Abstract
Aldo-keto reductase 1C3 (AKR1C3) has been shown to mediate the metabolism of sex hormones and prostaglandin D2 (PGD2), a lipid mediator that promotes skin inflammation in atopic dermatitis (AD). Since both play a role in skin function and pathology, we first sought to investigate the expression pattern of AKR1C3 in normal human epidermis. Immunofluorescence revealed a strong expression of AKR1C3 in the differentiated suprabasal layers compared with the basal layer. Western blot and quantitative PCR confirmed that AKR1C3 expression was also upregulated in differentiation-induced primary human keratinocytes (PHK). To investigate the functional role of AKR1C3 during PHK differentiation, its expression and activity (measured as PGD2 reduction to 9α,11β-PGF2 by ELISA) were impaired by siRNA or 2′-hydroxyflavanone, respectively. Cytokeratin 10 (K10) and loricrin expression were then examined by western blot revealing altered expression of these differentiation markers. Finally, following an observation that the AD-associated mediator, PGD2 upregulated AKR1C3 expression in PHK, we used immunofluorescence to examine AKR1C3 expression in AD and psoriasis lesions. AKR1C3 was found to be upregulated in AD but not in psoriasis lesions compared with non-lesional skin. Our work demonstrates a function for AKR1C3 in differentiation-associated gene regulation and also suggests a role in supporting inflammation in AD.
Introduction
Human skin is considered a steroidogenic organ because it locally synthesizes and metabolizes various steroid hormones and expresses their corresponding receptors (Kanda and Watanabe, 2005; Thiboutot et al., 2003; Zouboulis et al., 2007). Prostaglandins (PGs), a large family of arachidonic acid-derived lipid mediators, have also been shown to play a role in skin function and pathology, including keratinocyte proliferation and differentiation, skin cancer and allergic inflammation (Chun et al., 2010; He et al., 2010; Konger et al., 2009; Surh et al., 2011). Since specific enzymes contribute to the regulation of local concentrations of steroid hormones and PGs in the skin, their distribution and activity may indirectly affect skin function.
Several aldo-keto reductase 1C (AKR1C) enzymes have been shown to synthesize or metabolize steroid hormones and/or PGs (Penning et al., 2003; Penning et al., 2006), and recent work has also characterized their expression in cultured human keratinocytes and suggested a possible role for them in keratinocyte survival (Marin et al., 2009). AKR1C enzymes are part of a larger AKR superfamily that utilizes NAD(P)(H) as a cofactor to mediate the enzymatic reduction of aldehyde and ketone groups of various substrates to their corresponding alcohols (Jez et al., 1997a; Jez et al., 1997b). In humans there are four AKR1C enzymes, AKR1C1–AKR1C4, that share about 86% amino acid sequence identity, yet vary in their substrate binding specificities (Penning et al., 2000). AKR1C1 and AKR1C2 primarily convert specific potent steroid hormones into their less potent forms (Rizner et al., 2003; Rizner et al., 2006). In contrast, AKR1C3, also known as type 5 17β-hydroxysteroid dehydrogenase (17β-HSD) (Lin et al., 1997) and prostaglandin F synthase (Matsuura et al., 1998; Suzuki-Yamamoto et al., 1999), converts the weak androgen Δ4-androstene-3,17-dione to testosterone (potent androgen) and the weak estrogen estrone to its potent form, 17β-estradiol (Lin et al., 1997). Unlike other AKR1C members, AKR1C3 can also synthesize PGF2α from the cyclooxygenase product PGH2 and mediate one of its most favorable catalytic activities, the reduction of PGD2 to 9α,11β-PGF2 (Byrns et al., 2010; Lin et al., 1997; Matsuura et al., 1998; Penning et al., 2000; Penning et al., 2006; Suzuki-Yamamoto et al., 1999).
Human skin is a major site for synthesis of PGD2, where it is synthesized primarily by immune cells such as macrophages, langerhans cells and mast cells (Morrow et al., 1992; Shimura et al., 2010). In skin, PGD2 is mostly investigated in the context of allergic responses, particularly in supporting inflammation in AD lesions (Barr et al., 1988; Satoh et al., 2006; Shimura et al., 2010).
The involvement of AKR1C3 in both PG and steroid hormone metabolism clearly situates this enzyme at the intersection of several very important physiologic and pathologic signaling pathways in the skin. We therefore characterized its expression pattern in normal human epidermis and in epidermis from AD and psoriasis patients, as well as examined its regulation and function in cultured PHK.
Results
AKR1C3 is expressed in differentiated human epidermis and co-localizes with K10
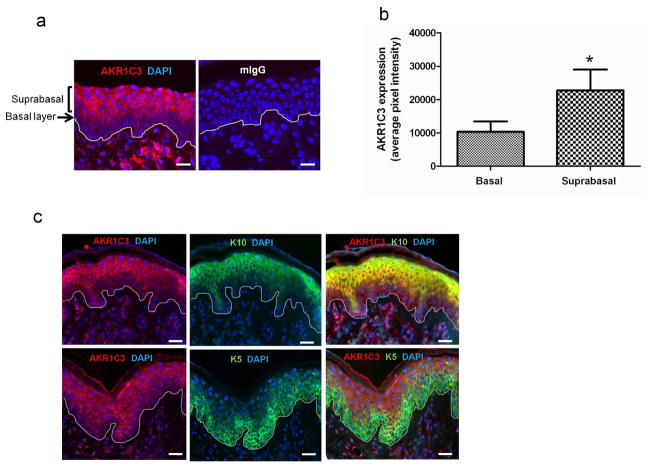
In order to define the expression pattern of AKR1C3 within the human epidermis, skin was evaluated by immunofluorescence using AKR1C3 antibody alone or in combination with layer-specific epidermal markers. AKR1C3 staining intensity varied within the epidermal layers, with the weakest intensity observed in the basal layer and stronger expression noted in suprabasal layers, especially in the stratum spinosum (figure 1a). On average, AKR1C3 expression was 2.3±0.07 fold higher in the suprabasal layers compared with the basal layer as determined by ImageJ analysis (figure 1b). In double immunolabeling experiments (figure 1c) using the basal layer marker keratin 5 (K5, green) or the early differentiation marker keratin 10 (K10, green) together with AKR1C3 (red), AKR1C3 co-localized with K10 (yellow), especially in the stratum spinosum, but did not appear to colocalize with K5 in the basal layer.
Figure 1. AKR1C3 is expressed in differentiated human epidermis and co-localizes with K10.
(a) AKR1C3 expression patterns were evaluated in normal human epidermis by immunofluorescence staining using AKR1C3 antibody and (b) quantified with ImageJ. Data is shown as the mean ±SEM pixel intensity after background subtraction (n=3, *P<0.05). (c) Normal human skin was subjected to double label immunofluorescence staining using AKR1C3 (red) and keratin 10 (K10, green) or keratin 5 (K5, green) antibodies. Labeling of each protein is shown separately in the middle and left panels. Yellow color in the merged pictures (right panels) suggests a co-localization of the 2 proteins. Bar=30μm, n=3.
AKR1C3 protein and mRNA are upregulated in calcium-induced PHK differentiation
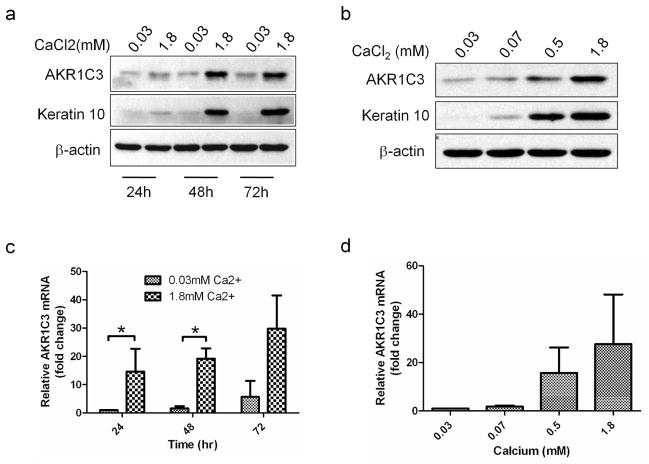
Since AKR1C3 showed stronger expression in differentiated epidermis, its regulation in response to calcium-induced differentiation was evaluated in PHK. Cultures treated with 1.8 mM calcium chloride or with equal volume of PBS were harvested at 24, 48 and 72 hours post treatment and AKR1C3 protein and mRNA levels were assessed using western blot or quantitative RT-PCR. Western blot analysis demonstrated a time-dependent upregulation of AKR1C3 protein in high calcium treated cells, and was similar to the expected upregulation trend of the early differentiation marker K10 (figure 2a). A 30–40 fold increase in AKR1C3 mRNA was also observed in high calcium treated cells (figure 2c). Next, cells were treated with the indicated calcium doses and harvested 48 hours post treatment. Western blot analysis revealed a dose-dependent upregulation of AKR1C3 protein, which again correlated with K10 expression (figure 2b). A dose-dependent upregulation of AKR1C3 mRNA of up to 28±20.5 fold over control was also noted (figure 2d).
Figure 2. AKR1C3 protein and mRNA are upregulated in response to calcium-induced PHK differentiation in a time and dose dependent manner.
(a) Western blot analysis demonstrating the regulation of AKR1C3 by PHK cultured under 1.8 mM or 0.03mM calcium conditions and harvested at intervals or (b) by cells treated with the indicated dose of calcium and harvested 48 hours post treatment (representative results of 3 separate experiments is shown). (c, d) RT-PCR analysis demonstrating the regulation of AKR1C3 mRNA by cells treated under the same conditions described in a or b, respectively. Data is expressed as means ± SEM fold change over the baseline (n=3, *P<0.015).
AKR1C3 siRNA specifically attenuates calcium-induced AKR1C3 expression
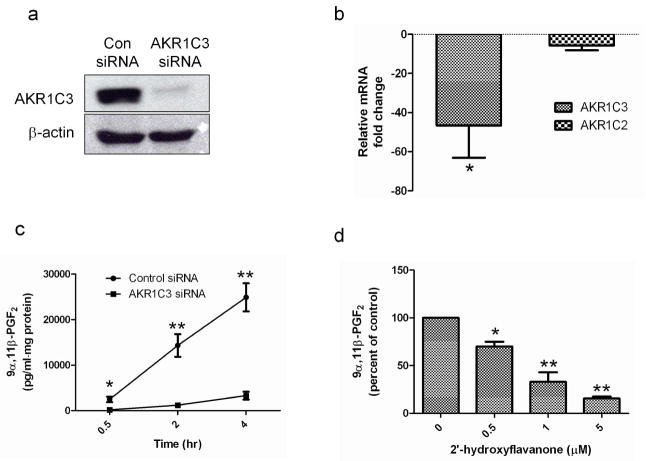
To investigate AKR1C3 function in keratinocytes, the effectiveness and specificity of an AKR1C3 siRNA construct were evaluated. PHK were transiently transfected with either 10nM of AKR1C3 or non-specific (Con) siRNA, allowed to differentiate in 1.8mM calcium for 48 hours and then harvested for protein and mRNA analysis. AKR1C3 protein expression, determined by western blot, was markedly reduced in AKR1C3 siRNA treated cells compared with controls, demonstrating the effectiveness of this treatment (figure 3a). AKR1C2 is a closely related family member of AKR1C3 which shares about 86% amino acid sequence homology (Jez et al., 1997a). In order to test the efficiency and specificity of the AKR1C3 siRNA construct, mRNA samples from AKR1C3 or control siRNA treated cells were collected and the relative change in AKR1C3 and AKR1C2, compared with controls was determined using RT-PCR (figure 3b). While AKR1C3 mRNA levels in AKR1C3 siRNA treated cells were reduced 46.6±19.1 fold over control siRNA, the reduction of AKR1C2 was not significant.
Figure 3. AKR1C3 siRNA and 2HFN attenuate AKR1C3 enzymatic activity.
(a) PHK transfected with 10nM of AKR1C3 or non-specific siRNA (Con siRNA) were allowed to differentiate for 48 hours and AKR1C3 protein was evaluated by western blot (n=3). (b) To examine AKR1C3 siRNA specificity, cells were treated as described in (a), mRNA was extracted and the transcription of AKR1C3 and AKR1C2 was evaluated by RT-PCR (means ± SEM, n=4, *P<0.05). (c) PHK were treated with AKR1C3 or control siRNA, allowed to differentiate for 48 hours and AKR1C3 enzymatic activity was evaluated at intervals by assessing 9α,11β-PGF2 in supernatants using ELISA (means ± SEM, n=4, *P<0.05, **P<0.01). (d) 2HFN dose-dependently reduced AKR1C3 activity in PHK, as determined by ELISA quantification of 9α,11β-PGF2 levels in supernatants, 2 hours post treatment (n=4, *P<0.05, **P<0.01).
AKR1C3 siRNA and 2HFN attenuated AKR1C3 enzymatic activity (measured as PGD2 reduction to 9α,11β-PGF2) in PHK
We next evaluated the effects of AKR1C3 protein reduction by siRNA or the specific AKR1C3 inhibitor 2′-hydroxyflavanone (2HFN) (Skarydova et al., 2009) on AKR1C3 enzymatic activity in PHK. Cells were transfected with 10nM AKR1C3 or control siRNA and induced to differentiate for 48 hours by calcium. Conversion of exogenously added PGD2 to 9α,11β-PGF2 was then assessed in the supernatants by ELISA. 9α,11β-PGF2 levels in supernatants from AKR1C3 siRNA treated cells were lower at all time points (14.7±2.4 fold on average) compared with control cells (figure 3c). Next, keratinocytes were induced to differentiate for 48 hours and 9α,11β-PGF2 generation from PGD2 in the presence of the indicated doses of 2HFN was assessed in supernatants two hours post treatment. The presence of 2HFN dose-dependently attenuated 9α,11β-PGF2 generation. The maximal 2HFN concentration tested (5μM) reduced 9α,11β-PGF2 levels to 15.4±2% of that measured in vehicle treated cells (figure 3d). These data confirmed that both AKR1C3 siRNA and 2HFN treatments resulted in the inhibition of this specific activity of AKR1C3 in PHK.
AKR1C3 siRNA and 2HFN treatment altered K10 and loricrin regulation during calcium-induced PHK differentiation
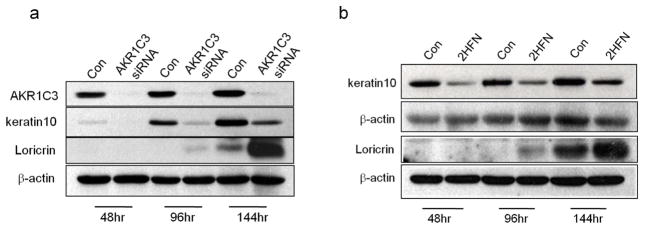
We postulated that AKR1C3 upregulation plays a role in differentiation-associated gene regulation during calcium-induced differentiation. To test this, AKR1C3 protein expression or enzymatic activity was attenuated during differentiation, and K10 and loricrin expression were evaluated at intervals by western blot (figure 4). PHK were transfected with AKR1C3 or non-specific siRNA and induced to differentiate for the indicated times (figure 4a). AKR1C3 siRNA treatment effectively reduced AKR1C3 protein expression at all tested time points, which also resulted in a marked reduction in K10 expression. Interestingly, in contrast to K10 reduction, AKR1C3 siRNA transfectants demonstrated an earlier and stronger expression of loricrin. Furthermore, cells that were induced to differentiate by calcium in the presence of 1μM of the AKR1C3 specific inhibitor 2HFN also demonstrated the same abnormal expression pattern of K10 and loricrin (figure 4b).
Figure 4. Treatment with AKR1C3 siRNA or the AKR1C3 inhibitor 2HFN results in abnormal K10 and loricrin regulation during PHK differentiation.
(a) PHK were treated with 10nM of control or AKR1C3 siRNA, or with (b) 1μM 2HFN or equal volume of DMSO (con). Cells from both experiments were induced to differentiate with 1.8mM calcium for the indicated times and the expression of K10 and loricrin was evaluated by western blot. A representative blot of 3 separate experiments is shown. K10 and loricrin are shown on 2 separate blots in panel b.
AKR1C3 is upregulated in response to PGD2 in PHK
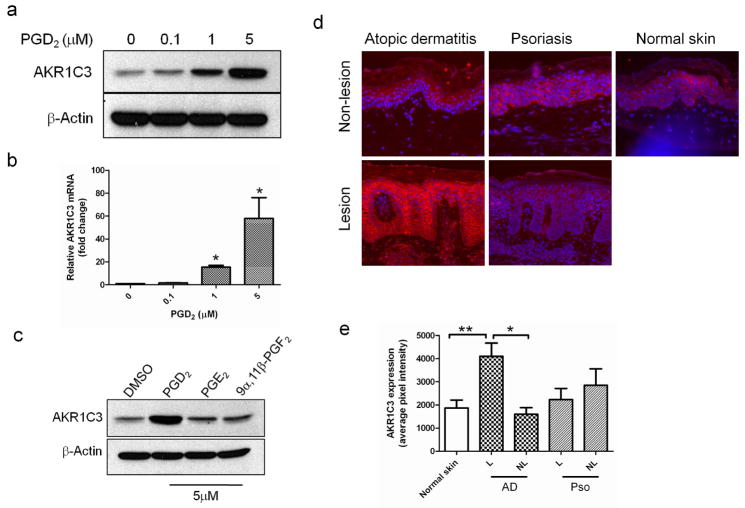
Since our data suggested that PHK utilize AKR1C3 to metabolize PGD2, we sought to further evaluate the effect of PGD2 on AKR1C3 expression. PHK were incubated in various concentrations of PGD2 (under low calcium conditions) for 24 hours and then harvested for analysis of AKR1C3 protein and mRNA. A dose-dependent upregulation of AKR1C3 in response to PGD2 was observed at the protein level (figure 5a), determined by western blot, and at the transcription level (figure 5b), determined by RT-PCR. AKR1C3 mRNA levels were increased by up to 58.5±18.1 fold in cells treated with 5μM PGD2. To evaluate the specificity of this response, cells were incubated in the presence of 5μM of either PGD2, PGE2 or 9α,11β-PGF2, and the expression of AKR1C3 was evaluated by western blot (figure 5c). The expected upregulation of AKR1C3 was observed in PGD2 treated cells but was absent in the other tested conditions, suggesting that this response was specific to PGD2. In addition, incubation of PHK in the presence of 5μM of Δ4-androstene-3,17-dione or estrone (other steroid hormone substrates of AKR1C3) did not alter the protein expression of AKR1C3 (data not shown).
Figure 5. AKR1C3 is upregulated in AD lesions and in PHK treated with PGD2.
(a) Dose-dependent upregulation of AKR1C3 protein and (b) mRNA by PHK treated with various doses of PGD2 and harvested after 24 hours, determined by western blot (n=3) and RT-PCR (means ± SEM, n=3, *P<0.01), respectively. (c) PHK were treated with 5μM of the indicated prostaglandin for 24 hours and the regulation of AKR1C3 was assessed by western blot (n=3). (d) Immunofluorescence staining showing the relative expression of AKR1C3 in epidermis of AD (n=4) psoriasis (n=3) and normal skin (n=4). One representative case is shown, images obtained at 10x magnification. (e) ImageJ analysis of AKR1C3 staining intensity. Data is presented as the mean ±SEM of 2 separate experiments where 4 normal skin samples, 4 AD and 3 psoriasis cases were evaluated. L=lesion, NL=non-lesion, *P<0.001, **P=0.012.
AKR1C3 expression is upregulated in skin lesions of atopic dermatitis but not in psoriasis
PGD2 plays a significant role in atopic dermatitis-associated inflammation (Satoh et al., 2006). Our data suggested that keratinocytes upregulate the expression of AKR1C3 in response to PGD2 and also utilize this enzyme to metabolize PGD2 to 9α,11β-PGF2. Since the regulation of AKR1C3 in atopic dermatitis may play a role in controlling the local concentration of this pro-inflamatory prostaglandin, we investigated the expression of AKR1C3 in lesional and non-lesional skin of AD and psoriasis patients by immunofluorescence (figure 5d). AKR1C3 was markedly upregulated in AD lesions compared with non-lesional matching controls. In contrast, no differences in AKR1C3 expression were observed in psoriasis lesions compared with nearby non-lesional skin. Quantification of AKR1C3 expression by ImageJ confirmed a 2.8±0.4 fold increase in AKR1C3 expression in AD lesions over matching non-lesional skin, while no change in expression was noted in psoriasis samples (figure 5e).
Discussion
AKR1C3 is the only AKR1C family member that also mediates 17β-HSD activity (Penning et al., 2001). Previous work investigating 17β-HSD activity in human skin sections localized its peak activity around the basement layer of the epidermis (Hikima and Maibach, 2007) while another study also suggested a functional role for several AKR1C isoforms in keratinocyte survival (Marin et al., 2009). The current work shows that AKR1C3 is differentially expressed in human epidermis, with the highest expression levels observed in mid-epidermis around the stratum spinosum. Other work documented multiple AKR1C3-associated 17β-HSD activities in HaCaT cells, as well as in PHK. Their data demonstrated that these enzymatic activities were relatively low in basal keratinocytes, peaked during early stages of differentiation and returned to baseline in terminally differentiated cultures, suggesting that the expression of steroid metabolizing enzymes in keratinocytes is differentiation-dependent (Gingras et al., 2003). Their work, however, did not evaluate the protein or transcriptional regulation of any 17β-HSD enzymes. The current work provides further evidence that the 17β-HSD enzyme, AKR1C3, is markedly upregulated in differentiated epidermis and in calcium-induced PHK differentiation.
AKR1C3 upregulation in differentiated PHK suggests a differentiation-associated function for this enzyme. To test this, a specific AKR1C3 siRNA and the specific AKR1C3 inhibitor, 2HFN were utilized. 2HFN has been shown to specifically inhibit recombinant AKR1C3 with an IC50 of 0.3μM (Skarydova et al., 2009). Our work further demonstrates the capacity of this flavanoid derivative to inhibit cellular AKR1C3 ketoreduction activity with an IC50 between 0.5–1μM, suggesting a good bioavailability for this drug under our experimental conditions.
The current work demonstrates a functional role for AKR1C3 in gene regulation during keratinocyte differentiation. Of interest is the observation that the interruption of AKR1C3 expression or activity attenuates K10 expression, while resulting in the upregulation of loricrin. In order to investigate whether the upregulation of loricrin was directly due to AKR1C3 inhibition or the subsequent decrease in K10 expression, we attempted to attenuate K10 expression with siRNA in PHK during differentiation; however, we were unable to examine loricrin expression due to cytotoxicity (data not shown). Based on our current data showing earlier and more profound loricrin expression, it is reasonable to assume that attenuating AKR1C3 activity during differentiation disrupted this delicate process at its early stages which hastened terminal differentiation. Future studies will be needed to evaluate the regulation of other differentiation-associated markers such as profilaggrin and involucrin. This data will provide further insights into whether altered loricrin expression was a direct outcome of AKR1C3 attenuation during keratinocyte differentiation, or was it just a part of an overall hastened terminal differentiation.
Since there is no evidence that AKR1C3 directly regulates gene expression, this action is probably mediated indirectly by its related PG and/or steroid metabolites, its contribution to the cellular redox state or its action in ketoreduction of an unknown substrate. Keratinocytes synthesize and secrete PGF2α and express its corresponding receptor FP (Pentland and Needleman, 1986; Scott et al., 2005). AKR1C3 upregulation during keratinocyte differentiation could result in increased PGF2α synthesis which may affect differentiation-associated gene regulation. However, this mechanism is not supported by our observations, since the addition of various doses of PGF2α to AKR1C3 siRNA treated cells failed to retrieve normal K10 expression during keratinocyte differentiation (data not shown). AKR1C3 can also convert steroid hormone precursors to their active form, but since the culture medium does not contain significant quantities of these precursors, this mechanism is unlikely. Increased expression of AKR1C3 during differentiation suggests a requirement for its enzymatic activity. Our work did not identify a specific substrate; however, since the ketoreduction activity mediated by AKR1C3 is also NAD(P)(H)-dependent, its increased enzymatic activity may affect cellular redox state. This action may indirectly alter the activity of redox-sensitive transcription factors which regulate the expression of differentiation-associated genes during keratinocyte differentiation (Meyer et al., 1994; Nakamura et al., 2007). Taken together, AKR1C3 is likely involved in differentiation-associated gene regulation in a PGF2α and hormone independent manner. However, the precise mechanism remains to be definitively resolved by future work.
Local release of PGD2 is known to play a major role in regulating Th2 chronic inflammation in atopic dermatitis, where it mainly serves as a chemoattractant for Th2-associated immune cells (Iwasaki et al., 2002; Satoh et al., 2006). Recent work has shown that in addition to PGD2, its stable metabolite, 9α,11β-PGF2 mediates the same Th2 inflammatory responses, but with much lower potency (Sandig et al., 2006). This suggests that both PGD2 and the accumulation of its stable metabolite 9α,11β-PGF2 may contribute to inflammation in lesional sites of atopic dermatitis.
Our studies show that PHK upregulate AKR1C3 specifically in response to PGD2 and utilize this enzyme to reduce PGD2 to 9α,11β-PGF2. In fact, our preliminary data also suggest that treating PHK with other steroidal substrates of AKR1C3 (i.e. estrone and Δ4-androstene-3,17-dione) or with their corresponding reduced products (17β-estradiol and testosterone) had no effect on AKR1C3 expression (data not shown). Additionally, treatment with other pro-inflammatory cytokines such as Interleukin-4, Interferon-γ and tumor necrosis factor-α had no effect on AKR1C3 expression by PHK (data not shown). PGD2 is a relatively unstable prostaglandin that has been shown to spontaneously dehydrate to 15-deoxy- Δ12,14-PGJ2 (15d-PGJ2) in vivo and in vitro (Shibata et al., 2002). 15d-PGJ2 is an anti-inflammatory lipid that mostly mediates its actions directly via activation of PPARγ and/or inhibition of NF-κB signaling in immune cells (Forman et al., 1995; Maggi et al., 2000; Straus et al., 2000; Watanabe et al., 2010). Previous data have shown that PPARγ activation attenuates allergen-induced inflammation in skin and lungs of mice (Dahten et al., 2008; Ward et al., 2006). This suggests that PPARγ activation by 15d-PGJ2 may play a role in suppressing inflammation in AD patients.
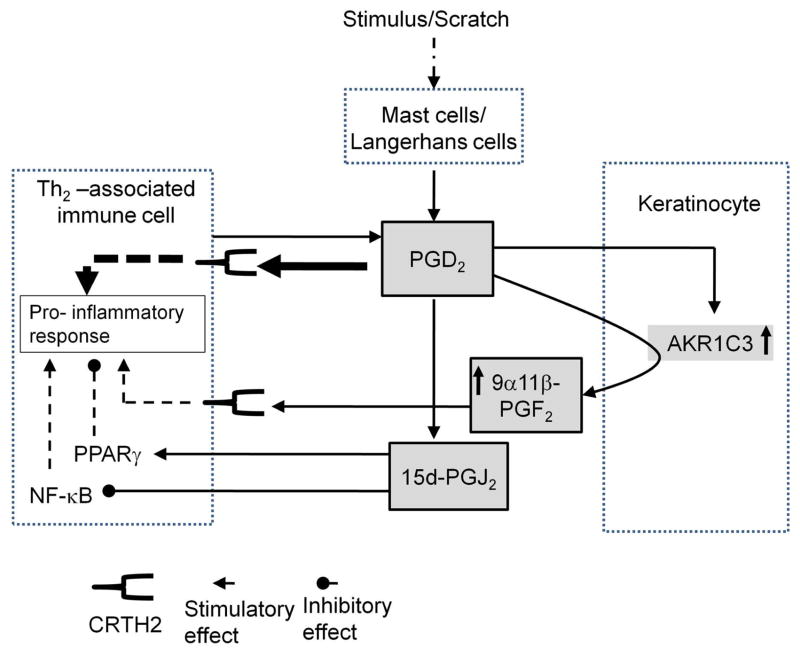
A specific role for AKR1C3 in AD is supported by our observation that AKR1C3 expression is markedly upregulated in lesions of this skin condition, but is unchanged in the Th1-mediated inflammatory lesions of psoriasis (Schlaak et al., 1994). We propose a model (figure 6) in which upregulation of AKR1C3 in AD lesions supports inflammation by directly causing an increase in 9α,11β-PGF2 synthesis rates and diverting the spontaneous generation of the potent anti-inflammatory mediator, 15d-PGJ2. This function of AKR1C3 has been previously implicated in HL-60 cells (Desmond et al., 2003) and in MCF-7 cells (Byrns et al., 2010). Thus, AKR1C3 activity and expression in AD lesions could determine the balance between pro- and anti-inflammatory prostaglandin mediators. This work suggests that inhibition of AKR1C3 may be a potential therapeutic target in atopic dermatitis-associated inflammation.
Figure 6. A role for AKR1C3 in promoting inflammation in AD (a suggested model).
Pruritus-induced scratching causes mast cell degranulation and rapid PGD2 synthesis. PGD2 attracts CRTH2-expressing immune cells which in turn can amplify its signaling by synthesizing and secreting more of this prostaglandin. Keratinocytes respond to high levels of PGD2 by upregulating AKR1C3 expression and utilize this enzyme to metabolize PGD2 to 9α,11β-PGF2, a weaker but very stable pro-inflammatory mediator. This activity of AKR1C3 competes with the spontaneous conversion of PGD2 to 15d-PGJ2, an anti-inflammatory/pro-apoptotic mediator. Upregulation of 9α,11β-PGF2 synthesis along with decreased formation of 15d-PGJ2, an agonist for PPARγ and an inhibitor of NF-κB, potentially situates AKR1C3 as an indirect pro-inflammatory player in AD.
Materials and Methods
Cell culture
Defatted skin, obtained from breast reduction or panniculectamies, placed in 0.25% trypsin in PBS for 5 hours at 37°C and 5% CO2. Epidermis was separated and epidermal cell suspension placed in T-75 flasks, pre-coated with 1:5 Purecol (Advanced Biomatrix, San Diego, California) in keratinocyte growth media (KGM) (Invitrogen, Grand Island, NY), supplemented with 5 ng/ml EGF, 20 μg/ml bovine pituitary extract (BPE) and antibiotics. The medium was routinely changed every 3–4 days and cells were passed at approximately 90% confluence. Cells were used at passage 3–6.
Reagents
Monoclonal mouse anti human AKR1C3 (ab49680), monoclonal rabbit anti human keratin 5 (ab52635) and polyclonal rabbit anti human β-actin were obtained from Abcam (Abcam, Cambridge, MA). Polyclonal rabbit anti human keratin 10 (PRB-159p-100) and rabbit anti human loricrin (PRB-145p) were purchased from Covance (Emeryville, CA). 2′-hydroxyflavanone, PGE2, PGD2 and 9α,11β-PGF2 were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in dimethyl sulfoxide (DMSO) as 10mM stocks.
Immunofluorescence
Biopsies were fixed in 10% formalin and then embedded in paraffin. Five millimeter sections were deparaffinized in xylene for 10 minutes followed by graded rehydration in EtOH (100, 95, 85, 70 and 50%) for 5 minutes each. Sections were then incubated in antigen retrieval buffer (10 mM Tris, 1 mM EDTA, 0.05% Tween 20, pH 9.0) at 95°C for 10 minutes. Samples were incubated in 1:50 anti AKR1C3, 1:2000 cytokeratin 10 or 1:2000 keratin 5 antibodies alone or in mixtures as indicated overnight at 4°C. Non specific mouse or rabbit IgG controls were performed in parallel. Samples were then incubated in 1:400 Fluorescein conjugated goat anti rabbit IgG, 1:200 Texas Red goat anti mouse IgG or a mixture of both as required for 1 hour at room temperature. Samples were visualized using a fluorescence microscope (Nikon Eclipse E800 equipped with a Spot RT3 camera). Pictures were obtained using Spot advanced software (Diagnostic Instruments).
ImagJ analysis
ImageJ version 1.44o was used. The averages of the fluorescent pixel intensity of 10 random fields per sample (rectangular tool) from the site of interest (i.e. epidermis or specific epidermal area) minus the averages of non-specific fluorescence (background) were recorded and averaged.
Western blot analysis
Cells were extracted in RIPA buffer containing 1:100 Protease Inhibitor Cocktail (Santa Cruz Biotechnology, Santa Cruz, CA). Protein (50μg) was separated on a 10% SDS-PAGE and transferred to nitrocellulose. Membrane was blocked, then incubated in the presence of either 1:1000 anti AKR1C3, 1:2000 anti keratin 10, 1:2000 anti loricrin or 1:4000 anti β-actin antibodies at 4°C overnight. Matching secondary horseradish peroxidase (HRP)-conjugated IgG at 1:2000 was applied and immunoreactive protein bands were detected using Western Blot Luminal Reagent (Santa Cruz Biotechnology, Santa Cruz, CA).
AKR1C3 siRNA transient transfection
Transfection was performed on cultures at 50% confluence, according to the manufacturer protocol, using 10nM AKR1C3 Stealth siRNA (oligo ID: HSS112670, Invitrogen) or non-specific siRNA (cat# 12935-200, Invitrogen). Transfection medium was aspirated after 6 hours and replaced by conditioned KGM media as indicated.
Evaluation of AKR1C3 enzymatic activity (PGD2 to 9α,11β-PGF2 conversion) in primary human keratinocytes
Cells were cultured in 6 well dishes as indicated. KGM without supplements (500μl per well), containing 1μM PGD2, was added to appropriate wells followed by incubation at 37°C for the indicated times. 9α,11β-PGF2 from each condition was evaluated in the supernatant by a specific 9α,11β-PGF2 kit (catalog: 516521, Cayman Chemical, Ann Arbor, MI) according to manufacturer protocol, and results were normalized to protein content. 9α,11β-PGF2 was detected in 1μM PGD2-containing media only after it had been incubated with cells, while no signal was detected in PGD2-containing media alone or in media exposed to cells that did not contain PGD2 (not shown).
Total RNA isolation and real time polymerase chain reaction
Total RNA was extracted using Trizol reagent (Invitrogen, Grand Island, NY) and cDNA was synthesized using SuperScript III First Strand cDNA synthesis kit (Invitrogen, Grand Island, NY). Real-time PCR primers for AKR1C3 (Fwd: 5′GAGAAGTAAAGCTTTGGAGGTCACA-3′, Rev: 5′-CAACCTGCTCCTCATTATTGTATAAATGA-3′) AKR1C2 (Fwd: 5′-CCTAAAAGTAAAGCTCTAGAGGCCGT-3′, Rev: 5′-GAAAATGAATAAGATAGAGGTCAACATAG-3′) and GAPDH (Fwd: 5′-CCACCCATGGCAAATTCC-3′, Rev: 5′-TGGGATTTCCATTGATGACAAG-3′) were designed using ABI Primer Express Software (Applied Biosystems, Foster City, CA). PCR products were amplified and detected using SYBR Green in an iCycler iQ real-time PCR system (Bio-Rad, Hercules, CA). Relative cDNA amounts were calculated on the basis of the threshold cycle (CT) value and were normalized to the amount of GAPDH by means of the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Atopic dermatitis, psoriasis and normal skin samples
All studies were approved by the Research Subject Review Boards at the University of Rochester Medical Center and/or by the Research Subject Review Boards at the Johns Hopkins University. All subjects gave written informed consent. The diagnosis of AD was made using the US consensus conference criteria (Eichenfield, 2004). All subjects underwent a 5 mm punch biopsy from a non-lesional site and an additional 5 mm biopsy from a lesional site. For the expression of AKR1C3 in normal skin, 6 mm biopsies were obtained from the buttock area of random volunteers who all gave written informed consent. All experiments were conducted in accordance with the Declaration of Helsinki Principles.
Statistical Analysis
The results are presented as means +/− SEM. The appropriate t-test was calculated using Microsoft Excel or GraphPad Prism. A P-value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Glynis Scott (Department of Dermatology, University of Rochester) for inspiring discussions and George Liu for providing editorial support for this manuscript. This work was supported by the NIH Grant 5 RO1CA117821.
Abbreviations
- AKR1C3
aldo-keto reductase family 1 member C3
- 17β-HSD
17-beta-hydroxysteroid dehydrogenase
- 2HFN
2′-hydroxyflavanone
- PG
prostaglandin
- 15d-PGJ2
15-deoxy- Δ12,14-PGJ2
- K10
cytokeratin 10
- K5
cytokeratin 5
- AD
atopic dermatitis
- PHK
primary human keratinocytes
- Th2
T-helper type 2
- PPARγ
peroxisome proliferator-activated receptor gamma
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- CRTH2
chemoattractant receptor-homologous molecule expressed on Th2 cells
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Barr RM, Koro O, Francis DM, Black AK, Numata T, Greaves MW. The release of prostaglandin D2 from human skin in vivo and in vitro during immediate allergic reactions. Br J Pharmacol. 1988;94:773–80. doi: 10.1111/j.1476-5381.1988.tb11588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrns MC, Duan L, Lee SH, Blair IA, Penning TM. Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer. J Steroid Biochem Mol Biol. 2010;118:177–87. doi: 10.1016/j.jsbmb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun KS, Lao HC, Langenbach R. The prostaglandin E2 receptor, EP2, stimulates keratinocyte proliferation in mouse skin by G protein-dependent and {beta}-arrestin1-dependent signaling pathways. J Biol Chem. 2010;285:39672–81. doi: 10.1074/jbc.M110.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahten A, Koch C, Ernst D, Schnoller C, Hartmann S, Worm M. Systemic PPARgamma ligation inhibits allergic immune response in the skin. J Invest Dermatol. 2008;128:2211–8. doi: 10.1038/jid.2008.84. [DOI] [PubMed] [Google Scholar]
- Desmond JC, Mountford JC, Drayson MT, Walker EA, Hewison M, Ride JP, et al. The aldo-keto reductase AKR1C3 is a novel suppressor of cell differentiation that provides a plausible target for the non-cyclooxygenase-dependent antineoplastic actions of nonsteroidal anti-inflammatory drugs. Cancer Res. 2003;63:505–12. [PubMed] [Google Scholar]
- Eichenfield LF. Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy. 2004;59(Suppl 78):86–92. doi: 10.1111/j.1398-9995.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Gingras S, Turgeon C, Brochu N, Soucy P, Labrie F, Simard J. Characterization and modulation of sex steroid metabolizing activity in normal human keratinocytes in primary culture and HaCaT cells. J Steroid Biochem Mol Biol. 2003;87:167–79. doi: 10.1016/j.jsbmb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Wang JY, Hodge MR, Jin H, Geha RS. The prostaglandin D receptor CRTH2 is important for allergic skin inflammation after epicutaneous antigen challenge. J Allergy Clin Immunol. 2010;126:784–90. doi: 10.1016/j.jaci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikima T, Maibach HI. Gender differences of enzymatic activity and distribution of 17beta-hydroxysteroid dehydrogenase in human skin in vitro. Skin Pharmacol Physiol. 2007;20:168–74. doi: 10.1159/000101386. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Nagata K, Takano S, Takahashi K, Ishii N, Ikezawa Z. Association of a new-type prostaglandin D2 receptor CRTH2 with circulating T helper 2 cells in patients with atopic dermatitis. J Invest Dermatol. 2002;119:609–16. doi: 10.1046/j.1523-1747.2002.01862.x. [DOI] [PubMed] [Google Scholar]
- Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldo-keto reductase superfamily. Biochem J. 1997a;326 (Pt 3):625–36. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol. 1997b;54:639–47. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 2005;38:1–7. doi: 10.1016/j.jdermsci.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Konger RL, Billings SD, Prall NC, Katona TM, Dasilva SC, Kennedy CR, et al. The EP1 subtype of prostaglandin E2 receptor: role in keratinocyte differentiation and expression in non-melanoma skin cancer. Prostaglandins Leukot Essent Fatty Acids. 2009;81:279–90. doi: 10.1016/j.plefa.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3 alpha-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3 alpha/17 beta-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–84. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- Maggi LB, Jr, Sadeghi H, Weigand C, Scarim AL, Heitmeier MR, Corbett JA. Anti-inflammatory actions of 15-deoxy-delta 12,14-prostaglandin J2 and troglitazone: evidence for heat shock-dependent and -independent inhibition of cytokine-induced inducible nitric oxide synthase expression. Diabetes. 2000;49:346–55. doi: 10.2337/diabetes.49.3.346. [DOI] [PubMed] [Google Scholar]
- Marin YE, Seiberg M, Lin CB. Aldo-keto reductase 1C subfamily genes in skin are UV-inducible: possible role in keratinocytes survival. Exp Dermatol. 2009;18:611–8. doi: 10.1111/j.1600-0625.2008.00839.x. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Shiraishi H, Hara A, Sato K, Deyashiki Y, Ninomiya M, et al. Identification of a principal mRNA species for human 3alpha-hydroxysteroid dehydrogenase isoform (AKR1C3) that exhibits high prostaglandin D2 11-ketoreductase activity. J Biochem. 1998;124:940–6. doi: 10.1093/oxfordjournals.jbchem.a022211. [DOI] [PubMed] [Google Scholar]
- Meyer M, Pahl HL, Baeuerle PA. Regulation of the transcription factors NF-kappa B and AP-1 by redox changes. Chem Biol Interact. 1994;91:91–100. doi: 10.1016/0009-2797(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Oates JA, Roberts LJ., 2nd Identification of skin as a major site of prostaglandin D2 release following oral administration of niacin in humans. The Journal of investigative dermatology. 1992;98:812–5. doi: 10.1111/1523-1747.ep12499963. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kawachi Y, Xu X, Sakurai H, Ishii Y, Takahashi T, et al. The combination of ubiquitous transcription factors AP-1 and Sp1 directs keratinocyte-specific and differentiation-specific gene expression in vitro. Exp Dermatol. 2007;16:143–50. doi: 10.1111/j.1600-0625.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1–AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Jez JM, Lin HK, Ma H, Moore M, et al. Structure-function aspects and inhibitor design of type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) Mol Cell Endocrinol. 2001;171:137–49. doi: 10.1016/s0303-7207(00)00426-3. [DOI] [PubMed] [Google Scholar]
- Penning TM, Jin Y, Heredia VV, Lewis M. Structure-function relationships in 3alpha-hydroxysteroid dehydrogenases: a comparison of the rat and human isoforms. J Steroid Biochem Mol Biol. 2003;85:247–55. doi: 10.1016/s0960-0760(03)00236-x. [DOI] [PubMed] [Google Scholar]
- Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Jin Y, Peehl DM, et al. Aldo-keto reductase (AKR) 1C3: role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–91. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Pentland AP, Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986;77:246–51. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizner TL, Lin HK, Penning TM. Role of human type 3 3alpha-hydroxysteroid dehydrogenase (AKR1C2) in androgen metabolism of prostate cancer cells. Chem Biol Interact. 2003;143–144:401–9. doi: 10.1016/s0009-2797(02)00179-5. [DOI] [PubMed] [Google Scholar]
- Rizner TL, Smuc T, Rupreht R, Sinkovec J, Penning TM. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol. 2006;248:126–35. doi: 10.1016/j.mce.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Sandig H, Andrew D, Barnes AA, Sabroe I, Pease J. 9alpha,11beta-PGF2 and its stereoisomer PGF2alpha are novel agonists of the chemoattractant receptor, CRTH2. FEBS Lett. 2006;580:373–9. doi: 10.1016/j.febslet.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–9. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–9. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- Scott G, Jacobs S, Leopardi S, Anthony FA, Learn D, Malaviya R, et al. Effects of PGF2alpha on human melanocytes and regulation of the FP receptor by ultraviolet radiation. Exp Cell Res. 2005;304:407–16. doi: 10.1016/j.yexcr.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K. 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J Biol Chem. 2002;277:10459–66. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- Shimura C, Satoh T, Igawa K, Aritake K, Urade Y, Nakamura M, et al. Dendritic cells express hematopoietic prostaglandin D synthase and function as a source of prostaglandin D2 in the skin. Am J Pathol. 2010;176:227–37. doi: 10.2353/ajpath.2010.090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarydova L, Zivna L, Xiong G, Maser E, Wsol V. AKR1C3 as a potential target for the inhibitory effect of dietary flavonoids. Chem Biol Interact. 2009;178:138–44. doi: 10.1016/j.cbi.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–9. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh I, Rundhaug J, Pavone A, Mikulec C, Abel E, Fischer SM. Upregulation of the EP1 receptor for prostaglandin E(2) promotes skin tumor progression. Mol Carcinog. 2011;50:458–68. doi: 10.1002/mc.20730. [DOI] [PubMed] [Google Scholar]
- Suzuki-Yamamoto T, Nishizawa M, Fukui M, Okuda-Ashitaka E, Nakajima T, Ito S, et al. cDNA cloning, expression and characterization of human prostaglandin F synthase. FEBS Lett. 1999;462:335–40. doi: 10.1016/s0014-5793(99)01551-3. [DOI] [PubMed] [Google Scholar]
- Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, et al. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) J Invest Dermatol. 2003;120:905–14. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- Ward JE, Fernandes DJ, Taylor CC, Bonacci JV, Quan L, Stewart AG. The PPARgamma ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulm Pharmacol Ther. 2006;19:39–46. doi: 10.1016/j.pupt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yokoyama Y, Kokuryo T, Kawai K, Kitagawa T, Seki T, et al. 15-deoxy-delta 12,14-prostaglandin J2 prevents inflammatory response and endothelial cell damage in rats with acute obstructive cholangitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G410–8. doi: 10.1152/ajpgi.00233.2009. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.