Abstract
Williams Syndrome is a developmental disorder with a genetic basis, which results in an uneven cognitive profile with relatively strong language skills and severely impaired visuospatial abilities. To better understand the brain structure underlying this profile, we compared individuals with Williams Syndrome to controls using multimodal neuroimaging data and new analytic methods (diffeomorphic mapping and atlas-based analysis). People with Williams Syndrome had basal ganglia atrophy, while the fusiform, the medium temporal gyri, and the cerebellar cortex were relatively preserved. The right superior longitudinal fasciculus, the left fronto-occipital fasciculus, the caudate, and the cingulum demonstrated increased fractional anisotropy, while the corticospinal tract revealed decreased values. These findings may be linked to the uneven cognitive profile evident in Williams Syndrome.
Keywords: Williams syndrome, atlas-based analysis, MRI, diffusion tensor imaging, DTI
INTRODUCTION
Individuals with Williams Syndrome (WS) generally have mild to moderate retardation, but they also present a unique profile of cognitive functioning. Their profile includes hypersociability and relatively strong language, along with severe deficits in visuospatial construction ability. This imbalance in cognitive ability has been associated with an uneven pattern of brain abnormalities, with relative loss of volume in specific brain regions. For instance, there is evidence of a disproportionate reduction in the occipital and superior parietal cortices with a relative preservation of the frontal lobes[1-4]. Other studies have detected increased cortical complexity[5,6] and differences in temporo-parietal gyrification[7, 8] specific to temporal and perisylvian cortical thickness[6]. White matter structure has not been examined extensively and is still poorly understood. Past diffusion tensor imaging (DTI)-based studies of Williams Syndrome have shown increased anisotropy in the right superior longitudinal fasciculus and a reduction of lateralization[9,10]. However, to the best of our knowledge, there has been no previous study investigating the anatomical status of the cortex, the basal ganglia, and the white matter within one framework that includes volumetric and intensity quantification among different MR contrasts.
We examined the gray and white matter in people with Williams Syndrome using conventional structural images (T1-weighted) and DTI. We utilized both structure-based (comparison of regions) and voxel-based (comparison of each voxel) whole-brain analyses. This study provides a comprehensive view of the structural brain differences in this disorder, and the method applied here has the potential to quantify anatomic-functional correlations and characterize individuals in the future.
METHODS
Subjects
Eight adolescents and young adults with Williams Syndrome (5F, age range: 14-27 years-old, mean: 18.6±4.4,) were tested. They were positively diagnosed by a geneticist and the FISH test (fluoride in situ hybridization) for a deletion in the Williams Syndrome region on chromosome 7[11]. Their profile was consistent with the typical Williams Syndrome profile reported in other studies, with a mean IQ of 67 ± 12 (Kaufman Brief Intelligence Test, KBIT-1), and relatively stronger performance on the Verbal subtest (i.e., picture naming) than on the Matrices subtest (picture based category matching) of the KBIT. They also showed the typical severe spatial impairment, as shown by performance below the 4th percentile (with one exception at the 12th percentile) on the Pattern Construction sub-test of the Differential Abilities Scales[12], which requires people to assemble copies of designs using individual blocks. Typically developing controls were also tested (5F, age range: 14-22 years-old, mean: 16.6±3.2). The controls were matched to the individuals with Williams Syndrome on gender and age, within a year except for one pair of adults (WS=27, control=22 years old). People with Williams Syndrome were recruited through the Williams Syndrome Association, and typically developing controls were recruited through local schools or other studies. This study was approved by the Johns Hopkins University Institutional Review Board.
Images
Images were acquired using a 3T MRI scanner and consisted of two DTI datasets (TR/TE=6600/70ms, EPI, 32 gradient directions, b=700 s/mm2), and an MPRAGE T1-WI (TR/TE=8/3.8ms) sequence. FOV, matrix, number of slices, and slice thickness were 212×212 mm, 96×96, 60, 2.2mm, respectively, for DTI; and 256×200mm, 256×256, 140, 1mm, respectively, for T1-WI. The DTI were processed using DtiStudio (www.MRIStudio.org) and the subsequent imaging process (Fig. 1) was performed using DiffeoMap and ROIEditor (www.MriStudio.org). One of the most commonly approaches used in imaging analysis is the voxel-based analysis, in which subject images are transformed (or mapped) to a common brain image (the atlas), and the differences are compared point-by-point, i.e., voxel-by-voxel. One issue is that there are many voxels to be analyzed (more than 1 million), which leads to high noise and low statistical power. In this study, we combined voxel-based analysis with an automated image parcellation (atlas-based analysis), which allowed studying the brain in a structure-by-structure basis. This was possible by using the large deformation diffeomorphic metric mapping (LDDMM)[14] as the normalization algorithm. LDDMM has a high accuracy on matching the shapes of different brains, which is not usually achieved with other frequently used normalization methods. By this approach, each individual brain was automatically divided into 211 anatomically defined regions, and the image parameters were analyzed both voxel-by-voxel and structure-by-structure (Fig.1).
Figure 1.
Schematic diagram of the image normalization and quantification process. The images were normalized to a single-subject atlas, extensively parcelled in 3 dimensions[13]. For the analysis of diffusivity and white matter regional volumes, the normalization process was based on DTI contrasts (fractional anisotropy (FA) and b0 images, red labels), while, for the gray matter volumetric analysis, the normalization process was based on T1-WIs (blue labels). For the “forward” transformation, the subject images were normalized to the template first linearly, then by a highly accurate elastic algorithm (LDDMM). After this procedure, all brains have a shape similar to that of the atlas, and it is possible to compare subjects and controls voxel-by-voxel (voxel-based analysis - VBA). For the “backward” transformation, the parcellation map defined in the atlas is reverse-transformed to the original MRI. This enabled the 3-D automated segmentation of the original images and the structure-by-structure analysis (atlas-based analysis - ABA).
The differences between individuals with Williams Syndrome and controls were assessed using the non-parametric Mann-Whitney test, at a p-value threshold of 0.05, corrected for multiple comparisons by the false discovery rate (FDR). Two types of volumetric analysis were performed: in the native space, and normalized by the brain volume. Normalizing the regional volumes by the total brain volume minimizes the effect of the global atrophy, allowing the identification of areas disproportionately reduced or relatively preserved (those that remain smaller or those that are detected as bigger, respectively, when all the brains are normalized to the same volume).
Manual measurements for accuracy assessment
The accuracy of the normalization and the automated parcellation was demonstrated in our previous publications for normal and populations with different types of pathology [15-18]. In the current study, the caudate volume, and the caudate and the corticospinal tract fractional anisotropy were found to be abnormal in people with Williams Syndrome. Since these results were unexpected, we validated them by asking two raters, blinded to diagnosis and previous findings, to manually delineate the caudate and to reconstruct the corticospinal tract by tractography[19]. We then compared these results with those derived from the automated parcellation.
RESULTS
Global volumetric analysis
The total white matter volume was 15% smaller in WS than in controls (WS: 542.32±42.68cm3; controls: 632.96±17.92cm3; p<0.001), which was greater than the total brain volume reduction of 11% (WS: 1135.23±106.97cm3; controls: 1267.16±54.95cm3; p=0.01). Note that the total white matter and gray matter volumes were calculated from the SPM8 intensity-based segmentation maps; therefore, some regions of the basal ganglia, particularly the thalamus, were included in the white matter compartment, which might have contributed to the greater proportion of white matter reduction, rather than gray matter, that we detected.
Regional volumetric analysis
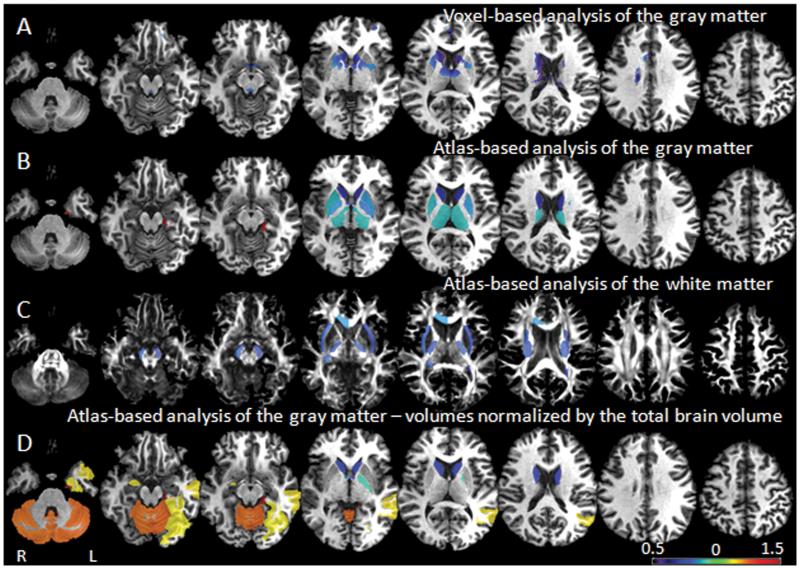
The voxel-based analysis detected small areas of atrophy in the basal ganglia, particularly in the caudate (Fig. 2A). The atlas-based analysis detected the same (Fig. 2B), but in a more extensive and symmetric pattern. The left parahippocampal gyrus was the only region that was larger in individuals with Williams Syndrome than in controls. In the white matter, using atlas-based analysis, we detected significant atrophy in the projection fibers (the cerebral peduncles, the posterior limb of the internal capsules, the external capsules, the anterior commissures) and commissural fibers (the corpus callosum, the left tapetum, and the right fornix/stria terminallis), which was again highly symmetric (Fig. 2C).
Figure 2.
Volumetric differences between groups. Colors code the ratio of Williams Syndrome / controls in areas with significant differences between groups. Using voxel-based analysis (A) we detected atrophy in a cluster of voxels in the basal ganglia. The atlas-based analysis of the gray matter (B) and the white matter (C) showed that the basal ganglia, deep white matter regions such as the internal and external capsules, and the commissural tracts such as the anterior commissure, the left tapetum, the right fornix, and the corpus callosum, were reduced in subjects with Williams Syndrome compared to controls. After normalization to a common brain volume, the atlas-based analysis (D) showed atrophy of the caudate and the left globus palladium in Williams Syndrome, and relative preservation of the gray matter of the cerebellum, the right amygdala, the left fusiform, parahippocampus, and the medial temporal gyri.
Analysis of “relative” volumes (regional volumes normalized by the total brain volume)
The atlas-based analysis (Fig. 2D) indicated that the gray matter of the cerebellum, the left fusiform, the parahippocampus, the medium temporal gyrus, and the right amygdala were relatively preserved in people with Williams Syndrome compared to controls, while the volume of the caudate and left globus pallidum were significantly smaller, even after the global volume reduction was considered. In addition, the anterior commissure volume was smaller in Williams Syndrome. Fig. 3 summarizes the significant results. The voxel-based analysis did not show significant differences between groups.
Figure 3.
Summary of the differences between Williams Syndrome and controls in terms of absolute volume (A), and relative volume (i.e., regional volumes normalized by the total brain volume, B), detected using atlas-based analysis. PHG=parahippocampal gyrus, Tap=tapetum, Caud=caudate, Put=putamen, Thal=thalamus, GP=globus pallidum, CP=cerebral peduncle, PLIC=posterior limb of internal capsule, EC=external capsule, GCC=genu of corpus callosum. Fx/ST=fornix/stria terminalis, cereb=gray matter of cerebellum, Fu=fusiform gyrus, MTG=medium temporal gyrus, Amyg=amygdala, AC=anterior comissure, L=left, R=right.
Validation of the automated parcellation by manual delineation of structures
The volume of the caudate, as measured by manual delineation, was also significantly smaller (p-value<0.05) in Williams Syndrome than in controls, in both native and volume-normalized spaces. The indices of agreement between automated and manual delineations were kappa=0.79, Jaccardi=0.67, Dice=0.8, specificity=0.99, and sensitivity=0.84, indicating the accuracy of the automated parcellation.
Analysis of diffusivity
The atlas-based analysis (Fig. 4) showed significantly higher fractional anisotropy on the right superior longitudinal fasciculus, the left superior fronto-occipital fasciculus, the cingulum, and the caudate in Williams Syndrome, while the fractional anisotropy of the projection tracts (corticospinal tract, the cerebral peduncles, and the posterior limb of the internal capsule) was significantly lower. The fractional anisotropy of the caudate, as manually delineated, and of corticospinal tract, as defined by tractography, was also significantly lower in Williams Syndrome (p-value<0.05), confirming the automated measurements. The right cerebral peduncle and the left corticospinal tract showed increased radial diffusivity (an index of axonal integrity and myelination status), which was decreased in the left cingulum and in the right anterior limb of the internal capsule. Axial diffusivity (related to axonal status and brain architectural organization) was significantly reduced in the left external capsule and in the right amygdala. The voxel-based analysis did not show significant differences between groups in terms of diffusivity indices.
Figure 4.
Differences in fractional anisotropy (FA) detected by the atlas-based analysis. Colors code the ratio of Williams Syndrome (WS) / normal controls (NC) in regions with statistically significant differences. The plots contain the actual fractional anisotropy data (WS=red, controls=blue). The bar graphic shows that, in controls, the superior longitudinal fasciculus (SLF) has a higher fractional anisotropy on the left, while, in people with Williams Syndrome, fractional anisotropy is higher on the right. The plot of fractional anisotropy in the right superior longitudinal fasciculus and the corticospinal tract shows total segregation between Williams Syndrome and controls. In the right bottom corner, Williams Syndrome / control ratios of radial and axial diffusivity are shown for the areas that displayed significant fractional anisotropy differences.
DISCUSSION
Volumetric Findings
The majority of the findings in our study confirm previous reports, attesting to the accuracy of our approach for measuring MRI contrasts in multiple brain regions in a single framework. In terms of volume, the areas most affected were the basal ganglia, the projection, and the commissural tracts, all of which were significantly decreased in people with Williams Syndrome compared to controls (Fig. 2). When the regional volumes were corrected for differences in the brain volume, we found relative preservation of the left fusiform gyrus, the parahippocampal gyrus, and the medial temporal gyrus, as well as the right amygdala and the gray matter of the cerebellum. Previous voxel-based morphometry studies had already shown a disproportionate reduction of commissural tracts, and preservation of these gyri[1,7,20-23] . Particularly in the left medium temporal gyrus, the abnormality is possibly related to abnormal gyrification in the planum temporale, associated with the loss of the normal hemispheric asymmetry[8] .
The disproportionate reductions in the basal ganglia were recently reported by a few studies[1, 3], but this result has not been emphasized previously. This is congruent with functional MRI indications of lower neural activity in Williams Syndrome in the bilateral striatum[24], suggesting striatal dysfunction. It is important to point out, however, that, despite the temptation to establish direct correlations between anatomy and function, it is difficult to connect the complex pattern of sparing and abnormality in our findings to cognitive function; alterations in either direction (thickening or thinning) may reflect deficiencies or compensatory activity.
Diffusivity findings
In terms of diffusivity, the fractional anisotropy increase in both the caudate and the cingulum was an unexpected finding. Previous studies found an increase of volume and number of fibers in the cingulum[1,10], but diffusivity alterations, that are thought to indicate white matter integrity, were not mentioned. However, the increased fractional anisotropy in the superior longitudinal fasciculus that we found has been recently reported[9,10]. Although not statistically significant, we also found a tendency toward increasing axial diffusivity in the right superior longitudinal fasciculus (Fig. 4). In addition to the increased fractional anisotropy in the left fronto-occipital fibers, this suggests the possibility of abnormal lateralization with preferential right hemisphere involvement in Williams Syndrome[25]. Consistent with Marenco et al.[10], we detected decreased fractional anisotropy in the corticospinal tract. In fact, the fractional anisotropy in the superior longitudinal fasciculus, combined with the fractional anisotropy in any segment of corticospinal tract, were striking characteristics of our Williams Syndrome participants, and distinguished them from controls (Fig. 4).
Methodological Notes: Differences between voxel- and atlas-based analyses
This study demonstrates that the atlas-based analysis can characterize 3-D volumetric and diffusivity abnormalities in Williams Syndrome more sensitively than the voxel-based analysis. In the atlas-based analysis, rather than comparing million of voxels, we are comparing only hundreds of structures, where each structure corresponded to a group of voxels with similar anatomic characteristics. Therefore, we are reducing the noise, increasing the statistical power, and facilitating the biological interpretation. The atlas-based analysis works as well as the manual definition of regions of interest,[15,17,18] with important advantages: is not time-consuming, since it is automated, is evaluator-independent, and captures the full anatomic pattern, because it covers the whole brain. Also, the measurements acquired with atlas-based analysis derive directly from the native images of each individual, reducing the imprecision produced by the imaging post-processing. Another advantage of this method is the possibility of transforming the subject image to the atlas or, inversely, the atlas (or the structural parcellation defined in this atlas) to the subject image (Fig. 1). Therefore we can perform voxel- and atlas-based analysis, which are complementary approaches, at the same time. It also allows characterizing each brain structure by many different features, such as volume and various intensities. This quantitative structural characterization might be ultimately related to clinical characteristics and different outcomes, potentially contributing to the understanding of a given disorder.
Limitations
The main limitation of this study is the small sample size. The brain imaging analysis typically involves multiple comparisons, and therefore the likelihood of false positive associations is high. To control for these “type I” statistical errors, we corrected the level of significance using the false discovery rate (FDR), as mentioned in the Methods. It is possible, however, that we still have false negatives, i.e., that we are failing to detect regional differences between groups, particularly in cortex, where the degree of inter-subject variability is higher. This could explain, for example, why we did not detect atrophy in the parietal and occipital cortex of individuals with Williams Syndrome, while other groups did [1-4] (we detected this tendency, though at a non-significant level). In favor of the reliability of our findings, we can point that our results are mostly in agreement with previous studies, they were validated by manual delineation of structures (gold-standard), and were corrected for false positives. False negatives, if they exist, can be further evaluated by increasing the sample size.
CONCLUSION
Using multiple MRI modalities, we observed that individuals with Williams Syndrome have atrophy in the basal ganglia and white matter, while fusiform, medium temporal gyri, and the cerebellar cortex are relatively preserved. The right superior longitudinal fasciculus, the left fronto-occipital fasciculus, the caudate, and the cingulum have increased fractional anisotropy (an index of white matter integrity), while the corticospinal tract shows decreased values.
Acknowledgments
Source of funding: This publication was made possible by NIH grants # UL1 RR 025005 (AVF); P41RR15241, RO1AG022012, and RO1NS058299 (SM), R21AG033774, P50AG05146, and R01HD065955-01A1 (KO), F32 HD42346, and KO1 081191 (KOH); RO1 NS 050876 (BL); and M01-RR00052 (KOH, BL). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of any of these Institutes.
Footnotes
Conflicts of interest: None declared
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, et al. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert MA, Hu D, Eliez S, Bellugi U, Galaburda A, Korenberg J, et al. Evidence for superior parietal impairment in Williams syndrome. Neurology. 2005;64:152–153. doi: 10.1212/01.WNL.0000148598.63153.8A. [DOI] [PubMed] [Google Scholar]
- 3.Chiang MC, Reiss AL, Lee AD, Bellugi U, Galaburda AM, Korenberg JR, et al. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage. 2007;36:1096–1109. doi: 10.1016/j.neuroimage.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, et al. IV. Neuroanatomy of Williams syndrome: a high-resolution MRI study. J Cogn Neurosci. 2000;12(Suppl 1):65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- 5.Gaser C, Luders E, Thompson PM, Lee AD, Dutton RA, Geaga JA, et al. Increased local gyrification mapped in Williams syndrome. Neuroimage. 2006;33:46–54. doi: 10.1016/j.neuroimage.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Eckert MA, et al. Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J Neurosci. 2005;25:4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert MA, Tenforde A, Galaburda AM, Bellugi U, Korenberg JR, Mills D, et al. To modulate or not to modulate: differing results in uniquely shaped Williams syndrome brains. Neuroimage. 2006;32:1001–1007. doi: 10.1016/j.neuroimage.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J Neurosci. 2006;26:5470–5483. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, et al. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci. 2007;27:11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marenco S, Siuta MA, Kippenhan JS, Grodofsky S, Chang WL, Kohn P, et al. Genetic contributions to white matter architecture revealed by diffusion tensor imaging in Williams syndrome. Proc Natl Acad Sci U S A. 2007;104:15117–15122. doi: 10.1073/pnas.0704311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, et al. Hemizigozity at the elastin locus in a developmental disorder, Williams Syndrome. Nat Genet. 1993;5:11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- 12.Elliott CD. Differential abilities scales. Harcourt Brace Jovanovich; San Diego, CA: 1990. [Google Scholar]
- 13.Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceritoglu C, Oishi K, Li X, Chou MC, Younes L, Albert M, et al. Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. Neuroimage. 2009;47:618–627. doi: 10.1016/j.neuroimage.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faria AV, Zhang J, Oishi K, Li X, Jiang H, Akhter K, et al. Atlas-based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and applications for automated abnormality detection. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participantstlas. Neuroimage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faria AV, Hoon A, Stashinko E, Li X, Jiang H, Mashayekh A, et al. Quantitative analysis of brain pathology based on MRI and brain atlases--applications for cerebral palsy. Neuroimage. 2011;54:1854–1861. doi: 10.1016/j.neuroimage.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, et al. Multi-contrast human neonatal brain atlas: Application to normal neonate development analysis. Neuroimage. 2011;56:8–20. doi: 10.1016/j.neuroimage.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boddaert N, Mochel F, Meresse I, Seidenwurm D, Cachia A, Brunelle F, et al. Parieto-occipital grey matter abnormalities in children with Williams syndrome. Neuroimage. 2006;30:721–725. doi: 10.1016/j.neuroimage.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt JE, Eliez S, Bellugi U, Reiss AL. Analysis of cerebral shape in Williams syndrome. Arch Neurol. 2001;58:283–287. doi: 10.1001/archneur.58.2.283. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt JE, Eliez S, Warsofsky IS, Bellugi U, Reiss AL. Enlarged cerebellar vermis in Williams syndrome. J Psychiatr Res. 2001;35:225–229. doi: 10.1016/s0022-3956(01)00024-3. [DOI] [PubMed] [Google Scholar]
- 23.Tomaiuolo F, Di Paola M, Caravale B, Vicari S, Petrides M, Caltagirone C. Morphology and morphometry of the corpus callosum in Williams syndrome: a T1-weighted MRI study. Neuroreport. 2002;13:2281–2284. doi: 10.1097/00001756-200212030-00022. [DOI] [PubMed] [Google Scholar]
- 24.Mobbs D, Eckert MA, Mills D, Korenberg J, Bellugi U, Galaburda AM, et al. Frontostriatal dysfunction during response inhibition in Williams syndrome. Biol Psychiatry. 2007;62:256–261. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]