Abstract
Schwannoma is a rare gastrointestinal mesenchymal tumor as the vast majority of gastric mesenchymal tumors are GISTs. In this study, we analyzed clinicopathologically 51 gastric schwannomas. These tumors predominantly occurred in older adults with a marked female predominance (40 women and 11 men, median and mean ages, 60 and 58 years). They variably presented with gastric discomfort, bleeding, or rarely by gastric outlet obstruction, and many were incidental findings during other medical procedures. The tumors ranged from 1–10.5 cm (median, 4.5 cm). The typical histologic features included spindle cells usually with microtrabecular architecture and focal nuclear atypia, and peritumoral lymphoid cuff, whereas features of soft tissue schwannomas, such as encapsulation, nuclear palisading, vascular hyalinization and dilatation, were absent or infrequent. Median mitotic count was 2/50 HPFs, with the highest count being 13/50 HPFs. No malignant variants were recognized, and long-term follow-up did not reveal recurrences or metastases. Immunohistochemically, all examined tumors were S100 protein positive and most were also GFAP positive, whereas CD34 and NF68 were encountered rarely and all tumors were negative for HMB45, KIT, DOG1/Ano 1, SMA, desmin, and synaptophysin. None of the 9 tumors studied contained GIST-specific KIT or PDGFRA mutations. FISH studies revealed multiple signals with BCR probe (chromosome 22) and centromeric probes for chromosomes 2 and 18 suggesting polyploidy. These findings indicate that gastric schwannoma is a distinctive form of peripheral nerve sheath tumor that in many ways differs from soft tissue schwannoma. It should be distinguished from GIST and other mesenchymal tumors of the gastrointestinal tract, such as the S100 protein-positive gastrointestinal clear cell sarcoma and metastatic melanoma.
Keywords: Schwannoma, stomach, nerve sheath tumor, S100 protein, GFAP, prognosis, chromosome 22, polyploidy
INTRODUCTION
Daimaru et al. reported 24 cases of benign schwannoma of the gastrointestinal tract, the first series of immunohistochemically-documented cases of this entity. 1 These tumors showed evidence for nerve sheath differentiation (S100 protein and GFAP-positive), and usually occurred in the stomach. Although the original report indicated benign nature, long-term follow-up information and histologic characterization is limited in the original11 and subsequent small series 2–6 and isolated cases. 7–15 The epidemiology and pathology of gastric schwannoma in comparison with peripheral soft tissue schwannoma are also incompletely analyzed. In the older literature, gastric schwannoma could not be reliably separated from the more common GISTs so that virtually all old reports on these tumors illustrate GISTs. 16–25 In this study we analyzed 51 gastric schwannomas clinically, histologically, immunohistochemically, and genetically, providing long-term follow-up data and confirming the benign nature of this tumor without being able to find malignant variants.
MATERIALS AND METHODS
All gastric mesenchymal tumors coded as leiomyomas, smooth muscle tumors of uncertain potential, leiomyosarcomas, nerve sheath tumors (any designation), and sarcomas of different types were retrieved from the Armed Forced Institute of Pathology files from 1970–1999, and partially up to 2005 (nerve sheath tumors). English language literature on gastric schwannoma was also reviewed, based on PubMed records.
A total of 51 tumors were histologically identified as gastric schwannomas and form the basis for this study. Clinical data and tumor size were recorded. Hematoxylin and eosin slides were reviewed on all cases. The examined histological features included: mitotic count per 50 fields (approximately 10 mm2 total area), presence of ulcer, lymphoid reaction, architecture (trabecular, solid), blood vessel changes, occurrence of xanthoma cells, nature of extracellular matrix (collagenous vs. myxoid), and encapsulation.
Immunohistochemical studies without epitope retrieval pretreatments were performed for S100 protein: polyclonal, 1:1600, Dako Cytomation, Carpinteria, California, HMB45: 1:50, Dako, α–smooth muscle actin: clone 1A4, 1:1600, Sigma Chemicals, St. Louis, MO, and synaptophysin: polyclonal, prediluted, Ventana Medical Systems, Tucson, AZ;
Pretreatments were used for GFAP: polyclonal, 1:4000, Dako, Sigma protease 1 for 8 min; KIT: polyclonal A4502, 1:250, Dako; DOG1/Ano 1: clone K9, 1:200, Novocastra/Leica Biosystems, Bannockburn IL; and desmin: clone D33, Dako, 1:50, and neurofilament 68, clone NR4, 1:50, Novocastra/Leica. Heat-induced epitope retrieval in an EDTA buffer was used for the latter four antibodies. Detection was performed using Ventana detection system and automation with diaminobenzidine as the chromogen.
Mutation analysis of GIST-specific KIT and PDGFRA mutational “hot spots” was performed as previously described. 26,27 Briefly, tumor DNA samples obtained from formalin-fixed and paraffin (FFPE) embedded tissue were PCR amplified, and the purified PCR-products were sequenced directly.
Interphase fluorescence in situ hybridization (FISH) studies were performed on standard 5-μm sections of FFPE tissues prepared for hybridization using SPoT-Light Cell Pretreatment Kit, following manufacturer’s protocol (Zymed, CA, USA). In lieu of a centromeric probe to chromosome 22, the BCR locus- specific probe (LSP) and the chromosome 2 and 18 centromeric enumeration probes (CEP) were assessed individually following the manufacturer’s recommended procedure (Vysis Inc., Downers Grove, IL). The images were analyzed with Olympus fluorescence microscope and captured by camera couple with SPOT Imaging Software (Diagnostic Instruments, Inc., Sterling Heights, MI).
RESULTS
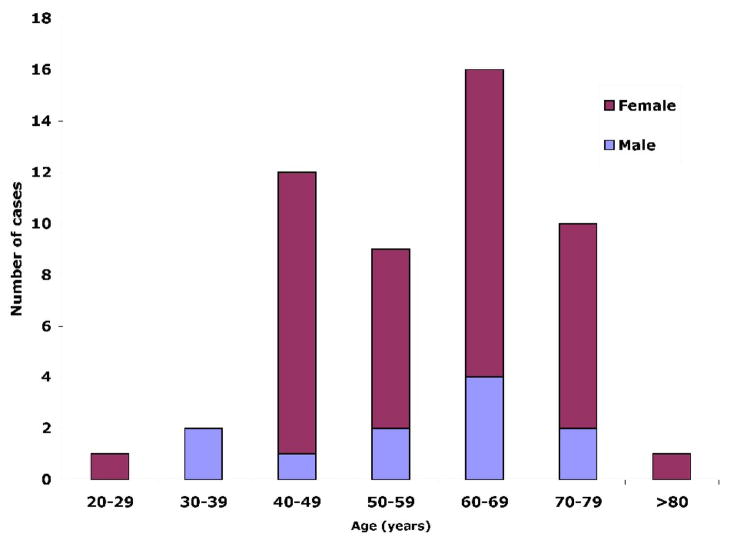
The clinical data are summarized in Table 1. Patient age ranged from 29 to 90 years (mean, 58; median, 60 years). There were 11 males and 40 females with an approximately 1:4 male to female ratio (Fig. 1).
Table 1.
Clinicopathologic summary of 51 gastric schwannomas
| Case no. | Age/Sex | Clinical presentation | Size (cm) | Mitoses/50 HPFs | Outcome |
|---|---|---|---|---|---|
| 1 | 57M | Epigastric discomfort | 1 | 0 | LTF |
| 2 | 76F | Incidental | 1.2 | 0 | DUNK, 1 year |
| 3 | 64M | Incidental | 1.5 | 1 | DUC, 7 years |
| 4 | 71F | N/A | 1.7 | 0 | LTF |
| 5 | 71F | Incidental | 1.7 | 0 | DUC, 8 years |
| 6 | 90F | Incidental | 1.8 | 0 | DUC, 2 years |
| 7 | 62F | Epigastric pain | 2.5 | 5 | ATSU, 20 years |
| 8 | 65F | Incidental | 2.5 | 0 | ANED, 24 years |
| 9 | 68F | N/A | 2.5 | 7 | DUNK, 20 years |
| 10 | 58F | GI bleeding | 2.8 | 6 | ATSU, 9.5 years |
| 11 | 66M | Incidental | 2.8 | 1 | ANED, 12.4 years |
| 12 | 70F | Incidental | 2.8 | 2 | ANED, 11.5 years |
| 13 | 36M | GI bleeding | 3 | 0 | LTF |
| 14 | 42F | ND | 3 | 2 | ANED, 9 years |
| 15 | 47F | Gastric pain | 3 | 0 | LTF |
| 16 | 54M | ND | 3 | 5 | ATSU, 15 years |
| 17 | 55F | ND | 3 | 1 | DUNK, 32 years |
| 18 | 56F | ND | 3 | 1 | ATSU, 15 years |
| 19 | 56F | Incidental | 3 | 1 | ATSU, 14 years |
| 20 | 73F | Incidental | 3 | 6 | ANED, 18 years |
| 21 | 46F | ND | 3.5 | 2 | ANED, 7 years |
| 22 | 72F | ND | 3.5 | 2 | DUC, 11 years |
| 23 | 38M | Detected during upper GI series for unknown symptoms | 4 | 3 | LTF |
| 24 | 42F | Nausea and gastric distress | 4 | 3 | ANED, 19.3 years |
| 25 | 47F | Asymptomatic | 4.5 | 0 | Autopsy finding |
| 26 | 68M | ND | 4.5 | 2 | ATSU, 2 years |
| 27 | 60F | Incidental | 4.7 | 1 | LTF |
| 28 | 56F | Unspecified gastric complaints | 5 | 1 | DUC, 15.5 years |
| 29 | 70F | Intermittent heartburn | 5 | 0 | DUC, 2 years |
| 30 | 50F | Hematemesis, melena | 6 | 1 | ATSU, 20 years |
| 31 | 54F | Incidental on CT for bladder cancer follow-up | 6 | 1 | ANED, 8 years |
| 32 | 60M | Occult blood in stool | 6 | 3 | DUC, 10.5 years |
| 33 | 66F | Abdominal pain | 6 | 1 | ANED, 5.5 years |
| 34 | 69F | Epigastric discomfort and mass palpated on routine visit | 6 | 3 | ANED, 6.5 years |
| 35 | 72M | Incidental on CT for colon cancer follow-up | 6.7 | 3 | ANED, 4 years |
| 36 | 66F | Epigastric pain | 6.8 | 0 | ANED, 13.7 years |
| 37 | 40F | Fullness of stomach | 7 | 2 | ANED, 14 years |
| 38 | 43F | Gastric pain and occult blood, operated with colon carcinoma | 7 | 13 | DUC, 5 years (Colon Cancer) |
| 39 | 43F | ND | 7 | 1 | ANED, 6 years |
| 40 | 63F | ND | 7 | 0 | ANED, 21.3 years |
| 41 | 64F | GI bleeding | 7.5 | 7 | DUNK, 9.5 years |
| 42 | 48F | ND | 7.9 | 2 | ANED, 11 years |
| 43 | 29F | ND | 8 | 4 | LTF |
| 44 | 45F | ND | 8 | 1 | ATSU, 34.9 years |
| 45 | 49F | ND | 8.5 | 2 | ANED, 14 years |
| 46 | 68F | Gastric pain | 8.5 | 1 | DUC, 15 years |
| 47 | 64F | ND | 10 | 2 | LTF |
| 48 | 67F | ND | 10 | 9 | ANED, 7 years |
| 49 | 74M | Gastric outlet obstruction, weight loss | 10.5 | 0 | DUC, 28 years |
| 50 | 44M | ND | ND | 0 | ANED, 16 years |
| 51 | 78F | Incidental | ND | 4 | DOPC |
ANED, no evidence of disease; ATSU, alive with tumor status unknown; DOPC, died of postoperative complications; DUC, died of unrelated causes; DUNK, died of unknown causes; LTF, lost to follow-up; ND, no data; Incidental, Incidental during unrelated surgery
Fig. 1.
Age and sex distribution of 51 gastric schwannomas.
One or more presenting complaints were documented in 20 cases. Gastrointestinal bleeding was seen in 7 patients (usually occult blood in stool or melena, and hematemesis in 1 patient). Other complaints included epigastric pain or discomfort (n=12) and weight loss due to gastric outlet obstruction (n = 1). Fifteen patients were asymptomatic and came to medical attention for an unrelated medical procedure. Eleven tumors were incidentally discovered during surgery for gallbladder disease (n = 4), various carcinomas (3 colon, 1 kidney, 1 ovary), appendectomy (n=1), and hernia repair (n=1). One case (3%) was seen at the time of upper gastrointestinal tract endoscopy performed for unknown reasons. Computer tomography done as part of follow-up examination after bladder and colon cancer identified two gastric schwannomas. One tumor was incidentally discovered during autopsy. The presentation was unknown for 15 patients. None of the patients had history of NF1 or NF2 syndrome.
The surgical treatment included partial gastrectomy (n=21), local excision or wedge resection (n=18), or biopsy (n =2). The excision type was not specified in 9 cases.
Follow-up
Follow-up revealed 18 patients to be alive without recurrences for 4–24 years (median, 11 years). Nine patients had died of unrelated causes during 2–28 years (median, 9 years). Causes of death included colon cancer (n = 3), bronchogenic carcinoma (n = 1), malignant lymphoma (n = 1), and malignant neoplasm of unknown origin (n = 1). Three patients died of non-neoplastic disease. One additional patient died of postoperative complications. Nine patients were known to be alive (2 – 34.9 years, median, 15 years), but tumor status could not be verified. Five patients died of unknown causes (1–32 years; median, 9.5 years). One tumor was diagnosed during an autopsy for unrelated disease. Eight patients were lost to follow-up.
Gross pathology
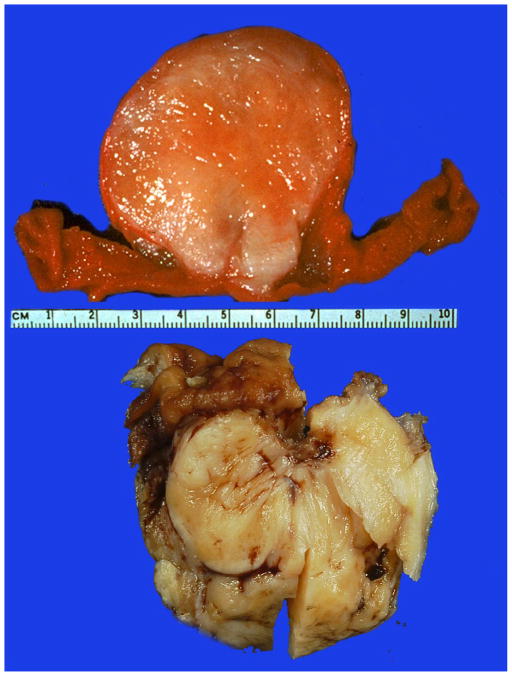
The greater curvature (8), lesser curvature (3), anterior stomach wall (2), cardia (1), and distal stomach (1) were specified as involved subregions of the stomach. The mural location was specified as submucosal in fourteen cases, and muscular wall, serosal and subserosal involvement was noted in a minority of cases. Two cases were described as sessile growths projecting into the stomach lumen (Fig 2).
Fig 2.
Gross appearance of two gastric schwannomas. The upper panel shows and endophytic, sessile polypoid tumor with a whitish to pale tan surface of sectioning of an unfixed specimen. The lower panel contains a portion of a fixed tumor with a yellowish surface on sectioning. Mucosa is seen on top left.
The maximum tumor diameters ranged from 1.0 to 10.5 cm (mean, 5.1 cm; median 4.5 cm). Tumors were described as ovoid or round mural masses, and gross mucosal ulceration and hemorrhage were recognized on seven and two occasions, respectively. No areas of cystic change or gross necrosis were reported in any of the cases.
At least six tumors were well circumscribed and a capsule-like boundary was noted in four cases. The tumors varied from fibrotic (13) to rubbery (4), fleshy (2), or gritty (1). On sectioning, the tumors were reported as white (4), yellow (6), yellow-white (9), yellow-pink (1), and yellow-gray (1), gray (2) or tan (5). Cut surface was glistening or shiny and described as whorled (4), bosselated (1), or variegated (1).
Histologic findings
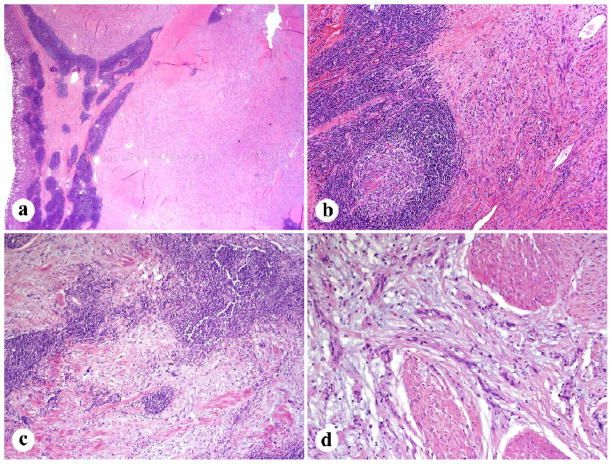
Of 41 cases including mucosa, an ulcer was appreciated either grossly or histologically in 20 cases. A lymphocytic peritumoral cuff was present in 48 (96%) occasions and germinal center formation was identified in 37 cases (74%). Diffuse intratumoral lymphoid infiltration was seen in all cases with plasma cells present in 30 (61%). Most tumors (80%) had an infiltrative spread between the muscularis propria elements and no definite true capsules were histologically detected (Fig. 3). All tumors were composed of spindle cells with six cases showing focal epithelioid morphology. A microtrabecular pattern was present in 41(82%) cases (prominently in 7, weakly in 2, and focally in 14). Cellularity was moderate in the great majority of the cases. Nuclear palisading was weak in the majority of the cases. Well-developed palisading was seen in only 10 cases and palisading was completely absent in four cases (Fig. 4). Well-developed Verocay bodies were observed in only 2 instances. Mitotic activity was low (median, 2/50 HPFs). Twenty-five tumors had 0–1, twenty had 2–5, five had 6–10, and one had 13 mitoses/50 HPFs.
Fig. 3.
Low magnification features of gastric schwannoma. A, B. Peripheral lymphoid cuff with occasional germinal centers. C. Lymphoid infiltration entrapped smooth muscle elements within the tumor. D. Tumor involves muscularis propria in an infiltrative manner.
Fig. 4.
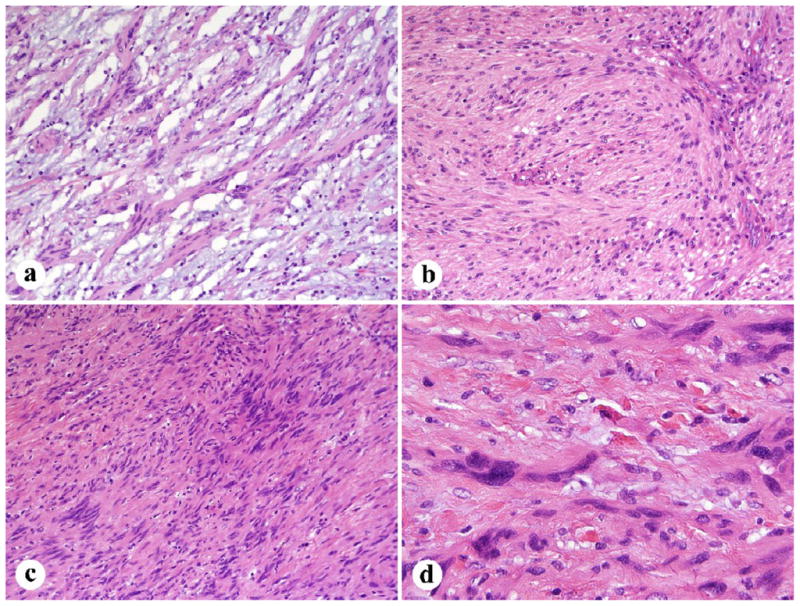
Histologic details of gastric schwannoma. A. Tumor cells show a microtrabecular architecture with interspersed fibromyxoid matrix. B. An example with a more solid to fascicular pattern. C. Focal nuclear palisading. D. Moderate nuclear atypia was often focally present.
All but two cases exhibited some degree of nuclear atypia. Mild atypia was identified in 32 tumors (14 focally), moderate or mild-moderate in 15 (3 focally), and moderate to high in 1 case. The matrix was at least minimally collagenized with the majority of the cases showing a moderate degree of collagen deposition. Fourteen cases had areas of myxoid change, one being prominent and another mainly perivascular (Fig. 4). Xanthoma cells and vascular hyalinization without luminal dilatation were observed in two and 15 cases, respectively.
Immunohistochemical findings
One or more immunohistochemical stains were available in all but eight cases. All 43 examined cases showed strong S100 protein positivity (Fig. 5). Eighteen of the 24 examined cases (75%) showed variable positivity for GFAP ranging from 25–100% of tumor cells (Fig. 5). Most cases (26/29) were negative for CD34. Three cases showed focal (<5%), 15% and 60% of CD34 positivity. All examined 22–38 cases were negative for smooth muscle actin (SMA), desmin, KIT, Dog1/Ano 1, and HMB-45. All cases evaluated for neurofilament 68 (n = 17) and synaptophysin (n = 7) were negative.
Fig 5.
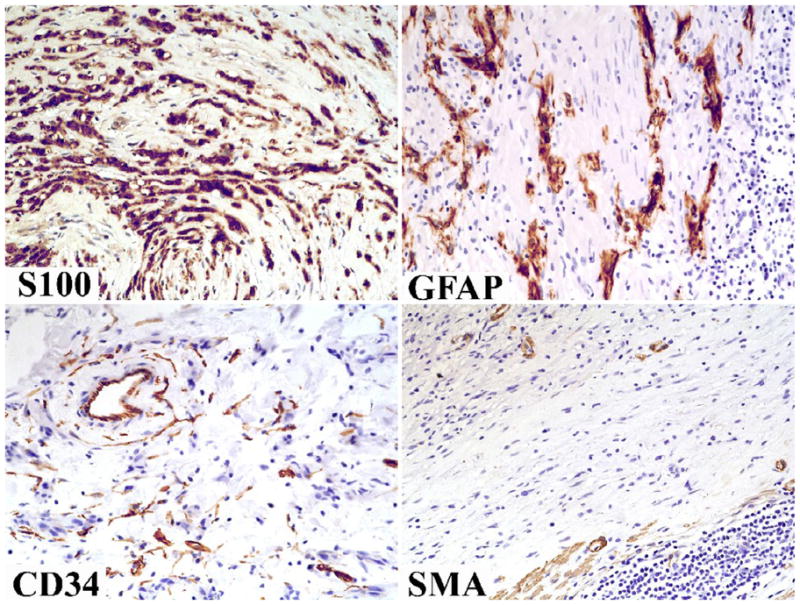
Immunohistochemically typical features include positivity for S100 protein and GFAP. Both markers highlight a microtrabecular pattern. Tumor cells are negative for CD34 and SMA; the positive cells represent non-tumor elements: fibroblasts/endothelial cells and smooth muscle cells/pericytes, respectively.
Genetic studies
KIT exons 9, 11, 13, and 17, and PDGFRA exons 12, 14, and 18, were evaluated in nine tumors. No mutations were detected in these regions known to be KIT and PDGFRA mutational “hot spots” for GISTs.
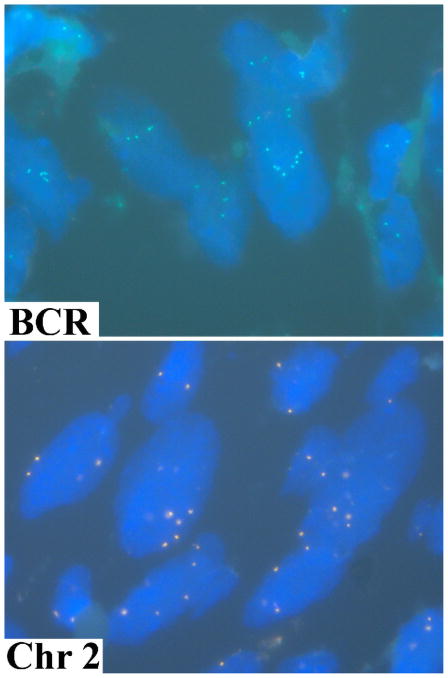
Chromosome 22 abnormalities were examined in 4 cases using a BCR locus-specific probe. All tumors contained cell subset with multiple (3 to14) BCR signals (Fig 6). Occurrence of single signals for BCR was rare arguing against monosomy of chromosome 22. Subsequently, one of the tumors with multiple BCR signals was evaluated with chromosome 2 and 18 CEPs. The multiple signals (up to 8 per cell) were detected with both probes suggesting numerical chromosomal changes (Fig. 6).
Fig. 6.
Both the BCR locus-specific probe and the chromosome 2 centromeric probe reveal multiple gene copies in large cells representing the neoplastic cells of gastric schwannoma.
DISCUSSION
In this study we evaluated 51 gastric schwannomas and reviewed the literature on these tumors. Based on our data, gastric schwannoma is much less common than gastrointestinal stromal tumor (GIST). We estimate that there are approximately 45 gastric GISTs for each gastric schwannoma. Some smaller studies have shown apparently higher frequencies on schwannoma in relation to GIST: only 8–14 gastric GISTs for each gastric schwannoma, although these numbers may not be fully comparable with our calculated frequency as they used different group specifications. 1,6 Our series, several previous series, and isolated cases demonstrate a variable female predominance, which was most marked in our series, the largest single series to date: nearly 4:1 (Table 2).
Table 2.
Collective data from the present and previous series and reported isolated cases of gastric schwannomas. *Includes one colonic schwannoma. **One plexiform schwannoma of stomach was excluded.
| Total cases | Male:Female | Age range and mean (years) | Size range and mean size (cm) | Association with NF1/NF2 | Reference/Data source |
|---|---|---|---|---|---|
| 24 | 9:15 | 36–78 (58) | 0.5–7 (2.8) | None | 10 |
| 5 | 1:4 | 47–72 (59) | 2.5–4 (2.7) | None | 35 |
| 16 | 7:9 | 38–86 (62) | 1.1–15.5 (3.7) | 1/16 | 1 |
| 3 | 1:2 | 56–73 (63) | 2–11 (5.5) | None | 33 |
| 4 | 1:3 | 47–52 (50) | 2.0–5 (3.2) | None | 18 |
| 16 | 6:10 | 32–79 (56) | 1.3–8 (3.8) | None | 15 |
| 10 | 4:6 | 24–73 (59) | 1.5–6 (4.3) | None | 5,6, 24,32,36,39 |
| 78 | 29:49 | 24–86 (58) | 0.5–15.5 (3.2) | Rare, 1/78 | All previously published cases shown above |
| 51 | 11:40 | 29–90 (58) | 1.0–10.5 (4.8) | None | Present series |
| 129 | 40:89 | 24–90 (58) | 0.5–15.5 (3.5) | Previously published and present cases |
Stomach is by far the most common site for schwannoma in the gastrointestinal tract in our experience (51 cases). We have seen similar tumors in every segment of the GI tract. The second most common site seems to be colon (20 cases). 28 In our experience, occurrence in small intestine and esophagus is rare: only 5 and 2 cases, respectively.
Typical histologic features that assist in recognition of gastric schwannoma are focally atypical spindle cells usually arranged in a microtrabecular-microfascicular pattern, and peritumoral lymphoid cuff, often with germinal centers. Immunohistochemical studies show universal positivity for S100 protein and frequent but variable immunoreactivity for GFAP. These morphologic and immunohistochemical findings, combined with negative results for KIT and smooth muscle markers, are confirmatory.
Gastric schwannoma differs histologically from soft tissue schwannoma in several ways. Gastric examples lack encapsulation as a result of their origin from the dispersed autonomic nerve Schwann cells, as opposed to being encased by epineurium, as seen in soft tissue schwannomas. Also, nuclear palisading, xanthoma cells and vascular hyalinization and dilatation, as commonly seen in soft tissue schwannomas, are rare in gastric schwannoma. Features comparable to gastric schwannoma are seen in colonic 28 and other GI schwannomas, in our experience. On the other hand, prominent lymphoid infiltration, microtrabecular architecture and frequent nuclear atypia are not features of soft tissue schwannomas.
Neither are the features of gastric schwannoma comparable with neurofibromas, which almost invariable contain a CD34-positive fibroblastic component not present in gastric schwannoma 29,30 and also residual neurofilament-positive axons, invariably absent in gastric schwannomas. Furthermore, in our experience and that of others, most gastrointestinal neurofibromas are plexiform neurofibromas. 1
Gastric schwannoma seems to have an invariably good prognosis, with no recurrences or metastases noted in our study. Similarly, previous follow-up studies have not identified malignant variants for this tumor. 1,2,5,6 Therefore, pathologic parameters such as tumor size and mitotic rate do not have prognostic signifcance, as in the case of GISTs. Although some gastric schwannomas exceed 10 cm in size, and a minority has mitotic rate > 5/50 HPFs, none of these tumors showed evidence for aggressive behavior. Despite this, we would advise caution for gastric schwannomas with higher mitotic rates > 10/50 HPFs, because experience on their long-term follow-up is limited. Complete excision and follow-up would be probably warranted whenever this can be reasonably accomplished. Some series including endoscopically detected tumors showed smaller tumor sizes probably reflecting earlier detection by endoscopic screening (Table 2). 1,4
Genetic features of gastric schwannoma include lack of KIT and PDGFRA mutations, in contrast with GIST. Somatic NF2 gene mutations, common in sporadic soft tissue schwannomas, are rare in gastric schwannomas.31 Nevertheless, one gastric schwannoma was reported to carry an NF2 exon 6 missense mutation. However, it is somewhat uncertain from the description whether this tumor was a typical gastrointestinal schwannoma, as it might have originated from the mesogastrium. 12 Although we did not find any of the cases associated with NF1 or NF2 syndromes, one gastric schwannoma was reportedly associated with NF1-syndrome.2
In this study, we explored chromosome 22 copy number changes using FISH and a BCR probe, especially to explore the possibility of monosomy of chromosome 22, known to be common in soft tissue schwannomas based on high resolution comparative genomic hybridization studies. 32 All cases examined by FISH lacked chromosome 22 monosomy and contained larger nuclei with multiple signals for BCR as well as for chromosome 2 and 18 centromers indicating polysomy, likely polyploidy. These findings, similar to previously reported lack of NF2 mutations31 , suggest that the fundamental genetic mechanism of gastric schwannoma may differ from that of soft tissue schwannoma.
Malignant schwannomas have been infrequently reported in the stomach, but most cases predate modern immunohistochemistry. In fact, by illustrated histologic features, two cases reported in NF1-patients probably represent GISTs rather than nerve sheath tumors. 33,34 We have not encountered definitive examples of malignant peripheral nerve sheath tumors in the stomach or any other parts of the gastrointestinal tract and found that tumors historically designated as such were mostly GISTs.
Rare variants of schwannomas or related tumors also occur in the stomach, and such tumors were not included in this study. Psammomatous melanotic schwannoma may occur in connection with Carney complex that also includes myxomas and Cushing syndrome among others. Three such gastric tumors were included in Carney’s study on 31 patients 35 and one solitary apparently non-syndromic example was also reported. 36 These tumors differ from the usual gastric schwannoma by their content of psammoma bodies and variable amounts of melanin pigment, as well as lack of lymphoid cuffing and microtrabecular architecture.
Plexiform schwannoma, especially its visceral subtype, is a rare variant that differs from the typical gastric schwannoma by its multinodular to multilobulated composition. In this study, there were no examples of this sub-entity. However, one example included in a previous series 36 and one gastric schwannoma reported in a patient with Lambert-Eaton myasthenic syndrome seems to represent this subtype. 37 Based on this, we estimate that the plexiform subtype represents no more than 1–2% of all gastric schwannomas.
Microcystic/reticular schwannomas are small, usually juxtaluminal nerve sheath tumors. 38,39 In this variant, the tumor cells are arranged in a distinctive microcystic or net-like pattern, and they lack peritumoral lymphoid tissue almost invariably seen in gastric schwannoma. In our experience, this sub-entity has a predilection to colon, as opposed to stomach.
Gastrointestinal tract variant of clear cell sarcoma is another S100 protein neoplasm that can involve stomach, although it is more common in small intestine. 40,41 These tumors typically show mural involvement, often with a nested pattern. By their higher cellularity and significant general nuclear atypia, they are usually easily distinguished from gastric schwannomas.
Metastatic malignant melanoma has sometimes been the submitted diagnosis for gastric schwannoma in our experience. To some degree, the microtrabecular-microfascicular pattern of gastric schwannoma resembles that of desmoplastic or spindle cell melanoma. However, metastatic melanoma usually manifests as a mucosa-based button-like lesion, shows greater degree of atypia and mitotic activity, and very rarely forms a solid intramural mass.
Gastrointestinal stromal tumor (GIST) can have a similar clinical and gross presentation, involve the same demographic group of older adults, and have certain overlapping histologic features with GI schwannoma. Because GISTs are much more common than gastric schwannomas, it is not surprising that most reports on gastric schwannoma prior to modern immunohistochemistry in fact describe GISTs, as found in our retrospective review of literature. 7–17 Among these cases are especially gastric spindle cell GISTs of the palisaded-vacuolated type, which is perhaps the most common distinctive histology subtype of gastric GIST. 42
Grossly GISTs differ from schwannomas by their predominantly tan-pink or hemorrhagic appearance 42, as opposed to yellow to yellow-white color typically seen in gastric schwannomas on sectioning. Histologically, some gastric GISTs, especially those of the palisaded-vacuolated subtype, have even more prominent nuclear palisading than most gastric schwannomas, but the perinuclear vacuolization is not seen in gastric schwannoma. Immunohistochemical studies easily separate GIST from schwannoma by the presence of KIT and DOG1/Ano 1 only in the former and S100 and often GFAP only in the latter. Furthermore CD34 is usually demonstrable in spindle cell GISTs and generally absent in gastric schwannomas.
In conclusion, we have reported 51 gastric schwannomas, the largest series to date. This tumor is much less common than gastric GIST and has a predilection for older adults with a female predominance. The clinical course is benign and neither our study nor our review of the literature identified malignant examples. These tumors differ from peripheral schwannomas histologically, and also genetically in their less common NF2 gene involvement. Based on these differences from peripheral schwannoma and similarities between schwannomas in various parts of the GI tract, the designation “gastrointestinal schwannoma” might be appropriate to for this unique subtype of schwannoma. Gastric schwannoma should be distinguished from GIST and malignant S100 positive tumors, especially gastrointestinal clear cell sarcoma and metastatic melanoma.
Acknowledgments
This research was supported in part by the intramural research program of the NIH and NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daimaru Y, Kido H, Hashimoto H, et al. Benign schwannoma of the gastrointestinal tract: A clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19:257–64. doi: 10.1016/s0046-8177(88)80518-5. [DOI] [PubMed] [Google Scholar]
- 2.Agaimy A, Markl B, Kitz J, et al. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphological variants. Virchows Arch. 2010;456:411–22. doi: 10.1007/s00428-010-0886-8. [DOI] [PubMed] [Google Scholar]
- 3.Hong HS, Ha HK, Won HJ, et al. Gastric schwannomas: radiologic features with endoscopic and pathologic correlation. Clinical Radiology. 2008;63:536–42. doi: 10.1016/j.crad.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Jung MK, Jeon SW, Cho CM, et al. Gastric schwannomas: endoscopic characteristics. Abdom Imaging. 2008;33:388–90. doi: 10.1007/s00261-007-9291-4. [DOI] [PubMed] [Google Scholar]
- 5.Prevot S, Bienvenu L, Vaillant JC, et al. Benign schwannoma of the digestive tract. A clinicopathologic and immunohistochemical study of five cases, including a case of esophageal tumor. Am J Surg Pathol. 1999;23:431–6. doi: 10.1097/00000478-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Sarlomo-Rikala M, Miettinen M. Gastric schwannoma- a clinicopathological analysis of six cases. Histopathology. 1995;27:355–60. doi: 10.1111/j.1365-2559.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 7.Chandra M, Mehrotra P, Mitra MK. Gastric schwannoma presenting as a gastric polyp with gastrointestinal bleeding. Indian J of Gastroenterol. 2002;21:31. [PubMed] [Google Scholar]
- 8.Chaudry NU, Zafar S. Benign nerve sheath tumor of stomach. J Coll Phys Surg Pak. 2007;17:277–279. [PubMed] [Google Scholar]
- 9.Jang KY, Park HS, Chung MJ, et al. Synchronous occurrence of primary adenocarcinoma and schwannoma in the stomach: a case report. Pathology. 2009;41:286–9. doi: 10.1080/00313020902756386. [DOI] [PubMed] [Google Scholar]
- 10.Khan AA, Schizas AMP, Cresswell AB, et al. Digestive tract schwannoma. Dig Surg. 2006;23:265–9. doi: 10.1159/000096159. [DOI] [PubMed] [Google Scholar]
- 11.Lin C, Hsu H, Tsai CH, et al. Gastric Schwannoma. Case report. J Chin Med Assoc. 2004;67:583–6. [PubMed] [Google Scholar]
- 12.Ogasawara N, Sasaki M, Ishiguro H, et al. Gastric schwannoma with adjacent external progression harbored aberrant NF2 gene. Dig Endosc. 2009;21:192–5. doi: 10.1111/j.1443-1661.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 13.Povoski SP, Chang WW. Gastric schwannoma found incidentally 19 years after a horizontal gastroplasty for morbid obesity. Obesity Surgery. 2001;11:762–5. doi: 10.1381/09608920160558740. [DOI] [PubMed] [Google Scholar]
- 14.Snyder RA, Harris E, Hansen EN, et al. Gastric schwannoma. Am Surg. 2008;74:753–6. [PubMed] [Google Scholar]
- 15.Yoon HY, Kim CB, Lee YH, et al. Gastric schwannoma. Yonsei Med J. 2008;49:1052–1054. doi: 10.3349/ymj.2008.49.6.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baty JA. Gastric neurilemmoma. Br J Surg. 1951;39:251–3. doi: 10.1002/bjs.18003915511. [DOI] [PubMed] [Google Scholar]
- 17.Bresnahan TJ, Brown FN. Neurilemmoma of stomach wall with peptic ulcer. Canad Med Assoc J. 1951;65:259. [PMC free article] [PubMed] [Google Scholar]
- 18.Dorfman M. Gastric Neurilemmoma. Gastroenterology. 1953;25:56–66. [PubMed] [Google Scholar]
- 19.Genova G, Maiorana AM, Agnello G, et al. Gastric Schwannoma after Nissen Fundoplication. A Rare Complication? The American Surgeon. 1989;55:495–7. [PubMed] [Google Scholar]
- 20.Imrie AH, Dunn RI. Neurilemmoma of the Stomach with Peptic Ulceration: Report of a Case. Glasgow Med J. 1955;36:168–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Janowitz P, Meier F, Reisig J. Gastric schwannoma as a rare differential diagnosis of pleural effusion. Z Gastroenterol. 2002;40:925–8. doi: 10.1055/s-2002-35416. [DOI] [PubMed] [Google Scholar]
- 22.Loffeld RJ, Balk TG, Oomen JL, et al. Upper gastrointestinal bleeding due to a malignant schwannoma of the stomach. Eur J Gastroenter Hepatol. 1998;10:159–62. doi: 10.1097/00042737-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Melvin WS, Wilkinson MG. Gastric schwannoma. Clinical and pathologic considerations. Am Surg. 1993;59:293–6. [PubMed] [Google Scholar]
- 24.Pinto A, Novo JA. Gastric neurilemmoma: a case report. Mt Sinai J Med. 1985;52:647–9. [PubMed] [Google Scholar]
- 25.Rutten APM. Neurogenic tumours of the stomach. Brit J Surg. 1965;52:920–5. doi: 10.1002/bjs.1800521204. [DOI] [PubMed] [Google Scholar]
- 26.Lasota J, Dansonka-Mieszkowska A, Sobin LH, et al. A great majority of GISTs with PDGFRA mutations represents gastric tumors of no or low malignant potential. Lab Invest. 2004;84:874–83. doi: 10.1038/labinvest.3700122. [DOI] [PubMed] [Google Scholar]
- 27.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245–66. doi: 10.1111/j.1365-2559.2008.02977.x. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum. A clinicopathologic and imunohistochemical study of 20 cases. Am J Surg Pathol. 2001;25:846–55. doi: 10.1097/00000478-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Chaubal A, Paetau A, Zoltick P, et al. CD34 immunoreactivity in nervous system tumors. Acta Neuropathol. 1994;88:454–8. doi: 10.1007/BF00389498. [DOI] [PubMed] [Google Scholar]
- 30.Weiss SW, Nickoloff BJ. CD34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol. 1993;17:1039–45. doi: 10.1097/00000478-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Lasota J, Wasag B, Dansonka-Mieszkowska A, et al. Evaluation of NF2 and NF1 tumor suppressor genes in distinctive gastrointestinal nerve sheath tumors traditionally diagnosed as benign schwannomas: a study of 20 cases. Lab Invest. 2003;83:1361–71. doi: 10.1097/01.lab.0000087591.29639.e3. [DOI] [PubMed] [Google Scholar]
- 32.Diaz se Ståhl T, Hansson CM, de Bustos C, et al. High-resolution array-CGH profiling of germline and tumor-specific copy number alterations on chromosome 22 in patients affected with schwannomas. Hum Genet. 2005;118:35–44. doi: 10.1007/s00439-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 33.Croker JR, Greenstein RJ. Malignant schwannoma of the stomach in a patient with von Recklinghausen disease. Histopathology. 1979;3:79–85. doi: 10.1111/j.1365-2559.1979.tb02983.x. [DOI] [PubMed] [Google Scholar]
- 34.Gennatas CS, Exarhakos G, Kondi-Pafiti A, et al. Malignant Schwannoma of the Stomach in a Patient with Neurofibromatosis. European Journal of Surgical Oncology. 1988;14:261–4. [PubMed] [Google Scholar]
- 35.Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol. 1990;14:206–22. [PubMed] [Google Scholar]
- 36.Chen YY, Yen HH, Soon MS. Solitary gastric melanotic schwannoma: sonographic findings. J Clin Ultrasound. 2007;35:52–4. doi: 10.1002/jcu.20279. [DOI] [PubMed] [Google Scholar]
- 37.Patanella AK, Bianco A, Federico F, et al. Lambert-Eaton myasthenic syndrome associated with gastric schwannoma. Muscle Nerve. 2010;41:569–70. doi: 10.1002/mus.21664. [DOI] [PubMed] [Google Scholar]
- 38.Chetty R. Reticular and microcystic schwannoma: distinctive tumor of the gastrointestinal tract. Ann Diagn Pathol. 2011;15:198–201. doi: 10.1016/j.anndiagpath.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Tozbikian G, Shen R, Suster S. Signet ring cell gastric schwannoma: report of a new distinctive morphological variant. Ann Diagn Pathol. 2008;12:146–52. doi: 10.1016/j.anndiagpath.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Stockman DL, Miettinen M, Spagnolo D, et al. Clinicopathological, immunohistochemical, ultrastructural, and molecular analysis of clear cell sarcoma (CCS)-like tumor of the gastrointestinal tract. Abstract Mod Pathol. 2011;23:21A. [Google Scholar]
- 41.Zambrano E, Reyes-Mugica M, Franchi A, et al. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: report of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11:75–81. doi: 10.1177/106689690301100202. [DOI] [PubMed] [Google Scholar]
- 42.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic studies of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]