Abstract
Lactation has long been recognized as a major determinant of interbirth intervals. The temporal pattern of nursing has been proposed as the mechanism behind lactational amenorrhea. We present a new model of the dynamic regulation of lactational amenorrhea that identifies maternal energy availability as the main determinant of ovarian resumption. Variation in the intensity of lactation remains a component of the model as a determinant of the absolute energetic cost of milk production. But maternal energy supply determines net energy availability; a larger energy supply leaves a greater net energy surplus than a smaller energy supply (lactation costs being equal).
We characterize the hormonal postpartum profile of 70 lactating Toba women of Argentina. We use C-peptide, which reflects maternal insulin production, as a measure of energy availability. Initially low, insulin production rises as the postpartum period progresses, reflecting the declining metabolic load of lactation. A short period of supernormal insulin production precedes menstrual resumption. The high levels of insulin may play a role in stimulating the resumption of ovarian activity, which in turn may help to resolve the transient period of insulin resistance. The dynamics of insulin sensitivity during lactation would aid in synchronizing the resumption of ovarian function with a reduction in the energy demands of milk production. This hypothesis is supported by the sustained weight gain experienced by lactating women during the months preceding the first postpartum menses. The link between fecundity and energy balance could serve as a mechanism for adjusting the duration of lactational amenorrhea to the relative metabolic load of lactation.
Introduction
The pacing of births is a key element of human life history strategies and a major regulator of female reproductive success. Human life history traits are characterized by shorter inter-birth intervals, earlier weaning times, and a higher total fertility rate relative to extant non-human primates (Hill, 1993; Mace, 2000; Robson and Wood, 2008). From an adaptive perspective, the ability to modulate the duration of interbirth intervals is crucial for the mother’s current and future offspring survival and, thus, for her lifetime reproductive success. Closely spaced births, i.e. less than 2 years apart, lead to an increased rate of infant mortality, not only of the first born child in a sequence but also of the second (Hobcraft et al., 1983; Mozumder et al., 2000). The mother’s energy supply may be severely affected by closely spaced births as well. A lactating woman who becomes pregnant while her infant is still young is burdened with the task of metabolizing for three, at least until the infant is completely weaned. Particularly notorious in developing countries where poor nutritional states and high female workloads are the norm, the “maternal depletion syndrome” (Jelliffe and Maddocks, 1964; Tracer, 1991) compromises maternal health and overall total fertility. On the other hand, spacing births too far apart represents lost opportunity and may reduce the mother’s lifetime fertility. Having such an important impact on female life history structure, the mechanisms underlying the duration of interbirth intervals are candidates for intense selective pressure (Sellen, 2006). After several decades of research, at different levels of analysis (from molecular to physiological to demographic) we are beginning to obtain a more focused picture of what those mechanisms are. The purpose of this work is to present a model that explains the transition to full postpartum ovarian functioning, and thus, of the pace of childbearing, as the result of maternal metabolic energy allocation decisions.
Being the most variable component of the interbirth interval (Wood, 1994), the period of postpartum amenorrhea is intrinsically associated with lactation practices. Lactation has long been recognized as a major determinant of postpartum fertility (Henry, 1961). Across different populations, regardless of subsistence patterns, women who do not breastfeed resume menstruation earlier than women who do breastfeed. As a result, natural fertility populations, in which long periods of lactation are the norm, are characterized by relatively long inter-birth intervals. The functional explanation for the birth-spacing role of lactation is the presumed need for mothers to spread out the metabolic demands of intense milk production and pregnancy. But what is the link between the physiology of lactation and postpartum ovarian function?
Until quite recently, the pituitary hormone prolactin was believed to be responsible for the suppressive effects of lactation on postpartum fecundity. Prolactin is released in response to nipple stimulation during breastfeeding and it promotes milk production by the mammary gland. Tyson (1977) found that nursing frequency is positively correlated with prolactin levels: within minutes of the infant latching on to the nipple, prolactin levels increase dramatically. Then, soon after the infant stops nursing, prolactin levels slowly drop. It was also well known by that time that hyperprolactenemia was usually associated with ovarian dysfunction. Tyson’s data suggested that the temporal pattern of suckling could be of importance in unmasking the link between lactation and ovarian function.
The pattern of nursing appeared to be so crucial that it served as the basis for a major hypothesis aimed at explaining the fertility-reducing effects of lactation. This hypothesis proposes that the “intensity” of nursing is the primary regulator of the duration of lactational amenorrhea (McNeilly, 1993; McNeilly, 2001; McNeilly et al., 1994). According to the nursing intensity hypothesis, variation in the duration of lactational infecundity reflects the diversity in nursing behavior across populations: the more intensive the breastfeeding, the longer the impact on fertility. Women in developing countries, showing intense prolonged breast feeding patterns, would have longer periods of lactational amenorrhea than women in industrialized societies where breast feeding, if practiced at all, was much more structured. Data from many field studies of natural fertility populations around the world appeared to support the nursing intensity hypothesis (Jones, 1989; Panter-Brick, 1991; Vitzthum, 1989; Wood et al., 1985; Konner & Worthman, 1980). While this hypothesis goes far in integrating these observations with the theoretical framework of life history theory, it does not explicitly incorporate energy allocation concepts. There are also numerous instances, both from field and clinical settings, in which variation in the duration of lactational amenorrhea could not be accounted for by the variation in nursing intensity patterns nor with variation in prolactin levels (Fink et al., 1992; Peng et al., 1998; Rahman et al., 2002; Tay et al., 1996; Worthman et al., 1993). These studies point to various aspects of maternal energy availability (e.g., maternal nutritional status, maternal body composition, introduction of solid food) as mediators in the return to postpartum fecundity.
Metabolic load model
We present a new model of the dynamic regulation of lactational amenorrhea that explicitly incorporates maternal energy allocation. The metabolic load model (Ellison, 1994a; Ellison, 2001; Valeggia and Ellison, 2001a; Valeggia and Ellison, 2004) proposes that the major determinant of postpartum ovarian function is maternal energy availability. Producing the same amount of milk would represent a higher relative metabolic load for women on negative energy balance than for women on a positive energy balance, even when nursing intensity is the same. Under our model, nursing intensity is still considered one key component of the duration of lactational amenorrhea because it serves as a proxy for the absolute cost of milk production. Thus, the relative metabolic load model subsumes many of the same observations thought to support the nursing intensity hypothesis while generating additional predictions. For example, the costs of lactation being equal, a woman with a higher energy supply (higher calorie intake and/or lower energy output) will transition to ovarian cyclicity sooner than a woman with a more limited energy supply. The introduction of supplements to the infant’s or to the mother’s diet is interpreted as a reduction in the relative metabolic load of producing milk. Therefore, as has been observed in numerous studies (Lunn et al., 1980; Lunn et al., 1984; Rahman et al., 2002; Simondon et al., 2003; Tay et al., 1996), supplementation leads to shorter periods of lactational amenorrhea.
The relative metabolic load model implies presumably direct channels of communication between energy metabolism and reproductive function. Considerable evidence is accumulating towards the existence of these channels at different endocrine and neuronal levels and at various sites along the hypothalamus-pituitary-gonadal (HPG) axis (Mircea et al., 2007; Schneider, 2004). Furthermore, some investigations propose that proper function of the HPG axis is “gated (italics added) by metabolic and nutritional factors” (Fernandez-Fernandez et al., 2006). Although the precise neuroendocrine circuits and molecular signals behind this regulation are only now beginning to be elucidated, it appears that the coordination of several pathways inform the body about energy allocation needs (Mircea et al., 2007). Hormones well known for their action on energy metabolism are among the usual suspects: thyroid hormones, cortisol, and insulin have all been shown to have direct effect on reproductive function. Low T3 and T4 levels influence ovarian function by decreasing levels of sex-hormone-binding globulin and increasing the secretion of prolactin (Poppe et al., 2008). Chronic high levels of cortisol, a catabolic hormone, have been associated with altered GnRH secretion and lower LH surges (Charmandari et al., 2004; Chrousos et al., 1998) and with low midluteal progesterone levels (Nepomnaschy et al., 2004). Insulin, an anabolic hormone synthesized by the pancreas, deserves a closer look because of its direct role in the regulation of glucose (i.e., metabolic energy) availability.
The role of insulin and insulin-like growth factors (IGFs) on reproductive function has been known for some time (Poretsky et al., 1999; Poretsky and Kalin, 1987). Briefly, insulin is necessary for the production of ovarian hormones. Acting both alone or in conjunction with gonadotropins, insulin stimulates the ovary to produce oestrogen and progesterone (Greisen et al., 2001; Willis et al., 1996). This is achieved through insulin signaling pathways that enhance the expression of genes and enzymes that are crucial for ovarian endocrine activity. Malfunction of these signaling pathways has been associated, for example, with polycystic ovarian syndrome, which is characterized by insulin resistance and ovarian disorders, including anovulation (Diamanti-Kandarakis et al., 2008; Greisen et al., 2001). Insulin sensing in the brain seems to be required for normal reproduction. Insulin receptors are expressed in the arcuate nucleus of the hypothalamus, which has been associated with the regulation of energy balance. Furthermore, insulin receptors are differentially expressed in the medial portion of the arcuate nucleus, the region that controls the release of neuropeptide Y, one of the proposed modulators of GnRH release (Hill et al. 2008). Although still an unsettled question, there is also evidence showing that hypothalamic GnRH-producing neurons have insulin receptors (Salvi et al. 2006) pointing to the sensitivity of the GnRH pulse generator to energetic stress.
Leptin, a peptide hormone produced by adipose cells, has received plenty of attention regarding its effects on reproduction (Barash et al., 1996; Conway and Jacobs, 1997; Cunningham et al., 1999; Holness et al., 1999; Matkovic et al., 1997; Schneider et al., 2000; Woodside et al. 2000). After several years of controversy about the true role of leptin, it is now commonly accepted that it functions as a modulator of reproduction rather than a direct actor (Schneider, 2004). Leptin seems to facilitate reproductive function via its ability to increase fuel oxidation (i.e., energy availability).
Among the recently discovered mediators for relaying the state of energy availability to hypothalamic centers governing reproduction, several studies have identified both peripheral gastric hormones such as ghrelin (Fernandez-Fernandez et al., 2006; Gambineri et al., 2005; Tena-Sempere, 2005), CCK (Perera et al., 1993), and polypeptide YY (Fernandez-Fernandez et al., 2006), and central mediators such as the hypothalamic kisspeptins (Castellano et al., 2006; Smith, 2008; Tena-Sempere, 2006) and neuropeptide Y (Woodside et al. 2002; Toufexis et al. 2002). Overall, these studies show that when energy is in short supply, several hormonal factors synergize to divert processes away from luxurious activities (e.g., reproduction) and towards energy conservation and survival.
In sum, there seems to be solid evidence for the proximate mechanisms underlying the energy management trade-offs implied in the metabolic load model. However, these findings do not necessarily help us differentiating the metabolic load model from the most frequently cited nursing intensity models. Under most circumstances, nursing intensity is a strong predictor of relative metabolic load. Hence, the principal explanatory variables are often confounded. Available data come mainly from two different settings: clinical studies and field studies. The clinical studies provided information on the response of mainly well-nourished women practicing some form of scheduled nursing (Diaz et al. 1988; Heinig et al. 1994). In these women, the mean duration of lactational amenorrhea is relatively short (6.3 months). The explanation provided by the nursing intensity hypothesis seems satisfactory: there is an early resumption of ovarian activity because nursing frequency is low and/or the intervals between nursing bouts are long. The metabolic load model would also explain satisfactorily these findings since the shorter period of amenorrhea might be the result of a lesser energetic stress that lactation implies for women in good nutritional status. Studies conducted among traditional societies (see Ellison, 1995 for a review) usually show the other extreme, i.e. the period of lactational amenorrhea in poorly nourished women practicing intense nursing is relatively long (mean = 20 months). Again, both models explain the results equally well.
Most studies of the determinants of postpartum fecundity published to date focused on breastfeeding women that were either 1) well-nourished with low nursing intensity (clinical studies), or 2) undernourished with high nursing intensity (field studies). We were able to test the predictions of the current models in a population of women for which both nursing intensity and energy availability are high. Thus, the two main confounding variables, nursing intensity and energy balance, would hypothetically not be working in conjunction with each other, but actually in opposite directions.
Testing the metabolic load model among lactating Toba women
The Toba are one of the three main indigenous groups living in the province of Formosa, in the border with Paraguay (Miller, 1999). Originally hunter-gatherers, most groups are now settled in “barrios” on the outskirts of larger towns. Our study was conducted in the barrio Namqom, which has a population of approximately 2800 people. Women’s activities include household chores, children caretaking, and basket weaving. A few women are employed temporarily as cooks, teaching assistants, or health agents while others go to the city once or twice a week to sell their weavings door to door.
Toba women are well-nourished and typically breastfeed their infants for 2–3 years, or until their next pregnancy is visibly recognizable (Valeggia and Ellison, 2003). In this population, semisolid and solid supplements are usually introduced around six months of age. The use of commercial formula and artificial contraception is infrequent.
We collected monthly interview data and anthropometric measurements (height and body mass) from 70 lactating women. In addition, we collected detailed behavioral data every other week. During these home visits, we recorded breastfeeding behavior, food intake, and duration and type of daily activities. We used noninvasive measures of ovarian function and metabolic energy availability to characterize progress from complete postpartum amenorrhea to fully competent cyclicity. Urinary C-peptide levels, reflecting maternal insulin production, and cortisol levels were used as a measure of maternal energy management and availability. Ovarian function was characterized by measuring urinary estrogen and progesterone metabolites (E1C and PdG). For a more detailed explanation of our methods see (Valeggia and Ellison, 2004).
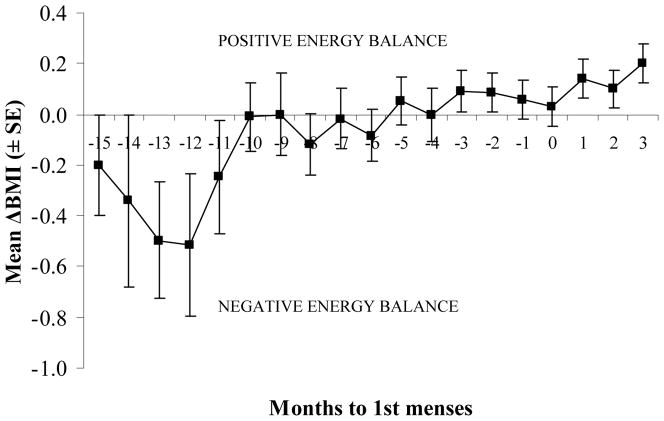
Toba women experienced a relatively short period of lactational amenorrhea (10.2 months ± 4.3). The resumption of post-partum menses was not associated with nursing behavior either taken as a summary index per woman or taken as a time-dependent variable. The body mass index (BMI) is a static measure of nutritional status, i.e. it represents a snapshot of the current nutritional status of a person. Interestingly, BMI was not a good predictor of duration of lactational amenorrhea either. However, when anthropometrics were analyzed as time-dependent variables, there was an association between those measures and time to resumption of menses (Figure 1). When we analyzed the women’s individual profiles for BMI changes over the postpartum period, we identified a conspicuous trend: lactating women tended to be in a sustained period of positive energy balance by the time they resume postpartum menses. We therefore calculated ΔBMI, a dynamic measure of nutritional status, as BMI(t) − BMI (t-1), i.e. the BMI corresponding to a given month minus the BMI corresponding to the month befor. There was a significant correlation between ΔBMI and time to resumption of menses. Figure 1 shows mean ΔBMI values as lactating women approach time of first postpartum menses.
Figure 1.
Changes in energy balance, as estimated using the change in body mass index (ΔBMI), in breastfeeding women as they approach their first postpartum menses (time 0).
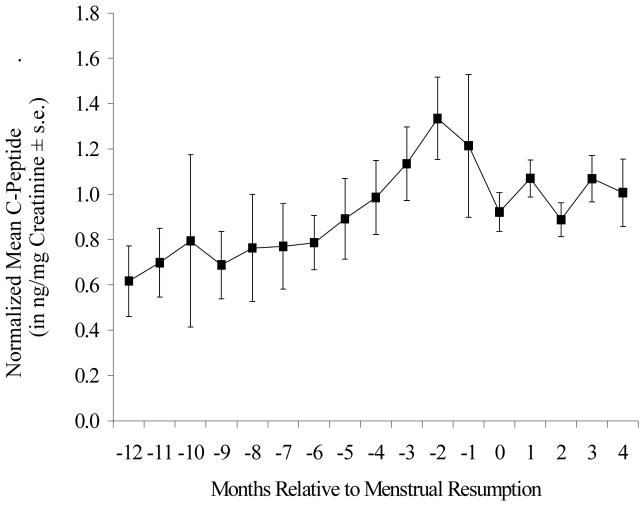
Early postpartum lactation was characterized by low insulin production and low C-peptide levels (Ellison and Valeggia, 2003). With time postpartum, average C-peptide levels tended to increase quite steadily. When C-peptide levels were realigned using month of resumption of menses as the anchor, a telling pattern emerged: there was a transient, but sharp increase in insulin production in the months immediately preceding menstrual resumption (Figure 2). Insulin levels then returned to normal after the first postpartum menses.
Figure 2.
Mean (± SE) values of individually normalized C-peptide values relative to month of menstrual resumption for all study subjects. Normalized C-peptide values are expressed as a percentage of the post-resumption mean valued for each subject (see text).
Urinary cortisol levels tend to increase until the fourth or fifth month before resumption of menses, when they seem to stabilize (Figure 3). Early lactation cortisol values were, on average, 60% of the values reached at the plateau and run parallel to those of urinary C-peptide until around the 5th month before resumption.
Figure 3.
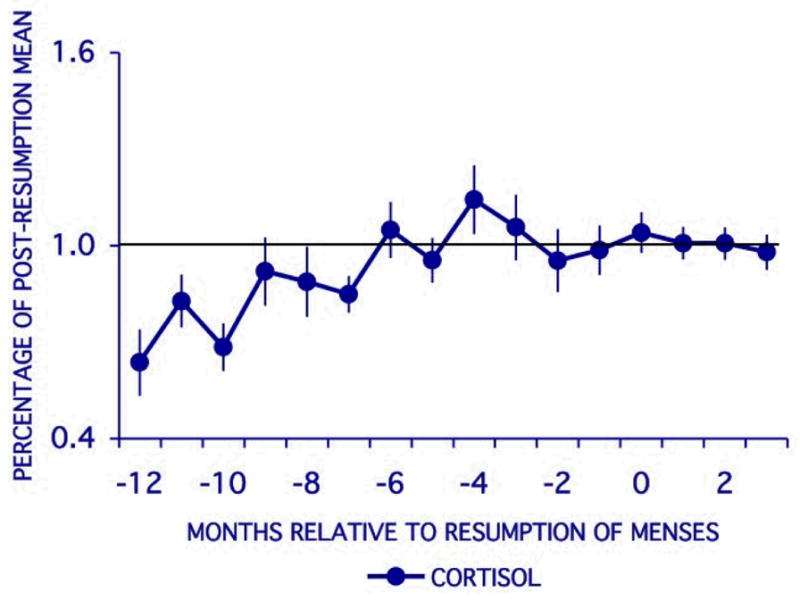
Mean (± SE) values of individually normalized cortisol values relative to month of menstrual resumption. Normalized cortisol values are expressed as a percentage of the post-resumption mean valued for each subject.
How do these findings fit in the model we set out to test? Lactation imposes a considerable energetic burden on the mother (Butte et al., 2001; Dewey, 1997). Furthermore, milk production is a significantly conserved process. During lactation, mechanisms are employed to ensure a preferential partitioning of nutrients to the mammary gland regardless of the nutritional status of the mother (Butte et al., 1999). Prolactin, for example, circulating at high levels early in the postpartum period, directs the allocation of energy towards milk production by up-regulating insulin receptors in the breast. The initially low C-peptide and insulin levels observed in lactating Toba during the early postpartum period likely reflect elevated carbohydrate utilization for milk production in lactating mothers. Low peripheral insulin concentration during early lactation has been also reported for other populations (Butte et al., 1999; Maliqueo et al., 2001; Tigas et al., 2002).
For our Toba population, we were able to monitor the progress of energy allocation during lactation beyond the first few weeks postpartum. Insulin production increased as the postpartum period progressed, reflecting a declining metabolic load of lactation. Rising C-peptide and insulin levels and falling glucose:insulin ratios would be consistent with the attenuation of this demand, particularly as supplemental foods are introduced into the baby’s diet, and by an increasing need for insulin secretion to maintain maternal glucose homeostasis. Superimposed to this mechanism, increasing levels of cortisol, a catabolic hormone, may help direct glucose away from storage and towards the mammary gland during the early postpartum period. As the infant grows and starts receiving supplements, other downstream metabolic mediators (see above) synergize to inform the mother’s reproductive axis of increasing levels of energy availability.
Particularly notable is the short period of supernormal insulin production (insulin resistance) that characterizes the months immediately preceding menstrual resumption. As mentioned before, insulin has been shown to stimulate ovarian hormonal production. The high levels of insulin during the insulin resistant phase in lactating women may play a role in stimulating the resumption of ovarian activity. Increasing levels of ovarian steroids, in turn, may help resolve the transient period of insulin resistance and restore peripheral insulin sensitivity.
Metabolic load model in context
The link between energy balance and reproduction has received considerable attention in non-human animal research. Models that assign a leading role to maternal nutrition in postpartum fecundity have become a staple in dairy cattle and other domestic animal literature (Chagas et al., 2007; Kiyma et al., 2004; Leroy et al., 2008a; Leroy et al., 2008b; Robinson et al., 2006). Numerous findings from this field have pointed to severe negative energy balance during the postpartum period as the primary cause of a delay in resumption of normal estrous cyclicity in high-yielding dairy cows. In these cases, lactation is very intense and there is concern about bringing the high-yielding dairy animals back into estrous. Furthermore, several studies have identified insulin and other energy management metabolites as key regulators of postpartum fecundity in several animal species (Leroy et al. 2008b). In ewes, for example, significant changes in circulating insulin, ghrelin, and glucose preceded restoration of pulsatile secretion of LH (Szymanski et al. 2007). These studies illustrate the trade-offs mammalsi have evolved to optimize during the postpartum period. Humans may be no exception. Our findings show that regardless of the intensity of nursing or the nutritional status of the mother, postpartum ovarian activity seems to be responding to metabolic signals that inform of a sustained period of positive energy balance.
Energy allocation decisions lie at the core of life history theory (Hill, 1993; Stearns, 1992). However, there are few examples on how these decisions can be actually exerted at a proximate, mechanistic level. Fortunately, there are several lines of research exploring the interface between energy metabolism and life history traits at a molecular and physiological level in several species of animals, including humans. The challenge is to build an integrative framework for understanding the implied trade-offs. The metabolic load model of lactational amenorrhea provides a good example of how this can be accomplished. Furthermore, it can be extended to other life history transitions, such as pubertal activation of ovarian functioning (Ellison, 2002; and more developed in Ellison, 2007). Interestingly, human girls go through a period of insulin resistance before menarche, which resembles the one observed in Toba women prior to the postpartum resumption of menses (Caprio, 1999; Travers et al., 1995). This parallelism between life history transitions brings about another layer that a robust integrative approach should incorporate: the role of early environmental influences on developmental trajectories, particularly those that shape the responses of the reproductive axis. Although developmental trajectories and responses have been a focus of scientific attention for quite some time, evidence has been accumulating relatively rapidly that shows that early life conditions (i.e. prenatal and early postnatal life) affect later physiological responses and health in general (Barker, 1994; Bateson et al., 2004; Gluckman et al., 2005). In fact, the association between early developmental influences, particularly nutrition, and adult trajectories has been extended to reproductive function and reproductive strategies in females (Kuzawa, 2007). The response of female reproductive physiology to energy availability (in most reproductive statuses and transitions: puberty, ovarian cycles, pregnancy, and postpartum amenorrhea) may be programmed early during development. Jasienska et al.(Jasienska et al., 2006a; Jasienska et al., 2006b), for example, have found that ponderal index, an index of energy availability in utero, predicts both adult estradiol levels and the response of the ovarian function to suppressive effects of negative energy balance. Furthermore, in a study of Bangladeshi migrants to the United Kingdom, Núñez-de la Mora et al. (2007) elegantly showed that the developmental window for shaping reproductive function seems to be before mid-childhood. In this study, women who migrated before 8 years of age had higher luteal progesterone than those who migrated after that age. The common theme of current models addressing how early development cues may affect life history strategies (including timing and tempo of reproduction) is the concept of energy-sensitive set points that can be modified during prenatal and early postnatal development (Gluckman and Beedle, 2007; Lipson, 2001; Vitzthum and Spielvogel, 2003). Energetic conditions in utero and during early childhood, mediated by metabolic gatekeepers such as insulin, leptin and other adiponectins, may determine the sensitivity of reproductive function to energy availability during puberty and adulthood. Chronically unfavorable environments would activate a cascade of metabolic mechanisms that optimize energy management and directly affect life history trade-offs, including growth and reproduction (Kuzawa, 2007). These developmental norm of reaction setting models would help explain, for example, an apparent paradox observed in human populations: women in developing countries, who endure long hardworking days in the field and/or a marginal nutritional status, are still able to average a total fertility rate of 7 or more live births (Vitzthum, 2001). Women in these populations tend to have later ages at menarche (Ellison, 1994b; Gluckman and Hanson, 2006), lower ovarian hormone levels (Ellison, 1994a and b; Jasienska and Jasienski, 2008; Vitzthum et al., 2002), and a more dramatic response to energetic constraints (Jasienska et al., 2006a). Still, they are able to conceive and have live births, even if at a slower pace. These life history characteristics can be seen as adaptive energy allocation responses to the local environment (Lipson, 2001). Furthermore, the programming that occurs during development (i.e., the organizational effect) would be superimposed with mechanisms that deal with acute (or short-term) fluctuations in energy balance (i.e., activational effects), resulting in optimal allocation strategies.
Energy allocation models also allow for the consideration of the effects of social, political, and cultural practices on fertility regulation. For example, infant feeding practices vary considerably across cultures affecting maternal energy availability and, thus, interbirth intervals. Women in societies in which supplementation of breast milk starts soon after birth, provided that they have enough calorie intake, can see her fertility return earlier than those in societies in which breastfeeding is exclusive for many months. Similarly, populations differ greatly in their patterns of postpartum physical activity, this being influenced by subsistence activities, cultural practices, and socioeconomic situation of the lactating mother. Furthermore, development programs that attempt to increase nutritional status of women may have dramatic fertility consequences (Valeggia and Ellison, 2001b). In rural Ethiopia, for instance, a program was initiated that aimed at improving women’s nutritional status by providing tap water, thus lessening the energetic expense of fetching water for women. An analysis done by Gibson and Mace (2002, 2006) eloquently shows that not only the energy-saving technology failed at improving maternal nutritional status, but it was also associated with increased birth rates and child malnutrition. The same appears to have occurred among the Xculoc Maya of the Yucatán Península in Mexico. In this case, the introduction of a water pump and a maize mill was followed by a reduction in the age at first birth and an increase in fertility measures (Kramer and McMillan, 1999). The surplus of energy provided by the reduction in energy expenditure was not channeled to energy storage but rather to investment in more children.
Conclusion
Our research supports the idea that the return to postpartum fecundity is an energy allocation problem: for a lactating mother, where is energy more optimally allocated, to an already born offspring through lactation or to future offspring, through resumption of ovulation? The relative metabolic load model proposes that the answer to this depends on how large the energy budget is compared to the cost of lactation. Although this model is gaining increasing empirical support, the original appeal of the nursing intensity model seems to persist. It should be stressed that the metabolic load model does not negate the importance of the suckling stimulus; it actually incorporates nursing intensity into the model. In fact, when the breastfeeding woman’s energetic budget of the woman is limited, e.g. under malnutrition or under high energetic output, the intensity of lactation can be taken as a proxy for the relative cost of lactation for that woman.
At a functional level, a reliance on the temporal pattern of nursing for the regulation of postpartum fecundity seems to be rather unlikely. Given the vast variation in nursing behavior across populations, from different work schedules, cultural attitudes towards breastfeeding, presence of other children, and individual maternal temperament, temporal nursing patterns may be a poor signal for the optimal resumption of postpartum fecundity. Nor does it incorporate any information about the mother’s energetic condition. If we take into account that pregnancy and lactation represent high energy investments for women, it makes more sense, from an evolutionary standpoint, for a woman to monitor her own energy availability rather than an imperfect proxy for infant demand in making the decision to embark on a new reproductive enterprise.
At a more mechanistic level, the dynamics of insulin sensitivity during lactation, as well as other metabolic mediators, may aid in synchronizing the resumption of ovarian function with a reduction in the energy demands of milk production. A better understanding on the regulation of postpartum fecundity will come with further research into the interface between energy metabolism and reproductive physiology.
Acknowledgments
Grant information: Financial support for this project was provided by NICHHD (R03HD37226), the Nestlé Foundation for Research, the Wenner-Gren Foundation, CONICET (Argentina), and the University of Pennsylvania Population Aging Research Center and Population Studies Center.
We thank Mhairi Gibson and Rebecca Sear for organizing the workshop and the IUSSP for sponsoring it. We are grateful to the Toba women of Namqom, the staff of the Centro de Salud Namqom, and the many field assistants that participated in this study for their support and collaboration. Eduardo Fernandez-Duque, Kevin Burke, and two anonymous reviewers provided useful and appreciated comments.
Footnotes
Marmosets and tamarins may be an exception, since they ovulate shortly after giving birth (to twins, most of the time) and lactating (Howie PW, McNeilly AS, Houston MJ, Cook a, Boyle H. 1981. Effect of supplementary food on suckling patterns and ovarian activity during lactation. Br Med J 283(757–759).. However, even in these species, nursing one or two offspring does have an impact on the duration of the (short) postpartum anovulation period (Ziegler et al., 1990)
References
- Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a Metabolic Signal to the Reproductive System. Endocrinology. 1996;137(7):3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- Barker DJP. The fetal and infant origins of adult disease. BMJ Publishing Group; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Unide B, Foley R, Gluckman PD, Godfrey K, Kirkwood T, Mirazón Lahr M, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Butte NF, Hopkinson JM, Mehta N, O'Brian Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- Butte NF, Wong W, Hopkinson JM. Energy requirements of lactating women derived from doubly labeled water and milk energy output. Journal of Nutrition. 2001;131:53–58. doi: 10.1093/jn/131.1.53. [DOI] [PubMed] [Google Scholar]
- Caprio S. Insulin: the other anabolic hormone of puberty. Acta Paediatrica. 1999;433:84–87. doi: 10.1111/j.1651-2227.1999.tb14410.x. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sanchez-Criado JE, Pellicer A, et al. Expression of KiSS-1 in Rat Ovary: Putative Local Regulator of Ovulation? Endocrinology. 2006;147(10):4852–4862. doi: 10.1210/en.2006-0117. [DOI] [PubMed] [Google Scholar]
- Chagas LM, Bass JJ, Blache D, Burke CR, Kay JK, Lindsay DR, Lucy MC, Martin GB, Meier S, Rhodes FM, et al. New Perspectives on the Roles of Nutrition and Metabolic Priorities in the Subfertility of High-Producing Dairy Cows. J Dairy Sci. 2007;90:4022–4032. doi: 10.3168/jds.2006-852. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Chrousos GP. Glucocorticoids and Their Actions. Ann N Y Acad Sci. 2004;1024:1–8. doi: 10.1196/annals.1321.001. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-ovarian axis and the female reproductive system. Ann Intern Med. 1998;1998(129):3. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Conway GS, Jacobs HS. Leptin: a hormone of reproduction. Hum Reprod. 1997;12(4):633–635. doi: 10.1093/humrep/12.4.633. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Clifton DK, Steiner RA. Leptin's Actions on the Reproductive Axis: Perspectives and Mechanisms. Biol Repro. 1999;60:216–222. doi: 10.1095/biolreprod60.2.216. [DOI] [PubMed] [Google Scholar]
- Dewey KG. Energy and Protein Requirements During Lactation. Annu Rev Nutr. 1997;17:19–36. doi: 10.1146/annurev.nutr.17.1.19. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Argyrakopoulou G, Economou F, Kandaraki E, Koutsilieris M. Defects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome (PCOS) The Journal of Steroid Biochemistry and Molecular Biology. 2008 doi: 10.1016/j.jsbmb.2008.03.014. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Diaz S, Miranda P, Brandeis A, Cardenas H, Croxatto HB. A Study on the Feasibility of Suppressing Ovarian Activity Following the end of Postpartum Amenorrhoea by Increasing the Frequency of Suckling. Clinical Endocrinology. 1988;28:525–535. doi: 10.1111/j.1365-2265.1988.tb03687.x. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Advances in Human Reproductive Ecology. Annu Rev Anthropol. 1994a;23:225–275. doi: 10.1146/annurev.an.23.100194.001351. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Salivary steroids and natural variation in human ovarian function. Ann N Y Acad Sci. 1994b;709:287–98. doi: 10.1111/j.1749-6632.1994.tb30417.x. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Breastfeeding, Fertility, and Maternal Condition. In: Stuart-Macadam P, Dettwyler KA, editors. Breastfeeding: Biocultural Perspectives. New York: Aldine de Gruyter; 1995. pp. 305–345. [Google Scholar]
- Ellison PT. On Fertile Ground. Cambridge, MA: Harvard University Press; 2001. [Google Scholar]
- Ellison PT. Puberty. In: Cameron N, editor. Human Growth and Development. New York: Academic Press; 2002. pp. 65–84. [Google Scholar]
- Ellison PT. The endocrinology of human life history transitions. In: Nesse R, editor. Evolutionary Medicine: How New Applications Advance Research and Practice. Henry Stewart Talks; London, UK: 2007. [Google Scholar]
- Ellison PT, Valeggia C. C-peptide levels and lactational amenorrhea among Toba women of northern Argentina. Fertil Steril. 2003;80(5):1279–1280. doi: 10.1016/s0015-0282(03)02158-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocr. 2006;(127):254–255. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Fink AE, Fink G, Wilson H, Bennie J, Carroll S, Dick H. Lactation, nutrition and fertility and the secretion of prolactin and gonadotrophins in Mopan Mayan women. J Biosoc Sci. 1992;24:35–52. doi: 10.1017/s0021932000006787. [DOI] [PubMed] [Google Scholar]
- Gambineri A, Pagotto U, De Lasio R, Meriggiola M, Costantino A, Patton L, Pelusi C, Pelusi G, Pasquali R. Short-term modification of sex hormones is associated with changes in ghrelin circulating levels in healthy normal-weight men. J Endocrinol Invest. 2005;28(3):241–246. doi: 10.1007/BF03345380. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Beedle A. Migrating Ovaries: Early Life Influences on Later Gonadal Function. PLoS Med. 2007;4(5):e90. doi: 10.1371/journal.pmed.0040190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Evolution, development and timing of puberty. Trends in endocrinology and metabolism: TEM. 2006;17(1):7–12. doi: 10.1016/j.tem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends in ecology & evolution (Personal edition) 2005;20(10):527–33. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Greisen S, Ledet TPO. Effects of androstenedione, insulin and luteininzing hormone on steroidogenesis in human granulosa luteal cells. Hum Reprod. 2001;16(10):2061–2065. doi: 10.1093/humrep/16.10.2061. [DOI] [PubMed] [Google Scholar]
- Heinig MJ, Nommsen- Rivers LA, Peerson JM, Dewey KG. Factors Related to Duration of Postpartum amenorrhoea Among USA Women with Prolonged Lactation. J Biosoc Sci. 1994;26:517–527. doi: 10.1017/s0021932000021647. [DOI] [PubMed] [Google Scholar]
- Henry L. Some Data on Natural Fertility. Eugenics Quarterly. 1961;8:81–91. doi: 10.1080/19485565.1961.9987465. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–32. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KR. Life History and Evolutionary Anthropology. Evolutionary Anthropology. 1993;2(3):78–88. [Google Scholar]
- Hobcraft J, Mcdonald JW, Rutstein S. Child-Spacing Effects on Infant and Early Child-Mortality. Population Index. 1983;49(4):585–618. [Google Scholar]
- Holness MJ, Munns MJ, Sugden MC. Current concepts concerning the role of leptin in reproductive function. Mol Cell Endocr. 1999;157:11–20. doi: 10.1016/s0303-7207(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Howie PW, McNeilly AS, Houston MJ, Cook A, Boyle H. Effect of supplementary food on suckling patterns and ovarian activity during lactation. Br Med J. 1981;283:757–759. doi: 10.1136/bmj.283.6294.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Jasienski M. Interpopulation, Interindividual, Intercycle, and Intracycle Natural Variation in Progesterone Levels: A Quantitative Assessment and Implications for Population Studies. Am J Hum Biol. 2008;20:35–42. doi: 10.1002/ajhb.20686. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Thune I, Ellison PT. Fatness at birth predicts adult susceptibility to ovarian suppression: an empirical test of the Predictive Adaptive Response hypothesis. Proc Natl Acad Sci U S A. 2006a;103(34):12759–62. doi: 10.1073/pnas.0605488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Ziomkiewicz A, Lipson SF, Thune I, Ellison PT. High ponderal index at birth predicts high estradiol levels in adult women. Am J Hum Biol. 2006b;18(1):133–140. doi: 10.1002/ajhb.20462. [DOI] [PubMed] [Google Scholar]
- Jelliffe DB, Maddocks I. Notes on the ecologic malnutrition in the New Guinea Highlands. Clinical Pediatric. 1964;3:432–438. doi: 10.1177/000992286400300710. [DOI] [PubMed] [Google Scholar]
- Jones RE. Breastfeeding and Postpartum Amenorrhea in Indonesia. Journal of Biosocial Science. 1989;21:83–100. doi: 10.1017/s0021932000017740. [DOI] [PubMed] [Google Scholar]
- Kiyma Z, Alexander BM, Van Kirk EA, Murdoch WJ, Hallford DM, Moss GE. Effects of feed restriction on reproductive and metabolic hormones in ewes. J Anim Sci. 2004;82(9):2548–2557. doi: 10.2527/2004.8292548x. [DOI] [PubMed] [Google Scholar]
- Konner MJ, Worthman C. Nursing frequency, gonadal funciton, and birth spacing among !Kung hunter-gatherers. Science. 1980;207:788–791. doi: 10.1126/science.7352291. [DOI] [PubMed] [Google Scholar]
- Kramer KL, McMillan GP. Women's labor, fertility, and the introduction of modern technology in a rural Maya village. J Anthropol Res. 1999;55(4):499–520. doi: 10.1086/jar.55.4.3631612. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW. Developmental origins of life history: growth, productivity, and reproduction. Am J Hum Biol. 2007;19(5):654–61. doi: 10.1002/ajhb.20659. [DOI] [PubMed] [Google Scholar]
- Leroy JL, Opsomer G, Van Soom A, Goovaerts IG, Bols PE. Reduced fertility in high-yielding dairy cows: are the oocyte and embryo in danger? Part I. The importance of negative energy balance and altered corpus luteum function to the reduction of oocyte and embryo quality in high-yielding dairy cows. Reproduction in domestic animals = Zuchthygiene. 2008a;43(5):612–22. doi: 10.1111/j.1439-0531.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- Leroy JL, Van Soom A, Opsomer G, Goovaerts IG, Bols PE. Reduced fertility in high-yielding dairy cows: are the oocyte and embryo in danger? Part II. Mechanisms linking nutrition and reduced oocyte and embryo quality in high-yielding dairy cows. Reproduction in domestic animals = Zuchthygiene. 2008b;43(5):623–32. doi: 10.1111/j.1439-0531.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- Lipson SF. Metabolism, maturation, and ovarian function. In: Ellison PT, editor. Reproductive Ecology and Human Evolution. New York: Aldine de Gruyter; 2001. pp. 235–248. [Google Scholar]
- Lunn PG, Austin S, Prentice AM, Whitehead RG. Influence of Maternal diet on Plasma-Prolactin Levels During Lactation. The Lancet. 1980:623–625. doi: 10.1016/s0140-6736(80)91119-8. [DOI] [PubMed] [Google Scholar]
- Lunn PG, Austin S, Prentice AM, Whitehead RG. The effect of improved nutrition on plasma prolactin concentrations and postpartum infertility in lactating Gambian women. The American Journal of Clinical Nutrition. 1984 February ;39:227–235. doi: 10.1093/ajcn/39.2.227. [DOI] [PubMed] [Google Scholar]
- Mace R. Evolutionary Ecology of Human Life History. Anim Behav. 2000;59:1–10. doi: 10.1006/anbe.1999.1287. [DOI] [PubMed] [Google Scholar]
- Maliqueo M, Sir-Petermann T, Salazar G, Pérez F, Racabarren SE, Wildt L. Resumption of ovarian function during lactational amenorrhea in breastfeeding women with polycystic ovarian syndrome: metabolic aspects. Hum Reprod. 2001;16(8):1598–1602. doi: 10.1093/humrep/16.8.1598. [DOI] [PubMed] [Google Scholar]
- Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, Klisovic D, Nahhas RW, Landoll JD. Leptin Is Inversely Related to Age at Menarche in Human Females. J Clin Endocrinol Meta. 1997;82(10):3239–3245. doi: 10.1210/jcem.82.10.4280. [DOI] [PubMed] [Google Scholar]
- McNeilly AS. Lactational Amenorrhea. Neuroendocrinology II. 1993;22(1):59–73. [PubMed] [Google Scholar]
- McNeilly AS. Lactational control of reproduction. Reproduction, Fertility and Development. 2001;13:583–590. doi: 10.1071/rd01056. [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Tay CCK, Glasier AF. Physiological Mechanisms Underlying Lactational Amenorrhea. In: Campbell KL, Wood JW, editors. Human Reproductive Ecology: Interactions of Environment, Fertility, and Behavior. New York: Annals of the new Yourk Academy of Sciences; 1994. [DOI] [PubMed] [Google Scholar]
- Miller E, editor. Peoples of the Gran Chaco. Westport, CT: Bergin & Garvey; 1999. [Google Scholar]
- Mircea CN, Lujan M, Pierson Ra. Metabolic Fuel and Clinical Implications for Female Reproduction. Journal of Obstetrics and Gynaecology Canada (JOGC) 2007;29(11):887–902. doi: 10.1016/S1701-2163(16)32661-5. [DOI] [PubMed] [Google Scholar]
- Mozumder AB, Barkat EK, Kane TT, Levin A, Ahmed S. The effect of birth interval on malnutrition in Bangladeshi infants and young children. J Biosoc Sci. 2000;32(3):289–300. doi: 10.1017/s0021932000002893. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch K, McConnell D, Strassmann BI, England BG. Stress and female reproductive function: A study of daily variations in cortisol, gonadotrophins, and gonadal steroids in a rural Mayan population. Am J Hum Biol. 2004;16(5):523–532. doi: 10.1002/ajhb.20057. [DOI] [PubMed] [Google Scholar]
- Nuñez de la Mora A, Chatterton R, Choudhury OA, Napolitano DA, Bentley GR. Childhood Conditions Influence Adult Progesterone Levels. PLoS Med. 2007;4(5):e167–e190. doi: 10.1371/journal.pmed.0040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter-Brick C. Lactation, birth spacing and maternal work-loads among two castes in rural Nepal. J Biosoc Sci. 1991;23:137–154. doi: 10.1017/s0021932000019179. [DOI] [PubMed] [Google Scholar]
- Peng YK, Hight-Laukaran V, Peterson AE, Perez-Escamilla R. Maternal nutritional status is inversely associated with lactational amenorrhea in Sub-Saharan Africa: results from demographic and health surveys II and III. J Nutr. 1998;128(10):1672–1680. doi: 10.1093/jn/128.10.1672. [DOI] [PubMed] [Google Scholar]
- Perera AD, Verbalis JG, Mikuma N, Majumdar SS, Plant TM. Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta) Endocrinology. 1993;132(4):1723–1728. doi: 10.1210/endo.132.4.8462472. [DOI] [PubMed] [Google Scholar]
- Poppe K, Velkeniers B, Glinoer D. Nature Clinical Practice Endocrinology & Metabolism. 2008. The role of thyroid autoimmunity in fertility and pregnancy. Published on line 27 May 2008. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Cataldo N, Rosenwaks Z, Giudice L. The Insulin-Related Ovarian Regulatory System in Health and Disease. Endocrine Reviews. 1999;20(4):535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Kalin M. The Gonadotropic Function of Insulin. Endocrine Reviews. 1987;8(2):132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- Rahman M, Mascie-Taylor C, Rosetta L. The Duration of Lactational Amenorrhea in Urban Bangladesh Women. J Biosoc Sci. 2002;34:75–89. [PubMed] [Google Scholar]
- Robinson JJ, Ashworth CJ, Rooke JA, Mitchell LM, McEvoy TG. Nutrition and fertility in ruminant livestock. Animal Feed Science and Technology. 2006;126:259–276. [Google Scholar]
- Robson SL, Wood B. Hominin life history: reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R, Castillo E, Voirol MJ, Glauser M, Rey JP, Gaillard RC, Vollenweider P, Pralong FP. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology. 2006;147:816–26. doi: 10.1210/en.2005-0728. [DOI] [PubMed] [Google Scholar]
- Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Zhou D, Blum RM. Leptin and Metabolic Control of Reproduction. Horm Behav. 2000;37:306–326. doi: 10.1006/hbeh.2000.1590. [DOI] [PubMed] [Google Scholar]
- Sellen DW. Lactation, Complementary Feeding, and Human Life History. In: Hawkes K, Paine R, editors. The Evolution of Human Life History. Santa Fe, New Mexico: School of American Research Press; 2006. pp. 155–196. [Google Scholar]
- Simondon KB, Delaunay V, Diallo A, Elguero E, Simondon F. Lactational amenorrhea is associated with child age at the time of introduction of complementary food: a prospective cohort study in rural Senegal, West Africa. Am J Clin Nutr. 2003;78:154–161. doi: 10.1093/ajcn/78.1.154. [DOI] [PubMed] [Google Scholar]
- Smith JT. Kisspeptin signalling in the brain: Steroid regulation in the rodent and ewe. Brain Research Reviews. 2008;57:288–298. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Szymanski LA, Schneider JE, Friedman MI, Ji H, Kurose Y, Blache D, Rao A, Dunshea FR, Clarke IJ. Changes in insulin, glucose and ketone bodies, but not leptin or body fat content precede restoration of luteinising hormone secretion in ewes. J Neuroendocrinol. 2007;19:449–60. doi: 10.1111/j.1365-2826.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- Tay CCK, Glasier AF, McNeilly AS. Twenty-four hour patterns of prolactin secretion during lactation and the relationship to suckling and the resumption of fertility in breast-feeding women. Hum Reprod. 1996;11(5):950–955. doi: 10.1093/oxfordjournals.humrep.a019330. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. Ghrelin: novel regulator of gonadal function. J Endocrinol Invest. 2005;28(5 Suppl):26–29. [PubMed] [Google Scholar]
- Tena-Sempere M. KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83(5–6):275–281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- Tigas S, Sunehag A, Haymond M. Metabolic adaptation to feeding and fasting during lactation in humans. J Clin Endocrinol Meta. 2002;87(1):302–307. doi: 10.1210/jcem.87.1.8178. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Yorozu S, Woodside B. Y1 receptor activation is involved in the effect of exogenous neuropeptide Y on pup growth and the early termination of lactational diestrus in the postpartum rat. J Neuroendocrinol. 2002;14:354–60. doi: 10.1046/j.0007-1331.2002.00785.x. [DOI] [PubMed] [Google Scholar]
- Tracer DP. Fertility-Related Changes in Maternal Body Composition Among the Au of Papua New Guinea. Am J Phys Anthropol. 1991;85:393–405. doi: 10.1002/ajpa.1330850404. [DOI] [PubMed] [Google Scholar]
- Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab. 1995;80(1):172–8. doi: 10.1210/jcem.80.1.7829608. [DOI] [PubMed] [Google Scholar]
- Valeggia C, Ellison PT. Lactation, Energetics, and Postpartum Fecundity. In: Ellison PT, editor. Reproductive Ecology and Human Evolution. New York: Aldine de Gruyter; 2001a. pp. 85–106. [Google Scholar]
- Valeggia C, Ellison PT. DRCLAS News. 2001b. Nutrition, breastfeeding, and fertility: Changing lifestyles and policy implications. [Google Scholar]
- Valeggia C, Ellison PT. Impact of breastfeeding on anthropometric changes in women of an indigenous population in transition in Formosa, Argentina. Am J Hum Biol. 2003;(15):717–724. doi: 10.1002/ajhb.10202. [DOI] [PubMed] [Google Scholar]
- Valeggia C, Ellison PT. Lactational amenorrhea in well nourished Toba women of Formosa, Argentina. J Biosoc Sci. 2004;36(5):573–595. doi: 10.1017/s0021932003006382. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. Nursing Behaviour and its Relation to Duration of Post-partum Amenorrhoea in an Andean Community. Journal of Biosocial Science. 1989;21:145–160. doi: 10.1017/s0021932000017843. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. Why not so great is still good enough: Flexible responsiveness in human reproductive functioning. In: Ellison PT, editor. Reproductive Ecology and Human Evolution. New York: Aldine de Gruyter; 2001. pp. 179–202. [Google Scholar]
- Vitzthum VJ, Bentley GR, Spielvogel H, Caceres E, Thornburg J, Jones L, Shore S, Hodges KR, Chatterton RT. Salivary progesterone levels and rate of ovulation are significantly lower in poorer than in better-off urban-dwelling Bolivian women. Hum Reprod. 2002;17(7):1906–1913. doi: 10.1093/humrep/17.7.1906. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ, Spielvogel H. Epidemiological transitions, reproductive health, and the Flexible Response Model. Economics and human biology. 2003;1(2):223–42. doi: 10.1016/S1570-677X(03)00037-6. [DOI] [PubMed] [Google Scholar]
- Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81(1):302–309. doi: 10.1210/jcem.81.1.8550768. [DOI] [PubMed] [Google Scholar]
- Wood JW. In: Dynamics of Human Reproduction: Biology, Biometry, Demography. Hrdy SB, editor. New York: Aldine de Gruyter; 1994. [Google Scholar]
- Wood JW, Lsi D, Johnson PL, Campbell KL, Maslar IA. Lactation and birth spacing in highland New Guinea. J Bios Sci Suppl. 1985;9:159–173. doi: 10.1017/s0021932000025190. [DOI] [PubMed] [Google Scholar]
- Woodside B, Beaule C, Lauay C. Chronic neuropeptide Y infusion during lactation suppresses pup growth and reduces the length of lactational infertility in rats. Horm Behav. 2002;41:59–69. doi: 10.1006/hbeh.2001.1737. [DOI] [PubMed] [Google Scholar]
- Woodside B, Abizaid A, Walker C. Changes in leptin levels during lactation: implications for lactational hyperphagia and anovulation. Horm Behav. 2000;37:353–65. doi: 10.1006/hbeh.2000.1598. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Jenkins CL, Stallings JF, Lai D. Attenuation of Nursing-Related Ovarian Suppression and High Fertility in Well-Nourished, Intensively Breast-Feeding Amele Women of Lowland Papua New Guinea. J Biosoc Sci. 1993;25:425–443. doi: 10.1017/s0021932000021817. [DOI] [PubMed] [Google Scholar]