Abstract
Semaphorins are secreted and membrane bound proteins involved in neural pathfinding, organogenesis, and tumor progression, through Plexin and neuropilins receptors. We recently reported that Plexin B1, the Semaphorin 4D receptor, is a tumor suppressor protein for melanoma, in part, through inhibition of the oncogenic c-Met tyrosine kinase receptor. In this report we show that Sema4D is a protective paracrine factor for normal human melanocyte survival in response to ultraviolet irradiation, that it stimulates proliferation, and regulates the activity of the c-Met receptor. c-Met receptor signaling stimulates melanocyte migration, in part through down-regulation of the cell adhesion molecule E-cadherin. Sema4D suppressed activation of c-Met in response to its ligand hepatocyte growth factor (HGF), and partially blocked the suppressive effects of HGF on E-cadherin expression in melanocytes and HGF-dependent migration. These data demonstrate a role for Plexin B1 in maintenance of melanocyte survival and proliferation in the skin, and suggest that Semaphorin 4D and Plexin B1 act cooperatively with HGF and c-Met to regulate c-Met dependent effects in human melanocytes. Because our data show that Plexin B1 is profoundly down-regulated by UVB in melanocytes, loss of Plexin B1 may accentuate HGF dependent effects on melanocytes, including melanocyte migration.
Keywords: melanocyte, Semaphorin, Plexin, c-Met
Introduction
Semaphorins are secreted or membrane bound proteins, and were originally described in the nervous system, but are also expressed in multiple organs, including lung, kidney, bone and lymph tissue (Takegahara et al., 2005; Yazdani and Terman, 2006). Plexins, transmembrane receptors for Semaphorins, are a family of highly conserved proteins, which alone or in cooperation with neuropilins, mediate effects of Semaphorins (Tamagnone and Comoglio, 2000; Castellani and Rougon, 2002; Puschel, 2002; Fujisawa, 2004). The Plexin B1 receptor binds Semaphorin 4D (Sema4D), a class IV Semaphorin whose functions include neo-vascularization of tumors, axon guidance, and immune regulation (Ch’ng and Kumanogoh et al., 2010; Elhabazi et al., 2003). Sema4D is cleaved by matrix metalloproteinases and is active in a membrane bound and soluble form (Basile et al., 2007; Zhu et al., 2007). Plexin B1 has R-Ras and M-Ras GTP-ase (GAP) activity (Oinuma et al., 2004; Negishi et al., 2005; Saito et al., 2009), and activates mitogen activated protein (MAP) kinase via Rho and integrin activation (Aurandt et al., 2006; Oinuma et al., 2006). Plexin B1 activation by Sema4D also participates in c-Met and ErbB receptor activation (Swiercz et al., 2008;Wickramasinghe et al., 2005; Conrotto et al., 2004; Giordano et al,. 2002).
Melanocytes are critically important in the skin because they produce the pigment melanin, mitigating effects photo-aging and photo-carcinogenesis (Bhawan et al., 1992; Tadokoro et al., 2003; Wulf et al., 2004). Many functions of melanocytes are regulated in part by growth factors produced by keratinocytes (Cardinali et al., 2005; Imokawa, 2004; Tada et al., 1998; Yaar et al., 1991), or by the melanocytes themselves (Abdel-Malek et al., 1999; Starner et al., 2010). A potentially important role for Plexin B1 in melanocytes is suggested by recent reports showing that Plexin B1 is a tumor suppressor protein for melanoma (Stevens et al., 2010; Argast et al., 2009). Plexin B1 expression is lost in melanoma in vivo, particularly in deeply invasive and metastatic tumors (Stevens et al., 2010) and introduction of Plexin B1 into human melanoma cell lines abrogates metastasis in a mouse model (Argast et al., 2009). While the mechanism by which Plexin B1 suppresses melanoma progression is still being defined, we showed that Plexin B1 signaling blocks activation of the tyrosine kinase receptor c-Met, by its ligand hepatocyte growth factor (HGF) (Stevens et al., 2010). c-Met controls multiple aspects of melanocyte function in response to HGF, which is upregulated by ultraviolet irradiation (UVR) in keratinocytes and fibroblasts (Brenner et al., 2005; Mildner et al., 2007). c-Met signaling suppresses expression of E-cadherin in melanocytes, melanoma, and other cell types, and loss of E-cadherin contributes to melanocyte migration, and progression of melanoma (Danen et al., 1996; Li et al., 2001; Davies et al., 2001; Desiderio et al., 2007).
In this report we examined the function of Plexin B1 in normal human melanocytes, and the expression of Sema4D in the skin. Our data point to a role for Plexin B1 in melanocyte survival and proliferation, and suggest that loss of Plexin B1 may promote early stages of melanoma formation through enhanced c-Met activation, resulting in loss of E-cadherin expression and increased c-Met dependent migration.
Results
Semaphorin 4D and Plexin B1 are expressed in the skin in vivo
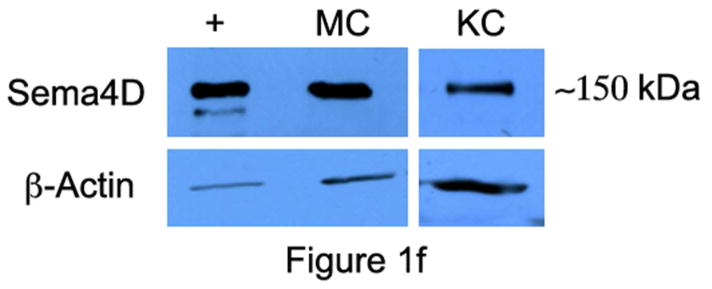
Immunocytochemical staining of skin shows strong expression of Sema4D in basal keratinocytes, closely apposed to melanocytes, which also expressed Sema4D, albeit weakly (Figure 1A and B); Sema4D was detected in dermal fibroblasts (not shown), lymphocytes (Figure 1C) and smooth muscle of blood vessels (Figure 1D), and nerves (Figure 1E), but not in endothelial cells. Keratinocytes and melanocytes expressed Sema4D in vitro (Figure 1F). Immunocytochemical staining of skin showed strong diffuse expression of Plexin B1 in keratinocytes, throughout all layers of the epidermis (Figure 1G). As previously reported, melanocytes express Plexin B1 in vivo (Figure 1H) as do endothelial cells (Stevens et al., 2010). Smooth muscle of blood vessels, and pilierector muscles, also expressed Plexin B1 (Figure 1I). Western blotting of melanocyte and keratinocytes showed a band of the expected size (~225 kDa) in both cell types (Figure 1J) for Plexin B1, as well as a lower band (~190 kDa) likely representing the proteolytically processed receptor (Artigiani et al., 2003). In some cases, a predominant band of ~100 kDa was observed in melanocytes, as previously reported (Stevens et al., 2010).
Figure 1. Semaphorin 4D and Plexin B1 are expressed in the skin.
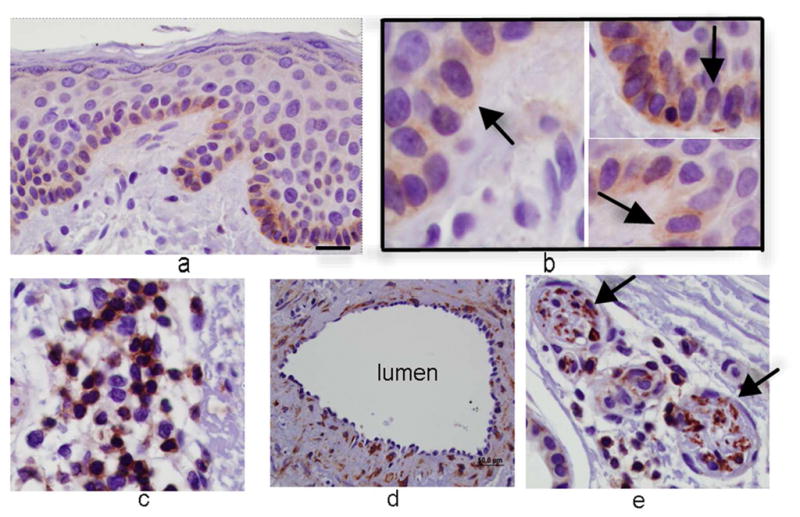
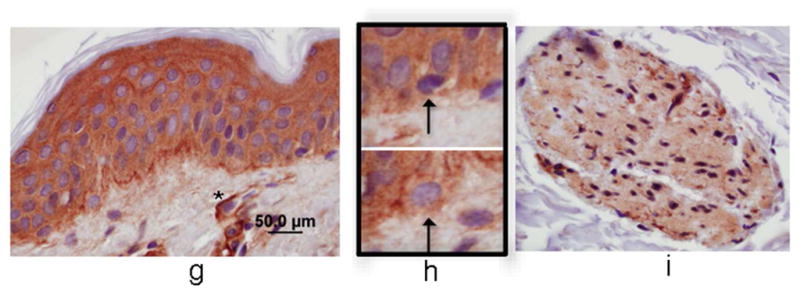
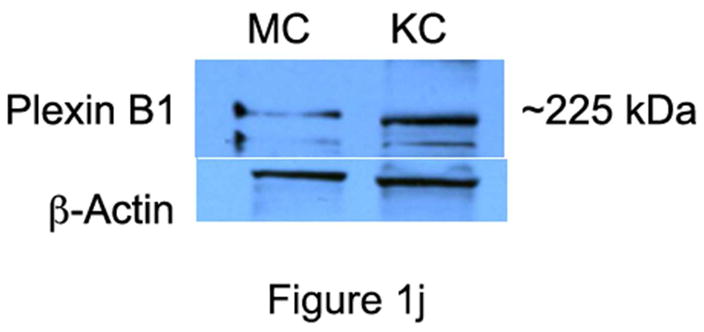
Basal keratinocytes strongly express Sema4D (A; bar=10μ). Melanocytes, characterized by peri-nuclear halos, also express Sema4D (arrows, inset; B). Lymphocytes (C), smooth muscle (D), and cutaneous nerves (E; arrows) express Sema4D. F) Total cell lysates of melanocytes (MC) and keratinocytes (KC) were blotted for Sema4D (80 μg loaded; 7.5% SDS-PAGE). A band of the expected size is detected in both cell types (representative of 4 experiments). Positive control (+) is a human squamous cell carcinoma cell line. The lower band (~190 kDa) likely represents the proteolytically cleaved portion of the protein. G) Plexin B1 is expressed throughout all layers of the epidermis (G; bar=50μ). Endothelial cells (asterisks) express Plexin B1, as do melanocytes (inset; H). Smooth muscle of pili-erector apparatus also express Plexin B1 (I). J) Total cell lysates of melanocytes (MC) and keratinocytes (KC) were resolved on 7.5% SDS-PAGE and blotted for Plexin B1 (40 μg loaded). A band of the expected size is detected in both cell types (representative of 4 experiments).
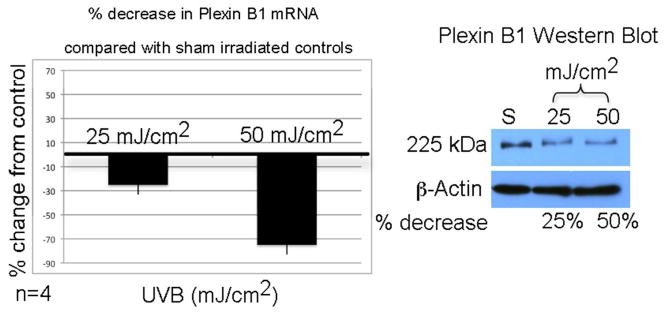
We next determined if UVB regulates Plexin B1 in melanocytes (Figure 2). Message levels of Plexin B1 were lowered by 25% and 50%, 18 hours after 25 mJ/cm2 and 50 mJ/cm2 UVB respectively, compared with sham irradiated controls. Twenty-four hours after irradiation, Plexin B1 at the protein level decreased 50% (25 mJ/cm2) and 75% (50 mJ/cm2) compared with sham irradiated controls. UVB had no effect on Plexin B1 expression in keratinocytes, or Sema4D expression in melanocytes (data not shown).
Figure 2. UVB down-regulates Plexin B1 in melanocytes.
Melanocytes (MC) were irradiated with UVB, or sham irradiated, and 18 hours later Plexin B1 was assessed by real-time PCR. Matched cultures were irradiated and Plexin B1 protein was measured 24 hours later by Western blotting. The bar graph shows the average percent decrease in Plexin B1 mRNA compared with sham irradiated cells, +/−SD (average of 4 cultures). The Western blot shows lysates from a representative culture resolved on 7.5% SDS-PAGE and blotted for Plexin B1. The numbers beneath the blot indicate the % decrease in Plexin B1 expression, normalized to β-actin.
Semaphorin 4D is a survival factor for melanocytes
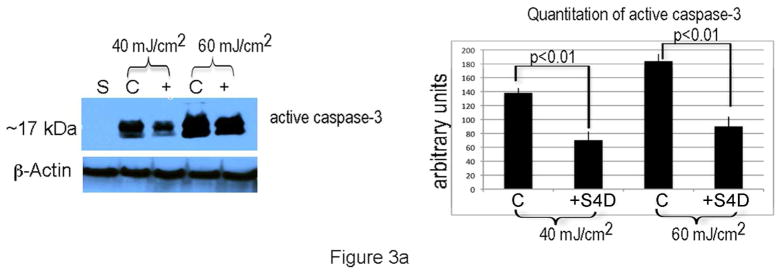
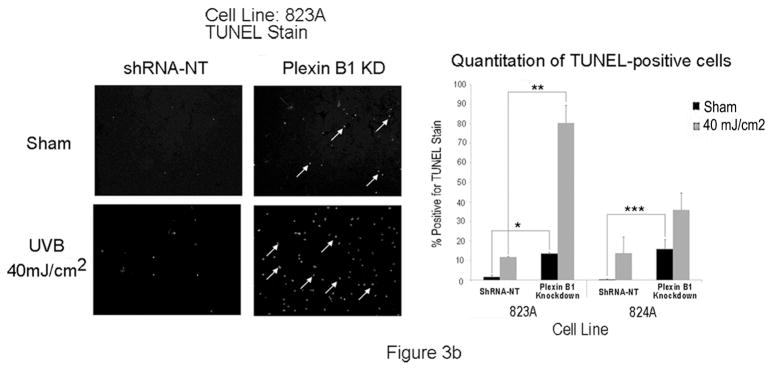
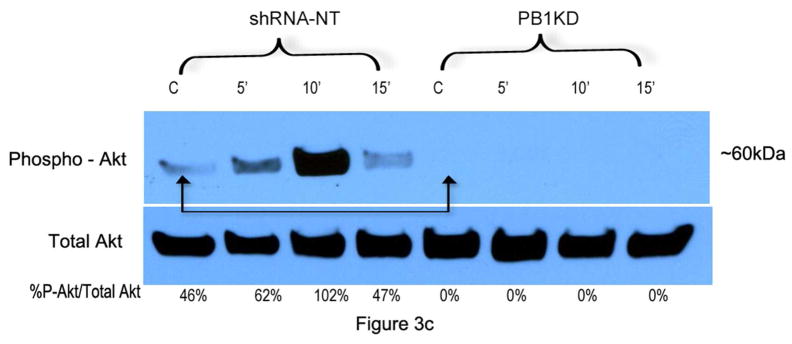
To determine if Sema4D protects melanocytes from UVB-induced apoptosis, melanocytes were placed in basal medium overnight, and were pre-treated with Sema4D (500 ng/ml) for 8 hours, then irradiated with UVB. Media was replaced with fresh Sema4D, and eighteen hours later, floating cells were collected and combined with adherent cells, and levels of cleaved (activated) caspase-3 were determined by Western blot (Figure 3A). Sema4D abrogated UV-dependent caspase-3 activation at both doses of UVB by > 30%. We knocked down Plexin B1 expression in melanocytes, and examined apoptosis by TUNEL assay in response to UVB. Plexin B1 knockdowns demonstrated significantly (p<0.01) more apoptosis in Sham irradiated cells compared with non-target controls, which was further increased by irradiation (Figure 3B), in the absence of exogenous Sema4D. Growth factor dependent activation of Akt suppresses apoptosis in melanocytes (Kadekaro et al., 2005; Larribere et al., 2004; Oka et al., 2004). Sema4D transiently induced Akt activation in serum-starved non-target controls, which peaked at 10 min. Knockdown of Plexin B1 completely blocked Sema4D-dependent Akt activation, indicating that Sema4D activates Akt through Plexin B1 (Figure 3C).
Figure 3. Sema4D protects melanocytes from apoptosis in response to UVB.
A) Sema4D (500 ng/ml) abrogated the effects of UVB-dependent caspase-3 activation. Shown are total cell lysates of melanocytes irradiated with UVB, and resolved on 10% SDS-PAGE and blotted for active caspase-3. Results are representative of 4 separate experiments. Densitometry analysis of averaged (n=4 experiments) caspase-3 levels normalized to β-actin, are shown.
B) Representative images of TUNEL staining of Plexin B1 knockdowns or controls (shRNA-NT) following UVR. Plexin B1 knockdowns show markedly higher levels of apoptosis, as shown by TUNEL positive nuclei (arrows), in the absence of ligand. Following UVB, a striking increase in TUNEL positive nuclei in Plexin B1 knockdowns was seen, compared with non-target controls. Quantitation of TUNEL positive nuclei from melanocyte cultures silenced for Plexin B1 (823A and 824A) show highly significant differences compared with shRNA-NT controls. Each column represents the average of 3 separate experiments +/−SD. *p=0.001; **p=0.0001; ***p=0.0013.
C) Treatment of melanocytes with Sema4D (500 ng/ml) stimulated Akt phosphorylation in control cells (shRNA-NT) but not in Plexin B1 knockdowns. Basal levels of phospho-Akt are lower in Plexin B1 silenced cells (arrows). Shown are total cellular lysates resolved on a 10% SDS-PAGE. Results are representative of 3 experiments.
Plexin B1 signaling promotes melanocyte proliferation
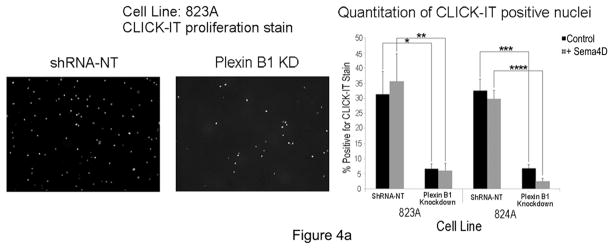
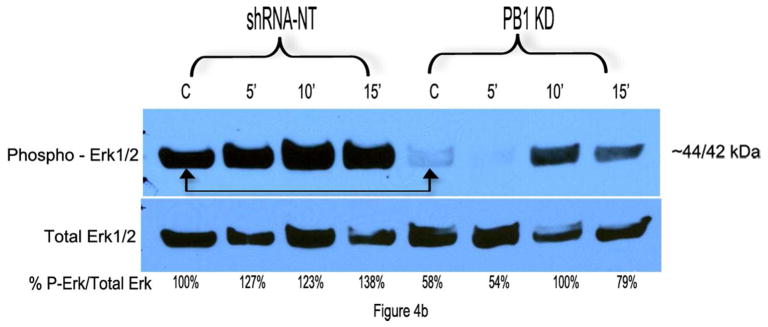
Plexin B1 knockdowns grew very slowly, suggesting that Plexin B1 stimulates melanocyte proliferation. To test this, Plexin B1 knockdowns, or non-target controls, were placed in basal media for 8 hours, and then treated with Sema4D (100 ng/ml) for 18 hours, and proliferation was assessed by CLlCK-IT assay (Figure 4A). Proliferation was significantly lower in Plexin B1 knockdowns compared with cells expressing non-target shRNA, even in the absence of Sema4D. The addition of Sema4D modestly increased proliferation in non-target controls, but this did not reach statistical significance, suggesting autocrine effects of Sema4D. Analysis of Erk1/Erk2 activation in response to Sema4D was performed in Plexin B1 knockdowns. Treatment of non-target controls with Sema4D activated Erk1/Erk2, which peaked at 10 min (Figure 4B). Plexin B1 knockdowns showed lower basal levels of activated Erk1/Erk2, consistent with their low proliferative rate under resting conditions, and delayed and lower levels of Erk1/Erk2 activation in response to Sema4D compared with non-target controls. Lack of complete blockade of Erk1/Erk2 activation in Plexin B1 knockdowns may be secondary to incomplete knockdown of the receptor (Supplemental Figure 1).
Figure 4. Plexin B1 stimulates proliferation of melanocytes.
A) Representative images of Plexin B1 knockdowns and control cells (shRNA-NT) from line 823A, stained to identify CLICK-IT uptake, and quantitation of CLICK-IT positive nuclei in both silenced cell lines (823A and 824A). Plexin B1 knockdowns showed a significant decrease in proliferation compared with controls. Each column represents the average of 3 separate experiments +/−SD. *p=0.005; **p=0.006; ***p=0.0004; ****p=0.0001.
B) Treatment of melanocytes with Sema4D (500 ng/ml) activates Erk1/Erk2, which peaks at 10′. Knockdown of Plexin B1 delays and lowers Erk1/Erk2 activation in response to Sema4D. Basal levels of phospho-Erk1/2 are lower in Plexin B1 silenced cells (arrows). Shown are total cell lysates resolved on 10% SDS-PAGE; results are representative of 3 experiments. Controls consisted of cells treated with Control Sema4D (“C”).
Sema4D blocks c-Met activation
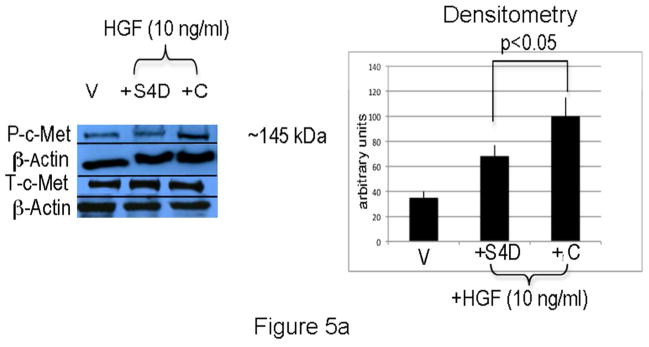
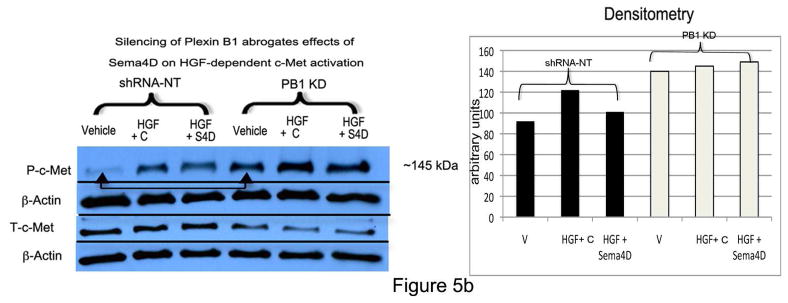
To determine if Sema4D blocks c-Met activation in melanocytes, cells were placed in basal medium overnight, pre-treated with Sema4D (100 ng/ml) for 30 min, and then treated with HGF (10 ng/ml for 15 min) in the presence of Sema4D or control Sema4D (Figure 5A). Cells treated with HGF in the presence of control Sema4D stimulated c-Met phosphorylation 2.5 fold. In contrast, c-Met phosphorylation was only 1.8-fold in cells treated with Sema4D and HGF, a difference which was significant (p<0.05). Treatment of melanocytes with Sema4D alone had no effect on c-Met activity (Supplemental Figure 1), similar to what we observed in melanoma (Stevens et al., 2010). Silencing of Plexin B1 blocked effects of Sema4D on HGF-dependent c-Met activation, and resulted in higher basal levels of activated c-Met (Figure 5B).
Figure 5. Sema4D inhibits HGF-dependent c-Met activation in melanocytes.
A) Melanocytes treated with HGF (10 ng/ml) for 15 minutes in the presence of Sema4D (“SD”;100 ng/ml) show less phosphorylated c-Met compared with cells treated with HGF and Control (“C”). Shown is a representative experiment in which total cell lysates were resolved on 10% SDS-PAGE. Densitometry levels of P-c-Met, normalized to β-actin, are shown. Each column represents the average of 4 separate experiments, +/−SD.
B) Control cells (shRNA-NT) or Plexin B1 knockdowns (PB1KD) were treated as described in “A”. Shown is a representative experiment (n=3) in which total cell lysates were resolved on 10% SDS-PAGE and blotted for P-c-Met. Densitometry analysis of P-c-Met, normalized to β-actin, is shown. Silencing of Plexin B1 blocked the suppressive effects of Sema4D on c-Met activation in response to HGF. In the absence of HGF (“Vehicle”, arrow), silencing of Plexin B1 led to constitutive activation of c-Met.
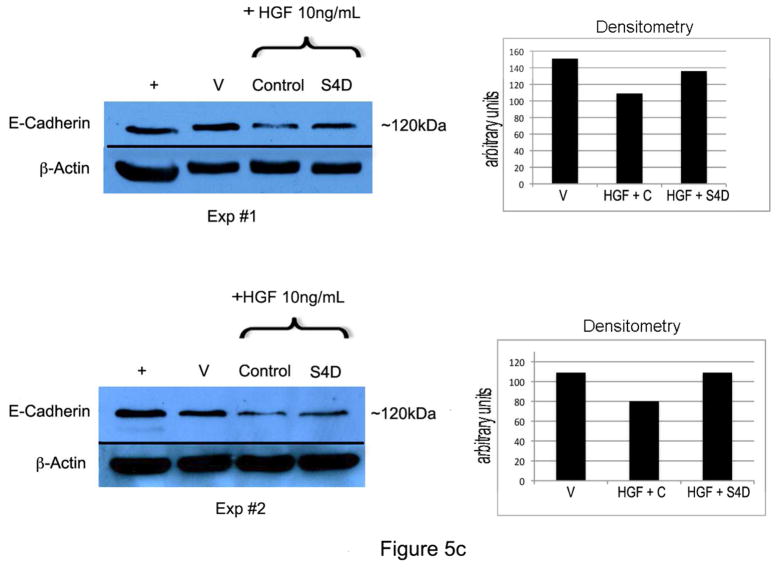
C) Melanocytes treated with HGF (10 ng/ml) for 24 hours show decreased E-cadherin expression compared with vehicle treated controls, which is partially rescued by Sema4D (100 ng/ml). Shown are 2 representative experiments of a total of 3, and densitometry in which E-cadherin is normalized to β-actin.
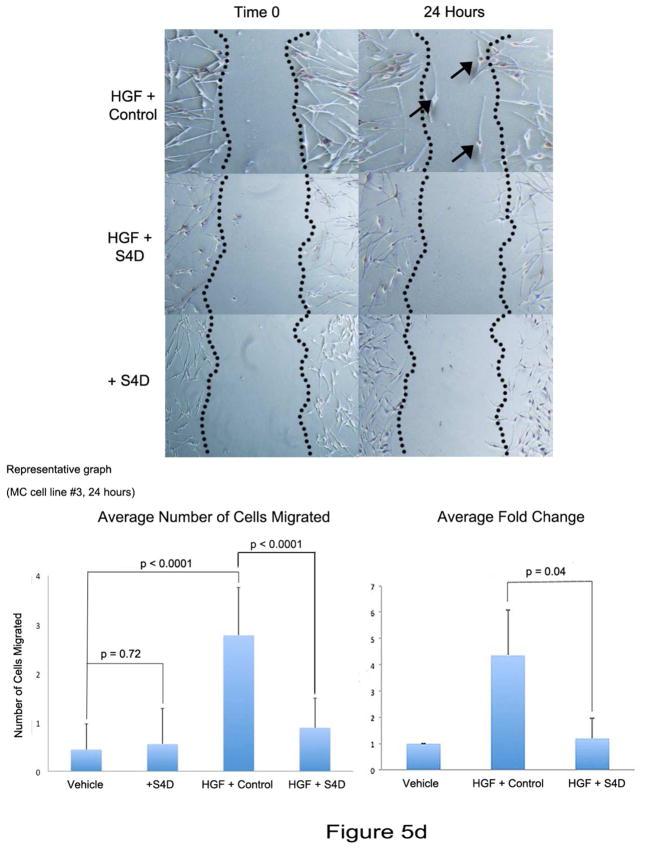
D) Representative images from melanocytes from a scratch assay of cells treated with vehicle, HGF (10 ng/ml) and Control, or HGF (10 ng/ml) and Sema4D (100 ng/ml). Cells treated with Sema4D alone are also shown (bottom). Melanocytes treated with HGF/Control show more cells migrating into the scratch area (arrows), compared with cells treated with HGF/Sema4D. Sema4D alone had no effect on migration. Each column represents the averaged number of cells migrated into the scratch, +/− SD. The graph to the right shows the average fold change in migration from 3 experiments, with vehicle treated cells set as “1” (+/−SD). Differences in fold change between HGF/C treated cells and HGF/Sema4D treated cells are significant (p=0.04).
HGF stimulates melanocyte migration, in part through down-regulation of E-cadherin expression (Li et al, 2001; Han et al., 2005). We next determined if Sema4D abrogates effects of HGF on E-cadherin expression and migration. A dose response for HGF-dependent suppression of E-cadherin showed that doses as low as 5 ng/ml completely suppressed E-cadherin expression in melanocytes (Supplemental Figure 2). We next treated melanocytes with HGF (10 ng/ml) in the presence of Sema4D (100 ng/ml) for 24 hours, and E-cadherin levels were analyzed (Figure 5C). Sema4D partially restored E-cadherin expression in melanocytes in response to HGF. We next determined if Sema4D blocks HGF-dependent migration of melanocytes (Figure 5D). Melanocytes were grown to 80% confluence in 6 well dishes, and migration was quantified by scratch assay at 24 hours. As expected, HGF (10 ng/ml) stimulated melanocyte migration with an average fold increase of 4.36 (+/− 1.72 SD) compared with vehicle treated cells. In contrast, in cells treated with HGF and Sema4D (100 ng/ml) migration was only 1.2-fold more (+/− 0.75 SD) compared with vehicle treated cells. The difference in fold change in migration between HGF/control and HGF/Sema4D treated cells was significant (p=0.04, n=3). Sema4D alone (100 ng/ml) had no effect on melanocyte migration.
Discussion
We were stimulated to do this study because Plexin B1 is a newly recognized tumor suppressor protein for melanoma (Argast et al., 2009; Stevens et al., 2010). Understanding the function of this receptor in melanocytes could provide insight into how loss of the receptor promotes melanoma initiation or progression. In this report we show that Plexin B1 expression is regulated by UVB in melanocytes, and that Plexin B1 signaling contributes to melanocyte growth and survival. Of particular interest was the observation that, similar to melanoma, Plexin B1 signaling suppresses c-Met activation and migration in response to HGF. Knockdown of Plexin B1 increased basal levels of c-Met activation, suggesting that chronic loss of Plexin B1 may promote HGF-dependent migration, and, potentially, early stages of melanoma progression, through enhanced c-Met activity.
Sema4D is expressed by keratinocytes in vivo, suggesting that Sema4D is a paracrine factor for melanocytes. Because Sema4D stimulates angiogenesis, T cell activation and B cell aggregation and viability, Sema4D may participate in new vessel formation or immune function in the skin, through effects on vascular endothelium and lymphocytes. Sema4D was also expressed, although at lower levels, by melanocytes in vivo and in vitro. In our previous report (Stevens et al., 2010) we did not identify Sema4D in melanocytes by Western blotting. In this report, we were able to uncover expression by loading a larger amount of protein (80–100 μg). Because of abundant expression of Sema4D by basal keratinocytes, it is likely that Sema4D is primarily a paracrine factor for melanocytes in vivo, although some autocrine effects may occur. Sema4D is released as an active product through the action of matrix metalloproteinases (Basile et al., 2007), therefore Sema4D from fibroblasts may function as a secreted paracrine factor for melanocytes, particularly following UVR, which activates matrix metalloproteinases (Brenneisen et al, 1996).
Sema4D protected melanocytes from UV-dependent apoptosis, as indicated by lower levels of cleaved caspase-3 in Sema4D treated cells, similar to effects of α-MSH on UV-dependent apoptosis in melanocytes (Bohm et al., 2005; Kadekaro et al., 2005). Akt is a major effector of growth factor dependent cell survival and was transiently activated by Sema4D in melanocytes. Whether transient activation of Akt by Sema4D plays a role in protecting melanocytes from UVB-dependent apoptosis remains to be determined, but in human keratinocytes, short term (1–2 hours), activation of Akt by insulin-like growth factor −1 delays the onset of UVB-dependent apoptosis at 48 hours (Decraene et al., 2002). A similar pathway may be involved in the protective effects of Sema4D on apoptosis in response to UVB in melanocytes. In melanoma, Plexin B1 signaling also activates Akt, and protects melanoma from apoptosis induced by cis-platin (Stevens et al., 2010). Therefore, Akt is a shared target of Plexin B1 in melanocytes and melanoma. Silencing of Plexin B1 profoundly inhibited proliferation in the absence of added Sema4D, suggesting potential autocrine effects, and treatment of melanocytes with Sema4D activated Erk1/Erk2 in a Plexin B1 dependent manner, consistent with a pro-proliferative effect of Plexin B1. In contrast, melanoma cell lines reconstituted with Plexin B1 proliferated slower than LacZ controls (Stevens et al., 2010). Therefore, fundamental differences in Plexin B1 signaling on downstream pathways that control proliferation exist in melanocytes and melanoma.
Our data show that Sema4D suppresses c-Met activation by HGF, and partially blocks HGF-dependent E-cadherin down-regulation and migration in melanocytes. Because Akt and Erk1/Erk2 are well known targets of c-Met, we tested the ability of Sema4D to suppress HGF-dependent Akt and Erk1/Erk2 phosphorylation, but did not observe detectable changes in activation of these targets (unpublished data). This may be because Plexin B1 and c-Met both activate Akt and Erk1/Erk2, masking suppressive effects of Sema4D on HGF-dependent phosphorylation of these targets. Silencing of Plexin B1 blocked the inhibitory effect of Sema4D on HGF-dependent c-Met phosphorylation, and increased basal levels of activated c-Met, indicating that Plexin B1 is necessary for regulation of c-Met activity. The observation that basal levels of active c-Met are higher in Plexin B1 knockdowns suggests that autocrine production of Sema4D regulates the activity of c-Met in normal melanocytes. Alternatively, chronic loss of Plexin B1 may lead to compensatory changes resulting in activation of c-Met, independent of ligand. The mechanism by which Plexin B1 inhibits c-Met activation in melanocytes remains to be defined. Several “co-receptors” for c-Met activation have been identified, including the adhesion molecules CD44v6 (Matzke et al., 2007) and ICAM-1 (Olaku et al., 2011), epidermal growth factor receptor (Bergstrom et al., 2000) and neuropilin-1/and neuropilin-2 (Sulpice et al., 2008), which potentiate the activation of c-Met. To our knowledge, Plexin B1 is the first receptor identified that stimulates and inhibits c-Met activation in a cell-type specific fashion. Because Plexin B1 shares significant homology with c-Met, it may compete with c-Met for binding to HGF, similar to neuropilin receptors (Sulpice et al., 2008). Alternatively, Plexin B1 may form a complex with c-Met, preventing tyrosine phosphorylation of residues necessary for docking of adaptor proteins necessary for c-Met downstream effects, such as migration. The mechanism by which Plexin B1 receptor signaling inhibits c-Met activation and cell migration will be the focus of a separate report.
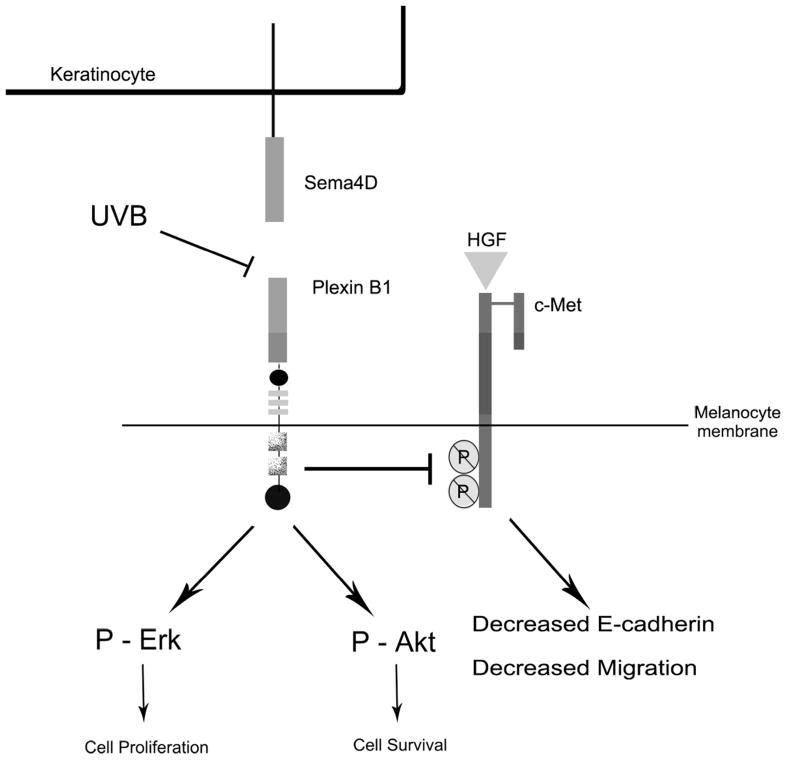
In our proposed model (Figure 6) we predict that Plexin B1 signaling in melanocytes is activated through Sema4D produced by basal keratinocytes and melanocytes, promoting survival and proliferation. Plexin B1 maintains a brake on c-Met signaling, and maintains levels of E-cadherin expression. Following irradiation, HGF production by keratinocytes and fibroblasts is increased (Brenner et al., 2005; Mildner et al., 2007) and Plexin B1 expression in melanocytes is suppressed, resulting in enhanced c-Met activity and loss of E-cadherin expression. Because loss of E-cadherin is an early event in transformation of melanocytes to melanoma, Plexin B1 could function as a tumor suppressor protein for early stages of melanoma by blocking effects of c-Met signaling on E-cadherin expression and melanocyte migration.
Figure 6. Proposed model of effects of Sema4D on human melanocytes.
The Plexin B1 receptor on the melanocyte membrane binds Sema4D, which is produced by basal keratinocytes. Plexin B1 signaling activates Akt and Erk1/Erk2, promoting melanocyte survival and proliferation, respectively. Exposure of melanocytes to UVB down-regulates Plexin B1 expression, releasing inhibitory signaling on the c-Met receptor, which is activated by HGF produced by keratinocytes and fibroblasts. This results in enhanced c-Met dependent loss of E-cadherin expression, promoting melanocyte migration and potentially, transformation to melanoma.
Material and Methods
Reagents
PureCol was from Inamed Biomaterials (Freemont, CA); the BCA Protein Assay kit was from Pierce Chemical (Rockford, IL). Rabbit polyclonal antibodies to β-actin were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA); rabbit polyclonal antibodies to Plexin B1 were purchased from ECM Biosciences (Versailles, KY). Mouse monoclonal antibodies to Sema4D were purchased from BD Transduction Laboratories (Sparks, MD). Mouse monoclonal antibodies to c-Met, rabbit monoclonal antibodies to phospho-Met (Y-1234/Y-1235), rabbit monoclonal antibodies to phospho-AKT and total AKT, rabbit monoclonal antibodies to phospho-Erk1/Erk2, mouse monoclonal antibodies to total Erk1/Erk2, and rabbit monoclonal antibodies to caspase-3 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against E-cadherin were purchased from R&D systems (Minneapolis, MN). Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse antibodies, and hepatocyte growth factor (HGF) were purchased from Sigma Co., (St Louis, MO). Full-range rainbow molecular weight marker was purchased from Amersham Life Sciences (Arlington Heights, IL). Polybrene was purchased from Santa Cruz Biotechnologies.
Cells and cell culture
Neonatal foreskins were obtained according to the University of Rochester Research Subjects Review Board guidelines and were the source of cultured human melanocytes and keratinocytes. Human melanocytes were cultured in Opti-MEM (Gibco-BRL) containing: 5% FBS (fetal bovine serum; Atlanta Biologicals; Lawrenceville, GA), 10−4 M iso-butyl-methylxanthine (IBMX), Anti-Anti (Gibco-BRL), 2.5 nM Cholera Toxin, 0.1 mM dbcAMP, 25 ng/ml phorbol ester. Keratinocytes were cultured in Keratinocyte Serum Free Media (Gibco-BRL). All supplements, except FBS and Anti-Anti were purchased from Sigma Co. With the exception of scratch assays and experiments in which Plexin B1 silenced cells were used, pooled cultures of melanocytes initiated from 2–3 foreskins were used at passage 4–8. Each experiment was repeated at least 3 times with separate pooled cultures of cells.
Silencing of Plexin B1 in human melanocytes
Melanocytes from individual foreskin cultures (designated “823A”, “824A”, “1230B”) were plated in MGM at 105 cells in a 6 well plate. Cells were infected at 2.5 Multiplicity of Infection (MOI) with MISSION Sigma Lentivirus particles expressing shRNA targeting 2 loci of human Plexin B1 (Clones TRCN0000061535 and TRCN0000061536). Cells infected with non-target shRNA (shRNA-NT) in Lentivirus were used as controls. Silenced cells were selected with puromycin dihydrochloride (Sigma Co., St Louis, MO) at 2.5 μg/ml. Silencing was confirmed by RT-PCR and Western blotting (Supplemental Figure 3).
Ultraviolet Irradiation
Cells were maintained in complete media and irradiation was carried out in sterile phosphate buffered saline (PBS) using a bank of 6 FS20 sun lamps (Westinghouse) that have more than 75% emission in the UVR range (280–320 nm), with a peak emission of 313 nm, and less than 25% UVA rays (>320 nm). Schott WGII filter was used to remove UVC rays. Irradiation times (in seconds) were calculated by multiplying the output of the FS20 lamps (Watts/cm2/10) determined using a IL1700 meter (Newberry Port, MA), by the desired dose (mJ/cm2). Total irradiation times ranged from 120 seconds (40 mJ/cm2) to 150 seconds (60 mJ/cm2).
Western Blotting
Cells were lysed in RIPA buffer (150 mM NaCl, 1%NP-40, 0.5% DOC, 0.1% SDS, 50 mM Tris-HCl) with protease inhibitors (Boehringer Mannheim, Gmbt, Germany) and protein was quantitated using bovine serum albumen (BSA) as a standard (Bio-Rad Laboratories, Hercules, CA). Protein was resolved on SDS-PAGE gels and blotted using standard procedures. Visualization of the immuno-reactive proteins was accomplished with an enhanced chemiluminescence reaction (Pierce Chemical, Rockford, IL).
Immunohistochemical Staining
Staining for Sema4D and Plexin B1 were carried out on sections of normal human adult skin from sun-exposed areas, which were obtained according to the University of Rochester Research Subjects Review Board guidelines. Staining for Plexin B1 was performed as previously described (Stevens et al., 2010). For Sema4D, an identical protocol was followed except that slides were incubated in a different antigen retrieval buffer (sodium citrate pH 6.0) and slides were incubated with anti-Sema4D mouse monoclonal antibodies (1/100). Negative controls consisted of mouse IgG or rabbit IgG (Sigma Co.) instead of the primary antibody.
CLICK-IT and TUNEL assays
Staining was performed on melanocytes cultured on PurCol coated coverslips. Analysis of proliferation was performed using CLICK-IT assay (Invitrogen, Carlsbad, CA). TUNEL uptake was detected using the DeadEnd Flurometric TUNEL System (Promega, Madison, WI). CLICK-IT or TUNEL-positive nuclei were visualized using a filter with excitation wavelength of 495 nm. Positive nuclei were counted in a minimum of 200 cells, and percent positive nuclei determined by dividing by total nuclei, identified by DAPI counter-staining and viewed with a filter with excitation wavelength of 341 nm.
Scratch Assay
Melanocytes were plated in full media at 3 × 105 cells in a 6 well dish and allowed to grow to near confluence (~80%). Three scratches were made on the bottom of each well using a sterile 200 microliter pipette tip. Wells were washed gently with PBS and replaced with media containing mitomycin C to prevent proliferation (5 μg/ml; Fisher Scientific, Pittsburg, PA) and either HGF (10 ng/ml) with Control, or HGF (10 ng/ml) with Sema4D (100 ng/ml). Controls consisted of cells treated with vehicle alone and mitomycin C. Digital images from an inverted phase contrast microscope were taken at time 0 and 24 hr later. Quantitation of migrated cells was performed by counting the number of cells (defined as cells with nuclei) that migrated from the edges of the scratches using ImageJ software, NIH. Three fields from each scratch (total of 9 fields per condition) was analyzed, and the numbers were averaged.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) and comparative real time PCR
Total RNA was isolated using the RNeasy Mini Kit (QIAgen, Valencia, CA) according to manufacturer’s instructions and reverse transcription and real-time PCR for Plexin B1 was performed as previously described (Stevens et al., 2010).
Recombinant Sema4D-Fc
Recombinant human Sema4D was expressed as an Fc-tagged protein in pEFBos vector (kindly provided by Hitoshi Kikutani, Osaka University, Osaka, Japan) in 293FT cells (Invitrogen; Carlsbad, CA). Sema4D was purified from culture supernatants as previously described (McClelland et al., 2010). Protein purity, quantity, and identity assessed by silver staining of gels and Western blotting (Supplemental Figure 4). Controls consisted of culture supernatants of 293FT cells that were treated identically as culture supernatants of transfected cells (“control”). Specifically, culture supernatants of un-transfected 293FT cells were collected, floating cells removed by centrifugation, and supernatant was incubated with protein-A conjugated sepharose beads. Following elution from the beads, the sample was assessed by silver staining of gels and Western blotting (Supplemental Figure 4).
Statistical Analysis
Differences between means were analyzed by two-tailed Student’s t-test. A p value <0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by R01CA136499 (GS) and NIH training grant 5T32AR007472
Abbreviations
- Sema4D
Semaphorin 4D
- SD
standard deviation
- HGF
hepatocyte growth factor
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Abdel-Malek Z, Suzuki I, Tada A, Im S, Akcali C. The melanocortin-1 receptor and human pigmentation. Annals of the New York Academy of Sciences. 1999;885:117–33. doi: 10.1111/j.1749-6632.1999.tb08669.x. [DOI] [PubMed] [Google Scholar]
- Argast GM, Croy CH, Couts KL, Zhang Z, Litman E, Chan DC, Ahn NG. Plexin B1 is repressed by oncogenic B-Raf signaling and functions as a tumor suppressor in melanoma cells. Oncogene. 2009;28:2697–709. doi: 10.1038/onc.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigiani S, Barberis D, Fazzari P, Longati P, Angelini P, van de Loo JW, Comiglio PM, Tamagnone L. Functional regulation of semaphorin receptors by proprotein convertases. J Biol Chem. 2003;278:10094–10101. doi: 10.1074/jbc.M210156200. [DOI] [PubMed] [Google Scholar]
- Aurandt J, Li W, Guan KL. Semaphorin 4D activates the MAPK pathway downstream of plexin-B1. The Biochemical journal. 2006;394:459–64. doi: 10.1042/BJ20051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JR, Gavard J, Gutkind JS. Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. The Journal of biological chemistry. 2007;282:34888–95. doi: 10.1074/jbc.M705467200. [DOI] [PubMed] [Google Scholar]
- Bergstrom JD, Westermark B, Heldin NE. Epidermal growth factor receptor signaling activates met in human anaplastic thyroid carcinoma cells. Exp Cell Research. 2000;259(1):293–299. doi: 10.1006/excr.2000.4967. [DOI] [PubMed] [Google Scholar]
- Bhawan J, Oh CH, Lew R, Nehal KS, Labadie RR, Tsay A, Gilchrest B. Histopathologic differences in the photoaging process in facial versus arm skin. Am J Dermatopathol. 1992;14:224–30. doi: 10.1097/00000372-199206000-00008. [DOI] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. The Journal of biological chemistry. 2005;280:5795–802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Oh J, Wlaschek M, Wenk J, Briviba K, Hommel C, Herrmann G, Sies H, Scharffetter-Kochaneck K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochemistry and photobiology. 1996;64:877–85. doi: 10.1111/j.1751-1097.1996.tb01851.x. [DOI] [PubMed] [Google Scholar]
- Brenner M, Degitz K, Besch R, Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br J Dermatol. 2005;153:733–739. doi: 10.1111/j.1365-2133.2005.06780.x. [DOI] [PubMed] [Google Scholar]
- Decraene D, Agostinis P, Bouillon R, Degreef H, Garmyn M. Insulin-like growth factor-1-mediated AKT activation postpones the onset of ultraviolet B-induced apoptosis, providing more time for cyclobutane thymine dimer removal in primary human keratinocytes. J Biol Chem. 2002;277:32587–32595. doi: 10.1074/jbc.M111106200. [DOI] [PubMed] [Google Scholar]
- Cardinali G, Ceccarelli S, Kovacs D, Aspite N, Lotti LV, Torrisi MR. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. The Journal of investigative dermatology. 2005;125:1190–9. doi: 10.1111/j.0022-202X.2005.23929.x. [DOI] [PubMed] [Google Scholar]
- Castellani V, Rougon G. Control of semaphorin signaling. Curr Opin Neurobiol. 2002;12:532–41. doi: 10.1016/s0959-4388(02)00357-4. [DOI] [PubMed] [Google Scholar]
- Ch’ng ES, Kumanogoh A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol Cancer. 2010;9:251. doi: 10.1186/1476-4598-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–7. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- Danen E, de Vries T, Morandini R, Ghanem G, Ruiter D, van Muijen G. E-cadherin expression in human melanoma. Melanoma Research. 1996;6:127–31. doi: 10.1097/00008390-199604000-00007. [DOI] [PubMed] [Google Scholar]
- Davies G, Jiang WG, Mason MD. HGF/SF modifies the interaction between its receptor c-Met, and the E-cadherin/catenin complex in prostate cancer cells. Int J Mol Med. 2001;7:385–8. doi: 10.3892/ijmm.7.4.385. [DOI] [PubMed] [Google Scholar]
- Desiderio MA. Hepatocyte growth factor in invasive growth of carcinomas. Cell Mol Life Sci. 2007;64:1341–54. doi: 10.1007/s00018-007-7050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhabazi A, Marie-Cardine A, Chabbert-de Ponnat I, Bensussan A, Boumsell L. Structure and function of the immune semaphorin CD100/SEMA4D. Crit Rev Immunol. 2003;23(1):65–81. doi: 10.1615/critrevimmunol.v23.i12.40. [DOI] [PubMed] [Google Scholar]
- Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59:24–33. doi: 10.1002/neu.10337. [DOI] [PubMed] [Google Scholar]
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4(9):720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- Han SU, Lee HY, Lee JH, Kim WH, Nam H, Kim H, Cho YK, Kim MW, Lee KU. Modulation of E-cadherin by hepatocyte growth factor induces aggressiveness of gastric carcinoma. Annals of Surgery. 2005;242(5):676–683. doi: 10.1097/01.sla.0000186171.85804.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Research. 2004;17:96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer research. 2005;65:4292–9. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Larribere L, Khaled M, Tartare-Deckert S, Busca R, Luciano F, Bille K, Valony G, Eychene A, Auberger P, Ortonne JP, Ballotti R, Bertolotto C. PI3K mediates protection against TRAIL-induced apoptosis in primary human melanocytes. Cell Death Differ. 2004;11:1084–91. doi: 10.1038/sj.cdd.4401475. [DOI] [PubMed] [Google Scholar]
- Li G, Schaider H, Satyamoorthy K, Hanakawa Y, Hashimoto K, Herlyn M. Downregulation of E-cadherin and Desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene. 2001;20:8125–35. doi: 10.1038/sj.onc.1205034. [DOI] [PubMed] [Google Scholar]
- Matzke A, Sargsyan V, Holtmann B, Aramuni G, Asan E, Sendtner M, Pace G, Howells N, Zhang W, Ponta H, Orian-Rousseau V. Haploinsufficiency of c-Met in cd44−/− Mice Identifies a Collaboration of CD44 and c-Met In Vivo. Mol Cell Biol. 2007 Dec;27(24):8797–8806. doi: 10.1128/MCB.01355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland L, Chen Y, Soong J, Kuo I, Scott G. Plexin B1 inhibits integrin-dependent pp125FAK and Rho activity in melanoma. Pigment Cell Melanoma Res. 2011;24:165–74. doi: 10.1111/j.1755-148X.2010.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner M, Mlitz V, Gruber F, Wojta J, Tschachler E. Hepatocyte growth factor establishes autocrine and paracrine feedback loops for the protection of skin cells after UV irradiation. The Journal of investigative dermatology. 2007;127:2637–44. doi: 10.1038/sj.jid.5700938. [DOI] [PubMed] [Google Scholar]
- Negishi M, Oinuma I, Katoh H. R-ras as a key player for signaling pathway of plexins. Mol Neurobiol. 2005;32:217–22. doi: 10.1385/MN:32:3:217. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–5. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating beta(1) integrin activity. The Journal of cell biology. 2006;173:601–13. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Kageyama A, Fukunaga M, Bito T, Nagai H, Nishigori C. Phosphatidylinositol 3-kinase/Akt-dependent and -independent protection against apoptosis in normal human melanocytes. The Journal of investigative dermatology. 2004;123:930–6. doi: 10.1111/j.0022-202X.2004.23454.x. [DOI] [PubMed] [Google Scholar]
- Olaku V, Matzke A, Mitchell C, Hasenauer S, Sakkaravarthi A, Pace G, Ponta H, Orian-Rousseau V. C-met recruits ICAM-1 as a co-receptor to compensate for the loss of CD44 in Cd44–null mice. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-02-0134. (EPub ahead of print, June 16 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschel AW. The function of neuropilin/plexin complexes. Adv Exp Med Biol. 2002;515:71–80. doi: 10.1007/978-1-4615-0119-0_6. [DOI] [PubMed] [Google Scholar]
- Saito Y, Oinuma I, Fujimoto S, Negishi M. Plexin-B1 is a GTPase activating protein for M-Ras, remodelling dendrite morphology. EMBO Rep. 2009;10:614–21. doi: 10.1038/embor.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starner RJ, McClelland L, Abdel-Malek Z, Fricke A, Scott G. PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Exp Dermatol. 2010;19:682–4. doi: 10.1111/j.1600-0625.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, McClelland L, Fricke A, Williamson M, Kuo I, Scott G. Plexin B1 suppresses c-Met in melanoma: a role for plexin B1 as a tumor-suppressor protein through regulation of c-Met. The Journal of investigative dermatology. 2010;130:1636–45. doi: 10.1038/jid.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. The Journal of biological chemistry. 2008;283:1893–901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- Sulpice E, Plouet J, Berge M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood. 2008;111(4):2036–2045. doi: 10.1182/blood-2007-04-084269. [DOI] [PubMed] [Google Scholar]
- Tada A, Suzuki I, Im S, Davis MB, Cornelius J, Babcock G, Nordland JJ, Abdel-Malek ZA. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998;9:575–84. [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003;17:1177–9. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- Takegahara N, Kumanogoh A, Kikutani H. Semaphorins: a new class of immunoregulatory molecules. Philos Trans R Soc Lond B Biol Sci. 2005;360:1673–80. doi: 10.1098/rstb.2005.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000;10:377–83. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe D, Kong-Beltran M. Met activation and receptor dimerization in cancer: a role for the Sema domain. Cell Cycle. 2005;4:683–5. doi: 10.4161/cc.4.5.1688. [DOI] [PubMed] [Google Scholar]
- Wulf HC, Sandby-Moller J, Kobayasi T, Gniadecki R. Skin aging and natural photoprotection. Micron. 2004;35:185–91. doi: 10.1016/j.micron.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Yaar M, Grossman K, Eller M, Gilchrest BA. Evidence for nerve growth factor-mediated paracrine effects in human epidermis. The Journal of cell biology. 1991;115:821–8. doi: 10.1083/jcb.115.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1621–6. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.