Introduction
Mesenchymal stem cells (MSC) represent emerging cell-based therapies for diabetes and associated complications (Volarevic et al., 2011). Ongoing clinical trials are using exogenous MSC to treat type 1 and 2 diabetes, cardiovascular disease and non-healing wounds due to diabetes (Fotino et al., 2010; Ankrum and Karp, 2010). The majority of these trials are aimed at exploiting the ability of MSC to release soluble mediators that reduce inflammation and promote both angiogenesis and cell survival at sites of tissue damage due to diabetic complications (Ankrum and Karp, 2010). Translational studies targeting the pancreas are also taking advantage of the immunosuppressive properties of MSC (Fotino et al., 2010).
Growing evidence suggests that MSC secretion of soluble factors is dependent on tissue microenvironment. To date, the majority of studies have investigated the effect of a hypoxic environment. Hypoxia has been shown to regulate MSC gene expression and protein secretion in vitro with reports of increased secretion of VEGF, FGF2, HGF, IGF-1, MCP1 and thymosin β4 (Annabi et al., 2003; Gnecchi et al., 2006; Hung et al., 2007; Potier et al., 2007) and reduced secretion of MMP2 (Annabi et al., 2003; Lee et al., 2009). Interestingly, hypoxic pre-conditioning appears to enhance the ability of MSC to mediate tissue repair in vivo. MSC-conditioned medium prepared under hypoxic conditions is more effective at accelerating wound closure (Lee et al., 2009) and improving cardiac function after myocardial infarction (Rosova et al., 2008). These studies clearly demonstrate that a hypoxic environment impacts MSC secretion and that these environment-induced changes have functional consequences in vivo.
Although less is known about the effect of the metabolic environment of diabetes on MSC secretion, there are reports that MSC secretion of growth factors is affected by exposure to elevated glucose. High glucose was shown to up-regulate MSC secretion of TGF-β1 protein levels (Ryu et al., 2010) but had no effect on levels of secreted VEGF, HGF and FGF2 (Weil et al., 2009). Despite the contribution of fatty acids to the metabolic environment of type 2 diabetes, almost nothing is known about their effects on MSC secretion of growth factors and cytokines. In this study, we determined the effect of two unsaturated fatty acids, linoleic and oleic acids, on human bone marrow-derived MSC. Our results show that exposure to either unsaturated fatty acid changed the MSC secretome at the level of both gene expression and protein secretion. In addition, linoleic and oleic acids affected MSC proliferation and chemotaxis.
Materials and Methods
Reagents
Linoleic and oleic acids were purchased from Nu-Chek Prep, Inc. (Elysian, MN). ELISA kits for Interleukin-6 (IL-6), Interleukin-8 (IL-8), and Vascular Endothelial Growth Factor (VEGF) were purchased from Assay Designs/Enzo Life Sciences (Plymouth Meeting, PA). Mouse anti-human phycoerythrin conjugated antibodies for CD19, CD34, CD45, HLA-DRII and the corresponding isotype controls were purchased from Molecular Probes (Invitrogen, Carlsbad, CA). Mouse anti-human phycoerythrin conjugated antibodies for CD11b, CD73, CD90, CD105, CD166 and the corresponding isotype controls were purchased from BD Biosciences (San Jose, CA). Propidium iodide was also purchased from BD Biosciences (San Jose, CA). The PPARγ antagonist, T0070907 (2-chloro-5-nitro-N-4-pyridinlybenzamide) was purchased from Enzo Life Sciences (Plymouth Meeting, PA).
Cell culture
The laboratory of Dr. Darwin Prockop at the Institute for Regenerative Medicine, Texas A&M Health Science Center kindly provided human bone marrow-derived MSC. On receipt, we confirmed that these cells express CD73, CD90, CD105 and CD166 and are negative for CD11b, CD19, CD34, CD45 and HLA-DR class II. Further validation showed that these cells have the capacity to differentiate into adipocytes and osteoblasts in standard in vitro differentiation assays.
For this study, MSC were expanded in complete culture medium (CCM) consisting of α-minimum essential medium supplemented with 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin (all from Invitrogen, Carlsbad, CA), 25mM HEPES (Calbiochem/EMD Chemicals Inc., San Diego, CA), and 16.3% fetal bovine serum (Atlanta Biologicals, Atlanta, GA). MSC were maintained at low density at 37°C in 5% CO2 and only passages 3–6 were used for experiments.
For all experiments, MSC were seeded at 60 cells/cm2 and grown for 7 days prior to the exposure to fatty acids. On day 7, cells were washed twice in PBS and then cultured in cultured in α-MEM, 2% FBS and 20 μM fatty acid for 7 days; medium was changed every other day with fresh fatty acid.
Cell proliferation assays: Cell counts and BrdU ELISA
Cell counts were performed on days 0, 3, 5 and 7 of exposure to fatty acids using a Vicell Cell Viability Analyzer (Beckman Coulter, Miami, FL) with trypan blue exclusion. DNA synthesis was measured using a BrdU colorimetric ELISA (Roche Applied Science, Indianapolis, IN). After 7 days of fatty acid exposure, BrdU labeling reagent was added into the cell culture medium (10μl per 100μl medium/well) and cells were incubated for a further 24 hours at 37°C and 5% CO2. Cells were then fixed and the DNA was denatured. An anti-BrdU-peroxidase antibody was then used to detect BrdU incorporated into newly synthesized DNA. Reaction of the peroxidase with TMB substrate produced a measurable color change, which was then measured at 370 nm with a reference wavelength of 492 nm using a SpectraMax Plus384 microplate reader using Softmax Pro version 4.7 (Molecular Devices Corporation, Sunnyvale, CA).
Flow cytometry
Propidium iodide staining was used to detect the number of dead cells after 20-hour exposure to increasing concentrations of fatty acid. Cells from the medium and the trypsinized cell layer were pooled, pelleted and resuspended in PBS containing 2% heat inactivated FBS and 0.1% sodium azide. Propidium iodide was added immediately prior to flow cytometry. The final concentration of propidium iodide was 1 μg/ml. A non-stained control was included for gating.
Flow cytometry was also used to determine the cell surface marker profile of MSC exposed to fatty acids. On day 7 of fatty acid exposure, MSC were harvested for flow cytometry as previously described (Fathke et al., 2004). In brief, the cells were rinsed twice with PBS, trypsinized and pelleted. The cell pellet was washed twice with PBS containing 2% heat inactivated FBS then resuspended in PBS containing 2% heat inactivated FBS and 0.1% sodium azide. Cells were first incubated on a rocker for 20 minutes at 4°C with 10% human serum to block Fc receptors and reduce non-specific staining. Cells were then incubated overnight at 4°C on a rocker in the dark with phycoerythrin-conjugated primary antibodies or their isotype control. Cells were then washed and resuspended in 0.5ml PBS. Flow cytometry was performed using a FACScan flow cytometer using CellQuest Pro version 4.0.1 (BD Biosciences, San Jose, CA).
Real time quantitative reverse-transcription PCR
RNA was extracted from MSC using Trizol Reagent (Invitrogen, Carlsbad, CA) followed by DNAse digestion on an RNeasy micro column (Qiagen, Valencia, CA). cDNA was then synthesized from total RNA using a Omniscript RT kit (Qiagen, Valencia, CA). Real time PCR was performed in an ABI Prism 7900 HT instrument (Applied Biosystems, Foster City, CA) using either the iTAQ SYBR green supermix with ROX (BioRad, Hercules, CA) or the Quantitect SYBR green PCR kit (Qiagen, Valencia, CA). Primers were designed to correspond to different exons to avoid amplification of genomic DNA and their sequences are listed in Table 1. A dissociation curve was generated for each primer set to ensure amplification of a single specific product. The comparative Ct method (2−ΔΔCt) was used to quantify gene expression levels where ΔΔCt = ΔCt(sample) – ΔCt(reference). In this study, the sample is MSC incubated in α-MEM, 2% FBS and 20 μM fatty acid with a final glucose concentration of 5.38 mM glucose. The reference is control MSC incubated in α-MEM, 2% FBS with a final glucose concentration of 5.38 mM glucose. Both sample and reference were normalized to the endogenous housekeeping gene, GAPDH; fatty acids had no significant effect on GAPDH cycle threshold. Data are reported as mRNA fold change.
Table 1.
Primer sequences used for real time PCR
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| GAPDH | ACGGGAAGCTTGTCATCAAT | TGGACTCCACGACGTACTCA |
| HGF | TGGATGCACAATTCCTGAAA | GAGTCACCTTCCCTCGATGA |
| IL-1B | GGGCCTCAAGGAAAAGAATC | TTCTGCTTGAGAGGTGCTGA |
| IL-6 | GGCACTGGCAGAAAACAACC | GCAAGTCTCCTCATTGAATCC |
| IL-8 | TCTGCAGCTCTGTGTGAAGG | AATTTCTGTGTTGGCGCAGT |
| VEGF | AAGGAGGAGGGCAGAATCAT | CACACAGGATGGCTTGAAGA |
Enzyme-linked immunosorbent assays
An ELISA Kit (Assay Designs/ENZO, Plymouth Meeting, PA) was used according to manufacturer instruction to detect cytokines and growth factors in the culture supernatant. Briefly, 100μl of either standard or sample was added to a well pre-coated with monoclonal antibody and then incubated at room temperature on a plate shaker for 1 hour at 500rpm. Standard or sample was aspirated and the well washed four times to remove unbound substances. After the last washing step, 100μl of polyclonal antibody was added to each well and the plate incubated at room temperature for 1 hour on a shaker. The plate was then washed to remove excess antibody. 100μl of HRP conjugate was added to each well and incubated at room temperature sealed on a plate shaker for 30 minutes at 500rpm. After this incubation, the plate was washed and 100μl TMB substrate solution was added to each well and then incubated at room temperature on a plate shaker for 15 minutes. The HRP-catalyzed substrate reaction generated a blue color and was finalized with 100μl of stop solution. The resulting yellow color was read at 450nm with a correction at 570nm on a SpectraMax Plus384 microplate reader using SoftMax Pro version 4.7.
Detection of human VEGF by ELISA was different from the other ELISA kits because it included an initial overnight incubation at 4°C to bind the monoclonal antibody. Each well was then washed seven times and 100μl of HRP labeled antibody was incubated for 30 minutes at 4°C. The wells were washed nine times and 100μl of TMB substrate solution was incubated for 30 minutes at room temperature in the dark. The enzyme reaction was stopped with 100μl stop solution and the plate was read at 450nm with a correction at 570nm on a SpectraMax Plus384 microplate reader using SoftMax Pro version 4.7. All ELISA data were normalized to cell number.
Nitric oxide assay
Total nitrite levels in the medium were measured as an index of nitric oxide (NO) production using a nitric oxide assay kit (Assay Designs, Inc., Ann Arbor, MI). This colorimetric assay is based on the interaction of nitrite with the Griess reagent. A SpectraMax Plus384 microplate reader was used to measured absorbance of the purple azo derivative at 540 nm. Total nitrite levels represented both total nitrate and nitrite concentrations because prior to addition of the Griess reagent nitrate was converted to nitrite by nitrate reductase. Data were normalized to cell number.
Chemotaxis assay
MSC chemotaxis was measured using a 24 well Boyden Chamber cell migration assay (Chemicon, Billerica, MA) according to the manufacturer’s protocol. In this assay, the Boyden chamber consists of a cell culture insert with a polycarbonate membrane (8μm pore size) placed into a well of a 24 well tissue culture plate. The top of the Boyden chamber (cell culture insert) was loaded with 3×105 MSC that had been exposed to fatty acids for 7 days, trypsinized and resuspended in serum free medium. The bottom chamber (well) contained the chemoattractant, 2% FBS in culture medium. Cell migration through the membrane was determined after 24 hour incubation at 37°C in 5% CO2. Cells that had migrated through the membrane were stained and quantified by measuring absorbance at 560 nm on SpectraMax Plus384 microplate reader using SoftMax Pro version 4.7.
NF-κB nuclear translocation
MSC were fixed for 20 minutes with 4% paraformaldehyde, permeabilized for 10 minutes with 0.05% Triton X100 in PBS and blocked for 1 hour in 1.3% normal goat serum and 0.1% BSA in PBS. Cells were then incubated overnight at 4°C with rabbit polyclonal antibody against the p-65 subunit of NF-κB (dilution 1:500; Cell Signaling Technology, Danvers, MA) in blocking solution. Cells were washed three times with PBS and then incubated for 30 minutes in the dark with Rhodamine Red-X goat anti-rabbit secondary antibody (dilution 1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Cells were then washed with PBS and incubated for 20 minutes with fluoroscein phalloidin (dilution 1:80; Invitrogen/Molecular Probes, Eugene, OR) to label the actin cytoskeleton. Cells were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in order to visualize the nucleus. Coverslips were mounted using ProLong Gold Antifade Reagent (Invitrogen/Molecular Probes, Eugene, OR). Digital images of the cells were captured with the Qimaging QICAM Fast 1394 Monochrome 12-bit cooled digital camera (10X and 20X) mounted on a Nikon TE300 Inverted microscope. Digital images were then imported into Adobe Photoshop CS2. There were no alterations to figures with image processing software other than the standard linear adjustment to brightness applied to the entire image. The compared images were the same magnification and adjusted for the same brightness and contrast.
Statistical analyses
Each experiment was performed a minimum of three times. A single representative experiment is shown with all data expressed as mean ± standard deviation (N≥3). The Student’s t-test was used to determine statistically significant differences between experimental and control samples. A P-value less than or equal to 0.05 was considered significant.
Results
Unsaturated fatty acids inhibit mesenchymal stem cell proliferation
For all experiments, human bone marrow-derived MSC were exposed to 20 μM unsaturated fatty acids in the presence of 5.38 mM glucose. Control MSC were cultured without the addition of fatty acids. The fatty acid concentration of 20 μM was selected based on dose response experiments that showed that concentrations greater than 40μM unsaturated fatty acids significantly decreased human MSC viability as measured by propidium iodide staining of dead cells (Figure 1). After a 24-hour incubation with 40μM linoleic acid, ~8% of the MSC stained positive for propidium iodide, which was significantly more than the 2% of dead cells in the control with no added linoleic acid (Figure 1A). The percentage of propidium iodide positive MSC increased to ~60% in the presence of 50 μM linoleic acid and almost all cells (~90%) were dead after incubation with 100 μM linoleic acid (Figure 1A). In contrast, the number of propidium iodide positive cells after incubation with 20 μM linoleic acid was negligible and not significantly different than the number of dead cells detected in the control MSC (Figure 1A). Oleic acid had similar effects on MSC viability. Oleic acid had no effect on MSC viability at 20 μM but decreased viability significantly at concentrations of 40 μM, 50 μM and 100 μM (Figure 1B).
Figure 1.
Unsaturated fatty acids reduce MSC viability. A. Human bone marrow derived-MSC exposed to increasing concentrations of linoleic acid for 24 hours. B. Human bone marrow derived-MSC exposed to increasing concentrations of oleic acid for 24 hours. At 24 hours, MSC from the medium and the cell layer were pooled and incubated with propidium iodide to stain dead cells. Flow cytometry was used to detect the percentage of MSC that are positive for propidium iodide staining. Data shown are the average of three independent experiments. (* P<0.05)
At 20 μM, both linoleic acid and oleic acid inhibited MSC proliferation (Figure 2). Exposure to either fatty acid resulted in a significant decrease in viable MSC number after 3, 5 and 7 days of fatty acid treatment (Figure 2A). For MSC exposed to linoleic acid there was no increase in cell number after day 3 (Figure 2A). Further investigation demonstrated that the fatty acid effect on viable MSC number was due to inhibition of MSC DNA synthesis (Figure 2B). BrdU incorporation into DNA was significantly reduced in MSC exposed to either linoleic or oleic acid for 7 days (Figure 2B).
Figure 2.
Unsaturated fatty acids inhibit MSC proliferation. Human bone marrow derived-MSC were exposed to 20 μM unsaturated fatty acids in the presence of 5.38 mM glucose. Control MSC were cultured without the addition of fatty acids. A. Total viable MSC were counted on days 3, 5 and 7. On day 3, there was a significant reduction in the number of viable MSC exposed to linoleic acid compared to control MSC († P<0.001). On days 5 and 7, both linoleic acid and oleic acid significantly inhibited MSC proliferation (* P<0.001). B. MSC DNA synthesis was measured using a BrdU colorimetric ELISA on day 8. Both unsaturated fatty acids inhibited MSC DNA synthesis significantly (* P<0.001). Data shown in A. and B. represent mean ± a standard deviation for a single representative experiment. A minimum of three independent experiments was performed with six wells for each condition.
Unsaturated fatty acids have no effect on the MSC cell surface marker profile
Using flow cytometry, we investigated whether fatty acid exposure altered the MSC cell surface marker profile as defined by the International Society for Cellular Therapy (Dominici et al., 2006). For this profile, greater than 95% of the cell population is positive for CD73, CD90 and CD105 expression and greater than 98% of the population is negative for CD11b or CD14, CD19 or CD79a, CD34, CD45 and HLA-DR surface molecules. In our study, human MSC exposed to unsaturated fatty acids for 7 days had a similar cell surface marker profile to control MSC. Linoleic acid treated MSC were positive for expression of CD73, CD90 and CD105 and negative for CD11b, CD19, CD34, CD45 and HLA-DR CLASS II (Table 2). A similar cell surface marker profile was observed for both control MSC and MSC exposed to oleic acid (Table 2). We also determined that exposure to linoleic and oleic acids for seven days did not induce MSC differentiation into either osteblasts or adipocytes. This control experiment was important because a high fat diet has been recently shown to increase bone marrow adiposity (Chen et al., 2010) and PPARγ, which acts downstream of unsaturated fatty acids, is known to promote MSC adipogenesis (Takada et al., 2009). In our study, there was no significant increase in mRNA levels for specific markers of either osteoblasts (RUNX2 and COL1A1) or adipocytes (LEP and PPARG) in MSC exposed to unsaturated fatty acids for seven days (data not shown).
Table 2.
Cell surface marker profile of MSC exposed to either linoleic or oleic acids for seven days.
| Cell Surface Marker | Control MSCa | Linoleic Acid MSCa | Oleic Acid MSCa |
|---|---|---|---|
| CD73 | 98.78±0.67 | 97.74±0.77 | 98.25±0.77 |
| CD90 | 99.39±0.81 | 99.11±0.80 | 99.90±0.10 |
| CD105 | 98.87±0.53 | 98.65±0.32 | 98.66±0.67 |
| CD11b | 0.13±0.01 | 0.10±0.03 | 0.31±0.11 |
| CD19 | 0.02±0.02 | 0.05±0.04 | 0.15±0.14 |
| CD34 | 0.04±0.04 | 0.06±0.06 | 0.08±0.04 |
| CD45 | 0.37±0.19 | 0.22±0.14 | 0.17±0.10 |
| HLA-DR class II | 0.32±0.12 | 0.13±0.07 | 0.09±0.08 |
Percentage of MSC positive for expression of specific cell surface marker. Data are expressed as mean±standard deviation (n=3). There was no significant difference between groups (P-value>0.05).
Unsaturated fatty acids alter MSC gene expression
Unsaturated fatty acids affected MSC expression of growth factors and cytokines (Figure 3). Seven-day exposure to linoleic acid resulted in MSC up-regulation of VEGF (Figure 3A), IL-1β (Figure 3B), IL-6 (Figure 3B), and IL-8 (Figure 3B) mRNA levels. Linoleic acid exposure also induced MSC to down-regulate expression of HGF (Figure 3A). Similar to linoleic acid, oleic acid exposure resulted in increased expression of VEGF (Figure 3A) and IL-6 (Figure 3B) and decreased mRNA levels for HGF (Figure 3A). In contrast to linoleic acid, oleic acid exposure had no significant effect on MSC expression of IL-1β and IL-8 (Figure 3B). It should also be noted that IL-6 mRNA levels were significantly higher in MSC exposed to oleic acid than in MSC exposed to linoleic acid. This gene expression study suggests that unsaturated fatty acids alter the MSC secretome given that the affected genes encode secreted proteins.
Figure 3.
Unsaturated fatty acids affect MSC expression of growth factors and cytokines. RNA was extracted from human bone marrow-derived MSC exposed to 20 μM unsaturated fatty acids for 7 days and control MSC cultured without the addition of fatty acids. A. Linoleic and oleic acids induced MSC to down-regulate HGF and up-regulate VEGF B (* P<0.005). Linoleic acid induced MSC to up-regulate IL-1β, IL-6 and IL-8 whereas oleic acid induced MSC to up-regulate only IL-6 (* P<0.005). Data shown in A. and B. represent mean ± a standard deviation for a single representative experiment. A minimum of three independent experiments was performed with six wells for each condition.
Unsaturated fatty acids affect MSC secretion of growth factors and cytokines
Further investigation determined that unsaturated fatty acids affect MSC secretion of VEGF, IL-6 and IL-8 proteins (Figure 4). Linoleic acid exposure for seven days induced MSC to increase secretion of VEGF (Figure 4A), IL-6 (Figure 4B) and IL-8 (Figure 4C). Oleic acid also induced MSC to increase secretion of VEGF (Figure 4A) and IL-6 (Figure 4B). Compared to MSC exposed to linoleic acid, IL-6 protein levels were significantly higher in oleic acid treated MSC (Figure 4B). In contrast to linoleic acid, oleic acid had no significant effect on MSC secretion of IL-8 (Figure 4C), which supports the gene expression data (Figure 4B). Collectively these data confirm the observed effect of linoleic and oleic acids on MSC gene expression.
Figure 4.
Unsaturated fatty acids induce MSC to increase secretion of growth factors and cytokines. Medium was collected from MSC exposed to fatty acids for 7 days and then analyzed by ELISA. A. Linoleic and oleic acids induced MSC to increase secretion of VEGF (* P<0.001). B. Linoleic and oleic acids induced MSC to increase secretion of interleukin-6 (IL-6) (* P<0.05). C. Linoleic acid induced MSC to increase secretion of interleukin-8 (IL-8) (* P<0.005) whereas oleic acid had no effect. Data shown have been normalized to cell number and represent mean ± a standard deviation for a single representative experiment. A minimum of three independent experiments was performed with three wells for each condition.
We also determined whether fatty acids affected MSC production of nitric oxide. Compared to control MSC, nitric oxide levels were significantly increased in the medium of MSC exposed to either linoleic acid or oleic acid for seven days (Figure 5). Again these data demonstrate that fatty acid exposure alters the MSC secretome.
Figure 5.
Unsaturated fatty acids induce MSC to increase nitric oxide production. Medium was collected from MSC exposed to fatty acids for 7 days and then analyzed by nitric oxide assay. Linoleic and oleic acids induced MSC to increase production of nitric oxide (* P<0.001). Data shown have been normalized to cell number and represent mean ± a standard deviation for a single representative experiment. A minimum of three independent experiments was performed with three wells for each condition.
Unsaturated fatty acids affect MSC chemotaxis
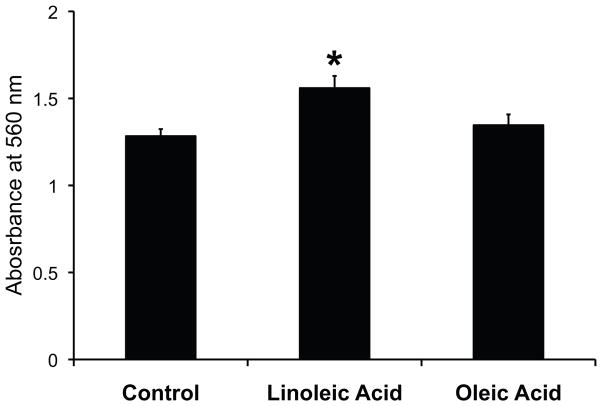
Exposure to unsaturated fatty acids also affected MSC chemotaxis in a colorimetric cell migration assay based on the Boyden chamber principle. In these experiments, human MSC were exposed to either linoleic acid or oleic acid for 7 days and then detached from the plate using trypsin. Equal numbers of control and fatty acid exposed MSC were placed in the top of the Boyden chamber. Culture medium supplemented with 2% FBS served as the chemoattractant in the bottom of the Boyden chamber. Compared to control MSC, linoleic acid treated MSC showed a significant increase in migration toward medium supplemented with 2% FBS (Figure 6). Oleic acid exposure had no significant effect on MSC chemotaxis (Figure 6).
Figure 6.
Unsaturated fatty acid exposure enhance MSC chemotaxis. MSC exposed to fatty acids for 7 days were washed extensively with PBS to remove residual fatty acid and then placed in the top chamber of a Boyden chamber with 2% FBS as the chemoattractant. After overnight incubation, MSC that had migrated through the membrane were stained and quantified by measuring absorbance at 560 nm. MSC exposed to linoleic acid showed increased migration towards the chemoattractant compared to both control and MSC exposed to oleic acid (* P<0.005). A minimum of three independent experiments was performed with six wells for each condition.
NF-κB and PPARγ signaling do not mediate the effects of unsaturated fatty acids on MSC
We investigated whether either NF-κB or PPARγ signaling mediated the effects of linoleic and oleic acids on human MSC. We chose to focus on these pathways because both transcription factors have been implicated as downstream effectors of fatty acid signaling. Saturated fatty acids have been reported to increase expression of inflammatory genes via an NF-κB dependent mechanism (Suganami et al., 2007; Haversen et al., 2009; Schwartz et al., 2009) and omega-3 polyunsaturated fatty acids activate PPARγ signaling (Marion-Letellier et al., 2009).
In our study, linoleic and oleic acids had no effect on NF-κB activation as measured by NF-κB nuclear translocation. NF-κB immunofluorescence was detected only in the cytoplasm of control MSC (Figure 7A) and MSC exposed to either linoleic acid (Figure 7B) or oleic acid (Figure 7C). In contrast, NF-κB was observed in the nucleus of the positive control MSC treated with TNF-α (Figure 7D).
Figure 7.
Unsaturated fatty acid exposure does not result in translocation of NF-κB to the MSC nucleus. Nuclear translocation of NF-κB was analyzed using an anti-p65 NF-κB antibody followed by a rhodamine red X-secondary antibody. A. NF-κB immunofluorescence was absent from the nuclei (arrows) but present in the cytoplasm of control MSC cultured without added fatty acids. B. NF-κB immunofluorescence was absent from the nuclei (arrows) but present in the cytoplasm of MSC exposed to linoleic acid. C. NF-κB immunofluorescence was absent from the nuclei (arrows) but present in the cytoplasm of MSC exposed to oleic acid. D. Positive Control: NF-κB immunofluorescence was detected in the nuclei (arrows) and cytoplasm of MSC treated with TNF-α (50 ng/ml) for 1 hour. Magnification Bar = 50 microns.
The effects of linoleic and oleic acids on MSC also appear to be independent of PPARγ signaling. MSC were exposed to the unsaturated fatty acids in the presence of 1μM T0070907, a PPARγ antagonist for 7 days. T0070907 had no significant effect on total viable MSC number in control and fatty acid treated MSC (Figure 8). Importantly, MSC proliferation was still inhibited by the unsaturated fatty acids in the presence of T0070907. These data suggest that PPARγ does not mediate the effect of linoleic and oleic acids on MSC proliferation.
Figure 8.
The effect of linoleic and oleic acids on MSC proliferation is independent of PPARγ signaling. Human bone marrow derived-MSC were exposed to 20 μM unsaturated fatty acids in the presence or absence of 1 μM T0070907, a PPARγ antagonist for 7 days. Control MSC were cultured without the addition of fatty acids. Total viable MSC were counted on day 7. T0070907 had no significant effect on MSC number for either control or fatty acid treated MSC. As previously shown, linoleic and oleic acids inhibited MSC proliferation (* P<0.005). Data shown represent mean ± a standard deviation for a single representative experiment.
Discussion
In this study, we have determined that two unsaturated fatty acids common in Western diets (Institute of Medicine, Food and Nutrition Board) alter the phenotype of human bone marrow-derived MSC. Linoleic acid, an omega-6 polyunsaturated fatty acid, and oleic acid, a monounsaturated fatty acid, affect MSC proliferation, gene expression, protein secretion, and migration. Collectively these data suggest that exposure to fatty acids may have functional consequences for MSC therapy. Fatty acids may affect: 1) MSC engraftment to injured tissue and 2) MSC secretion of cytokines and growth factors that regulate local cellular responses to injury.
MSC engraftment requires cell survival and proliferation at the site of injury (Karp and Leng Teo, 2009). It also requires homing to the site of injury (Karp and Leng Teo, 2009) for MSC therapy delivered systemically. This study suggests that linoleic and oleic acids may have both positive and negative effects on MSC engraftment. Our chemotaxis data indicate that MSC exposed to unsaturated fatty acids migrate faster, which may be beneficial for MSC homing to the damaged tissue. In contrast, linoleic and oleic acids had detrimental effects on MSC viability and proliferation and may therefore impair MSC survival and proliferation at the site of injury. Overall these results suggest that fatty acids may impact the efficiency of MSC engraftment to the injured tissue. Directly relevant to the potential effects of fatty acids on MSC engraftment are studies demonstrating that the efficiency of hematopoietic stem cell homing and engraftment is increased by pre-treatment with prostaglandin E2, which is derived from arachidonic acid, an omega-6 polyunsaturated fatty acid (Goessling et al., 2011; Pelus et al., 2011). Further investigation is clearly needed to determine whether fatty acids affect bone marrow-derived MSC engraftment in vivo.
Exposure to unsaturated fatty acids induced MSC to increase secretion of known mediators of angiogenesis such as VEGF, IL-1β, IL-8 and nitric oxide (Koch et al., 1992; Rizk et al., 2004; Ara and Declerck, 2010; Sung et al., 2010). These data suggest that MSC exposed to fatty acids may be more effective at promoting angiogenesis. A recent study supports this hypothesis by demonstrating that a derivative of docosahexaenoic acid, an omega-3 polyunsaturated fatty acid, enhanced the effects of bone marrow-derived MSC on angiogenesis in a mouse model of diabetic wound repair (Tian et al., 2011). Similar to our results, this fatty acid derivative induced MSC to increase secretion of VEGF. Collectively these findings have implications for MSC therapy because they suggest that exposure of MSC to unsaturated fatty acids prior to administration may enhance their beneficial effects of angiogenesis in vivo. This idea of pre-conditioning MSC for therapy is not new, for example hypoxic pre-conditioning has been used to enhance both MSC survival at the site of injury and MSC induced angiogenesis (Rosova et al., 2008; Lee et al., 2009; Chacko et al., 2010; Leroux et al., 2010; Peterson et al., 2011).
To our knowledge, the effect of linoleic and oleic acids on human bone marrow-derived MSC has not been previously described. However, the effects of these unsaturated fatty acids on cell proliferation, gene expression and protein secretion have been reported for other cell types. The effect on cell proliferation appears to be dependent on cell type. For example, linoleic acid induces proliferation of mouse embryonic stem cells (Kim et al., 2009) but inhibits proliferation of endothelial progenitor cells (Guo et al., 2008). Linoleic and oleic acids have also been reported to regulate expression of angiogenic mediators in other cell types. Both unsaturated fatty acids increase secretion of VEGF and IL-1β by neutrophils (Pereira et al., 2008) and IL-6 by intestinal epithelial cells (Yoshida et al., 2001). Also consistent with our data is the report that linoleic and oleic acids have different effects on production of IL-8 (Leik and Walsh, 2005). Secretion of IL-8 by vascular smooth muscle cells was increased by linoleic acid but unaffected by oleic acid. Such studies support our findings on the effect of unsaturated fatty acids on MSC expression of these specific angiogenic mediators.
The finding that linoleic and oleic acids at concentrations greater than 40 μM were cytotoxic to human bone marrow-derived MSC was surprising. Most studies report that saturated rather than unsaturated fatty acids induce cell death (Brookheart et al., 2009). However there is evidence that linoleic and oleic acids may also be cytotoxic. Treatment with either unsaturated fatty acid significantly reduces macrophage viability (Aronis et al., 2008) and linoleic acid augments saturated fatty acid-induced hepatocyte death (Wei et al., 2007). Further investigation is required to determine whether the effect of linoleic and oleic acids on MSC viability is due to lipotoxicity, which is characterized by activation of oxidative stress, activation of the ER stress response and apoptosis (Brookheart et al., 2009). More experiments are also needed to determine whether bone marrow-derived MSC have a lipotoxic response in vivo. It is also important to note that we were unable to make any conclusions about the effect of plasma fatty acid levels on MSC therapy in vivo because MSC were not viable in fatty acid concentrations seen in type 2 diabetes, which are typically greater than 600 μM throughout the entire day (Roden, 2004; Mathew et al., 2010).
Further investigation is required to identify the downstream effectors responsible for mediating the effects of linoleic and oleic acids on MSC. Potential candidates known to mediate fatty acid signaling include NF-κB (Schwartz et al., 2009; Dasu and Jialal, 2011), PPARγ (Tontonoz and Spiegelman, 2008; Marion-Letellier et al., 2009), reactive oxygen species (Giacco and Brownlee, 2010) and prostaglandins (Riccioti and Fitzgerald, 2011). Our results suggest that the effects of linoleic and oleic acids on MSC are independent of both NF-κB and PPARγ signaling. NF-κB was not translocated to the nucleus of bone marrow-derived MSC exposed to either linoleic or oleic acids and T0070907, a PPARγ antagonist failed to block the effect of these unsaturated fatty acids on MSC proliferation. Furthermore recent preliminary data shows that linoleic acid is still able to induce MSC to up-regulate expression of VEGF and IL-6 in the presence of T0070907 (data not shown). Hypoxia-inducible factor-1α (HIF-1α) is also a possible downstream effector of linoleic and oleic acids in MSC because hypoxia has been shown to induce MSC expression of VEGF and MSC migration (Rosova et al., 2008; Busletta et al., 2011). However, preliminary data suggests that linoleic and oleic acids do not signal via the HIF-1α pathway. These fatty acids had no significant effect on HIF-1α protein levels in MSC (data not shown).
Understanding the effect of the metabolic environment on MSC phenotype is essential for optimizing MSC therapy targeting diabetes and associated complications. Circulating metabolites should also be considered because of the current focus on systemic delivery of MSC therapy (Ankrum and Karp 2010). In this study, we chose to investigate the effects of fatty acids on MSC phenotype because of their significant contribution to the metabolic environment of individuals with impaired glucose tolerance, insulin resistance and type 2 diabetes (Roden, 2004; Wilding, 2007). In these patient populations, plasma fatty acids are maintained at elevated levels throughout the day (Roden, 2004; Wilding, 2007). The most abundant fatty acids in human plasma are linoleic, oleic and palmitic acids (Richieri and Kleinfeld, 1995; Wang et al., 2003; Patel et al., 2010). In contrast to linoleic and oleic acids, palmitic acid is a saturated fatty acid, which has been positively associated with risk of diabetes (Wang et al., 2003; Patel et al., 2010). Increased levels of palmitic acid have been detected in the plasma of patients with diabetes (Laaksonen et al., 2002; Wang et al., 2003; Patel et al., 2010). Palmitic acid has also been reported to affect viability (Gehrmann et al., 2009; Takahashi et al., 2012), proliferation (Ezure and Amano, 2011) and secretion of cytokines (Shi et al., 2006; Bunn et al., 2011) in a number of different cell types. It is clear therefore, that elucidating the effects of palmitic acid on MSC phenotype is an important future direction for our study.
In conclusion, linoleic and oleic acids affect human bone marrow-derived MSC proliferation, gene expression, protein secretion and chemotaxis. This study indicates that MSC are responsive to fatty acids in the metabolic environment. It also suggests that fatty acid preconditioning may enhance therapeutic MSC induction of angiogenesis at the site of injury.
Acknowledgments
We thank Dr. Nicole Gibran for her intellectual contribution throughout this project. A Pilot and Feasibility Award from the University of Washington Diabetes Endocrine Research Center (DERC) to Anne Hocking PhD supported this research. The DERC is funded by NIH NIDDK grant P30 DK-17047. The human bone marrow-derived mesenchymal stem cells employed in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH, Grant # P40 RR-017447.
Literature Cited
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J, Béliveau R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;2:337–347. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronis A, Madar Z, Tirosh O. Lipotoxic effects of triacylglycerols in J774.2 macrophages. Nutrition. 2008;24:167–176. doi: 10.1016/j.nut.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab. 2009;10:9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn RC, Cockrell GE, Ou Y, Thrailkill KM, Lumpkin CK, Jr, Fowlkes JL. Palmitate and insulin synergistically induce IL-6 expression in human monocytes. Cardiovasc Diabetol. 2010;9:73. doi: 10.1186/1475-2840-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Wu X, Tong Y, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Obesity reduces bone density associated with activation of PPARγ and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS One. 2010;5:e13704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab. 2011;300:E145–54. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Ezure T, Amano S. Negative regulation of dermal fibroblasts by enlarged adipocytes through release of free fatty acids. J Invest Dermatol. 2011;131:2004–2009. doi: 10.1038/jid.2011.145. [DOI] [PubMed] [Google Scholar]
- Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev Diabet Stud. 2010;7:144–157. doi: 10.1900/RDS.2010.7.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann W, Elsner M, Lenzen S. Role of metabolically generated reactive oxygen species for lipotoxicity in pancreatic β-cells. Diabetes Obes Metab. 2010;12(Suppl 2):149–158. doi: 10.1111/j.1463-1326.2010.01265.x. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Goessling W, Allen RS, Guan X, Jin P, Uchida N, Dovey M, Harris JM, Metzger ME, Bonifacino AC, Stroncek D, Stegner J, Armant M, Schlaeger T, Tisdale JF, Zon LI, Donahue RE, North TE. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WX, Yang QD, Liu YH, Xie XY, Wang-Miao, Niu RC. Palmitic and linoleic acids impair endothelial progenitor cells by inhibition of Akt/eNOS pathway. Arch Med Res. 2008;39:434–442. doi: 10.1016/j.arcmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Håversen L, Danielsson KN, Fogelstrand L, Wiklund O. Induction of proinflammatroy cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis. 2009;202:382–393. doi: 10.1016/j.atherosclerosis.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Hsueh HW, Zhou Z, Whelan J, Allen KG, Moustaid-Moussa N, Kim H, Claycombe KJ. Stearidonic and eicosapentaenoic acids inhibit interleukin-6 expression in ob/ob mouse adipose stem cells via toll-like receptor-2-mediated pathways. J Nutr. 2011;141:1260–1266. doi: 10.3945/jn.110.132571. [DOI] [PubMed] [Google Scholar]
- Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the P13K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. doi: 10.1016/s0002-8223(02)90346-9. [ http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_level=3&tax_subject=274&topic_id=1323&level3_id=5145&level4_id=0&level5_id=0&placement_default=0] [DOI] [PubMed]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kim MO, Kim YH, Kim JS, Han HJ. Linoleic acid induces mouse embryonic stem cell proliferation via Ca2+/PKC, PI3K/Akt, and MAPKs. Cell Physiol Biochem. 2009;23:53–64. doi: 10.1159/000204090. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Lakka TA, Lakka HM, Nyyssönen K, Rissanen T, Niskanen LK, Salonen JT. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med. 2002;19:456–464. doi: 10.1046/j.1464-5491.2002.00707.x. [DOI] [PubMed] [Google Scholar]
- Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH. Hypoxia-enhanced wound healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- Leik CE, Walsh SW. Linoleic acid, but not oleic acid, upregulates production of interleukin-8 by human vascular smooth muscle cells via arachidonic acid metabolites under conditions of oxidative stress. J Soc Gynecol Investig. 2005;12:593–598. doi: 10.1016/j.jsgi.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Leroux L, Descamps B, Tojais NF, Séguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamazière JM, Dufourcq P, Couffinhal T, Duplàa C. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18:1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion-Letellier R, Déchelotte P, Iacucci M, Ghosh S. Dietary modulation of peroxisome proliferator-activated receptor gamma. Gut. 2009;58:586–93. doi: 10.1136/gut.2008.162859. [DOI] [PubMed] [Google Scholar]
- Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. 2010;92:1214–1222. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- Pelus LM, Hoggatt J, Singh P. Pulse exposure of hematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011;44(Suppl 1):22–29. doi: 10.1111/j.1365-2184.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LM, Hatanaka E, Martins EF, Oliveira F, Liberti EA, Farsky SH, Curi R, Pithon-Curi TC. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem Funct. 2008;26:197–204. doi: 10.1002/cbf.1432. [DOI] [PubMed] [Google Scholar]
- Peterson KM, Aly A, Lerman A, Lerman LO, Rodriguez-Porcel M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: role of oxidative stress. Life Sci. 2011;88:65–73. doi: 10.1016/j.lfs.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk M, Witte MB, Barbul A. Nitric oxide and wound healing. World J Surg. 2004;28:301–306. doi: 10.1007/s00268-003-7396-7. [DOI] [PubMed] [Google Scholar]
- Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News Physiol Sci. 2004;19:92–96. doi: 10.1152/nips.01459.2003. [DOI] [PubMed] [Google Scholar]
- Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JM, Lee MY, Yun SP, Han HJ. High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J Cell Physiol. 2010;224:59–70. doi: 10.1002/jcp.22091. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioslcler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- Sung HK, Michael IP, Nagy A. Multifaceted role of vascular endothelial growth factor signaling in adult tissue physiology: an emerging concept with clinical implications. Curr Opin Hematol. 2010;17:206–212. doi: 10.1097/MOH.0b013e32833865e6. [DOI] [PubMed] [Google Scholar]
- Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Cambiaghi TD, Luchessi AD, Hirabara SM, Vinolo MA, Newsholme P, Curi R. Activation of survival and apoptotic signaling pathways in lymphocytes exposed to palmitic acid. J Cell Physiol. 2012;227:339–350. doi: 10.1002/jcp.22740. [DOI] [PubMed] [Google Scholar]
- Tian H, Lu Y, Shah SP, Hong S. 14S, 21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J Biol Chem. 2011;286:4443–4453. doi: 10.1074/jbc.M110.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH ARIC Study Investigators. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:91–98. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem. 2007;303:105–113. doi: 10.1007/s11010-007-9461-2. [DOI] [PubMed] [Google Scholar]
- Weil BR, Abarbanell AM, Herrmann JL, Wang Y, Meldrum DR. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1735–1743. doi: 10.1152/ajpregu.90876.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med. 2007;24:934–945. doi: 10.1111/j.1464-5491.2007.02186.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Miura S, Kishikawa H, Hirokawa M, Nakamizo H, Nakatsumi RC, Suzuki H, Saito H, Ishii H. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr. 2001;131:2943–2950. doi: 10.1093/jn/131.11.2943. [DOI] [PubMed] [Google Scholar]