Abstract
The objective of the present study was to determine the prevalence and variation of natural gastrointestinal nematode (GIN) infections in lambs according to birth type, gender and breed based on individual faecal egg counts (FEC) from various regions in Germany. A total of 3,924 lambs (3 to 15 months old) with different genetic backgrounds (Merinoland, German Blackhead Mutton, Rhoen, Texel and Merino long-wool) were individually sampled during the grazing period between 2006 and 2008. Furthermore, pooled faecal samples from each of the farms were cultured in order to differentiate the third-stage larvae of the nematode spp. Sixty-three percent of the lambs were infected with GIN. The infections were mostly low to moderate and involved several nematode species. The Trichostrongylus spp. was the predominant species based on the percentage of larvae in faecal cultures. Only 11.4% of the lambs were free of Eimeria oocysts. Tapeworm eggs were encountered in 13.2% of all samples. The prevalence of GIN infections varied significantly (P < 0.001) among farms. A significantly higher FEC (P < 0.05) was observed in multiple-born lambs when compared with singletons. Moreover, male lambs were more susceptible to infection than females (P < 0.001). No significant differences (P > 0.05) were observed between breeds regarding FEC. Inter-individual variations were higher than inter-breed differences, which may indicate the possibility of selection within these breeds for parasites resistance as described in earlier studies.
Introduction
Infections with gastrointestinal nematodes (GIN) can negatively affect the health and the overall productivity of infected animals (Levya et al. 1982; Holmes 1987; Suarez et al. 2009). Therefore, they can be a major cause of economic losses in small ruminant production (Coop et al. 1977). The common clinical signs of an infection with GIN are anorexia, diarrhoea, emaciation and anaemia (Steel et al. 1982; Behrens et al. 2001). The high level infections with nematodes may lead to the death of the infected animals. Under field conditions, most infections are usually mixed and include different species of nematodes. However, the impact of nematode infections on the animal is dependent on the intensity of infection as well as the physiological and immunological status of the host. Growing lambs and periparturient ewes are most susceptible to the infection by nematodes (Bishop and Stear 2001; Shubber et al. 1981). Today, the control of GIN in sheep has become less effective due to the development of anthelmintic resistance (Fleming et al. 2006). Moreover, there is a potent orientation towards organic farming, where the use of anthelmintics is limited Anonym (2010). Furthermore, the climate change will probably lead to changes in epidemiology and intensity of parasite infections (Hudson et al. 2006; van Dijk et al. 2010). Due to these facts, the economic impact of nematode infections will possibly increase in sheep farms in the future.
Most studies focussing on nematode infestations in Germany have reported a moderate to high prevalence of infection (51% and 100%) in sheep (Benesch 1993; Grzonka et al. 2000; Moritz 2005). The most common nematode genera infecting sheep in Germany are Haemonchus, Trichostrongylus, Telodorsagia, Nematodirus and Cooperia (Benesch 1993; Rehbein et al. 1996). It might be that some sheep breeds in Germany are more or less resistant to nematode infections than others. Gauly et al. (2002) reported that Merinoland had a lower FEC compared with Rhoen sheep following an experimental infection with H. contortus. However, differences between sheep breeds under conditions of natural infection in Germany have not yet been shown.
Therefore, the objective of the present study was to determine the prevalence of GIN in naturally infected lambs of five German sheep breeds based on individual faecal egg counts and to evaluate the predictable influence of birth type, gender, and breed on faecal nematode egg output.
Materials and methods
Animals and study areas
A total of 3,924 lambs of different breeds aged from 3 to 15 months were used in the study. Breeds used were Merinoland (ML), German Blackhead Mutton (GBM), Texel (TX), Rhoen (RH) and Merino long-wool (MLW) (Table 1). Data collection took place on various farms. Those were located in different federal states of Germany (Lower Saxony, Saxony-Anhalt, Thuringia, Baden-Wuerttemberg, Brandenburg and Hesse). The samples were collected once during the grazing seasons in 2006, 2007 and 2008. The farms were visited either once (ten farms), twice (nine farms) or three times (two farms) during the study period. Four farms kept more than one sheep breed. For statistical analysis, grazing season was divided into two periods (summer: June to August; autumn: September to December). The lambs were not dewormed at least 45 days before the sampling time.
Table 1.
Total number of lambs and farms used in the study over 3 years and their breed
| a | 2006 | 2007 | 2008 | Total no. of lambs | |
|---|---|---|---|---|---|
| ML | No. of farms | 2 | 6 | 6 | 1455 |
| No. of lambs | 198 | 625 | 632 | ||
| GBM | No. of farms | 3 | 4 | 6 | 851 |
| No. of lambs | 63 | 193 | 595 | ||
| TX | No. of farms | 1 | 4 | 2 | 377 |
| No. of lambs | 16 | 208 | 153 | ||
| RH | No. of farms | 1 | 3 | 4 | 557 |
| No. of lambs | 71 | 157 | 329 | ||
| MLW | No. of farms | 0 | 2 | 1 | 684 |
| No. of lambs | 0 | 287 | 397 |
ML Merinoland, GBM German Blackhead Mutton, TX Texel, RH Rhoen, MLW Merino long-wool
aThe farms were visited either once (ten farms), twice (nine farms) or three times (two farms) during the study period. Four farms kept more than one sheep breed
Parasitological measurements
Fresh faecal samples were taken once directly from the rectum of the individual lambs. Eggs per gram of faeces (FEC) were determined using a modified McMaster method (Maff 1986) to quantify FEC with saturated NaCl as the flotation fluid (specific gravity of 1.2 kg/m3). The eggs were counted with a sensitivity of 50 eggs per gram of faeces. Intensity of coccidia infection was semi-quantitatively scored via a four-score scaling system. The scaling evaluated samples as class 1 (coccidian-free), class 2 (<1,800 oocysts per gram (OPG)), class 3 (1,800 to 6,000 OPG) and class 4 (>6,000 OPG). For tapeworm infections, lambs were differentiated as non-infected and infected.
For the identification of nematode spp., 25 to 50 g of pooled faeces from each breed/farm were cultured for third-stage larvae (L3). For each pooled sample, 100 of these L3 were enumerated.
Data analyses
Individual FEC were loge (n + 10) transformed to produce approximately normally distributed data. All the statistical analyses were performed with SAS (9.1). The prevalence rates for the eggs of endoparasites in faecal samples and the confidence intervals were calculated with the FREQ procedure. Pearson's correlation coefficients between FEC and OPG as well as between FEC and age of lambs were determined using the CORR procedure.
Differences in FECs were analysed using the GLIMMIX procedure by using the following model:
Where Y ijklmnop = observed value; μ = overall mean; G i = fixed effect of breed (i = ML, GBM, TX, RH, MLW); G(F)j = fixed effect of breed nested within farms; S k = fixed effect of sex (k = male, female); B l = fixed effect of birth type (l = singleton, multiple); SE(AN)m = fixed effect of season nested within years; A n = age of lambs as covariate; FA(B)o = sire nested within breeds as random; e ijklmnop = random error.
ML lambs of one farm were not infected with GIN, and therefore, the data of this farm were not used for comparison of breeds, sex and birth type. Likewise, 600 lambs were excluded from the analyses due to missing values of fixed effects.
Results and discussion
Faecal egg counts
Table 2 summarises the results of faecal examinations. The prevalence of GIN infection is relatively high, where 62.8% of lambs were infected with at least one GIN species. However, the variations among farms were high and ranged from 0% to 100%. The infection level of GIN based on FEC was various and ranged from 50 to 17,000 with a mean value of 315.3 (±776.8) eggs per gram of faeces. Eleven samples had more than 5,000 eggs. Only 11.4% of the samples were free of Eimeria oocysts. Tapeworm eggs (Moniezia spp.) were encountered in 13.2% of the samples.
Table 2.
The prevalence of internal parasite eggs in faecal samples from lambs, as well as the 95% confidence interval of prevalence estimates (CI), the mean, standard deviation (SD) and the maximum value
| Prevalence (% positive) | CI | Mean | SD | Max. value | |
|---|---|---|---|---|---|
| FEC | 62.8 | 61.3–64.4 | 315.3 | 776.8 | 17,000 |
| Nematodirus spp. | 13.0 | 11.9–14 | 15.52 | 63.67 | 1,374 |
| Trichuris spp. | 3.4 | 2.9–4.1 | 2.99 | 19.78 | 432 |
| Strongyloides papillosus | 0.7 | 0.5–1 | 0.79 | 11.93 | 400 |
| OPG | 88.6 | 87.6–89.6 | – | – | – |
| Tapeworm eggs | 13.2 | 12.2–14.3 | – | – | – |
Earlier studies which were performed in Germany reported prevalences of GIN in sheep to be greater than 50% (Benesch 1993; Grzonka et al. 2000; Moritz 2005). Similar findings were obtained in the present study. Schwenk (1998) found Eimeria prevalence of 70% and for Moniezia spp., 6%. In other studies, all animals older than 10 weeks were infected with Eimeria spp. (Barutzki 1990); likewise, high infections with Moniezia spp. (57%) were reported by Graenzer et al. (1979). Between- and within-study variations in the infection rate of parasites under natural field conditions are not unexpected. These may be due to an inequality of the contamination of pasture with infective larvae, seasonal climatic fluctuations (Suttle 1994; Torina et al. 2004; Al-shaibani et al. 2008), feeding quality (Coop and Holmes 1996), husbandry systems (Wassmuth et al. 2001; Gauly et al. 2004) and genetic backgrounds of animals (Gray 1995; Gauly and Erhardt 2001; Reeg et al. 2005).
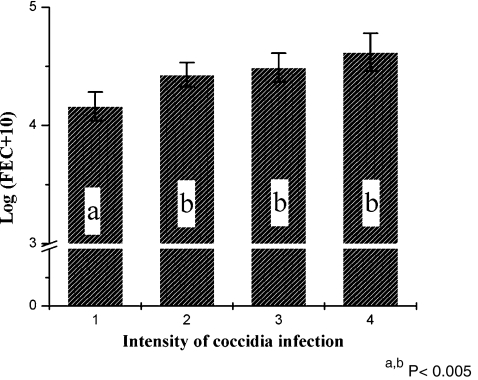
The samples of Eimeria oocyst-free lambs had less nematode eggs when compared with those of infected lambs (Fig. 1). No significant differences could be observed between the FEC values in the other classes of Eimeria-infection. However, FEC seemed to increase partially with increasing Eimeria infection. The phenotypic correlation between Eimeria oocysts and FEC values was 0.05 (P = 0.001). But the semi-quantitative estimation of coccidial infection limits the informational value. Kanyari (1988) reported a negative relation between the coccidial and helminth infection in goat. The author suggested that this negative relationship is a logical result of deworming against GIN and no treatment against coccidia. However, a positive correlation was estimated in sheep and goats in Kenya (Kanyari 1993).
Fig. 1.
Least-squares means and standard error of transformed FEC of lambs according to intensity of their coccidia infection
Larval cultures
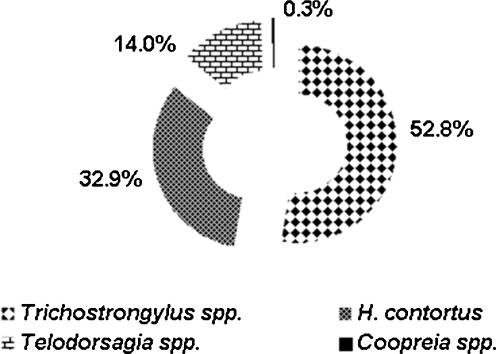
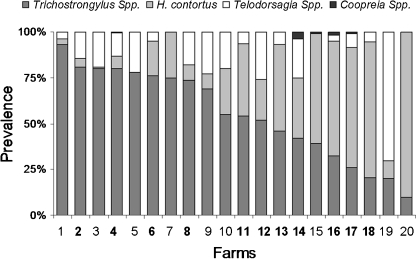
Depending on the results of larval cultures, Trichostrongylus spp. were the predominant nematode species with 52.8% of all the larvae (Fig. 2), whereas 32.9% of third-stage larvae were H. contortus, 14% Teladorsagia spp. and only 0.3% Cooperia spp. The distribution of nematode species by farm is given in Fig. 3. Larvae of Trichostrongylus spp. were found in all farms, with at least with 10% of the larvae. H. contortus was predominant in six farms, and only one farm was free of H. contortus. In three farms, Teladorsagia spp. was not detected while they were predominant in one farm. Cooperia spp. larvae were found in five farms. The larval culture method is used to determine the genera of worms and their proportion. However, the results of larval cultures may not describe the proportion of worms exactly (Amarante 2000). This may be due to the different fecundities between nematode species (Coyne et al. 1991), the pooled faecal samples (Amarante 2000) and the differential survival rates of larvae in faecal culture for species (Dobson et al. 1992).
Fig. 2.
Contribution of the nematode genera Trichostrongylus spp., Teladorsagia spp., H. contortus and Cooperia spp. according to the L3 differentiation
Fig. 3.
Prevalence of various genera of gastrointestinal nematode in lambs depending on the results of larval cultures by farms. Bold numbers indicate the farms were visited more than once during the study period. Lambs of one farm were not infected, and therefore this farm was not presented in this figure
Effect of sex, type of birth and age of lambs
Female lambs were less susceptible to infections with GIN (P < 0.001) compared with male lambs (Table 3). Many studies reported that male lambs are more susceptible to natural or experimental nematode infections around or after age of puberty when compared with females. Courtney et al. (1985) and Barger (1993) reported that host sex may have no consistent effect on susceptibility to infection in pre-pubertal lambs. Differences between females and males in susceptibility to parasite infection are probably caused by a difference in behaviour, morphology or physiological status of sex (Zuk and McKean 1996). Gauly et al. (2006) suggested that the different hormonal status of sexes may affect the immunological responses of lambs to H. contortus.
Table 3.
Least squares means and standard error (SE) of transformed FEC of lambs considering sex and birth type
| Log (FEC + 10) | SE | |
|---|---|---|
| Sex | ||
| Female | 4.15* | 0.10 |
| Male | 4.66* | 0.12 |
| Type of birth | ||
| Single | 4.35** | 0.11 |
| Multiple | 4.46** | 0.10 |
*p < 0.001; **p < 0.05
FEC values were affected by birth type of lambs (Table 3). Multiple-born lambs had higher FEC when compared with singleton-born lambs (P < 0.05). Similar findings were reported by Romjali et al. (1997) and Haile et al. (2007). These authors explained the differences by better rearing and nutrition conditions in the singletons in comparison to the multiples. However, Gauly and Erhardt (2001) as well as McManus et al. (2009) did not find a significant effect of birth type on FEC following natural infections.
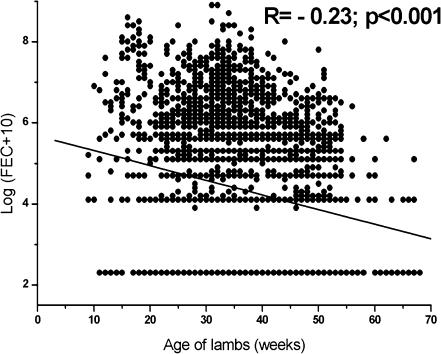
The faecal egg counts were negatively correlated with age of lambs (−0.23; P < 0.001) as indicated in Fig. 4. Development of acquired immunity to nematode infection is positively related with age of animals (Kambara et al. 1993; Kambara and McFarlane 1996). Furthermore, weaning, live weight, fat reserves or nutritional factors that are associated with age might also contribute to the positive relation between age and parasite resistance (McClure 2000).
Fig. 4.
The relationship of transformed fecal egg counts with age of lambs
Between- and within-breed variations
The MLW lambs were not compared with any other breeds because of the small number of farms included in the study and the time of sample collection month (December 2007 and 2008 only). No significant differences (P = 0.42) were observed between the four breeds ML, GBM, TX and RH (Table 4), where the LS-means of log (FEC + 10) ranged from 4.40 (±0.18) to 4.92 (±0.25). Very little information has been published about the variation of nematode resistance between sheep breeds in Germany. Gauly et al. (2002) reported that Merinoland had a lower FEC compared with Rhoen sheep following an experimental infection with H. contortus. However, worm burden, hematocrit and IgG antibody of the breeds did not differ significantly. In the present study, no differences between these two breeds in FEC appeared. A possible reason for that could be the differences in the infection (artificial, natural), such as the availability and sources of infectious larvae. Differences in the responses of lambs to mono- or mixed-infection may also be a determinant.
Table 4.
Least squares means, standard error (SE) and variation coefficient of FEC of lambs by breed
| Log (FEC + 10) | SE | CV% | |
|---|---|---|---|
| ML | 4.50 | 0.19 | 45.22 (225.28) |
| GBM | 4.40 | 0.18 | 39.97 (288.09) |
| TX | 4.92 | 0.25 | 34.34 (167.56) |
| RH | 4.48 | 0.32 | 33.00 (131.89) |
| MLW* | 3.77 | 0.16 | 37.77 (164.32) |
*The MLW lambs were not compared with any other breeds because of the small number of farms included in the study and the time of sample collection month; numbers within brackets represent the variation coefficient of untransformed FEC
ML Merinoland, GBM German Blackhead Mutton, TX Texel, RH Rhoen, MLW Merino long-wool
In Ireland, it has been reported that Texel sheep are less susceptible to natural nematode challenge when compared with Suffolk sheep (Good et al. 2006). In the present study, Texel lambs had in tendency greater FEC compared with the other breeds (P > 0.05). Rhoen sheep are a local breed, whereas Merinoland and German Blackhead Mutton resulted from crossing native sheep breeds with Merinos and British meat breeds, respectively. Furthermore, the ML and GBM breeds are known in Germany since the eighteenth and nineteenth century, while the Texel sheep were imported to Germany in the 1960s (Roesicke et al. 2007). Accordingly, this may have led to a natural selection and adaptation of RH, ML and GBM breeds.
High variation coefficients in FECs within breed were found (132–288%). The transformation of FEC reduced the variations (33–45%) but nevertheless remained high. The high variation in FEC within breeds has been early reported in different breeds and regions (Bisset et al. 1996; Bouix et al. 1998; Gauly and Erhardt 2001). Animals under natural conditions are unevenly exposed to nematode infection due to the differences in pasture contamination with infectious larvae. The animals in this study were kept in various farms with different feeding quality and husbandry systems. These factors could promote this variation in infection within breeds (Coop and Holmes 1996, Wassmuth et al. 2001). Even so, the obtained variation of FEC in this study might indicate partly the differences in host animal resistance against gastrointestinal nematode infections, which can be explained by the variety in the genetic basis of animals. Therefore, selection for resistance to nematode infections in German sheep under natural conditions might be possible.
Conclusion
The results showed that the natural infections with gastrointestinal nematodes in lambs in Germany are common, vary between farms, and are influenced by age, birth type and gender of lambs. A high prevalence of Eimeria infection was detected, and coccidia-free lambs appear to be less susceptible to nematode infections. The infection level of GIN based on FEC was low to moderate and involved multi-species infections. Trichostrongylus spp., H. contortus and Teladorsagia spp. were the predominant species. Inter-individual variations in susceptibility to natural nematode infections were found to be more important than inter-breed differences.
Acknowledgements
The authors would like to thank all the sheep farmers who helped us to make this study possible. Special thanks go to Mr. Toenges and Mr. Salzmann for their invaluable assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- FEC
Faecal egg counts
- GBM
German Blackhead Mutton
- GIN
Gastrointestinal nematode
- LS-means
Least square means
- ML
Merinoland
- MLW
Merino long-wool
- OPG
Oocysts per gram of faeces
- RH
Rhoen
- TX
Texel
References
- Al-shaibani IRM, Phulan MS, Arijo A, Qureshi TA. Contamination of infective larvae of gastrointestinal nematodes of sheep on communal pasture. Int J Agri Biol. 2008;10:653–657. [Google Scholar]
- Amarante A. Relationship between faecal egg counts and total worm counts in sheep infected with gastrointestinal nematodes. Brazil J Vet Parasitol. 2000;9:45–50. [Google Scholar]
- Anonym (2010) Verordnung (EG) Nr. 889/2008 der Kommission vom 5. September 2008 mit Durchführungsvorschriften zur Verordnung (EG) Nr. 834/2007 des Rates über die ökologische/ biologische Produktion und die Kennzeichnung von ökologischen/biologischen Erzeugnissen hinsichtlich der ökologischen/ biologischen Produktion, Kennzeichnung und Kontrolle ABl. L 250 (18.09.2008)
- Barger IA. Influence of sex and reproductive status on susceptibility of ruminants to nematode parasitism. Int J Parasitol. 1993;23:463–469. doi: 10.1016/0020-7519(93)90034-V. [DOI] [PubMed] [Google Scholar]
- Barutzki D. Parasitoses of sheep and goats in Germany. Tieraerztl Praxis. 1990;18:245–250. [PubMed] [Google Scholar]
- Behrens H, Ganter M, Hiepe T. Lehrbuch der Schafkrankheiten. 4. Berlin: Parey Verlag; 2001. [Google Scholar]
- Benesch C (1993) Parasiten des Magen-Darm-Traktes von Schafen in Hessen: eine Sektionsstudie. Dissertation, University of Giessen, Germany
- Bishop SC, Stear MJ. Inheritance of faecal egg counts during early lactation in Scottish Blackface ewes facing mixed, natural nematode infections. Anim Sci. 2001;73:389–395. [Google Scholar]
- Bisset SA, Morris CA, Squire DR, Hickey SM. Genetics of resilience to nematode parasites in young Romney sheep—use of weight gain under challenge to assess individual anthelmintic treatment requirements. NZ J Agr Res. 1996;39:313–323. doi: 10.1080/00288233.1996.9513191. [DOI] [Google Scholar]
- Bouix J, Krupinski J, Rzepecki R, et al. Genetic resistance to gastrointestinal nematode parasites in Polish long-wool sheep. Int J Parasitol. 1998;28:1797–1804. doi: 10.1016/S0020-7519(98)00147-7. [DOI] [PubMed] [Google Scholar]
- Coop RL, Holmes PH. Nutrition and parasite interaction. Int J Parasitol. 1996;26:951–962. doi: 10.1016/S0020-7519(96)80070-1. [DOI] [PubMed] [Google Scholar]
- Coop RL, Sykes AR, Angus KW. The effect of a daily intake of Ostertagia circumcincta larvae on body weight, food intake and concentration of serum constituents in sheep. Res Vet Sci. 1977;23:76–83. [PubMed] [Google Scholar]
- Courtney CH, Parker CF, Mcclure KE, Herd RP. Resistance of exotic and domestic lambs to experimental infection with Haemonchus contortus. Int J Parasitol. 1985;15:101–109. doi: 10.1016/0020-7519(85)90107-9. [DOI] [PubMed] [Google Scholar]
- Coyne MJ, Smith G, Johnstone C. Fecundity of gastro-intestinal trichostrongylid nematodes of sheep in the field. Am J Vet Res. 1991;52:1182–1188. [PubMed] [Google Scholar]
- Dobson RJ, Barnes EH, Birclijin SD, Gill JH. The survival of Ostertagia circumcincta and Trichostrongylus colubriformis in faecal culture as a source of basis in apportioning egg counts to worm species. Int J Parasitol. 1992;22:1005–1008. doi: 10.1016/0020-7519(92)90060-X. [DOI] [PubMed] [Google Scholar]
- Fleming SA, Craig T, Kaplan RM, Miller JE, Navarre C, Rings M. Anthelmintic resistance of gastrointestinal parasites in small ruminants. J Vet Intern Med. 2006;20:435–444. doi: 10.1111/j.1939-1676.2006.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gauly M, Erhardt G. Genetic resistance to gastrointestinal nematode parasites in Rhon sheep following natural infection. Vet Parasitol. 2001;102:253–259. doi: 10.1016/S0304-4017(01)00530-1. [DOI] [PubMed] [Google Scholar]
- Gauly M, Kraus M, Vervelde L, van Leeuwen MAW, Erhardt G. Estimating genetic differences in natural resistance in Rhoen and Merinoland sheep following experimental Haemonchus contortus infection. Vet Parasitol. 2002;106:55–67. doi: 10.1016/S0304-4017(02)00028-6. [DOI] [PubMed] [Google Scholar]
- Gauly M, Reeg J, Bauer C, Erhardt G. Influence of production systems in lambs on the Eimeria oocyst output and weight gain. Small Ruminant Res. 2004;55:159–167. doi: 10.1016/j.smallrumres.2004.02.001. [DOI] [Google Scholar]
- Gauly M, Schackert M, Hoffmann B, Erhardt G. Influence of sex on the resistance of sheep lambs to an experimental Haemonchus contortus infection. Deut Tierarztl Woch. 2006;113:178–181. [PubMed] [Google Scholar]
- Good B, Hanrahan JP, Crowley BA, Mulcahy G. Texel sheep are more resistant to natural nematode challenge than Suffolk sheep based on faecal egg count and nematode burden. Vet Parasit. 2006;136:317–327. doi: 10.1016/j.vetpar.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Graenzer W, Graunke WD, Sendke A. Zur Epidemiologie der Parasitosen beim Schaf in Bayern. Bayer Landwirtsch Jahrb. 1979;26:398–402. [Google Scholar]
- Gray GD. Genetic variation in resistance to parasites. In: Gray GD, Woolaston RR, Eaton BT, editors. Breeding for resistance to infectious diseases in small ruminants. Canberra: ACIAR; 1995. pp. 43–52. [Google Scholar]
- Grzonka E, Kaulfuss KH, Schliephake A, Pfeifer F. Studies on endoparasitic infection in sheep flocks in Sachsen-Anhalt. Tierarztl Umschau. 2000;55:658–662. [Google Scholar]
- Haile A, Tibbo M, Baker RL, Rege JEO. Effects of non-genetic factors on responses to gastro-intestinal nematode infections in Ethiopian sheep. Trop Anim Health Prod. 2007;39(6):411–417. doi: 10.1007/s11250-007-9034-0. [DOI] [PubMed] [Google Scholar]
- Holmes PH. Pathophysiology of nematode infections. Int J Parasitol. 1987;17:443–451. doi: 10.1016/0020-7519(87)90120-2. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Cattadori IM, Boag B, Dobson AP. Climate disruption and parasite–host dynamics: patterns and processes associated with warming and the frequency of extreme climatic events. J Helminth. 2006;80:175–182. doi: 10.1079/JOH2006357. [DOI] [PubMed] [Google Scholar]
- Kambara T, McFarlane RG. Changes in T cell subpopulations of sheep due to age and dietary protein intake, association with protective immunity to Trichostrongylus colubriformis. Vet Immunol Immunopathol. 1996;51:127–135. doi: 10.1016/0165-2427(95)05513-4. [DOI] [PubMed] [Google Scholar]
- Kambara T, McFarlane RG, Abell TJ, McAnulty RW, Sykes AR. The effect of age and dietary protein on immunity and resistance in lambs vaccinated with Trichostrongylus colubriformis. Int J Parasitol. 1993;23:471–476. doi: 10.1016/0020-7519(93)90035-W. [DOI] [PubMed] [Google Scholar]
- Kanyari PW. Experimental infections with coccidiosis and serum antibody quantitation in two breeds of goats. Vet Parasitol. 1988;28:11–18. doi: 10.1016/0304-4017(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Kanyari PW. The relationship between coccidial helminth infections in sheep and goats in Kenya. Vet Parasitol. 1993;51(1–2):137–141. doi: 10.1016/0304-4017(93)90204-Z. [DOI] [PubMed] [Google Scholar]
- Levya V, Henderson AE, Sykes AR. Effect of daily infection with Ostertagia circumcincta larvae on food intake, milk production and wool growth in sheep. J Agric Sci. 1982;99:249–260. doi: 10.1017/S0021859600030008. [DOI] [Google Scholar]
- Maff (1986) Manual of veterinary parasitological laboratory techniques. Ministry of Agriculture, Fisheries and Food. Technical Bulletin No. 18, HMSO, London, UK
- McClure S (2000) Sheep immunity to gastrointestinal nematode parasites—review. CSIRO Livestock Industries. http://www.csiro.au/proprietaryDocuments/McClure_Review2000.pdf
- McManus C, Louvandini H, Paiva SR, de Oliveira AA, Azevedo HC, de Melo CB. Genetic factors of sheep affecting gastrointestinal parasite infections in the Distrito Federal. Brazil Vet Parasit. 2009;166:308–313. doi: 10.1016/j.vetpar.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Moritz EI (2005) Ein Beitrag zum Befall mit Endoparasiten und zum Nachweis von Benzimidazolresistenzen bei Magen-Darm-Strongyliden der Schafe in Niedersachsen. Dissertation, Tieraerztliche Hochschule Hannover, Germany
- Reeg KJ, Gauly M, Bauer C, Mertens C, Erhardt G, Zahner H. Coccidial infections in housed lambs: oocyst excretion, antibody levels and genetic influences on the infection. Vet Parasitol. 2005;127:209–219. doi: 10.1016/j.vetpar.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Rehbein S, Kollmannsberger M, Visser M, Winter R. The helminth fauna of slaughtered sheep from upper Bavaria: 1. Species composition, prevalence and worm counts. Berl Muench Tieraerztl Wschr. 1996;109:161–167. [Google Scholar]
- Roesicke E, Seuser K, Dittrich K (2007) Schaf- und Ziegenrassen. AID infodienst Ernaehrung, Landwirtschaft, Verbraucherschutz e.V., Bonn. ISBN 978-3-8308-0482-6
- Romjali E, Pandey VS, Gatenby RM, Doloksaribu M, Sakul H, Wilson A, Verhulst A. Genetic resistance of different genotypes of sheep to natural infections with gastro-intestinal nematodes. Anim Sci. 1997;64:97–104. doi: 10.1017/S1357729800015599. [DOI] [Google Scholar]
- Schwenk R (1998) Untersuchungen über Abgangs- und Krankheitsursachen bei Schafen: Auswertungen der Untersuchungsergebnisse des Tiergesundheitsamtes Bonn der Landwirtschaftskammer Rheinland von 1986–1995. Dissertation, Tieraerztliche Hochschule Hannover, Germany
- Shubber AH, Lloyd S, Soulsby EJL. Infection with gastrointestinal helminths. Effect of lactation and maternal transfer of immunity. Z Parasitenkd. 1981;65:181–189. doi: 10.1007/BF00929184. [DOI] [PubMed] [Google Scholar]
- Steel JW, Jones WO, Symons LEA. Effects of a concurrent infection of Trichostrongylus colubriformis on the productivity and physiological and metabolic responses of lambs infected with Ostertagia circumcincta. Aust J Agr Res. 1982;33:131–140. doi: 10.1071/AR9820131. [DOI] [Google Scholar]
- Suarez VH, Cristel SL, Busetti MR. Epidemiology and effects of gastrointestinal nematode infection on milk productions of dairy ewes. Parasite. 2009;16:141–147. doi: 10.1051/parasite/2009162141. [DOI] [PubMed] [Google Scholar]
- Suttle NF. Seasonal infections and nutritional status. Proc Nutr Soc. 1994;53:545–555. doi: 10.1079/PNS19940064. [DOI] [PubMed] [Google Scholar]
- Torina A, Ferrantelli V, Sparagano OA, Reale S, Vitale F, Caracappa S. Climatic conditions and gastrointestinal nematode egg production: observations in breeding sheep and goats. Ann NY Acad Sci. 2004;1026:203–209. doi: 10.1196/annals.1307.031. [DOI] [PubMed] [Google Scholar]
- Van Dijk J, Sargison ND, Kenyon F, Skuce PJ. Climate change and infectious disease: helminthological challenges to farmed ruminants in temperate regions. Animal. 2010;4:377–392. doi: 10.1017/S1751731109990991. [DOI] [PubMed] [Google Scholar]
- Wassmuth R, Moors E, Langholz H. Vigour and endoparasite incidence of outdoor sheep. Arch Tierz Dummerstorf. 2001;44:230–239. [Google Scholar]
- Zuk M, McKean KA. Sex differences in parasite infections: patterns and processes. Int J Parasitol. 1996;26:1009–1023. [PubMed] [Google Scholar]