Abstract
Viruses play an important role in acute otitis media (AOM) pathogenesis and live viruses may cause AOM in absence of pathogenic bacteria. Detection of AOM pathogens generally relies on bacterial culture of middle ear fluid. When viral culture is used and live viruses are detected in the middle ear fluid of children with AOM, the viruses are generally accepted as AOM pathogens. Because viral culture is not sensitive and does not detect the comprehensive spectrum of respiratory viruses, polymerase chain reaction (PCR) assays are commonly used to detect viral nucleic acids in the middle ear fluid. While PCR assays have greatly increased the viral detection rate, new questions arise on the significance of viral nucleic acids detected in the middle ear because nucleic acids of multiple viruses are detected simultaneously, and nucleic acids of specific viruses are detected repeatedly and in a high proportion of asymptomatic children. This article first reviews the role of live viruses in AOM and presents the point-counter point arguments on whether viral nucleic acids in the middle ear represent an AOM pathogen or a bystander status. While there is evidence to support both directions, helpful information for interpretation of the data and future research direction are outlined.
Keywords: Acute otitis media, viral PCR, middle ear fluid, viral upper respiratory tract infection
BACKGROUND
(Dr. Chonmaitree)
Acute otitis media (AOM) occurs most commonly as a continuum of viral upper respiratory tract infection (URI) (1). AOM is defined by 3 criteria: acute onset of symptoms (e.g. fever, irritability, earache), signs of inflammation of the tympanic membrane and presence of middle ear fluid (MEF). Evidence to date suggests the importance of respiratory viruses in AOM pathogenesis, as well as their role as AOM pathogens. When live bacteria and/ or viruses are detected in the MEF of children with AOM, the microbial organisms are generally accepted as AOM pathogens. Detection of viruses in the MEF, however, had been dampened by the insensitivity of the conventional viral assays (viral culture and antigen detection). While molecular diagnostic techniques have increased detection of viruses in the MEF, they have generated new research questions on pathogenicity of the detected viruses. This review presents background information on the role of viruses in AOM and the problems with detection of pathogens in the MEF leading to discussion on interpretation of the findings ‘presence of viral nucleic acids in the middle ear’.
Role of viruses in AOM pathogenesis and effects of viral infection of the middle ear
Bacterial pathogens of AOM including Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis are those that colonize the child’s nasopharynx without causing symptoms until there is an acute inflammation of the nasopharynx that is mainly caused by viral URI. The mechanisms by which viral URI induces AOM have been partially elucidated based on data obtained from experimental animal studies, from studies of adult volunteers infected with respiratory viruses, and from studies of children with URI and AOM.
Virus-induced inflammation of the nasopharynx, which also extends to the Eustachian tube (ET), occurs mainly by way of production and release of cytokines and inflammatory mediators. Inflammation provokes ET dysfunction by reduction of the tube’s protective and transport functions (2) resulting in loss of pressure equilibrium between the nasopharynx and the middle ear, impairment of the protective function of the tube, and reduced clearance by the tube; these allow bacteria and/or virus to invade the middle ear (3). The mechanisms by which the colonized bacteria are activated to become pathogens in the middle ear are still under investigation.
Viruses also interact with bacteria and concurrent viral infection of the middle ear or the respiratory tract significantly worsens the clinical course of bacterial AOM. In chinchillas, the incidence of experimental OM was higher in animals with combined bacterial and viral infection than those with either bacterial or viral infection alone (4). Severity of OM was also greater in animals with combined infection than in those with bacterial or viral infection alone (5). In children with AOM, bacteriologic failure 2-4 days into antibiotic therapy occurred in a significantly higher proportion of patients whose MEF contained both bacteria and virus, than in the group with bacteria alone (6); concurrent viral infection of the respiratory tract or the middle ear is associated with poor treatment outcomes (7). These data were derived from studies using conventional viral diagnostics (culture and antigen detection). One explanation for poor AOM outcome associated with viral infection of the middle ear is virus-induced inflammation. In MEF from children with AOM, concentrations of interleukin-8, histamine and leukotriene B4, have been found to be higher in MEF containing both bacteria and virus, than in MEF containing bacteria or virus alone (8, 9). Another explanation related to the ability of the viruses to induce local inflammation and immunologic reactions that interfere with penetration of antibiotic or dilute antibiotic concentration in the MEF; evidence to support these observations come from both data obtained in chinchilla experiments and in children with AOM (10, 11).
Viruses as AOM pathogens
While live viruses induce the initial process that leads to infection of the middle ear by bacteria, they may also enter the middle ear during AOM development. Importantly, viruses alone, without presence of bacteria can cause AOM, both in experimental animals and in infants and children. In a study in chinchillas (4), S. pneumoniae and/ or influenza A virus were inoculated into the animals intranasally. Bacteria alone induced AOM in 21% of animals, while both bacteria and virus induced AOM in 31%. Influenza A virus alone induced AOM in 4% of animals; the MEF was culture positive for influenza A. In another study involving chinchillas (2), intranasal inoculation of adenovirus type 1, in the absence of bacteria, caused mild to moderate tympanic membrane inflammation. Studies of infants and children with AOM for bacterial and viral etiology using conventional viral diagnostic methods have shown viruses in the MEF of 11-25% of cases. In 5-15% of these cases, only viruses (without bacteria) were detected in the MEF (12). Detection of viruses in the MEF has not been performed routinely in AOM studies, mainly because of technical difficulties, costs, and unavailability of treatment for broad spectrum of respiratory viruses.
Detection of AOM pathogens
AOM pathogen detection relies mainly on bacterial culture of the MEF; isolation of bacterial pathogens varies between 70% and 84% of cases and ‘sterile’ MEFs account for up to 30% (13). When both conventional bacterial and viral diagnostics were used (e.g. bacterial and viral cultures, with or without viral antigen detection), bacteria alone were detected in the MEF of 55% of AOM cases; viruses and bacteria together in 15%; viruses alone in 5%; no pathogen was detected in 25% of cases (12) (Figure 1A). It is clear that conventional assays under-detect viruses and may also under-detect bacteria. Furthermore, conventional viral diagnostics can’t detect specific respiratory viruses that commonly cause URI such as coronavirus, human metapneumovirus and bocavirus. Because respiratory viruses have been shown to play an important role in AOM pathogenesis and viruses alone can cause AOM, researchers have used more sensitive assays to detect viruses. Molecular diagnostics such as polymerase chain reaction (PCR) assays have been used to detect viral nucleic acids in the MEF of children with AOM (14-17). In a study using comprehensive diagnostic methods including conventional and molecular testing for bacteria and broad spectrum of respiratory viruses, bacteria alone was detected in the MEF in 27% of cases, bacteria and viruses together in 66%, viruses alone in 4% (18). The proportion of cases with no pathogen in the MEF was only 4%, a substantial difference from 25% reported previously (Figure 1B).
Figure 1.
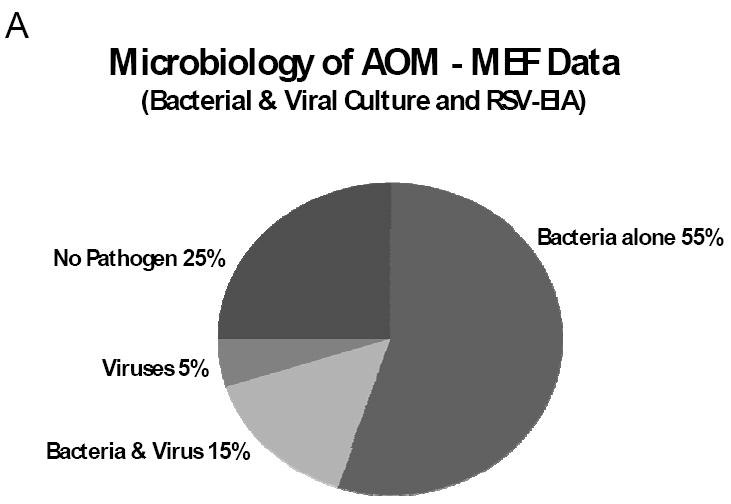
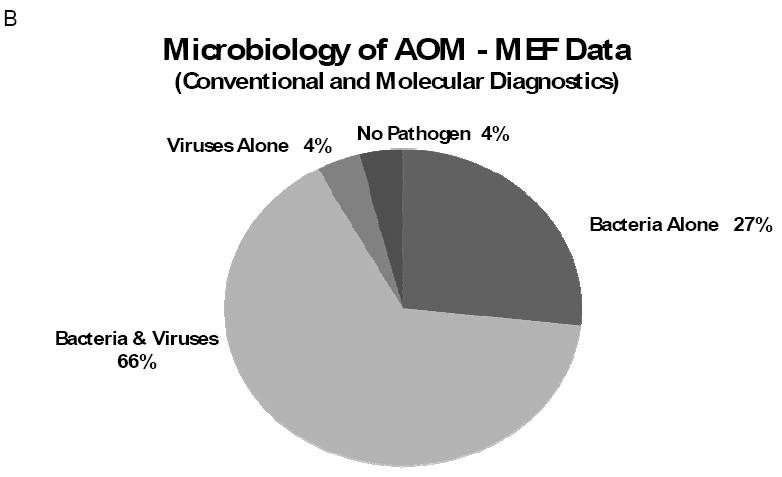
Microbiology of AOM; bacterial and viral findings of the MEF from children with AOM. Figure 1 A: data compiled from 5 studies (708 children) using bacterial and viral culture and respiratory syncytial virus-Ag detection by enzyme immunoassay (ref. 12). Figure 1B: data from a study of 79 children (with acute ear infection and drainage through existing tympanostomy tubes) using comprehensive microbiologic assays, including conventional and molecular viral diagnostics (ref. 18).
Molecular diagnostic results generate new research questions
While molecular diagnostics increase sensitivity for viral detection and enable detection of viruses that are not detected by culture, findings from these tests have generated new important questions on pathogenicity of the detected virus. First, nucleic acids of multiple viruses are detected simultaneously raising the question on which virus is the true ‘pathogen’ (1, 19). Second, viral nucleic acids of specific viruses such as adenovirus, bocavirus, enterovirus and rhinovirus are found repeatedly in nasopharyngeal samples collected sequentially from children suggesting prolonged presence of viruses in the respiratory tract (20-22). Lastly, nucleic acids of many respiratory viruses are detected in the nasopharynx in high proportion (up to 56%) of asymptomatic children (23-27).
While current data support the importance of viral infection of the middle ear (as represented by the presence of live viruses in the MEF), there has been no study to associate the presence of viral nucleic acids and the degree of middle ear inflammation. The point-counterpoint discussions below share the viewpoints on interpretation of the finding whether presence of viral nucleic acids in the MEF of children with AOM indicates their role as AOM ‘pathogen’ or it is simply the ‘bystander’.
POINT: Viral Nucleic Acids in the Middle Ear Indicates AOM “PATHOGEN”
(Dr. Ruohola)
Plenty of evidence supports the concept that presence of viral nucleic acids in the MEF indicates that viruses are pathogens. It must be acknowledged, however, that the evidence is indirect and to date there there has been no attempt to prove or negate the concept directly. The supportive evidence is presented below.
Presence of bacterial nucleic acids in the middle ear indicates their role as pathogens
Bacterial studies have shown that the findings of bacterial nucleic acids in the MEF are related to live metabolically active microbes. Based on an animal study, bacterial nucleic acids from killed bacteria disappeared rapidly from the middle ear; only nucleic acids of live bacteria stayed longer than a few days (28). In a landmark study, Rayner at el. examined MEF samples taken from children at the time of tympanostomy tube insertion (29); they demonstrated messenger-RNA of H. influenzae in 28 of 29 samples. These samples were culture negative but PCR positive for H. influenzae. Since the half-life of messenger-RNA is only seconds to minutes, this was strong evidence that the detection of nucleic acids is related to live metabolic active microbes.
Viral nucleic acids as etiologic agents in other infections
First, it could be asked why the detection of viral nucleic acids in MEF would not be a proof of pathogenic role of viruses in AOM as it is in several other infections such as meningitis, encephalitis, hepatitis, and HIV.
PCR has become the method of choice to detect the etiologic agents of central nervous system infections (30, 31) The superiority of PCR over viral culture was shown in a study analyzing 301 cerebrospinal fluid samples from clinical practice. Enteroviral etiology could be determined in only 6% of patients by viral culture while in 18% of samples enteroviral nucleic acids were detected by PCR (32).
Viral nucleic acids as etiologic agents in respiratory infections
Respiratory viral nucleic acids are often detected by PCR from nasopharyngeal samples from children and adults with acute respiratory symptoms. In more recent cross-sectional studies, PCR also detects respiratory viral nucleic acids from nasopharyngeal samples of asymptomatic individuals. A careful longitudinal study was performed in Charlottesville, Virginia, with weekly sampling from children aged 1-9 years (33). This study showed that although viral nucleic acids could be detected occasionally in children who presented with no symptoms, positive viral findings were seen almost always within one week before or three weeks after onset of symptoms. Thus, the vast majority of positive viral findings were actually related to symptomatic infections. Furthermore, the shedding of viral nucleic acids was episodic and no chronic shedding was detected (Figure 2). On the other hand, Jartti et al. demonstrated that children with prolonged symptoms actually had serial viral infections instead of prolonged infections (34) pointing out the importance of viral nucleic acid detection.
Figure 2.
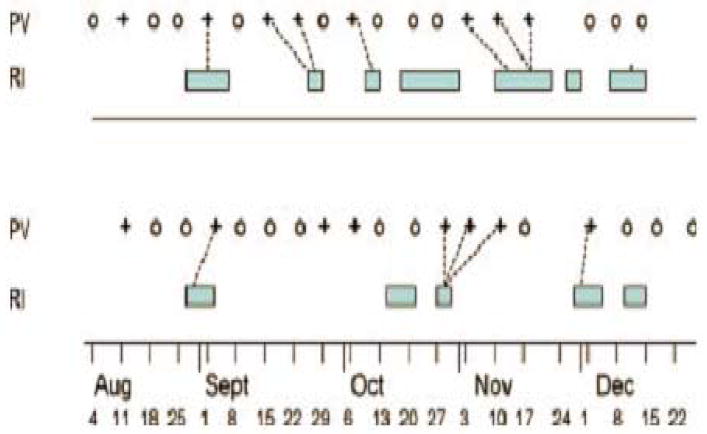
Timeline of two siblings ages 4 years (above) and 5 (below). PV, Picornavirus RT-PCR; O, negative test; +, Picornavirus positive; RI, Respiratory illness; Bar, span of reported symptoms, dashed line-connects positive samples to associated illness. Reprinted from J. Med. Virol. 78:644-650, 2006. Picornavirus RNA was detected in 42% of weekly samples of nasopharyngeal secretions.
In another study, the research group in Charlottesville has also shown that viral nucleic acid detection indicates true infection even in asymptomatic children (35). Following children with weekly pneumatic otoscopy and periodic sampling for viral PCR, otitis media was diagnosed in 46% of symptomatic and in 33% of asymptomatic children, all of whom had positive viral PCR detections. The researchers concluded that otitis media is a complication of viral infection both with and without concurrent symptoms, thus downwardly biasing coincidence estimates that use symptomatic illnesses as the denominator.
Serologic response is regarded as a definite evidence of infection. One of the strongest evidence that viral nucleic acids are a proof of etiology comes from a cohort of 258 children hospitalized because of acute wheezing (36). Comprehensive viral diagnostics was performed yielding 19% of nasopharyngeal aspirates to be positive for human bocavirus by PCR. Of these bocavirus positive children, 71% showed serologic response while only 6% of children not harboring bocavirus in their nasopharynx showed serologic response.
In summary, nucleic acid detection of respiratory viruses from nasopharyngeal samples has been widely accepted in diagnosis of respiratory infections (37). Viruses enter the middle ear via Eustachian tube from the nasopharynx and there is not a rationale that their role as infectious agents would be different in nasopharynx than that in the middle ear.
COUNTERPOINT: Presence of Viral Nucleic Acids Represents only “BYSTANDER”
(Dr. Hendley)
Although it seems to me that the moderator of this debate favors the “pathogen” role for viral nucleic acids in the middle ear during AOM, I would argue that the “bystander” role is more likely, based on three points.
Picornavirus nucleic acids are commonly present in nasopharynx of children
This point was demonstrated in a longitudinal surveillance study in healthy children in which nasal/ nasopharyngeal secretions were obtained weekly and tested for picornavirus RNA with RT-PCR (33). Respiratory illnesses were diagnosed from a daily symptom record maintained by the parent(s). The findings pertinent to this point are illustrated in Figure 2, in which timelines from two siblings are shown. During the five months of surveillance the 4-year old had 4 picornavirus illnesses and one infection without illness which coincided with an infection without illness in her sibling. The 5-year old had 3 picornavirus illnesses and 2 infections without reported illness. Her third infection (2 consecutive positives) occurred at the time of picornavirus illness in her sibling. The picornavirus infections in these girls were marked by PCR positivity for 1 to 3 weeks followed by PCR negative samples in subsequent weeks. In view of the fact that an episode of picornavirus infection occurred in healthy children in this study (33) once every two months, picornavirus RNA would be present in nasopharyngeal secretions for one to three weeks out of every eight to reach the middle ear during swallowing and yawning (see below).
Virus in the middle ear during AOM is also present in the nasopharynx
Virus present in the middle ear during AOM (detected by RT-PCR or time-resolved fluoroimmunoassay) is usually present in nasopharyngeal secretions obtained the same time (Table 1). In three Finnish studies (15, 38) in which virus was detected in the MEF obtained by tympanocentesis or myringotomy with aspiration, the identical virus was found in the nasopharynx in 65 to 80% of children.
Table 1.
Viruses Detected in Middle Ear (ME) during AOM: Frequency of Detection of Identical Virus in Nasopharynx (NP)
| Virus in ME | Identical Virus in NP | Frequency | Viruses Soughta | Reference |
|---|---|---|---|---|
| 46b | 35b | 75% | Picorna, RSV, Corona | 15 |
| 252 | 163 | 65% | Broad spectrum | 38c |
| 579 | 466 | 80% | Broad spectrum | 38 d |
Viral presence determined by PCR or Time-resolved fluoroimmunoassay
Number of children with AOM with virus present in middle ear or nasopharynx
Finn Cohort Study
Finn Vaccine Study
The Eustachian tube allows air to pass to the middle ear during swallows/yawns
The middle ear can be thought of as a box with the tympanic membrane comprising the lateral wall (Figure 3). Air in the middle ear cavity is connected to mastoid air cells by way of a tube labeled aditus. Air reaches the middle ear via the Eustachian tube which originates at an opening on the lateral wall of the nasopharynx at the level of the hard palate. In children the tube is shorter and more horizontal than in adults, and the pharyngeal orifice is slit-like. About one-third of the middle ear cavity and the entire Eustachian tube are lined with mucociliary epithelium so that particulate matter including bacteria can be transported back to the nasopharynx. The nasopharyngeal orifice of the Eustachian tube is normally closed. For equilibration of the air pressure in the middle ear with ambient pressure the Eustachian tube orifice is opened passively by action of the levator and tensor veli palatini muscles during swallowing and yawning. This allows air from the nasopharynx to pass through the tube to the middle ear. It has been thought that secretion from the nasopharynx does not reach the middle ear by passage through the tube when the tympanic membrane is intact due to the narrowness of the mid portion of the tube and the cushion of air in the middle ear.
My belief that secretion from the nasopharynx frequently reaches the middle ear in normal humans is based on a study (39) of three healthy adults in whom radiopaque contrast dye was delivered to the orifice of the Eustachian tube while they were in a lateral decubitus position. After each dye aliquot the subject swallowed and yawned. The same procedure was carried out with the contralateral nasal cavity in the dependent position before CT scanning to determine whether the contrast had reached the middle ear. In two of the three subjects contrast was present in the middle ear in addition to presence in the Eustachian tubes (39).
Although the number of subjects in this study was small, it seems reasonable to conclude that secretion from the nasopharynx reaches the middle ear during sleep in adults (and children). The mucociliary clearance by the Eustachian tube is the factor responsible for keeping the ear “sterile”. Milk otitis certainly occurs in infants drinking from a bottle while recumbent (40). Viral nucleic acids may be transiently present in the middle ear during AOM after arriving during pressure equilibration rather than a resident causing infection of the middle ear mucosa.
In summary, the evidence supporting “Bystander” includes: 1) virus in middle ear during AOM is present in the nasopharynx 75% of the time; 2) secretions containing virus from the nasopharynx may reach the middle ear during swallows/yawns via the Eustachian tube; and 3) virus in the middle ear may simply be “passing through”, to be returned to the nasopharynx by mucociliary clearance of the Eustachian tube.
CONCLUSIONS / FUTURE RESEARCH
(Dr. Chonmaitree)
In AOM research, use of molecular techniques to detect viral nucleic acids in the MEF is unavoidable because of the importance of respiratory viruses in AOM pathogenesis, insensitivity of the conventional viral diagnostics and their inability to detect specific respiratory viruses. The above discussions argue for positive PCR findings to represent both roles as pathogen and bystander. Therefore, positive findings of viral nucleic acids in the middle ear during AOM must be interpreted with caution.
Specific information may help with the interpretation of results. The type of virus nucleic acids detected is important; adenovirus, rhinovirus, enterovirus, and bocavirus nucleic acids are found repeatedly in respiratory secretions collected frequently from children while respiratory syncytial virus, influenza, and coronavirus are not (20-22, 33). These data suggest either repeated infections or tendency for prolonged presence / persistence for some respiratory viruses but not all. Therefore, PCR data from previous and recent samples, when available, can be helpful. Detection of single virus as opposed to multiple in symptomatic children also suggests the importance of the finding. Quantitative PCR (viral load) has been use as a way to help indicate the significance of the detected viruses and there have been suggestions to use viral load cut off values to separate virus cause from persistence (27, 41), but the issue is still controversial. While many studies reported overall positive correlation between viral load and symptom severity (21, 42-44), others did not find significant relationship between the quantity of specific viruses and selected clinical outcomes (45). Viral load and symptom correlation may depend on specific viral types or clinical diagnoses and there is still a need to determine the significance of viral load in URI and the risk for AOM development.
Further studies are required for better understanding of the value of molecular diagnostics in symptomatic and asymptomatic URI, as well as to determine the impact of the presence of viral nucleic acids in the MEF on the degree of middle ear inflammation and its clinical implication.
Acknowledgments
This work was supported in part by the National Institutes of Health grant R01DC005841.
Footnotes
Presented in part at the 10th International Symposium on Recent Advances in Otitis Media, New Orleans, LA, June 8, 2011
Disclosures: The authors have no conflict of interest.
References
- 1.Chonmaitree T, Revai K, Grady JJ, Clos A, Patel JA, Nair S, Fan J, Henrickson KJ. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakaletz LO, Daniels RL, Lim DJ. Modeling adenovirus type 1-induced otitis media in the chinchilla: Effect on ciliary activity and fluid transport function of eustachian tube mucosal epithelium. J Infect Dis. 1993;168:865–872. doi: 10.1093/infdis/168.4.865. [DOI] [PubMed] [Google Scholar]
- 3.Chonmaitree T. Viral Otitis Media. In: Alper, Bluestone, Casselbrant, Dohar, Mandel, editors. Advanced Therapy of Otitis Media. Hamilton, Ontario: B.C Decker Inc.; 2004. pp. 63–68. [Google Scholar]
- 4.Giebink GS, Berzins IK, Marker SC, et al. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect Immun. 1980;30:445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K, Bakaletz LO. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun. 1994;62:1710–8. doi: 10.1128/iai.62.5.1710-1718.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chonmaitree T, Owen MJ, Howie VM. Respiratory viruses interfere with bacteriological response to antibiotic in patients with acute otitis media. J Infect Dis. 1990;162:546–549. doi: 10.1093/infdis/162.2.546. [DOI] [PubMed] [Google Scholar]
- 7.Chonmaitree T, Owen MJ, Patel JA, Hedgpeth D, Horlick D, Howie VM. Effect of viral respiratory tract infection on outcome of acute otitis media. J Pediatr. 1992;120:856–862. doi: 10.1016/s0022-3476(05)81950-x. [DOI] [PubMed] [Google Scholar]
- 8.Chonmaitree T, Patel JA, Lett-Brown MA, Uchida T, Garofalo R, Owen MJ, Howie VM. Virus and bacteria enhance histamine production in middle ear fluids of children with acute otitis media. J Infect Dis. 1994;169:1265–1270. doi: 10.1093/infdis/169.6.1265. [DOI] [PubMed] [Google Scholar]
- 9.Chonmaitree T, Patel JA, Garofalo R, Uchida T, Sim T, Owen MJ, Howie VM. Role of leukotriene B4 and interleukin-8 in acute bacterial and viral otitis media. Ann Otol Rhinol Laryngol. 1996;105:968–974. doi: 10.1177/000348949610501207. [DOI] [PubMed] [Google Scholar]
- 10.Jossart GH, Canafax DM, Erdmann GR, et al. Effect of Streptococcus pneumoniae otitis media. Pharm Res. 1994;11:860–4. doi: 10.1023/a:1018933925707. [DOI] [PubMed] [Google Scholar]
- 11.Canafax DM, Yuan Z, Chonmaitree T, Deka K, Russle HQ, Giebink GS. Amoxicillin middle ear fluid penetration and pharmacokinetics in children with acute otitis media. Pediatr Infect Dis J. 1998;17:149–56. doi: 10.1097/00006454-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Chonmaitree T. Viral and bacterial interaction in acute otitis media. Pediatr Infect Dis J. 2000;19:S24–30. doi: 10.1097/00006454-200005001-00005. [DOI] [PubMed] [Google Scholar]
- 13.Bluestone CD, Klein JO. Otitis media in infants and children. 4. Hamilton, Ontario: BC Decker; 2007. Microbiology; pp. 101–126. [Google Scholar]
- 14.Chonmaitree T, Henrickson KJ. Detection of respiratory viruses in the middle ear fluids of children with acute otitis media by multiplex reverse transcription-polymerase chain reaction assay. Pediatr Infect Dis J. 2000;19:258–60. doi: 10.1097/00006454-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Pitkaranta A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102:291–295. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 16.Williams JV, Tollefson SJ, Nair S, Chonmaitree T. Association of human metapneumovirus with acute otitis media. Int J Pediatr Otorhinolaryngol. 2006;70:1189–93. doi: 10.1016/j.ijporl.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beder LB, Hotomi M, Ogami M, et al. Clinical and microbiological impact of human bocavirus on children with acute otitis media. Eur J Pediatr. 2009 Nov;168(11):1365–72. doi: 10.1007/s00431-009-0939-7. [DOI] [PubMed] [Google Scholar]
- 18.Ruohola A, Meurman O, Nikkari S, et al. Microbiology of acute otitis media in children with tympanostomy tubes: prevalences of bacteria and viruses. Clin Infect Dis. 2006;43:1417–22. doi: 10.1086/509332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulut Y, Güven M, Otlu B, et al. Acute otitis media and respiratory viruses. Eur J Pediatr. 2007;166:223–228. doi: 10.1007/s00431-006-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalu SU, Loeffelholz M, Beck E, Patel JA, Revai K, Fan J, Henrickson KJ, Chonmaitree T. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J. 2010;29:746–750. doi: 10.1097/INF.0b013e3181d743c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin ET, Fairchok MP, Kuypers J, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis. 2010;201(11):1625–32. doi: 10.1086/652405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72(4):695–9. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 23.van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis. 2005;41(4):490–7. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Zalm MM, Uiterwaal CS, Wilbrink B, et al. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr Infect Dis J. 2009;28(6):472–6. doi: 10.1097/inf.0b013e318195e26e. [DOI] [PubMed] [Google Scholar]
- 25.Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82(7):1282–90. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore HC, Jacoby P, Taylor A, et al. The interaction between respiratory viruses and pathogenic bacteria in the upper respiratory tract of asymptomatic Aboriginal and non-Aboriginal children. Pediatr Infect Dis J. 2010;29(6):540–5. doi: 10.1097/INF.0b013e3181d067cb. [DOI] [PubMed] [Google Scholar]
- 27.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent Detection of Respiratory Viruses without Symptoms: Toward Defining Clinically Relevant Cutoff Values. J Clin Microbiol. 2011;49(7):2631–6. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Post JC, Aul JJ, White GJ, Wadowsky RM, Zavoral T, Tabari R, et al. PCR-based detection of bacterial DNA after antimicrobial treatment is indicative of persistent, viable bacteria in the chinchilla model of otitis media. Am J Otolaryngol. 1996;17(2):106–111. doi: 10.1016/s0196-0709(96)90005-8. [DOI] [PubMed] [Google Scholar]
- 29.Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279(4):296–299. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 30.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171(4):857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 31.Kupila L, Vuorinen T, Vainionpää R, Hukkanen V, Marttila RJ, Kotilainen P. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006;66(1):75–80. doi: 10.1212/01.wnl.0000191407.81333.00. [DOI] [PubMed] [Google Scholar]
- 32.Vuorinen T, Vainionpää R, Hyypia T. Five years’ experience of reverse-transcriptase polymerase chain reaction in daily diagnosis of enterovirus and rhinovirus infections. Clin Infect Dis. 2003;37(3):452–455. doi: 10.1086/376635. [DOI] [PubMed] [Google Scholar]
- 33.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J Med Virol. 2006;78(5):644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 34.Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF, Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32(2):314–320. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winther B, Alper CM, Mandel EM, Doyle WJ, Hendley JO. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119(6):1069–1075. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 36.Söderlund-Venermo M, Lahtinen A, Jartti T, Hedman L, Kemppainen K, Lehtinen P, et al. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis. 2009;15(9):1423–1430. doi: 10.3201/eid1509.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray Pr, Witebsky FG. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. The Clinician and the Microbiology Laboratory; pp. 258–260. [Google Scholar]
- 38.Nokso-Koivisto J, Räty R, Blomqvist S, et al. Presence of specific viruses in the middle ear fluids and respiratory secretions of young children with acute otitis media. J Med Virol. 2004;72:241–248. doi: 10.1002/jmv.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winther B, Gwaltney J, Phillips CD, Hendley JO. Radiopaque contrast dye in nasopharynx reaches the middle ear during swallowing and/ or yawning. Acta otolaryngologica. 2005;125:625–628. doi: 10.1080/00016480510027466. [DOI] [PubMed] [Google Scholar]
- 40.Tully SB, Bar-Haim Y, Bradley RL. Abnormal tympanography after supine bottle feeding. J Pediatrics. 1995;126(6):S105–11. doi: 10.1016/s0022-3476(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 41.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008;27:1103–7. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 42.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–10. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis. 2010;201:1404–13. doi: 10.1086/651662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48:239–45. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houben ML, Coenjaerts FE, Rossen JW, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–71. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]


