Abstract
Rosacea is a frequent chronic inflammatory skin disease of unknown etiology. Because early rosacea reveals all characteristics of neurogenic inflammation, a central role of sensory nerves in its pathophysiology has been discussed. Neuroinflammatory mediators and their receptors involved in rosacea are poorly defined. Good candidates may be transient receptor potential (TRP) ion channels of vanilloid type (TRPV), which can be activated by many trigger factors of rosacea. Interestingly, TRPV2, TRPV3, and TRPV4 are expressed by both neuronal and non-neuronal cells. Here, we analyzed the expression and distribution of TRPV receptors in the various subtypes of rosacea on non-neuronal cells using immunohistochemistry, morphometry, double immunoflourescence, and quantitative real-time PCR (qRT-PCR) as compared with healthy skin and lupus erythematosus. Our results show that dermal immunolabeling of TRPV2 and TRPV3 and gene expression of TRPV1 is significantly increased in erythematotelangiectatic rosacea (ETR). Papulopustular rosacea (PPR) displayed an enhanced immunoreactivity for TRPV2, TRPV4, and also of TRPV2 gene expression. In phymatous rosacea (PhR)-affected skin, dermal immunostaining of TRPV3 and TRPV4 and gene expression of TRPV1 and TRPV3 was enhanced, whereas epidermal TRPV2 staining was decreased. Thus, dysregulation of TRPV channels also expressed by non-neuronal cells may be critically involved in the initiation and/or development of rosacea. TRP ion channels may be targets for the treatment of rosacea.
Keywords: rosacea, neuroimmunology, skin, blood vessel, mast cell, gene array, inflammation, fibrosis, TRPV Cation Channels
Introduction
Rosacea is a common chronic skin disease of uncertain etiology which affects mainly the central facial region. Because the pathophysiology of rosacea is still unclear, a causal therapy is still poor (Powell, 2005; van Zuuren et al, 2011, Steinhoff et al, 2011).
Rosacea can be categorized into four different subtypes (Wilkin et al, 2002; Elewski et al, 2011). Subtype I, erythematotelangiectatic rosacea (ETR), involves flushing - often after trigger factors such as temperature changes, spicy food, hot beverages, UV exposure, exercising, emotional stress, or alcohol - facial erythema and telangiectasia. Subtype II, papulopustular rosacea (PPR), is associated with papules and/or pustules in addition to erythema. Subtype III, phymatous rosacea (PhR), is characterized by skin fibrosis and glandular hyperplasia leading to phymata, mainly rhinophyma. Another differentiated subtype is ocular rosacea.
Etiopathological factors discussed are dysfunction of the innate immune system, dermal matrix degeneration, chemical irritants, or microbial organisms (Yamasaki and Gallo, 2009; Zhang et al., 2011a). Because the symptoms of rosacea such as vasodilation, flushing, increased skin sensitivity, and lower pain thresholds (Guzman-Sanchez et al., 2007) are caused by neuromediators (e.g., PACAP (pituitary adenylate cyclase-activating polypeptide); Seeliger et al., 2010), the involvement of the skin nervous system can be anticipated. As classical neuronal receptors can also be expressed by non-neuronal cells such as keratinocytes, endothelial cells, and immune cells, a dysregulation of these receptors on non-neuronal cells in rosacea has to be considered as well.
TRPV (transient receptor potential vanilloid subfamily) receptors are a subgroup of the heterogeneous TRP cation channels with markedly diverse functions. The TRPV subfamily consists of four nonselective cation channels (TRPV1, TRPV2, TRPV3, and TRPV4) and two highly Ca2+ selective channels (TRPV5 and TRPV6; Nilius et al., 2007; Aubdool and Brain, 2011).
TRPV1 is expressed on neuronal cells and has an important role in nociception (Basbaum et al., 2009) and neurogenic inflammation (Roosterman et al., 2006). In addition, in non-neuronal cells, e.g., keratinocytes, TRPV1 is discussed to be expressed (Pecze et al., 2008). It is activated by capsaicin (‘‘spicy food’’), heat (>42 °C), or under inflammatory conditions. TRPV2 was found on neuropeptide-positive C-fibers and many other non-neuronal cells, e.g., keratinocytes (Axelsson et al., 2009) and macrophages (Link et al., 2010). It is suggested to have a role in innate immunity, inflammation, nociception, and sensing of noxious heat. TRPV3 is localized on neuronal tissue, skin, and blood vessels (Earley et al., 2010). TRPV3 is activated by innocuous warm temperatures (32–39 °C) and is involved in thermosensation and keratinocyte differentiation (Cheng et al., 2010). TRPV4 is widely spread on neuronal and nonneuronal cells, including keratinocytes and endothelium. It is activated by moderate heat (24–34 °C), hypotonic cell swelling, and inflammatory metabolites. It may serve as an osmoreceptor, promote vasodilation, and cause mechanical and inflammation-evoked hyperalgesia (Vennekens et al., 2008).
According to the fact that TRPV channels can be activated by typical trigger factors of rosacea, these receptors may have a role in the pathophysiology of this disease by ‘‘sensing’’ exogenous and endogenous trigger factors in the skin. Therefore, the aim of this study was to (1) investigate the distribution of TRPV2, TRPV3, and TRPV4 in non-neuronal cells of rosacea patients by immunohistochemistry and double immunoflourescence, (2) perform a semiquantitative analysis of the immunohistochemical data, (3) determine the expression levels of TRPV1, TRPV2, TRPV3, and TRPV4 in rosacea-affected skin by quantitative real-time PCR (qRT-PCR), and (4) compare these results with skin tissues from healthy donors and patients with lupus erythematosus (LE).
Results
Dermal immunoreactivity of TRPV2 is enhanced in ETR and PPR
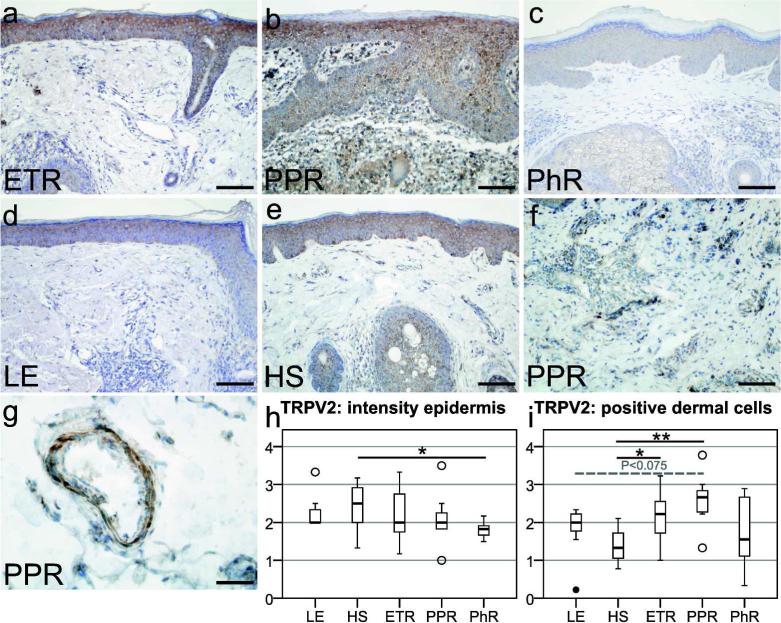
Immunohistochemical investigation of human skin revealed immunoreactivity for TRPV2 in the epidermis of all study groups (Figure 1a–e). Epidermal TRPV2 staining intensity was rated semiquantitatively (Figure 1h) and showed a decrease in PhR-affected skin (P<0.05), as compared with healthy skin (HS). Thus, TRPV2 immunoreactivity was only reduced in keratinocytes of PhR patients, and never increased in any group.
Figure 1.
Localization of transient receptor potential vanilloid subfamily 2 (TRPV2) in erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PhR), lupus erythematosus (LE), and healthy skin (HS), followed by semiquantitative analysis of TRPV2 immunoreactivity in the epidermal or dermal compartment. (a–e) TRPV2 immunostaining was observed in keratinocytes in all study groups. (a, b, f) Strong dermal labeling was found on immune cells in ETR and PPR. Fibroblasts and endothelial cells in (a) ETR, and smooth muscle cells of blood vessels in (g) PPR also showed immunoreactivity. (h) Semiquantitative examination of epidermal TRPV2 staining displayed a decreased intensity in PhR (P<0.05) as compared with HS. (i) Semiquantitative analysis of TRPV2-positive immune cells displayed an increased staining in PPR (P<0.01) and ETR (P<0.05) as compared with HS. Additionally, PPR revealed an enhanced immunoreactivity as compared with LE (P<0.075). Bar=100 µm (a–f) and 50 µm (g); unfilled circles represent outliers; *P<0.05, **P<0.01.
Dermal staining for TRPV2 was observed in all investigated groups. TRPV2 was localized on immune cells, fibroblasts, and smooth muscle cells of blood vessels (Figure 1g). Remarkably, multinucleated giant cells (Figure 1b), formed by the union of several macrophages, showed marked immunoreactivity for TRPV2. Semiquantitative scoring (Figure 1i) of positive dermal cells showed increased staining in PPR (P<0.01), ETR (P<0.05), and no statistical significance in LE or PhR, as compared with HS. In addition, PPR revealed increased dermal immunoreactivity as compared with LE (P<0.075). Thus, TRPV2 immunoreactivity is enhanced in dermal cells of rosacea patients.
TRPV2 is colocalized with CD68 and MC tryptase and is upregulated in rosacea tissue
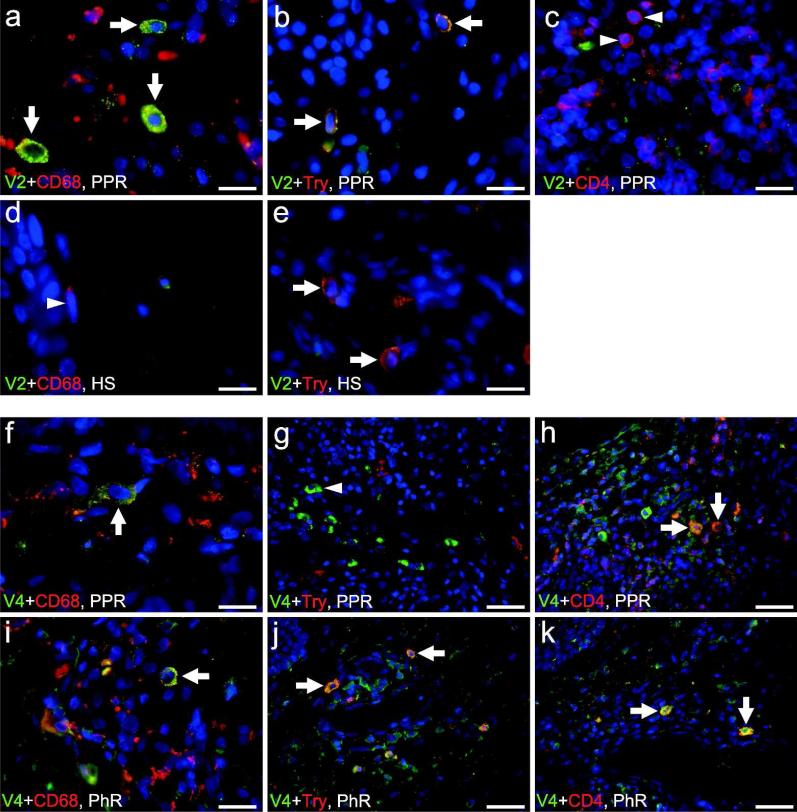
Double immunoflourescence reveals colocalization of TRPV2 with CD68+ macrophages and mast cells (MCs) in PPR (Figure 4a and b). In addition, immunolabeling for TRPV2 in macrophages and MCs is enhanced in rosacea tissue as compared with HS (Figure 4d and e). CD4+ T helper cells show no convincing colocalization with TRPV2 in PPR (Figure 4c).
Figure 4.
Double immunofluorescence of transient receptor potential vanilloid subfamily 2 (TRPV2) and TRPV4 in patients with rosacea. TRPV2 reveals colocalization with (a) CD68+ macrophages and (b) mast cell (MC) tryptase (Try) in papulopustular rosacea (PPR). (c) CD4+ T helper cells show no convincing colocalization with TRPV2 in PPR (arrowheads). (d) In healthy skin (HS), macrophages showed only background staining for TRPV2 (arrowhead). (e) Colocalization of TRPV2 in MCs in healthy skin was observed, and immunolabeling for TRPV2 in macrophages and MCs is enhanced in PPR as compared with HS. TRPV4 shows colocalization with (f, i) CD68 (macrophages) and (h, k) CD4 (T helper cells). (g) No colocalization of TRPV4 with MC tryptase was found in PPR (arrowhead), (j) whereas TRPV4 shows colocalization with MCs surrounding a blood vessel in phymatous rosacea (PhR). Bar=40 µm (g, h, j, k), 20 µm (i), 15 µm (a–e, f), arrows indicate colocalization.
Dermal immunostaining of TRPV3 is increased in ETR and PhR
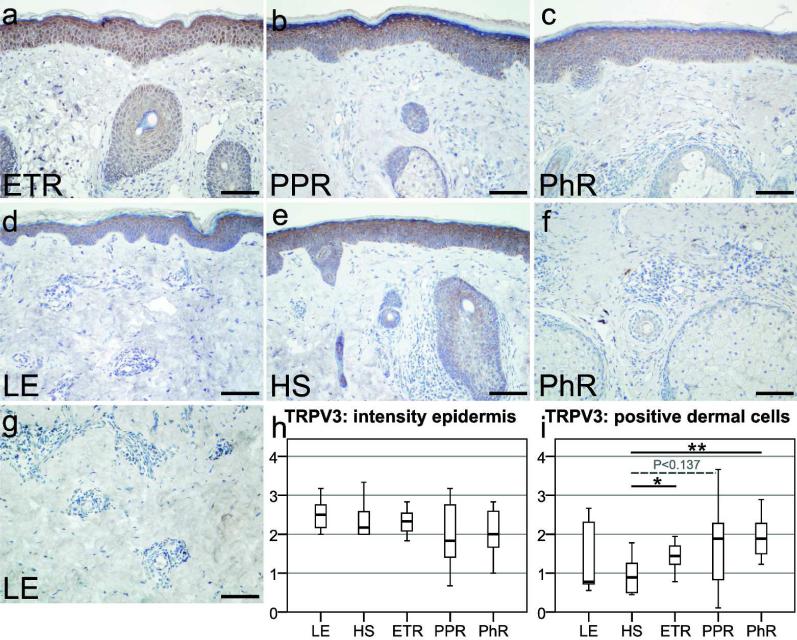
Immunohistochemical staining of TRPV3 in human skin showed positive staining for keratinocytes in all study groups (Figure 2a–e). Semiquantitative scoring of epidermal TRPV3 staining intensity (Figure 2h) did not reveal any statistically significant differences between skin affected by rosacea or LE, as compared with HS. Thus, TRPV3 immunoreactivity can be found in keratinocytes, but no differences were observed between the study groups.
Figure 2.
Localization of transient receptor potential vanilloid subfamily 3 (TRPV3) in erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PhR), lupus erythematosus (LE), and healthy skin (HS), followed by semiquantitative analysis of TRPV3 immunoreactivity in the epidermal or dermal compartment. (a–e) TRPV3 immunostaining was observed in epidermal keratinocytes in all study groups. (a–g) Dermal labeling was also evident in some immune cells, fibroblasts, and only reached intensity of background staining in endothelial cells and smooth muscle cells. (h) Semiquantitative scoring of epidermal staining revealed a slight attenuated staining in ETR and PPR as compared with HS, but did not reach statistical significance. (i) After semiquantitative examination of positive-stained dermal immune cells or fibroblasts, statistically significant increased scores were found in ETR (*P<0.05) and PhR (**P<0.01) as compared with HS. Counterstaining with hematoxylin, Bar=100 µm (a–g).
In the dermis, immunohistochemical data demonstrate immunostaining for some fibroblasts and immune cells in rosacea. Semiquantitative investigations (Figure 2i) of positive-stained dermal cells confirmed statistically significant increased dermal staining in ETR (P<0.05) and PhR (P<0.01) as compared with HS. Staining in PPR for TRPV3 was also abundant (P<0.137), but did not reach statistical significance. In sum, increased immunostaining for TRPV3 in immune cells and fibroblasts was observed in rosacea as compared with HS.
Dermal immunoreactivity of TRPV4 is enhanced in PPR and PhR
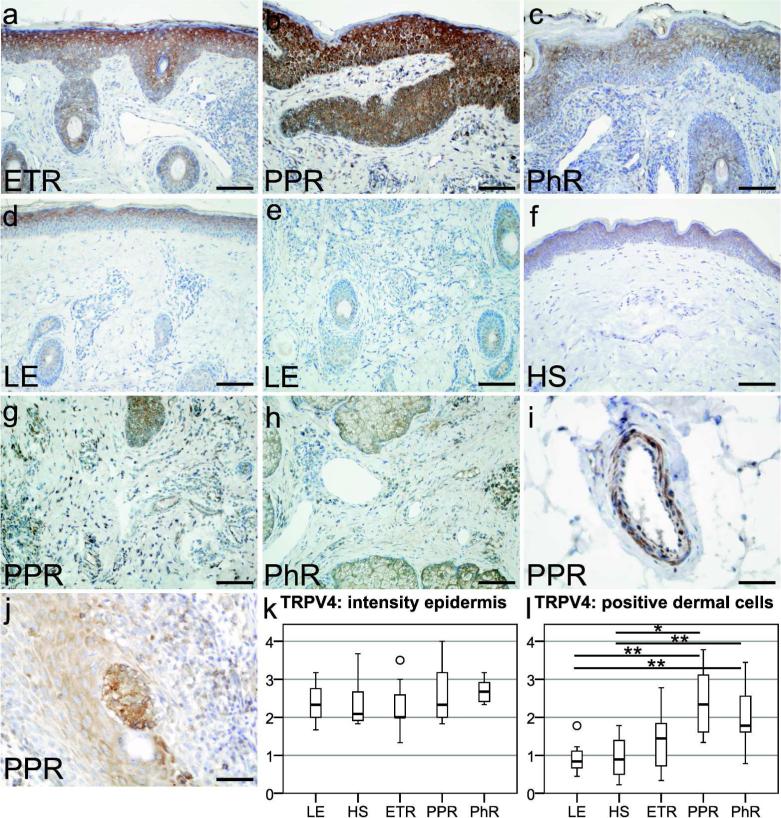
Immunohistochemical investigation of human skin revealed TRPV4 labeling in the epidermis and dermis of all study groups (Figure 3a–f). Epidermal TRPV4 staining intensity was rated semiquantitatively (Figure 3k) and displayed a tendency for enhanced immunostaining in PhR, but semiquantitative analysis did not show any statistically significant differences between the study groups.
Figure 3.
Localization of transient receptor potential vanilloid subfamily 4 (TRPV4) in erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PhR), lupus erythematosus (LE), and healthy skin (HS), followed by semiquantitative analysis of TRPV4 immunoreactivity in the epidermal or dermal compartment. (a–f) TRPV4 immunostaining was located on keratinocytes in all study groups, on dermal immune cells of rosacea, and in LE patients. Certain immune cells were strongly positive in (g) PPR and (h) PhR, whereas TRPV4-positive immune cells were only rarely found in (e) LE. Interestingly, in PPR endothelial cells, (i) smooth muscle cells and (j) Langerhans cells showed immunolabeling. (k) Semiquantitative analysis of epidermal staining revealed no significant differences. (l) Semiquantitative scoring of positive dermal cells confirmed an increased staining for TRPV4 in PPR (P<0.01) and PhR (P<0.05) as compared with HS and LE. Bar=100 µm (a–h) and 50 µm (i–j); unfilled circles represent outliers; *P<0.05, **P<0.01.
We also found dermal immune cells to be positive for TRPV4 in rosacea-affected skin (Figure 3g and h), as compared with LE or HS (Figure 3d–f), which showed no or very weak immunostaining on dermal immune cells. In PPR, endothelial cells, smooth muscle cells (Figure 3i), and Langerhans cells (Figure 3j) showed immunolabeling for TRPV4. In sum, semiquantitative examination (Figure 3l) revealed increased staining for TRPV4 in immune cells of PPR (P<0.01) and PhR (P<0.05), indicating an upregulation of TRPV4 in dermal immune cells in comparison with HS and LE.
The role of neuronally expressed TRPV channels is part of a different study using thick cryosections in order to quantify TRPV-positive nerves in rosacea tissues (Sulk et al, in preparation).
TRPV4 is colocalized with CD4, CD68, and MC tryptase in rosacea tissue
Double immunoflourescence reveals colocalization of TRPV4 with CD4+ T helper cells in PPR and interestingly in PhR around a dilated blood vessel (Figure 4h and k). CD68+ macrophages occasionally show colocalization with TRPV4 in PPR and PhR (Figure 4f and i). Interestingly, MCs show only colocalization with TRPV4 in PhR, whereas MCs in PPR exhibit no colocalization (Figure 4j and g).
qRT-PCR reveals differential expression of TRPV1-4 genes in rosacea
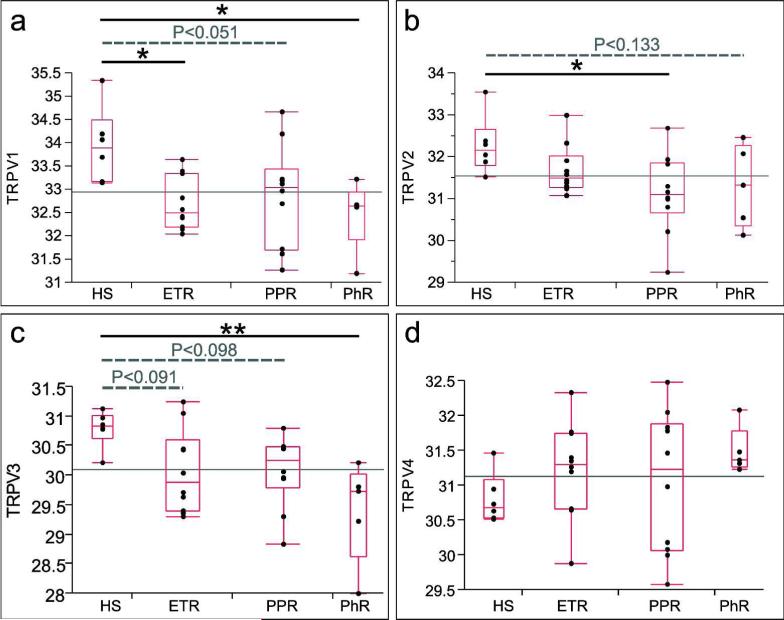
Expression levels of TRPV1–4 mRNA from skin biopsies of rosacea and healthy patients were examined (Figure 5). Our data demonstrate increased gene expression levels for TRPV1 mRNA in all rosacea subtypes, especially in ETR and PhR as compared with HS. Gene expression analysis for TRPV2 mRNA showed a general decrease of Ct values, indicating increased expression for all rosacea patients, especially in PPR. Expression level for TRPV3 mRNA was found to be enhanced in all rosacea subtypes, especially in PhR. TRPV4 gene expression levels were diminished in all rosacea subtypes, although without statistical significance as compared with HS. In sum, TRPV1, TRPV2, and TRPV3 mRNA levels were found to be upregulated in rosacea, although with differences among the various subtypes, whereas TRPV4 mRNA levels were not changed in rosacea patients.
Figure 5.
Normalized Ct values of individual genes (transient receptor potential vanilloid subfamily (TRPV)1–4) in healthy skin (HS) compared with erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), and phymatous rosacea (PhR). The ends of the box indicate the lower and upper 25% quartiles. The line across the middle of the box identifies the median sample value. The width of the box is proportional to the number of observations. The central line in each subfigure identifies the global mean. The P-values of the comparisons of gene expression levels in the different subtypes of rosacea versus HS are indicated. In sum, TRPV1, TRPV2, and TRPV3 mRNA levels were found to be upregulated in rosacea, although with differences among the various subtypes, whereas TRPV4 mRNA levels were not changed with statistical significance in rosacea patients. *P<0.05, **P<0.01.
Discussion
In this study, we analyzed the distribution of TRPV2, TRPV3, and TRPV4 receptors in non-neuronal cells of rosacea patients as compared with HS and patients with LE. Semiquantitative analysis of dermal and epidermal TRPV2, TRPV3, and TRPV4 immunolabeling revealed differences between skin of patients suffering from rosacea and unaffected skin. Using qRT-PCR technology, we showed a modulation of TRPV1, TRPV2, TRPV3, and TRPV4 genes in rosacea patients (Supplementary Table S1 online). The complex role of neuronal and non-neuronal TRPV1 is part of a separate study (Sulk et al, in preparation).
TRPV2
Semiquantitative immunohistochemical analysis of TRPV2 revealed decreased epidermal staining intensity in PhR and increased immunoreactivity of dermal immune cells in all investigated rosacea subtypes.
Epidermal staining for TRPV2 was found previously (Radtke et al., 2011) and a decrease in PhR might be based on compensatory mechanisms due to UV radiation or inflammation, but the role of TRPV2 on keratinocytes still remains unclear.
Cardiac fibroblasts were described to express TRPV2 (Hatano et al., 2009). We also found immunoreactivity for TRPV2 in some fibroblasts of rosacea patients, but the role of TRPV2 on fibroblasts, myofibroblasts, and extracellular matrix regulation needs further investigation.
Blood vessels also exhibited immunostaining for TRPV2 in rosacea tissue, especially in smooth muscle cells. It is noteworthy that TRPV2 activation has already been associated with modulation of smooth muscle cell function in other tissues related to vasodilation (Baylie and Brayden, 2011). The precise role of TRPV2 in cutaneous vasodilation has to be determined in the future.
Previously, TRPV2 has been suggested to modulate immune cell function. In particular, others found TRPV2 on MCs (Kim et al., 2010), neutrophil granulocytes (Heiner et al., 2003), lymphocytes (Saunders et al., 2007; Wenning et al., 2011), and macrophages. Accordingly, macrophages and MCs were shown to colocalize with TRPV2 and, in addition, to be upregulated in PPR as compared with HS (Figure 4a, b, d and e), which could explain the abundant immunolabeling of TRPV2 in the dermal compartment of rosacea patients, whereas CD4+ T helper cells revealed no colocalization with TRPV2 in PPR. It is assumed that TRPV2 has an important role in MC degranulation (Stokes et al., 2004; Zhang et al., 2011b) and activation (Giudice et al., 2007), whereas in neutrophils and lymphocytes its role remains elusive. Recently, it was revealed that TRPV2 in macrophages is essential for phagocytosis (Link et al., 2010), as well as tumor necrosis factor-a and IL-6 production (Yamashiro et al., 2010). TRPV2 expression was also shown in monocyte-related cell types such as Langerhans cells (Shimohira et al., 2009; Link et al., 2010) and phagocytic retinal pigment epithelial cells where TRPV2 may regulate vascular endothelial growth factor-A secretion (Cordeiro et al., 2010). The precise characterization of TRPV2-expressing immune cells in rosacea has to await further investigation.
According to our semiquantitative immunohistochemical and double-immunoflourescence analysis, mRNA levels for TRPV2 were also enhanced in all rosacea subtypes. In particular, in PPR, expression rates were significantly increased as compared with HS, which could be explained by an increased number of TRPV2-expressing immune cells, and an upregulation of TRPV2 mRNA in macrophages and MCs may be due to inflammation (Shimosato et al., 2005).
In sum, non-neuronal TRPV2 is expressed by various skin and immune cells, and may be involved in vasoregulation and immunomodulation, as described in mice (Link et al., 2010). In rosacea, upregulated TRPV2 can be activated by trigger factors (e.g., noxious heat), and thus could have a role in the pathophysiology of rosacea.
TRPV3
In rosacea-affected skin, increased immunostaining for TRPV3 was observed in the dermal compartment, whereas the epidermal compartment showed no differences, as compared with HS. In addition, qRT-PCR revealed enhanced TRPV3 gene expression levels in all rosacea subtypes. Keratinocytes express functional TRPV3 and release IL-1α (Xu et al., 2006), prostaglandin E2 (Huang et al., 2008), transforming growth factor-a (Cheng et al., 2010), and nitric oxide (Miyamoto et al., 2011) after TRPV3 activation. Thus, TRPV3 on keratinocytes may modulate inflammation, fibroblast function, keratinocyte differentiation, vasodilation, thermotransduction, and nociception. In addition, TRPV3 may be involved in the pathophysiology of atopic dermatitis and elevation of MC numbers (Asakawa et al., 2006; Yoshioka et al., 2009). As temperature changes and MCs have an important role in rosacea pathophysiology (Aroni et al., 2008; Schwab et al., 2011), one may speculate that TRPV3 has a role in inflammatory and nociceptive processes in rosacea, perhaps by regulating keratinocyte and MC function.
Furthermore, some fibroblasts in PhR showed immunoreactivity for TRPV3. Accordingly, carvacrol, a well-known TRPV3 agonist, induces type I collagen gene expression (Lee et al., 2008). Thus, TRPV3 may also be involved in fibrotic processes in rosacea.
Recent findings indicate a role of TRPV3 in regulating lymphocyte function. In particular, B and T lymphocytes were described to express TRPV3 (Inada et al., 2006; Wenning et al., 2011). We also found increased immunolabeling of TRPV3 on dermal immune cells in all rosacea subtypes. However, the precise role of TRPV3 as an immunomodulator in inflammatory skin diseases and the exact distribution of TRPV3 in specific dermal immune cells in rosacea are still unknown and need further exploration.
Blood vessels and especially endothelial cells were found to express functional TRPV3 and mediate vasodilation (Earley et al., 2010). However, we found only weak immunoreactivity of dermal endothelial cells in our study in rosacea tissue; thus, a potential role of TRPV3 as a vasoregulator in rosacea has to await further functional studies.
Upregulation of dermal TRPV3 and TRPV3 mRNA levels may be associated with nociception (Gopinath et al., 2005; Bang et al., 2011), tissue injury (Facer et al., 2007), inflammation (Hu et al., 2006), and probably fibrosis. In sum, non-neuronal TRPV3 is able to be activated by trigger factors of rosacea, and thus could mediate and maintain typical symptoms of rosacea.
TRPV4
In rosacea-affected skin, increased immunostaining for TRPV4 was found in the dermal compartment, whereas the epidermal compartment showed no differences as compared with HS and also LE. In particular, PPR and PhR showed enhanced immunostaining in dermal cells, whereas qRT-PCR revealed no statistically significant changes with respect to TRPV4 gene expression levels.
Recently, it was demonstrated that it is functionally expressed in keratinocytes, and may contribute to barrier integrity (Sokabe and Tominaga, 2010). According to recent studies (Baylie and Brayden, 2011), also in rosacea endothelial cells and smooth muscle cells showed TRPV4-immunoreactivity, and thus TRPV4 may mediate vasodilation in rosacea patients. Furthermore, TRPV4 is assumed to have a role in edema (Willette et al., 2008), angiogenesis due to shear stress (Schierling et al., 2011), inflammation (Fiorio Pla et al., 2011), and extracellular matrix deformation (Thodeti et al., 2009).
In our study, increased localization of TRPV4 was also found on dermal immune cells in rosacea-affected skin. According to recent findings (Stokes et al., 2004; Kim et al., 2010), we found that MCs express TRPV4, and a functional role of TRPV4 in MC degranulation is suggested (Yang et al., 2007).
In addition, TRPV4 has been described in macrophages (Hamanaka et al., 2010) and lymphocytes (Inada et al., 2006; Spinsanti et al., 2008; Wenning et al., 2011), which is supported by our findings in rosacea patients (Figure 4). To our knowledge, this is the first description of TRPV4 localized on Langerhans cells. Thus far, a functional role of TRPV4 was found for macrophage activation (Hamanaka et al., 2010) However, a functional role of TRPV4 in skin immune cells needs further investigation. Thus, increased dermal immunostaining in rosacea could be considered as MC, macrophage, and/or CD4+ T helper cell related. Inflammation induced by histamine (Cenac et al., 2010), proteases (Grant et al., 2007), and prostaglandin E2 (Alessandri-Haber et al., 2003) may lead to sensitization of TRPV4 on these cells and thus modulate inflammatory processes, especially in patients with rosacea. As we found differences of TRPV4 expression on the protein level but not on the RNA level, one may assume that TRPV4 is regulated on the protein level rather than on the transcriptional level in rosacea patients. Regulation of (neuronal) TRPV4 on the translational level has been described under inflammatory conditions (Cenac et al., 2010; D'Aldebert et al., 2011; Fiorio Pla et al., 2011), which may be due to desensitization and trafficking after repeated stimulation (Guler et al., 2002). Another explanation could be different sensitivities of immunohistochemistry and PCR, respectively. We tried to determine the specificity on the protein level by using two different primary antibodies against TRPV2, TRPV3, and TRPV4, which all showed a similar staining pattern (Supplementary Figure S1 online).
In sum, TRPV4 can be activated by rosacea trigger factors and under inflammatory conditions. We found increased immunostaining of TRPV4 in various dermal cells in rosacea as compared with HS and—interestingly—LE. Therefore, TRPV4 may be involved in inflammatory processes in rosacea.
TRPV1 mRNA
The qRT-PCR analysis of TRPV1 expression of rosacea-affected skin was performed, which revealed increased TRPV1 mRNA expression levels in all rosacea subtypes when compared with HS, indicating a role of TRPV1 in rosacea. Thus, the functional relevance of our qRT-PCR findings in rosacea needs to be clarified. Because of its potential as a future target in rosacea therapy (Steinhoff et al., 2011), the role of TRPV1 protein and RNA in rosacea as compared with other skin diseases with and without treatment is currently under investigation (Sulk et al, in preparation).
In sum, non-neuronal TRPV channels are shown to be differentially regulated and distributed among the various rosacea subtypes. In ETR, dermal immunolabeling of TRPV2 and TRPV3 and also TRPV1 gene expression are significantly increased. Clinically, ETR is characterized by flushing after triggers such as temperature changes, spicy food, or UV/sun exposure, and erythema. Histopathologically, an infiltrate of lymphocytes and MCs was demonstrated (Marks and Harcourt-Webster, 1969; Schwab et al., 2011). Flushing after trigger factors could be mediated directly by TRPV2-positive blood vessels or indirectly via activation and degranulation of TRPV2-positive MCs. The precise role of TRPV2- and TRPV3- positive lymphocytes and the increased TRPV1 gene expression in ETR has to be further investigated. The expression of TRPV4 remained unchanged, although TRPV4 on blood vessels and MCs might have a role in ETR. TRPV2 and TRPV3 immunostaining in ETR showed no significant changes as compared with LE, which might be due to a similar lymphocytic infiltrate in LE.
PPR displayed an enhanced dermal immunolabeling for TRPV2, TRPV4, and also of TRPV2 gene expression. Clinically, PPR is associated with papules and/or pustules in addition to erythema. Histologically, an inflammatory infiltrate of T cells, macrophages, MCs, and, occasionally, neutrophils or B cells (Steinhoff et al., 2011) was described. In PPR, inflammation could be mediated by macrophages and MCs. TRPV2 and TRPV4 were found to be expressed by these cells (Figure 4), whereas in the case of TRPV2 an upregulation in rosacea tissue on macrophages was demonstrated. As discussed above, a role of TRPV2 and TRPV4 in mediating macrophage and MC functions is assumed, and thus trigger factors of TRPV2 and TRPV4 could initiate and maintain inflammation. The impact of TRPV4-positive staining on CD4+ T helper cells in PPR needs further investigation. In addition, mRNA of TRPV1 and TRPV3 and also dermal immunolabeling for TRPV3 were increased, which could be due to immunopositive lymphocytes. Interestingly, dermal immunostaining of TRPV2 and TRPV4 in PPR was increased, as compared with LE. This might indicate a specific role for TRPV2 and TRPV4 in PPR, and the analysis of higher numbers of specimen may be directive.
PhR shows an enhanced dermal immunostaining of TRPV3 and TRPV4 and gene expression of TRPV1 and TRPV3, whereas epidermal TRPV2 staining was decreased. Clinically, PhR is characterized by skin fibrosis and glandular hyperplasia. Histopathologically, vasodilation, angiogenesis, fibrosis, edema and infiltration of MCs, lymphocytes, plasmocytes, and macrophages were described (Aloi et al., 2000; Schwab et al., 2011; Steinhoff et al., 2011). As noted above, endothelial TRPV4 is assumed to mediate edema, angiogenesis, inflammation, and extracellular matrix deformation, which could all maintain or initiate symptoms of PhR. In addition, TRPV4 on macrophages and MCs (Figure 4) might modulate inflammatory and fibrotic processes via macrophage activation and MC degranulation (Hermes et al., 2001; Chujo et al., 2009). The role of TRPV4 and TRPV3 in lymphocytes, as well as the upregulation of TRPV1 mRNA, and the decreased staining of epidermal TRPV2 need further investigations. Interestingly, dermal immunostaining of TRPV4 in PhR was increased as compared with LE. This might indicate a specific role for TRPV4 in PhR.
Clinically, LE patients can be characterized by a photosensitive malar (butterfly) rash with erythematous macules or infiltrated plaques with a tendency for confluence; histologically, a lymphocytic infiltrate is observed (Obermoser et al., 2010). Dermal upregulation of TRPV2 and TRPV3 as compared with HS could be explained by an enhanced number of immunopositive lymphocytes. Despite this, TRPV2–4 immunolabeling is increased in rosacea tissue as compared with LE, which is caused by further immunopositive dermal cells such as MCs, macrophages, and blood vessels.
In particular, in PPR, TRPV2 and TRPV4 and in PhR, TRPV4 are upregulated as compared with LE, which might indicate a specific role for TRPV2 and TRPV4 in rosacea.
In sum, non-neuronal TRPV1–4 cation channels are differentially regulated in rosacea tissue and may thus have an important role in the pathophysiology of this chronic disease. After activation through typical rosacea trigger factors (e.g., temperature changes, toxins, and spices), they are able to mediate and maintain symptoms of rosacea, such as inflammation, flushing, hypersensitive skin, MC activation, and even fibrosis, and thus may be targets for specific therapy. Nevertheless, further investigations are necessary to reveal the precise function of TRPV-positive non-neuronal cells in rosacea pathophysiology. In addition, the functional relevance of TRPV channels in human inflammatory skin diseases still needs to be clarified, and the precise role of non-neuronal TRPV ion channels in rosacea still remains unclear.
Materials and methods
Tissue collection
For (double) immunohistochemistry, 36 diagnostic skin biopsies from patients with different subtypes of rosacea (ETR: n=7; PPR: n=7, and PhR: n=7), LE (n=7), and HS (n=8) were investigated. The clinical diagnosis of rosacea subtypes was performed according to the classification system of the National Rosacea Society (Wilkin et al., 2002). For qRT-PCR, 40 patients were examined and skin from healthy patients was obtained following plastic surgery (n=12). Skin biopsies of patients with rosacea subtype ETR (n=11), PPR (n=11), and PhR (n=6) were performed. Patients were informed about the possible use of tissue leftover from surgery for investigation, and they gave their written consent. Permission for human studies was given by the Ethics Committee of the University of Münster, Germany, in accordance with the ethical standards of the 1964 Declaration of Helsinki Principles.
Immunohistochemistry
Punch biopsies were embedded in paraffin and 7μm sections were cut and mounted. Standard procedures were used as previously described (Bocheva et al., 2009). Tissues were incubated with antibodies for TRPV2, TRPV3, and TRPV4 (Supplementary Table S2 online). As negative controls, the primary antibodies were omitted (Supplementary Figure S1g–i online). Pictures of the epidermis and dermis were taken from three representative areas within each section. For specificity control, a second primary antibody was used, which showed a similar staining pattern for each area (Supplementary Figure S1a–f online).
Semiquantitative image analysis
Epidermal intensity and the number and area of immunostained dermal cells were rated on a scale of 0–4 (0=absent, 1=weak/low, 2=moderate, 3=strong, and 4=very strong staining).
Statistical analysis of immunohistochemistry
Statistical analysis was performed using SPSS (SPSS, Chicago, IL). Statistical significance was determined using Student’s t-test and differences were considered significant at a P-value of <0.05.
Double-immunoflourescence
For TRPV4, three rosacea tissues (PPR: n=2, PhR: n=1) and for TRPV2 five PPR tissues and two HS samples were investigated qualitatively. Costaining was performed together with antibodies against CD4, CD68, and MC tryptase (Supplementary Table S1 online). As negative controls, preabsorption of antibodies at working dilutions with an excess of the respective antigen (10-5 mol l-1, 48 hours before reaction) was performed.
RNA-Extraction and qRT-PCR
RNA-extraction and qRT-PCR were performed as previously described (Seeliger et al, 2010). In short, RNA was extracted, quantity was measured, quality was monitored and 800 ng RNA was used for synthesizing complementary DNA. Gene expression analysis was performed using Ready-to-use TaqMan Gene Expression Assays (Applied Biosystems, Courtaboeuf, France; numbers are listed in Supplementary Table S3 online). A triplicate determination was performed for each sample. qRT-PCR was performed on ABI7900HT (Applied Biosystems) with 50 ng synthesized complementary DNA. PCR threshold cycle (Ct) numbers were defined and normalized Ct values were calculated. mRNA expressionwas determined using the ΔCt method. Fold modulation of gene expression of rosacea samples versus samples of HS was defined as 2(mean CtHS – mean CtRo), with CtHS and CtRo depicting the Ct values of HS and rosacea samples, respectively.
Statistical analysis
To identify significantly modulated genes, one-way analysis of variance with Benjamini–Hochberg multiplicity correction was performed using JMP7.0.1 (SAS Institute, Cary, NC) and irMF3.5 (National Institute of Statistical Sciences (NISS), Research Triangle Park, NC) software.
Supplementary Material
Distribution and Expression of Non-Neuronal Transient Receptor Potential (TRPV) Ion Channels in Rosacea
Mathias Sulk, Stephan Seeliger, Jerome Aubert, Verena D. Schwab, Ferda Cevikbas, Michel Rivier, Pawel Nowak, Johannes J. Voegel, Jörg Buddenkotte, and Martin Steinhoff
Supplementary Information
Supplementary Figure S1
Supplementary Tables S1-S3
Figure S1: Demonstration of antibody specificity for TRPV2, TRPV3, and TRPV4. We determined the specificity on the protein level by using another different primary antibody against TRPV2 (a, d), TRPV3 (b, e), TRPV4 (c, f), which all showed a similar staining pattern in PhR as compared to Figures 1-3. As negative controls, primary antibodies were omitted and immunostaining was absent for rabbit-TRPV2 in ETR (g), sheep-TRPV3 in LE (h) and rabbit-TRPV4 in ETR (i). Pre-immune absorption control for double-immunoflourescence shows absence of TRPV2 (j) and TRPV4 (k) in PPR demonstrating specificity of immunostaining. Scale bars: 100μm (a-c, g-i), 50μm (d-f), 40μm (j-k).
Supplementary Table 1: Summary of TRPV-staining in rosacea-affected skin compared to normal skin
E = semiquantitative analysis of epidermal staining intensity; D = semiquantitative analysis of immunoreactivity in the dermal compartment; qRT-PCR = gene expression. (+ = upregulation, P<0.4; ++ = P<0.2, +++ = P<0.05; - = downregulation, P<0.4; -- = P<0.2; --- = P<0.05, n.i. = not investigated/performed, N = normal/no changes)
Supplementary Table 2:
Antibodies used for (double-) immuohistochemistry
Supplementary Table 3:
Ready-to-use Taqman® Gene Expression Assay-Numbers
Acknowledgements
The technical help of Heike Hinte, Christian Meß, Andrea Poppe, Nani Osada, Sandy Wise, Pascale Reiniche and Luigi Russo is gratefully acknowledged. Work was supported by NIH (NIAMS), National Rosacea Society (NRS), German Research Foundation (DFG STE 1014/2-2, SFB 492), IZKF Münster (Stei3/034/09), CE.R.I.E.S, Paris, West Havens foundation (to M.S.), and DFG (CE165/1-1, to F.C.).
Abbreviations
- ETR
erythemato-telangiectatic rosacea
- HS
healthy skin
- LE
lupus erythematosus
- MC
mast cell
- PPR
papulo-pustular rosacea
- PhR
phymatous rosacea
- qRT-PCR
quantitative real-time polymerase chain reaction
- TRPV
transient receptor potential vanilloid subfamily
Footnotes
Conflict of Interests
J.A., M.R. and J.J.V. are employees of Galderma.
The other authors state no conflict of interest.
References
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Aloi F, Tomasini C, Soro E, et al. The clinicopathologic spectrum of rhinophyma. J Am Acad Dermatol. 2000;42:468–72. doi: 10.1016/s0190-9622(00)90220-2. [DOI] [PubMed] [Google Scholar]
- Aroni K, Tsagroni E, Kavantzas N, Patsouris E, Ioannidis E. A study of the pathogenesis of rosacea: how angiogenesis and mast cells may participate in a complex multifactorial process. Arch Dermatol Res. 2008;300:125–31. doi: 10.1007/s00403-007-0816-z. [DOI] [PubMed] [Google Scholar]
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126:2664–72. doi: 10.1038/sj.jid.5700468. [DOI] [PubMed] [Google Scholar]
- Axelsson HE, Minde JK, Sonesson A, Toolanen G, Hogestatt ED, Zygmunt PM. Transient receptor potential vanilloid 1, vanilloid 2 and melastatin 8 immunoreactive nerve fibers in human skin from individuals with and without Norrbottnian congenital insensitivity to pain. Neuroscience. 2009;162:1322–32. doi: 10.1016/j.neuroscience.2009.05.052. [DOI] [PubMed] [Google Scholar]
- Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Invest Dermatol Symp Proc. 2011;15:33–39. doi: 10.1038/jidsymp.2011.8. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain. 2011;152:1156–64. doi: 10.1016/j.pain.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 2011;203(1):99–116. doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocheva G, Rattenholl A, Kempkes C, Goerge T, Lin CY, D'Andrea MR, et al. Role of matriptase and proteinase-activated receptor-2 in non-melanoma skin cancer. J Invest Dermatol. 2009;129:1816–23. doi: 10.1038/jid.2008.449. [DOI] [PubMed] [Google Scholar]
- Cenac N, Altier C, Motta JP, d'Aldebert E, Galeano S, Zamponi GW, et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010;59:481–8. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141:331–43. doi: 10.1016/j.cell.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro S, Seyler S, Stindl J, Milenkovic VM, Strauss O. Heat-sensitive TRPV channels in retinal pigment epithelial cells: regulation of VEGF-A secretion. Invest Ophthalmol Vis Sci. 2010;51:6001–8. doi: 10.1167/iovs.09-4720. [DOI] [PubMed] [Google Scholar]
- Chujo S, Shirasaki F, Kondo-Miyazaki M, Ikawa Y, Takehara K. Role of connective tissue growth factor and its interaction with basic fibroblast growth factor and macrophage chemoattractant protein-1 in skin fibrosis. J Cell Physiol. 2009;220(1):189–95. doi: 10.1002/jcp.21750. [DOI] [PubMed] [Google Scholar]
- D'Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–85. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol. 2010;77:612–20. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewski BE, Draelos Z, Dreno B, Jansen T, Layton A, Picardo M. Rosacea - global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188–200. doi: 10.1111/j.1468-3083.2010.03751.x. [DOI] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorio Pla A, Ong HL, Cheng KT, Brossa A, Bussolati B, Lockwich T, et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2011 doi: 10.1038/onc.2011.231. 2011 doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice ED, Rinaldi L, Passarotto M, Facchinetti F, D'Arrigo A, Guiotto A, et al. Cannabidiol, unlike synthetic cannabinoids, triggers activation of RBL-2H3 mast cells. J Leukoc Biol. 2007;81:1512–22. doi: 10.1189/jlb.1206738. [DOI] [PubMed] [Google Scholar]
- Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, et al. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–33. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–14. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Sanchez DA, Ishiuji Y, Patel T, Fountain J, Chan YH, Yosipovitch G. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800–5. doi: 10.1016/j.jaad.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, et al. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L353–62. doi: 10.1152/ajplung.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiner I, Eisfeld J, Luckhoff A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium. 2003;33:533–40. doi: 10.1016/s0143-4160(03)00058-7. [DOI] [PubMed] [Google Scholar]
- Hermes B, Welker P, Feldmann-Böddeker I, Krüger-Krasagakis S, Hartmann K, Zuberbier T, et al. Expression of mast cell growth modulating and chemotactic factors and their receptors in human cutaneous scars. J Invest Dermatol. 2001;116(3):387–93. doi: 10.1046/j.1523-1747.2001.01284.x. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, et al. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208:201–12. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–37. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano N, Itoh Y, Muraki K. Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci. 2009;85:808–14. doi: 10.1016/j.lfs.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Inada H, Iida T, Tominaga M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem Biophys Res Commun. 2006;350:762–7. doi: 10.1016/j.bbrc.2006.09.111. [DOI] [PubMed] [Google Scholar]
- Kim KS, Shin DH, Nam JH, Park KS, Zhang YH, Kim WK, et al. Functional Expression of TRPV4 Cation Channels in Human Mast Cell Line (HMC-1). Korean J Physiol Pharmacol. 2010;14:419–25. doi: 10.4196/kjpp.2010.14.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Jung E, Yu H, Kim Y, Ha J, Kim YS, et al. Mechanisms of carvacrol-induced expression of type I collagen gene. J Dermatol Sci. 2008;52:160–9. doi: 10.1016/j.jdermsci.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–9. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks R, Harcourt-Webster JN. Histopathology of rosacea. Arch Dermatol. 1969;100:683–91. [PubMed] [Google Scholar]
- Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat Commun. 2011;2:369. doi: 10.1038/ncomms1371. doi: 10.1038/ncomms1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Obermoser G, Sontheimer RD, Zelger B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus. 2010;19(9):1050–70. doi: 10.1177/0961203310370048. [DOI] [PubMed] [Google Scholar]
- Pecze L, Szabo K, Szell M, Josvay K, Kaszas K, Kusz E, et al. Human keratinocytes are vanilloid resistant. PLoS One. 2008;3:e3419. doi: 10.1371/journal.pone.0003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FC. Clinical practice. Rosacea. N Engl J Med. 2005;352:793–803. doi: 10.1056/NEJMcp042829. [DOI] [PubMed] [Google Scholar]
- Radtke C, Sinis N, Sauter M, Jahn S, Kraushaar U, Guenther E, et al. TRPV channel expression in human skin and possible role in thermally induced cell death. J Burn Care Res. 2011;32:150–9. doi: 10.1097/BCR.0b013e318203350c. [DOI] [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- Saunders CI, Kunde DA, Crawford A, Geraghty DP. Expression of transient receptor potential vanilloid 1 (TRPV1) and 2 (TRPV2) in human peripheral blood. Mol Immunol. 2007;44:1429–35. doi: 10.1016/j.molimm.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Schierling W, Troidl K, Apfelbeck H, Troidl C, Kasprzak PM, Schaper W, et al. Cerebral Arteriogenesis is Enhanced by Pharmacological as Well as Fluid-Shear-Stress Activation of the Trpv4 Calcium Channel. Eur J Vasc Endovasc Surg. 2011;41:589–96. doi: 10.1016/j.ejvs.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Schwab VD, Sulk M, Aubert J, Seeliger S, Novak P, Mess C, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Invest Dermatol Symp Proc. 2011;15:53–62. doi: 10.1038/jidsymp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger S, Buddenkotte J, Schmidt-Choudhury A, Rosignoli C, Shpacovitch V, von Arnim U, et al. Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo. Am J Pathol. 2010;177:2563–75. doi: 10.2353/ajpath.2010.090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohira D, Kido MA, Danjo A, Takao T, Wang B, Zhang JQ, et al. TRPV2 expression in rat oral mucosa. Histochem Cell Biol. 2009;132:423–33. doi: 10.1007/s00418-009-0616-y. [DOI] [PubMed] [Google Scholar]
- Shimosato G, Amaya F, Ueda M, Tanaka Y, Decosterd I, Tanaka M. Peripheral inflammation induces up-regulation of TRPV2 expression in rat DRG. Pain. 2005;119:225–32. doi: 10.1016/j.pain.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Tominaga M. The TRPV4 cation channel: A molecule linking skin temperature and barrier function. Commun Integr Biol. 2010;3:619–21. doi: 10.4161/cib.3.6.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinsanti G, Zannolli R, Panti C, Ceccarelli I, Marsili L, Bachiocco V, et al. Quantitative Real-Time PCR detection of TRPV1-4 gene expression in human leukocytes from healthy and hyposensitive subjects. Mol Pain. 2008;4:51. doi: 10.1186/1744-8069-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Aubert J, Novak P, Sulk M, Schwab V, et al. Clinical, Cellular and Molecular Aspects in the Pathophysiology of Rosacea. J Invest Dermatol Symp Proc. 2011;15:2–11. doi: 10.1038/jidsymp.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AJ, Shimoda LM, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med. 2004;200:137–47. doi: 10.1084/jem.20032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123–30. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuuren EJ, Kramer S, Carter B, Graber MA, Fedorowicz Z. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262. doi: 10.1002/14651858.CD003262.pub4. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des. 2008;14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- Wenning AS, Neblung K, Strauss B, Wolfs MJ, Sappok A, Hoth M, et al. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochim Biophys Acta. 2011;1813:412–23. doi: 10.1016/j.bbamcr.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, et al. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584–7. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther. 2008;326:443–52. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–35. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K, Sasano T, Tojo K, Namekata I, Kurokawa J, Sawada N, et al. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem Biophys Res Commun. 2010;398:284–9. doi: 10.1016/j.bbrc.2010.06.082. [DOI] [PubMed] [Google Scholar]
- Yang WZ, Chen JY, Yu JT, Zhou LW. Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem Photobiol. 2007;83:979–84. doi: 10.1111/j.1751-1097.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129:714–22. doi: 10.1038/jid.2008.245. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu X, Rao NV, Argyle B, McCoard L, Rusho WJ, et al. Novel sulfated polysaccharides disrupt cathelicidins, inhibit RAGE and reduce cutaneous inflammation in a mouse model of rosacea. PLoS One. 2011;6:e16658. doi: 10.1371/journal.pone.0016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Spielmann A, Wang L, Ding G, Huang F, Gu Q, et al. Mast-cell degranulation induced by physical stimuli involves the activation of Transient-Receptor-Potential Channel TRPV2. Physiol Res. 2011 May 16; doi: 10.33549/physiolres.932053. 2011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution and Expression of Non-Neuronal Transient Receptor Potential (TRPV) Ion Channels in Rosacea
Mathias Sulk, Stephan Seeliger, Jerome Aubert, Verena D. Schwab, Ferda Cevikbas, Michel Rivier, Pawel Nowak, Johannes J. Voegel, Jörg Buddenkotte, and Martin Steinhoff
Supplementary Information
Supplementary Figure S1
Supplementary Tables S1-S3
Figure S1: Demonstration of antibody specificity for TRPV2, TRPV3, and TRPV4. We determined the specificity on the protein level by using another different primary antibody against TRPV2 (a, d), TRPV3 (b, e), TRPV4 (c, f), which all showed a similar staining pattern in PhR as compared to Figures 1-3. As negative controls, primary antibodies were omitted and immunostaining was absent for rabbit-TRPV2 in ETR (g), sheep-TRPV3 in LE (h) and rabbit-TRPV4 in ETR (i). Pre-immune absorption control for double-immunoflourescence shows absence of TRPV2 (j) and TRPV4 (k) in PPR demonstrating specificity of immunostaining. Scale bars: 100μm (a-c, g-i), 50μm (d-f), 40μm (j-k).
Supplementary Table 1: Summary of TRPV-staining in rosacea-affected skin compared to normal skin
E = semiquantitative analysis of epidermal staining intensity; D = semiquantitative analysis of immunoreactivity in the dermal compartment; qRT-PCR = gene expression. (+ = upregulation, P<0.4; ++ = P<0.2, +++ = P<0.05; - = downregulation, P<0.4; -- = P<0.2; --- = P<0.05, n.i. = not investigated/performed, N = normal/no changes)
Supplementary Table 2:
Antibodies used for (double-) immuohistochemistry
Supplementary Table 3:
Ready-to-use Taqman® Gene Expression Assay-Numbers