco J, Ahrendt SA, Koch W, Sidransky D. p16 and p16 beta are potent growth suppressors of head and neck squamous carcinoma cells in vitro. Cancer Res. 1996;56:4119–4123. [PubMed] [Google Scholar]
- Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Tomicic MT, Thust R, Sobol RW. DNA polymerase mediates protection of mammalian cells against ganciclovir-induced cytotoxicity and DNA breakage. Cancer Res. 2001;61:7399. [PubMed] [Google Scholar]
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu S, Li CY, Yuan F. A novel method for viral gene delivery in solid tumors. Cancer Res. 2005;65:7541–7545. doi: 10.1158/0008-5472.CAN-05-1112. [DOI] [PubMed] [Google Scholar]
- Westerink MA, Smithson SL, Srivastava N, Blonder J, Coeshott C, Rosenthal GJ. ProJuvant (Pluronic F127/chitosan) enhances the immune response to intranasally administered tetanus toxoid. Vaccine. 2001;20:711–723. doi: 10.1016/s0264-410x(01)00423-6. [DOI] [PubMed] [Google Scholar]
- Wortmann A, Vöhringer S, Engler T, Corjon S, Schirmbeck R, Reimann J, Kochanek S, Kreppel F. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Molecular Therapy. 2007;16:154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]