Abstract
Many different species of acidophilic prokaryotes, widely distributed within the domains Bacteria and Archaea, can catalyze the dissimilatory oxidation of ferrous iron or reduction of ferric iron, or can do both. Microbially mediated cycling of iron in extremely acidic environments (pH < 3) is strongly influenced by the enhanced chemical stability of ferrous iron and far greater solubility of ferric iron under such conditions. Cycling of iron has been demonstrated in vitro using both pure and mixed cultures of acidophiles, and there is considerable evidence that active cycling of iron occurs in acid mine drainage streams, pit lakes, and iron-rich acidic rivers, such as the Rio Tinto. Measurements of specific rates of iron oxidation and reduction by acidophilic microorganisms show that different species vary in their capacities for iron oxido-reduction, and that this is influenced by the electron donor provided and growth conditions used. These measurements, and comparison with corresponding data for oxidation of reduced sulfur compounds, also help explain why ferrous iron is usually used preferentially as an electron donor by acidophiles that can oxidize both iron and sulfur, even though the energy yield from oxidizing iron is much smaller than that available from sulfur oxidation. Iron-oxidizing acidophiles have been used in biomining (a technology that harness their abilities to accelerate the oxidative dissolution of sulfidic minerals and thereby facilitate the extraction of precious and base metals) for several decades. More recently they have also been used to simultaneously remediate iron-contaminated surface and ground waters and produce a useful mineral by-product (schwertmannite). Bioprocessing of oxidized mineral ores using acidophiles that catalyze the reductive dissolution of ferric iron minerals such as goethite has also recently been demonstrated, and new biomining technologies based on this approach are being developed.
Keywords: acidophiles, iron, oxidation, reduction
Energetic Constraints on the Oxido-Reduction of Iron at Low pH
Iron is the most abundant transition metal in the lithosphere, as well as the most abundant element (on a weight basis) in planet earth. In contrast, the concentration of soluble, bio-available iron often limits the growth of primary producing organisms in many aquatic (e.g., marine) and terrestrial (e.g., calcareous soils) environments. However, extremely acidic (pH < 3) environments tend to buck this trend, though there are exceptions, such as acidic sites that develop in calcareous substrata due to the microbiological oxidation of hydrogen sulfide (e.g., in the Frasissi cave system, Italy; Macalady et al., 2007). There are two main reasons why iron is more bio-available (often to the point at which it becomes toxic to most life forms) at low pH: (i) extremely acidic environments, both natural and man-made, are often associated with the oxidative dissolution of sulfide minerals, many of which contain iron (including the most abundant of all such minerals, pyrite; FeS2), and (ii) both ionic forms of (uncomplexed) iron are far more soluble (especially ferric iron) at low pH than at circum-neutral pH. Metallic (zero-valent) iron is rare in the lithosphere, while the dominant non-complexed ionic form of this metal in the environment is mostly dictated by its aeration status, with ferrous iron [iron (II)] being more relatively abundant in anoxic environments and ferric iron [iron (III)] in aerobic sites. This is also the case in extremely acidic environments, though the causative agents of ferrous iron oxidation are often very different in the two situations. At pH 7, the spontaneous (abiotic) rate of oxidation in an oxygen-saturated solution containing 100 mg ferrous iron L−1 is 8.4 mg min−1, while at pH 2 the corresponding rate is 8.4 × 10−7 μg min−1 (using the equation described in Stumm and Morgan, 1981). Spontaneous chemical oxidation of (uncomplexed) iron can, therefore, be rapid at circum-neutral pH, though it is much slower where oxygen concentrations are low, e.g., the oxidation rate of the same hypothetical solution (pH 7, containing 100 mg ferrous iron L−1) is much slower (0.47 mg min−1) when the dissolved oxygen concentration is 0.5 mg L−1, as opposed to 9 mg L−1. An important consequence of this is that acidophilic iron-oxidizing bacteria and archaea can exploit ferrous iron as a resource in oxygen-saturated waters where, in contrast to their neutrophilic counterparts, abiotic iron oxidation is not competitive.
The greatly enhanced solubility of ferric iron at low pH derives chiefly from the fact that ferric (oxy-)hydroxide phases have very small solubility products. For example, the log Ksp of Fe(OH)3 is −38.6 at 25°C (Monhemius, 1977) so that the theoretical maximum concentrations of non-complexed ferric iron at pH 2 is 140 mg L−1, compared to 140 ng L−1 at pH 4 and 0.14 fg L−1 at pH 7. However, the most significant ferric iron mineral phases that form in sulfate-containing low pH environments are jarosites [e.g., KFe3(SO4)2(OH)6] and schwertmannite [ideal formula Fe8O8(OH)6SO4; Bigham et al., 1996] the solubility products of which have not been determined. Schwertmannite is metastable with respect to goethite (α-FeO.OH) and recrystallizes to the latter in an acid-generating reaction:
At very low pH values, and in the presence of monovalent cations, schwertmannite is also metastable with respect to jarosite (Regenspurg et al., 2004). In addition, ferric iron forms complexes with both sulfate and hydroxyl ions, and the dominant soluble forms of this ionic species in acidic, sulfate liquors are Fe(SO4)2− and Fe(SO4)+ rather than uncomplexed Fe3+ (Welham et al., 2000), which influences its solubility.
The standard redox potential of the ferrous/ferric couple at pH 2 is generally quoted as +770 mV. However, the fact that ferric iron is complexed by sulfate ions in most natural and man-made extremely acidic environments influences this value, and the standard redox couples of both the Fe(SO4)2− and Fe(SO4)+/Fe2+ couples are more electronegative than that of the uncomplexed metal. In sulfate solutions at pH 1, the standard potential of the ferrous/ferric couples has been calculated to be +697 mV (Welham et al., 2000), and it is slightly more electropositive (∼ + 720 mV) at pH 3 (O’Hara and Johnson, unpublished data). Although significant, these more electronegative redox potentials do still not allow the oxidation of iron to be coupled, at low pH, to potential alternative electron acceptors to oxygen, such as nitrate (the redox potential of the nitrate/nitrite couple is +430 mV). This bioenergetic constraint is one of the factors that differentiates the metabolic diversities of acidophilic and neutrophilic iron-oxidizing bacteria (Hedrich et al., 2011). However, the more electronegative redox potential of the ferrous/ferric sulfate complex couple, together with the more electropositive potential of the oxygen/water couple in acidic liquors (1.12 V at pH 2) than at pH 7 (+820 mV), means that the net potential difference between ferrous iron as electron donor and oxygen as electron acceptor is ∼420 mV at pH 2, which is far greater than values (50–350 mV) that are often quoted.
Biodiversity of Prokaryotes that Catalyze Redox Transformations of Fe at Low pH
Acidophilic microorganisms, as a generic group, have wide metabolic diversities, including the abilities to use solar and chemical (organic and inorganic) sources of energy, a variety of electron acceptors, and to use both inorganic and organic sources of carbon (and, in some cases, both). In phylogenetic terms, iron-metabolizing acidophiles are highly diverse, occurring in the domain Bacteria within the Proteobacteria (alpha-, beta-, and gamma-classes), Nitrospirae, Firmicutes, Actinobacteria, and Acidobacteria phyla, and in the domain Archaea within the Crenarchaeota and Euryarchaeota phyla. They also vary in their response to temperature, and include species that are psychrotolerant (e.g., Acidithiobacillus ferrivorans), mesophilic (e.g., Ferrimicrobium acidiphilum), thermotolerant/moderately thermophilic (e.g., Sulfobacillus acidophilus), and extremely thermophilic (e.g., Sulfolobus metallicus).
Chemolithotrophy (using inorganic chemicals as sole or major electron donors) is a particularly widespread and much studied trait among acidophilic bacteria and archaea (Johnson and Hallberg, 2009). Reduced forms of sulfur (including elemental sulfur) are used by diverse genera of acidophilic bacteria (e.g., Acidithiobacillus spp.) and archaea (e.g., Sulfolobus spp.), and some species of acidophiles can also use hydrogen as an energy source (e.g., Drobner et al., 1990). The first ferrous iron-oxidizing acidophile to be characterized was Acidithiobacillus (At.) ferrooxidans (strains variously named at the time as Thiobacillus ferrooxidans, Ferrobacillus ferrooxidans, and Ferrobacillus sulfooxidans). These were all described as chemo-autotrophic bacteria that appeared to vary in their capacities for oxidizing reduced sulfur. Subsequently, many different species of acidophilic bacteria, though fewer acidophilic archaea, have also been shown to be able to utilize the energy derived from oxidizing ferrous iron to support their growth, including species that are obligate and facultative heterotrophs, as well as other autotrophic species.
In contrast to iron oxidation at low pH, the ability of acidophiles to grow via the dissimilatory reduction of ferric iron was only discovered in the late 1980s/early 1990s, with this trait being first reported for an autotrophic bacterium (At. ferrooxidans; Pronk et al., 1991) and heterotrophic bacteria (Acidiphilium spp.; Johnson and McGinness, 1991). Currently, all acidophiles that are known to use ferric as an electron acceptor to support their growth are facultative reducers, in that all of them can also reduce molecular oxygen. Electron donors that are coupled to iron reduction are inorganic (sulfur or hydrogen) in the case of chemolithotrophic acidophiles such as At. ferrooxidans, and organic (e.g., glucose or glycerol) in the case of heterotrophic acidophiles, such as Acidiphilium spp.
Table 1 groups acidophiles that catalyze the dissimilatory redox transformations of iron into three categories, based in their abilities to oxidize ferrous iron, to reduce ferric iron or (depending on environmental conditions) to do both. One interesting fact is that the majority of iron-oxidizing bacteria can also reduce ferric iron and, in cases where this has been examined, use the latter to support their growth. Exceptions to this include Leptospirillum spp. and “Ferrovum (Fv.) myxofaciens,” though this is explained by the fact that these two genera are known to use only ferrous iron as an electron donor, thereby restricting them to an aerobic life-style and negating the possibility that they can grow by ferric iron respiration. In the case of the currently mis-named acidophile “Thiobacillus (T.) prosperous” (proposed to be re-classified as “Acidihalobacter prosperous”; Nicolle et al., 2009) there are currently no published data showing that this salt-tolerant acidophilic iron- and sulfur-oxidizer can grow anaerobically using sulfur as electron donor and ferric iron as electron acceptor. For all other iron-oxidizing acidophilic bacteria, the co-existence of iron oxidation and iron reduction capabilities suggests that the electron shuttle pathways involved might have some components in common. This, however, is patently not the case for heterotrophic acidophiles, such as Acidiphilium spp., that catalyze iron reduction but not iron oxidation. Also, Ohmura et al. (2002) found that At. ferrooxidans grown anaerobically using ferric iron as electron donor synthesized large amounts of an acid-stable c-type cytochrome whose reduced form was re-oxidized by ferric iron. Interestingly, neither species of Acidithiobacillus that oxidizes sulfur but not ferrous iron (At. thiooxidans and At. caldus) can grow anaerobically by ferric iron respiration. Although Brock and Gustafson (1976) had reported that At. thiooxidans can reduce ferric iron, Hallberg et al. (2001) showed that this only occurred in cell suspensions that were incubated aerobically, and that there was no corresponding increase in cell numbers. This suggests that iron reduction may have been mediated abiotically by a metabolite produced during sulfur oxidation and that At. thiooxidans cannot grow by ferric iron respiration. Interestingly, the situation with iron-oxidizing archaea is less clear, though it appears that the ability to couple the oxidation of sulfur to the reduction of ferric iron is commonplace among the Sulfolobales (Paul Norris, Warwick University, unpublished data). Brock and Gustafson (1976) also reported that a Sulfolobus sp. [quoted to be Sulfolobus (S.) acidocaldarius, though this is unlikely as this archaeon does not grow autotrophically as described in the paper] coupled sulfur oxidation to ferric iron reduction. Both classified genera of iron-oxidizing euryarchaeotes (Ferroplasma and Acidiplasma) can also reduce ferric iron, though the electron donor here is organic (e.g., yeast extract) rather than elemental sulfur (Dopson et al., 2004; Golyshina et al., 2009).
Table 1.
Acidophilic bacteria and archaea that have been reported to catalyze the dissimilatory oxidation of ferrous iron or reduction of ferric iron.
| Iron-oxidizing acidophiles | Iron-reducing acidophiles | Iron-oxidizing/reducing acidophiles |
|---|---|---|
| BACTERIA | ||
| Leptospirillum (L.)1 | Acidiphilium (A.)2/3 | Acidithiobacillus (At.)1 |
| L. ferrooxidans | A. cryptum2 | At. ferrooxidans |
| L. ferriphilum | A. acidophilum3 | At. ferrivorans |
| “L. ferrodiazotrophum” | A. angustum/rubrum2 | Acidiferrobacter thiooxydans1 |
| “Ferrovum myxofaciens”1 | A. organovorum2 | Ferrimicrobium acidiphilum2 |
| “Thiobacillus prosperous”1 | A. multivorum2 | Acidimicrobium ferrooxidans3 |
| Acidocella (Ac.)2 | Ferrithrix thermotolerans2 | |
| Ac. facilis | Sulfobacillus (Sb.)3 | |
| “Ac aromatica” | Sb. acidophilus | |
| Acidobacterium2 | Sb. thermosulfidooxidans | |
| Acb. capsulatum | Sb. benefaciens | |
| Acidobacterium spp. | Alicyclobacillus (Alb.) | |
| Alb. tolerans | ||
| Alb. ferrooxydans | ||
| Alb. aeris | ||
| Alb. pohliae | ||
| Alicyclobacillus sp. GSM | ||
| ARCHAEA | ||
| Sulfolobus (S.) | Ferroplasma (Fp.) spp. | |
| S. metallicus | Fp. acidiphilum | |
| S. tokodaii | “Fp. acidarmanus” | |
| Metallosphaera | Acidiplasma (Ap.) | |
| sedula | Ap. cupricumulans | |
| Ap. aeolicum | ||
1Obligate autotrophs; 2obligate heterotrophs; 3facultative autotrophs.
New insights into the nature of iron-oxidizing acidophiles and how they interact with each other and with other acidophilic microorganisms have arisen from the detailed microbiological and molecular studies carried out at the abandoned Richmond mine at Iron Mountain, California (e.g., Denef et al., 2010). Among other things, this research has highlighted the important role of the iron-oxidizing/reducing archaeon “Ferroplasma (Fp.) acidarmanus” in the dissolution of sulfide minerals in extremely acidic (pH < 1) moderately thermal (∼40°C) environments, and identified very small (<0.45 μm diameter) pleomorphic cells within acidic biofilms as novel archaeal lineages (designated “ARMAN”: Archaeal Richmond Mine Acidophilic Nanoorganisms; Baker et al., 2006).
Biochemical Mechanisms of Dissimilatory Oxidation and Reduction of Fe at Low pH
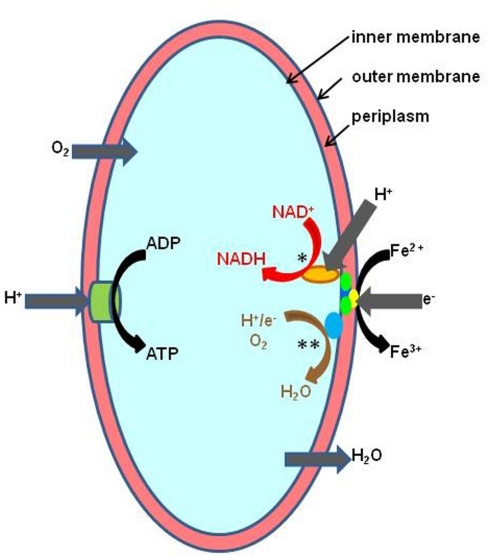
The pathway of dissimilatory iron oxidation has been elucidated for At. ferrooxidans, and genomic and proteomic studies have provided revealing insights of how this is mediated in other acidophiles (reviewed in Bonnefoy and Holmes, 2011). By maintaining a near-neutral cytoplasmic pH and living in acidic solutions, acidophiles have a “ready-made” trans-membrane pH gradient for generating ATP. However, continued influx of protons would lead to severe acidification of the cytoplasm and cell death if this was not counterbalanced by equivalent amounts of negatively charged ions or particles. Electrons derived from the oxidation of ferrous iron satisfy this requirement, and the reduction of molecular oxygen completes the energy-synthesizing (“downhill electron flow”) pathway (Figure 1). Electrons derived from ferrous iron are also used to generate reducing equivalents (e.g., NADH) but since the redox potential of the ferrous/ferric couple is far more electropositive than that of NAD+/NADH (−320 mV), energy is required to fuel this reaction (“uphill electron flow”) which is thought to derive from the proton motive force (Bonnefoy and Holmes, 2011). For mixotrophic and heterotrophic iron-oxidizers, the latter appears not to be the case as reducing equivalents can be generated from the oxidation of organic carbon.
Figure 1.
Schematic representation of ferrous iron oxidation by the Gram-negative autotrophic acidophile, At. ferrooxidans. Controlled influx of protons is used to generate ATP via the membrane-bound ATP synthetase complex (ATPase). Iron oxidation is mediated by a cytochrome located on the outer membrane and electrons transferred via periplasmic cytochromes and rusticyanin either to a terminal oxidase (“downhill pathway,” indicated in brown text and by **) where they are used to reduce oxygen, or used to reduce NAD+ (“uphill pathway,” indicated red text and by *) in a reaction also driven by the proton motive force across the inner membrane (Bonnefoy and Holmes, 2011).
The “uphill” and “downhill” electron transport pathways run concurrently in all iron-oxidizing chemoautotrophs, but the molecular complexes involved are often quite different, suggesting that iron oxidation in acidophiles has evolved independently on several occasions (Bonnefoy and Holmes, 2011). For example, the rus operon, which contains genes coding for the blue copper protein rusticyanin and several cytochromes, mediates ferrous oxidation in iron-oxidizing acidithiobacilli and, seemingly, in the salt-tolerant gammaproteobacterium “T. prosperous” (Nicolle et al., 2009). The role of another high potential iron-sulfur protein found in At. ferrivorans (at least one strain of which does not appear to contain rusticyanin) encoded by the iro gene, has yet to be resolved (Amouric et al., 2011). Interestingly, Ferroplasma spp. also contain a blue copper protein (sulfocyanin) which is also thought to be involved in iron oxidation in this euryarchaeote (Bonnefoy and Holmes, 2011). In contrast, rusticyanin/sulfocyanin has not been detected in Leptospirillum spp., where the electron shuttle pathway is thought to involve four or five different cytochromes (Bonnefoy and Holmes, 2011). In the iron-oxidizing crenarchaeotes [S. metallicus, S. tokodaii, and Metallosphaera (M.) sedula], the fox [Fe(II) oxidation] cluster, which houses genes coding for cytochrome b, electron transporters, and a terminal oxidase, appears to have an analogous role to the rus operon in At. ferrooxidans, an hypothesis strengthened by the fact that this cluster has not been found in Sulfolobus spp. (e.g., S. acidocaldarius) that do not oxidize ferrous iron.
Relatively little is known about how prokaryotes catalyze ferric iron reduction at low pH, for example whether this is mediated enzymatically (via “iron reductases”), indirectly via metabolic intermediates, or both. All Acidiphilium spp. are known to reduce ferric iron, though most strains do not grow under strictly anoxic conditions in the presence of ferric iron, (Johnson and McGinness, 1991; Bridge and Johnson, 2000) and growth coupled to ferric iron reduction is most readily observed in cultures inoculated under micro-aerobic conditions (Johnson and McGinness, 1991; Bridge and Johnson, 2000). However, Küsel et al. (2002) reported that one strain [Acidiphilium (A.) cryptum JF-5] could reduce ferric iron in oxygen-saturated media. Johnson and Bridge (2002) showed that ferric iron reduction was constitutive in obligately heterotrophic Acidiphilium SJH (also identified as a strain of A. cryptum) but was inducible in the facultative autotroph A. acidophilum. Low oxygen concentrations, rather than ferric iron, appeared to induce a putative “iron reductase” in A. acidophilum. Whole cell protein profiles of Acidiphilium SJH were similar for cells grown under oxygen-saturated or micro-aerobic conditions, while in contrast three additional proteins were detected in SDS-PAGE profiles of A. acidophilum cells that could reduce ferric iron compared to those where iron reduction had not been induced. A. organovorum and A. multivorum were also reported to have constitutive iron reduction systems, while iron reduction appeared to be inducible in A. rubrum. Interestingly, Küsel et al. (2002) reported that the ferric iron reduction enzyme(s) were not constitutive in A. cryptum JF-5, a strain that is closely related to Acidiphilium SJH. Recent annotation of the draft genome of another Acidiphilium strain (PM) has failed to identify a putative iron reductase gene (San Martin-Uriz et al., 2011). Elsewhere, an outer membrane cytochrome c has been implicated as having a role in ferric iron reduction by A. cryptum JF-5 (Magnuson et al., 2010), which is intriguing in the light of the induced production of an acid-stable c-type cytochrome by At. ferrooxidans when respiring on ferric iron (Ohmura et al., 2002). Whether c-type cytochromes are also involved in iron reduction by other species of heterotrophic bacteria that are known to reduce ferric iron (e.g., Acidocella and Acidobacterium; Coupland and Johnson, 2008) is unknown.
Measurement and Interpretation of Specific Rates of Iron Oxidation and Iron Reduction in Acidophiles
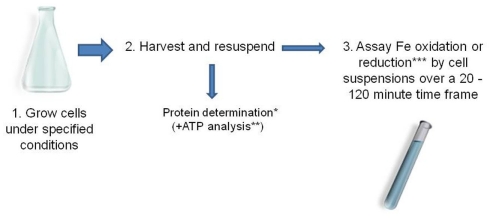
Determining specific rates of iron oxidation and iron reduction by acidophilic microorganisms is a relatively simple procedure. The data obtained illustrate how different species vary in their capacities for iron oxido-reduction, and how this is influenced by growth histories and environmental parameters. Such information is also of fundamental importance for the design of biotechnological processes that utilize microbial iron oxidation or reduction in low pH liquids (see following sections). The technique involves growing biomass under controlled conditions, harvesting and concentrating cells and assaying their abilities to either oxidize ferrous iron or reduce ferric iron (typically 1 mM of either) under suitable conditions, e.g., in the presence of a suitable electron donor, and under anoxic conditions, for iron reduction (Figure 2). Biomass measurements are simultaneously carried out on sub-samples of the concentrated cells. Usually, this involves determination of protein contents using, for example, the Bradford assay (Bradford, 1976) but measurements of active biomass (e.g., of ATP; Okibe and Johnson, 2011) are more relevant where the total biomass includes a significant proportion of dead or moribund cells. The time taken to determine rates of iron oxidation and reduction is also important and needs to be much smaller than the culture doubling time of the organism being assayed in order for there to be no significant increase in biomass during the assay period. Typically, 20 min to 2 h provides sufficient time to allow sufficient iron oxidation or reduction by cell suspensions while avoiding significant biomass perturbations (acidophiles generally have culture doubling times varying between 6 and 12 h). Details of the method used to determine specific rates of ferrous iron oxidation are given in Hallberg et al. (2011a). Specific rates of ferric iron reduction referred to in Table 2 were measured using a similar protocol, with nitrogen replacing air (to create anoxic conditions in the cell suspensions) and a suitable electron donor (organic or inorganic) supplied in stoichiometric excess to ferric iron.
Figure 2.
Typical protocol used for determining specific rates of ferrous iron oxidation and ferric iron reduction by acidophilic prokaryotes (*Bradford, 1976; **Okibe and Johnson, 2011; *** e.g., using the ferrozine assay, Lovley and Phillips, 1987).
Table 2.
Specific rates of ferrous iron oxidation determined for different species of acidophilic bacteria and the archaeon Ferroplasma.
| Acidophile | Fe2+ oxidation (mg min−1 g protein−1) |
|---|---|
| (I) AUTOTROPHIC SPECIES | |
| Iron-oxidizing acidithiobacilli | |
| Group I (At. ferrooxidansT) | 484 ± 3.3 |
| Group II (ATCC 33020) | 440 ± 11.5 |
| Group III (At. ferrivoransT) | 192 ± 14 |
| Group IV (JCM 7812) | 446 ± 6.8 |
| Leptospirillum spp. | |
| L. ferrooxidansT | 426 ± 6.5 |
| L. ferriphilumT* | 484 ± 88 |
| Others | |
| Acd. thiooxidansT | 457 ± 20 |
| “Fv. myxofaciens”T | 532 ± 23 |
| (II) MIXOTROPHIC/HETEROTROPHIC SPECIES | |
| Ferrimicrobium acidiphilum | |
| (25 mM Fe2+/100 μM glycerol) | 191 ± 7.5 |
| (100 μM Fe2+/5 mM glycerol) | 7.7 ± 0.14 |
| Sulfobacillus spp.* | |
| Sb. thermosulfidooxidans | 449 ± 4.0 |
| Sb. acidophilus | 236 ± 11 |
| Sb. benefaciens | 341 ± 16 |
| Ferroplasma acidiphilum* | 371 ± 4.0 |
(i) All specific iron oxidation rates were determined at 30°C (except those indicated *, which were determined at 37°C); (ii) all prokaryotes were grown in 25 mM ferrous iron medium, supplemented (for mixotrophs and heterotrophs) with 0.02% (w/v) yeast extract (the media used for Fm. acidophilum were as indicated in the Table).
Table 2 shows specific rates of iron oxidation by different species of autotrophic acidophiles, and facultatively and obligately heterotrophic species, using this approach. Most of the autotrophic species show similar specific rates of oxidation, varying between mean values of 428 and 484 mg ferrous iron oxidized min−1 g protein−1. The two outliers in Table 2 are At. ferrivorans and the betaproteobacterium “Fv. myxofaciens.” Two other strains of At. ferrivorans (Peru6 and CF27) also displayed lower rates (236 ± 2.4 and 312 ± 6.6 mg ferrous iron oxidized min−1 g protein−1, respectively) than the other three “Groups” of iron-oxidizing acidithiobacilli (based on the delineation described by Amouric et al., 2011) suggesting that this is a species-specific trait. “Fv. myxofaciens” displayed the fastest specific rate of iron oxidation of all bacteria (and the sole archaeon) examined, but since the isolate tested (P3G; the nominated type strain of the species) is the only strain currently available in pure culture, the question of whether this is a genus- or species-specific characteristic remains to be verified. All of the mixotrophic and heterotrophic prokaryotes tested, with the exception of Sulfobacillus (Sb.) thermosulfidooxidans, displayed significantly lower specific rates of iron oxidation than the mean value found with autotrophic species, even though these assays were carried out at 37°C rather than at 30°C [as for all of the autotrophic strains, except Leptospirillum (L.) ferriphilum], since most mixotrophic iron-oxidizing acidophilic bacteria are thermotolerant or moderate thermophiles. In the case of the mesophilic heterotroph Fm. acidiphilum which was, like the autotrophic strains, assayed at 30°C, the specific rate of iron oxidation was very much dictated by the growth medium used, in particular the ratio of ferrous iron to organic carbon source (Table 2).
Specific rates of ferric iron reduction by the heterotroph Acidiphilium SJH and two autotrophic Acidithiobacillus spp. (At. ferrooxidansT, and Acidithiobacillus sp ATCC 33020, the proposed type strain of “Group II” iron-oxidizing acidithiobacilli), again using the approach described above, are shown in Table 3. These were much smaller (by about one order of magnitude) than typical rates of iron oxidation found in acidophiles. Data obtained for Acidiphilium SJH suggested that the electron donor had little influence on the specific rate of ferric iron reduction, with the value being similar when either glycerol or galactose was supplied. Ferric iron reduction data shown in Table 3 for the two iron-oxidizing Acidithiobacillus spp. are for hydrogen-grown cultures grown either aerobically, or under anoxic conditions with ferric iron as electron acceptor. There was evidence of suppression of the potential for ferric iron reduction by At. ferrooxidansT with protracted aerobic growth on hydrogen, and the reverse when it was grown anaerobically on hydrogen and ferric iron. While similar tends were apparent for Acidithiobacillus spp. ATCC 33020, differences in specific rates of iron reduction were far less pronounced.
Table 3.
Specific rates of ferric iron reduction by heterotrophic (Acidiphilium) and autotrophic (Acidithiobacillus spp.) acidophiles.
| Bacterium | Growth conditions (incubation time) | Fe3+ reduction (mg min-1 g protein−1) |
|---|---|---|
| A. cryptum SJH | Glycerol/Fe3+ | 32 ± 3.7 |
| Galactose/Fe3+ | 40 ± 8.0 | |
| At. ferrooxidansT | H2/O2 (7 days) | 46 ± 10 |
| H2/O2 (21 days) | 23 ± 5.0 | |
| H2/Fe3+* (5 days) | 84 ± 2.3 | |
| Acidithiobacillus | H2/O2 (7 days) | 63 ± 7.3 |
| sp. ATCC 33020 | H2/O2 (21 days) | 53 ± 1.9 |
| H2/Fe3+* (5 days) | 76 ± 2.1 |
*Cultures grown under anoxic conditions; the electron donors used to grow the acidophiles (glycerol, galactose, or hydrogen) were also those used for the measurements of specific rates of ferric iron reduction.
The effect of growing iron-oxidizing acidithiobacilli on alternative electron donors to ferrous iron on the specific rates of iron oxidation is shown in Table 4. Data shown for tetrathionate-grown cultures are of cells harvested at early stationary phase (generally after ∼5 days) from cultures grown in 5 mM with varying concentrations of ferrous iron, while for both elemental sulfur and hydrogen more protracted growth was possible by providing excess elemental sulfur or by continuously replenishing the hydrogen supply. These results showed that specific rates of iron oxidation were lower for both At. ferrooxidansT and Acidithiobacillus sp. ATCC 33020 when grown on electron donors other than ferrous iron, though this varied with the electron donor used and incubation period. In the case of At. ferrooxidansT, the specific rate of iron oxidation for sulfur-grown cells was about 10% of that of iron-grown cells after 10 days, and was not detectable after 3 weeks of growth on this electron donor. In contrast, the specific rate of ferrous iron oxidation by cells grown aerobically on hydrogen for 3 weeks was still about 50% of that of ferrous iron-grown cells. Ferrous iron has been reported to induce expression of the rus operon in At. ferrooxidans (Amouric et al., 2009), and the lack of ferrous oxidation ability in sulfur-grown cultures can be explained by the ferrous iron originally present in the cultures being rapidly oxidized (within 1 day) to ferric. However, the same would have been true for hydrogen-grown biomass grown with excess oxygen, so why the rus operon was still apparently being expressed in these cultures is unclear. Similar results were obtained for Acidithiobacillus sp. ATCC 33020 (Table 4). Both Acidithiobacillus spp. displayed significant rates of iron oxidation when grown anaerobically on hydrogen (even after 14 days, in the case of Acidithiobacillus sp. ATCC 33020) though this can be explained that ferrous iron would have accumulated in these cultures as a result of dissimilatory reduction of ferric iron, causing the rus operon to be expressed.
Table 4.
Effects of different electron donors and culture conditions on the specific rates of ferrous iron oxidation by Acidithiobacillus spp.
| Bacterium and culture medium | Fe2+ oxidation (mg min−1 g protein-1) |
|---|---|
| AT. FERROOXIDANST | |
| (i) -grown cultures | |
| +0 Fe2+ | 69 ± 1.0 |
| +10 μM Fe2+ | 219 ± 2.0 |
| +100 μM Fe2+ | 210 ± 4.0 |
| +1000 μM Fe2+ | 190 ± 7.5 |
| (ii) S0-grown cultures | |
| +0 Fe2+; 10 days | 44 ± 8.0 |
| +10 μM Fe2+; 10 days | 24 ± 0.6 |
| +100 μM Fe2+; 10 days | 38 ± 0.95 |
| +1000 μM Fe2+; 10 days | 32 ± 1.5 |
| +0 Fe2+; 21 days | <0.01 |
| (iii) H2-grown cultures | |
| 7 days | 149 ± 2.0 |
| 21 days | 210 ± 3.1 |
| +25 mM Fe3+; 5 days* | 178 ± 8.3 |
| ACIDITHIOBACILLUS SP. ATCC 33020 | |
| (i) -grown culture | |
| +100 μM Fe2+ | 45 ± 2.0 |
| (ii) H2-grown cultures | |
| 7 days | 132 ± 4.8 |
| 21 days | 225 ± 7.5 |
| +25 mM Fe3+; 5 days* | 139 ± 12 |
| +25 mM Fe3+; 14 days* | 130 ± 7.0 |
All cultures were grown aerobically, except those indicated by *, which were grown under anoxic conditions.
In natural and man-made (biomining) environments, pyrite is a major energy resource for chemo-autotrophic acidophiles. Ferric iron attack on this mineral releases both ferrous iron and soluble reduced sulfur species (Rohwerder et al., 2003) so that bacteria that can use both as electron donors, such as At. ferrooxidans, may use one or the other in preference, or both simultaneously. Comparison of specific rates of iron oxidation of bacteria grown on pyrite with those of the same strain grown on ferrous iron or reduced sulfur can provide insights into what substrate is being utilized. Results for the type strains of At. ferrooxidans and L. ferrooxidans grown in pure culture in 1% (w/v) pyrite liquid medium, in which planktonic-phase cells and those attached to the mineral were harvested separately using the protocol described by Okibe and Johnson (2004), are shown in Table 5. After 1 week of incubation, the specific rates of ferrous iron oxidation by planktonic-phase cells of both acidophiles were actually somewhat greater than those of ferrous iron-grown cells, and much greater than of those of sulfur-grown cells, implying that both bacteria were oxidizing ferrous iron rather than reduced sulfur species. However, by week 2, corresponding values of both had fallen by about 50%. While this might suggest a switch from iron to sulfur oxidation, this could not be the case for L. ferrooxidans which does not use reduced sulfur as an electron donor. Measurements of ATP concentrations in harvested cells showed that these had also fallen by about 50% from week 1 to 2 relative to protein contents, implying that much of the bacterial populations in these cultures had become moribund or had died. Indexed relative to ATP concentrations, specific rates of ferrous iron oxidation were similar on weeks 1 and 2, suggesting that both bacteria were continuing to use ferrous iron as their preferred electron donor. The specific rates of iron oxidation by planktonic-phase and attached cells of At. ferrooxidans sampled at the same time were found to be similar, suggestion that bacteria attached to the mineral phase were also using ferrous iron as their major energy source.
Table 5.
Specific rates of ferrous iron oxidation by the type strains of L. ferrooxidans and At. ferrooxidans grown on pyrite.
| Bacterium | Fe2+ oxidation (mg min−1 g−1 protein) | RLU* (μg protein−1) |
|---|---|---|
| L. FERROOXIDANS (PYRITE; PLANKTONIC CELLS) | ||
| Week 1 | 527 ± 7.8 | 5,112 |
| Week 2 | 283 ± 6.1 | 2,291 |
| AT. FERROOXIDANS (PYRITE; PLANKTONIC CELLS) | ||
| Week 1 | 562 ± 1.0 | 5,486 |
| Week 2 | 240 ± 1.0 | 2,444 |
| At. ferrooxidans: pyrite | ||
| Planktonic cells | 224 ± 1.0 | n.d. |
| Attached cells | 307 ± 1.0 | n.d. |
*Relative light units (measurement of ATP concentration).
The conclusion that bacteria such as At. ferrooxidans use ferrous iron in preference to reduced sulfur when oxidizing pyrite correlates with similar observations reported when these bacteria are grown in liquid or on solid media that contain both ferrous iron and a soluble (usually tetrathionate) sulfur oxy-anion (Johnson, 1995; Yarzábal et al., 2004). Using ferrous iron in preference to reduced sulfur would appear not to make thermodynamic sense, given that the free energies (ΔG values) of elemental sulfur and tetrathionate (−507 and −1225 kJ mol−1, respectively) far exceed that of ferrous iron (−30 kJ mol−1; Kelly, 1978). However, the specific rates of tetrathionate oxidation tend to be much slower than ferrous iron oxidation for the acidithiobacilli (Table 6; data for Acidithiobacillus sp. ATCC 33020) so that the rates at which these bacteria can generate energy by oxidizing ferrous iron or tetrathionate are very similar. In addition, whereas ferrous iron oxidation by At. ferrooxidans involves a relatively short electron transport chain (comprising three cytochromes and rusticyanin), tetrathionate metabolism by this acidophile requires the synthesis of several enzymes (tetrathionate hydrolase, sulfur dioxygenase, thiosulfate quinone oxidoreductase, sulfite oxidoreductase, and rhodanese; Kanao et al., 2007), in addition to trans-membrane electron transport proteins. The additional energy expenditure required to synthesize these enzymes coupled with the fact that energy yields in unit time are similar for both tetrathionate and ferrous iron oxidation help explain why ferrous iron is used as a preferential electron donor by Acidithiobacillus spp. Additional supporting evidence for ferrous iron being the preferred electron donor for At. ferrooxidans comes from the observation that the rus operon is not repressed by elemental sulfur when ferrous iron is present (Amouric et al., 2009).
Table 6.
Comparison of the specific rates of ferrous iron and tetrathionate oxidation by Acidithiobacillus sp. ATCC 33020, and conversion of these into specific rates of energy generation in unit time.
| Electron donor | Oxidation rate (mmol min−1 g protein−1) | Energy generation (J min−1 g protein−1) |
|---|---|---|
| Fe2+ | 7.9 ± 0.21 | 237 ± 6 |
| 0.21 ± 0.027 | 260 ± 33 |
Environmental Aspects of Oxido-Reduction of Iron in Low pH Environments
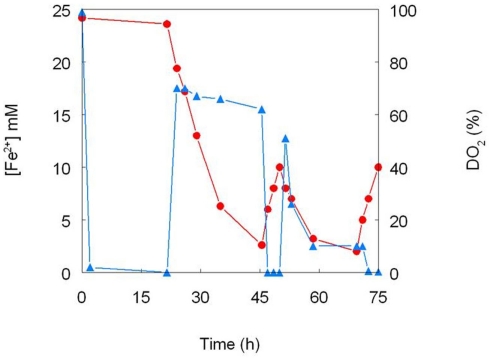
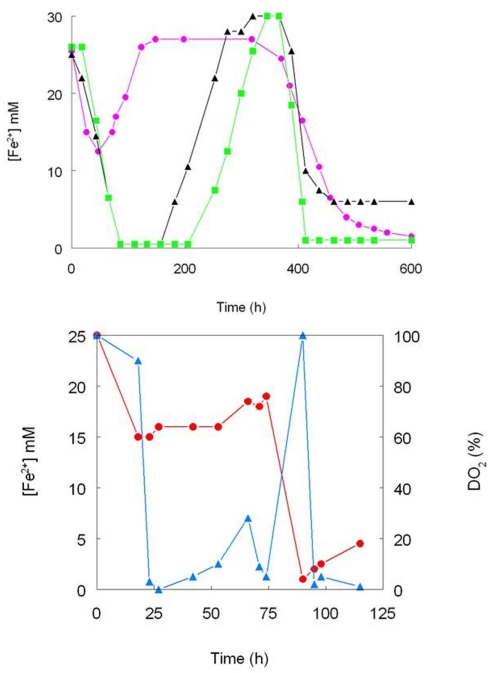
One of the first reports of active iron cycling in an acidic environment was by Johnson et al. (1993) who noted that 30 cm deep acid streamer growths found within an abandoned pyrite mine were stratified in terms of their color and consistency, and that these correlated with variations in concentrations of dissolved oxygen and redox potentials, the latter reflecting relative concentrations of ferrous and ferric iron (Table 7). Later work identified the major iron-oxidizing acidophile in the surface streamers as “Fv. myxofaciens,” while iron-reducing Acidiphilium spp. and novel Firmicutes were more abundant in the lower depths of the streamer growths (Kimura et al., 2011). Johnson et al. (1993) also demonstrated that iron cycling by a mixed bioreactor culture of At. ferrooxidans and Acidiphilium SJH could be controlled by varying concentrations of dissolved oxygen (Figure 3). Pure cultures of moderate thermophiles [Sulfobacillus spp. and Acidimicrobium (Am.) ferrooxidans] also displayed iron cycling in vitro (Figure 4). Stratified acid streamer/mat growths have also been reported in an adit draining an abandoned small-scale copper mine (Cantareras) in Spain (Figure 5). Fragments of acid streamers from the site were shown to both oxidize and reduce iron in vitro, and acidophiles known to catalyze the oxido-reduction of iron were identified by molecular techniques and isolated from the streamer growths (Rowe et al., 2007). The Cantareras streamers have also been materials from which acidophilic sulfate-reducing bacteria have been isolated. The latter can also mediate ferric iron reduction in acidic environments indirectly via the hydrogen sulfide they generate as a waste product (H2S + 2 Fe3+ → S0 + 2 Fe2+ + 2H+) and possibly also directly, using ferric iron as an alternative electron acceptor to sulfate.
Table 7.
Depth-related chemical changes in acid streamer growths found within the abandoned Cae Coch pyrite mine.
| Streamer depth (cm) | pH | DO2 (% of ambient) | Eh (mV) | Fe2+ (g L−1) | Fe3+ (g L−1) | SO42− (g L−1) |
|---|---|---|---|---|---|---|
| 0–10 | 2.35 | 82 | +734 | 0.25 | 1.15 | 5.9 |
| 10–20 | 2.40 | 10 | +634 | 1.21 | 0.39 | 6.0 |
| 20–30 | 2.45 | <0.1 | +484 | 2.16 | <0.01 | 5.2 |
Figure 3.
Cycling of iron by a mixed culture of At. ferrooxidans and Acidiphilium SJH. The culture medium (pH 2.0) contained (initially) 25 mM ferrous sulfate and 10 mM glycerol, and was maintained at 30°C under controlled aeration. Ferrous iron concentrations are shown in red and concentrations of dissolved oxygen in blue (modified from Johnson et al., 1993).
Figure 4.
Iron cycling by pure cultures of moderately thermophilic acidophiles. (Top) -▲- Sb. thermosulfidooxidans (strain TH1), - - Sb. acidophilus (strain THWX), -
- Sb. acidophilus (strain THWX), - - Acidimicrobium sp. (strain HPTH; all cultures grown in pH 2.0 medium containing 25 mM ferrous sulfate, 10 mM glucose and 0.02% yeast extract; no aeration control); (bottom) the effect of controlling concentrations of dissolved oxygen iron cycling by Sb. acidophilus (strain THWX) grown at pH 2.0 in a medium containing 25 mM ferrous sulfate, 10 mM glycerol and 0.02% yeast extract; ferrous iron concentrations are shown in red and concentrations of dissolved oxygen in blue (modified from Johnson et al., 1993).
- Acidimicrobium sp. (strain HPTH; all cultures grown in pH 2.0 medium containing 25 mM ferrous sulfate, 10 mM glucose and 0.02% yeast extract; no aeration control); (bottom) the effect of controlling concentrations of dissolved oxygen iron cycling by Sb. acidophilus (strain THWX) grown at pH 2.0 in a medium containing 25 mM ferrous sulfate, 10 mM glycerol and 0.02% yeast extract; ferrous iron concentrations are shown in red and concentrations of dissolved oxygen in blue (modified from Johnson et al., 1993).
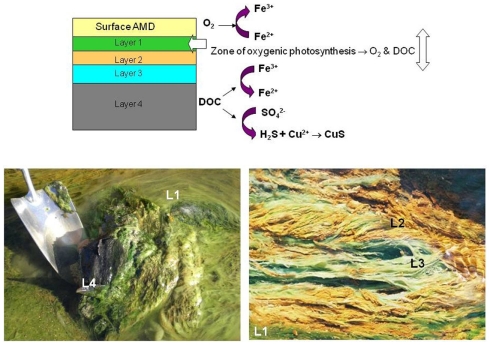
Figure 5.
Redox transformations of iron and sulfur in stratified streamer/mat growths found in a channel draining ad abandoned copper mine (Cantareras, Spain). The differently colored streamer/mat layers are indicated in the top diagram, and shown (as L1, L2 etc.) in the lower photographs.
The Rio Tinto is a 100 km long river located in south-west Spain, characterized by being extremely acidic (pH 1.5–3.1) throughout its length and containing elevated concentrations of soluble iron (both ferrous and ferric, the latter conferring the characteristic burgundy red color of the river). The microbiology of this extensive extreme environment has been researched in depth by Ricardo Amils and colleagues at the Centro de Astrobiologia/Universidad Autonoma in Madrid. They found that the dominant bacteria in the Tinto river were the iron-metabolizing acidophiles At. ferrooxidans, L. ferrooxidans, and Acidiphilium spp., and that other prokaryotes that also catalyze the oxido-reduction of iron at low pH (Ferrimicrobium and Ferroplasma spp.) were present in smaller numbers (González-Toril et al., 2003). Iron cycling, involving ferrous iron oxidation mediated primarily by At. ferrooxidans and L. ferrooxidans in the oxic zones of the river and ferric iron reduction by Acidiphilium spp. and At. ferrooxidans in the anoxic zones, maintain the iron dynamics of the river from its source at the Peña de Hierro in the Iberian Pyrite Belt to Huelva, where it enters the Atlantic Ocean. Since the oxidation of ferrous to (soluble) ferric iron is a proton-consuming reaction, while the reverse reaction (as well as ferric iron hydrolysis) generates protons, the net effect of ongoing oxido-reduction of iron is to maintain the extremely low pH of the Tinto river throughout its length.
Extensive opencast mining of lignite in Lusatia and other regions of Germany has left a legacy of a large number of abandoned and flooded sites (“pit lakes”), many of which are moderately or extremely acidic and contain elevated concentrations of soluble iron and sulfate, resulting from the oxidation of pyrite and other minerals present in the disturbed substrata (Geller et al., 1998). Microbially mediated cycling of both iron and sulfur has been described in these pit lakes (Küsel, 2003; Meier et al., 2004). Oxidation of reduced sulfur and of ferrous iron (coupled with hydrolysis of ferric iron) in oxic surface waters causes these to be acidic (pH as low as 2.0) whereas the reductive dissolution of schwertmannite and microbial sulfidogenesis in the anoxic lake sediments generates net alkalinity, results in these zones having higher pH values (typically about 5.5; Küsel et al., 1999). The major iron-oxidizing bacteria identified in the lignite pit lakes are the acidophiles At. ferrooxidans, L. ferrooxidans, and Ferrimicrobium (Küsel, 2003; Lu et al., 2010) whereas the neutrophile Geobacter as well as acidophilic species (Acidiphilium, Acidobacteria, Acidocella, and Acidithiobacillus) have been implicated in mediating ferric iron reduction in these ecosystems (Lu et al., 2010).
Ferrous iron oxidation is an exergonic reaction, but for cycling of iron to perpetuate in acidic streams, rivers and lakes, energy is required to fuel the reduction of ferric iron, which may be microbially mediated or an abiotic process. Both organic and inorganic electron donors can be used by iron-reducing acidophiles. The latter may arise from extraneous sources (e.g., leaf detritus in surface waters) or derive from the indigenous primary producers present. These include chemautotrophic bacteria such as Leptospirillum and Acidithiobacillus spp., and photoautotrophic acidophiles, which are predominantly eukaryotic micro-algae (Gross, 2000). A proportion of the inorganic carbon fixed by both groups is released into the environments as organic compounds (cell exudates and lysates) that can be utilized by heterotrophic and mixotrophic iron-reducing prokaryotes. The nature and composition of organic exudates arising from acidophilic primary producers varies from species to species, however, and this impacts their potential utilization by organotrophic acidophiles. For example, glycolic acid was identified by Ňancucheo and Johnson (2010) as a significant exudate (accounting for up to 24% of total dissolved organic carbon) in cultures of iron- and sulfur-oxidizing acidophiles but was shown to be utilized by relatively few species (mostly Firmicutes) of organotrophic acidophiles. In contrast, monosaccharides (fructose, glucose and mannitol) identified in exudates of the acidophilic algae Euglena mutabilis and Chlorella protothecoides var. acidicola were found to support the growth of both iron-reducing and sulfate-reducing acidophiles (Ňancucheo and Johnson, 2011). These findings suggest that reduction of both ferric iron and sulfate can be more active processes in surface acidic environments where acidophilic algae are active (such as at Cantareras) than in subterranean sites like the Cae Coch mine. Reduced sulfur and hydrogen can also act as inorganic electron donors for iron reduction by some species of acidophiles (Johnson and Hallberg, 2009). The former can arise by a variety of routes in extremely acidic environments. For example, elemental sulfur and sulfur oxy-anions are formed as by-products of the abiotic oxidation of pyrite and other sulfide minerals by (microbially generated) ferric iron (Rohwerder et al., 2003), and hydrogen sulfide may be generated by sulfur- and sulfate-reducing prokaryotes in anaerobic sediments in acid lakes and streams. However, dissimilatory sulfur- and sulfate-reduction also requires a suitable electron donor, which may again be either organic or inorganic (hydrogen). Hydrogen, therefore, has a potentially important (though currently unknown) role in fueling ferric iron reduction (directly or indirectly) in acidic environments, where it may be formed via the acid dissolution of metals (e.g., relics of mining activities) and some minerals.
Ferric iron reduction in acidic waters can also, however, be mediated by solar energy. Diez Ercilla et al. (2009) showed that solar radiation was a major driver of diel changes in ferrous iron concentrations (10–90 μM) recorded in an acidic (pH 2.8) pit lake in the Iberian pyrite belt. The kinetics of photo-reduction of iron were shown to be zero-order and to be a function of temperature and the intensity of solar radiation, but were found to be independent of ferric iron concentration. Wavelengths within the UV-A spectrum and part of the visible spectrum (400–700 nm) were both shown to be active in iron reduction in the acidic lake. This is a particularly significant observation as it explains how iron cycling can perpetuate in acidic waters, such as acid mine drainage streams and pit lakes, in the absence of either inorganic or organic reductants, or of iron-reducing and sulfate-reducing bacteria. In addition, photochemical regeneration of ferrous iron explains why numbers of iron-oxidizing acidophiles can be maintained or even increase when their primary energy source (e.g., pyrite) is exhausted.
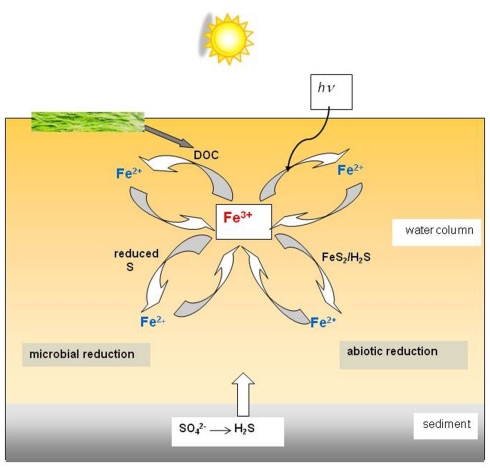
Iron cycling in acidic waters, highlighting the various possible routes of ferric iron reduction, is summarized schematically in Figure 6.
Figure 6.
Schematic of the different microbial and abiotic routes of ferric iron reduction in an acidic (pH < 3) stream, river, or pit lake. Abiotic iron oxidation proceeds slowly in these environments, and is primarily mediated by prokaryotic microorganisms.
Technological Applications of Microbial Iron Oxido-Reduction at Low pH
Acidophilic iron-metabolizing prokaryotes are increasingly used in biotechnologies, the longest established of which is “biomining.”
Technologies utilizing acidophilic iron-oxidizers
The discovery of the first bacterium (At. ferrooxidans) shown, in the late 1940s, to oxidize iron in acidic liquors and, several years later, demonstrated to accelerate the oxidative dissolution of pyrite and other sulfide minerals, was relatively quickly followed up by the first full-scale industrial application that used this acidophile (a dump copper leaching operation in Utah, in the mid-1960s). In reality, human civilisations had been exploiting the abilities of acidophiles to solubilize metals from ores for many years, recovering bioleached metals (chiefly copper, via cementation) from mine waters at the Rio Tinto mine in Spain, Mynydd Parys in Wales, the Song Guo Mountain and other locations in China, and probably elsewhere in the world. Since the 1960s there have been many developments and refinements in both the engineering design and in understanding the mineral bioprocessing. Biomining technologies have also diversified into recovering metals other than copper, including gold, uranium, nickel, cobalt and zinc. More details accounts of biomining can be found in Rawlings (2002); Rawlings and Johnson (2007a); and Johnson (2010).
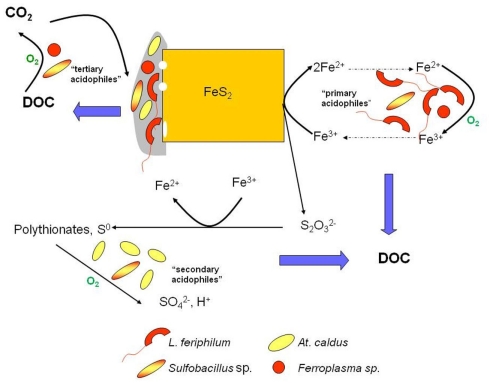
Ferric iron is a powerful oxidizing agent in aqueous solutions, and can degrade a variety of minerals, including metal sulfides. It follows, therefore, that any prokaryote that can oxidize ferrous iron in an extremely acidic (pH < 3) medium should, in theory, be able to accelerate the dissolution of such minerals. This indeed has proven to be the case, with all of the iron-oxidizing bacteria listed in Table 1 having been reported to also oxidize pyrite, though some (e.g., Fm. acidiphilum) only do this when supplied with organic carbon, which may originate from an extraneous source (e.g., added yeast extract) or from a sulfur-oxidizing autotroph in a mixed culture. The most numerous iron-oxidizing prokaryotes in commercial operations tend, however, to be autotrophs. For example L. ferriphilum, has frequently been reported to be the major primary mineral-degrader in stirred tanks that operate between 35° and 45°C. Although iron-oxidizing prokaryotes are the major players in the bioprocessing of sulfide ores and concentrates, these are members of microbial consortia in commercial operations. These consortia include “secondary” sulfur-oxidizing (acid-generating) prokaryotes, and “tertiary” heterotrophic or mixotrophic acidophiles that degrade the small molecular weight organic compounds, such as glycolic acid, that are excreted by autotrophic iron- and sulfur-oxidizers and which could otherwise accumulate to inhibitory concentrations, especially in tank leaching operations, as well as the “primary” iron-oxidizers (Rawlings and Johnson, 2007b). Some acidophiles can assume more than one role in such consortia, for example the heterotrophic iron-oxidizer Ferroplasma (Figure 7). Although, as also indicated in Figure 7, dissolution of sulfide minerals by ferric iron does not involve molecular oxygen, the process does not continue unless the ferrous iron generated by this reaction is re-oxidized to ferric. This microbially catalyzed reaction requires molecular oxygen, and oxygen is also consumed during the oxidation of reduced sulfur intermediates and the catabolism of organic materials, and therefore the process is described as “oxidative mineral dissolution.”
Figure 7.
The oxidative dissolution of pyrite in acidic liquors, showing the major microorganisms identified in the biooxidation of refractory gold concentrates and the bioleaching of a cobaltiferous pyrite concentrate processed at ca. 40°C. Those reactions that consume oxygen are indicated; “DOC” is dissolved organic carbon.
The concept of using iron-oxidizing acidophiles to remediate mine waters has a long history, but only recently has such a system been demonstrated at pilot-scale level. Many streams and ground waters associated with the mining of coals and metals are enriched with soluble ferrous iron. The most effective way of removing soluble iron is first to oxidize ferrous iron to ferric, thereby facilitating the formation of ferric minerals such as schwertmannite and ferrihydrite, which may require some additional input of alkaline materials. As noted previously, abiotic rates of ferrous iron oxidation in low pH waters tend to be very slow, but can be greatly accelerated by iron-oxidizing acidophiles. Since autotrophic species use the energy from ferrous iron oxidation to support their growth and have minimal nutritional requirements, the use of these acidophiles to remediate iron-contaminated waters is particularly attractive. Specific rates of iron oxidation (previous section) can provide important fundamental data when determining the size of a reactor required for this purpose. The maximum rate of iron oxidation by a mesophilic bacterium such as At. ferrooxidans is ∼5 × 10−8 μg cell−1 min−1 (based on data shown in Table 2). Acidic mine water discharged at 10 L s−1 and containing 500 mg ferrous iron L−1 would require a reactor containing ∼6 × 1015 iron-oxidizing bacteria. Cell numbers of autotrophic iron-oxidizers are typically ∼5 × 107 mL−1 in media containing 500 mg iron L−1, so that a minimum bioreactor volume of just over 10 m3 would be required to oxidize all of the iron present. In reality, a much larger reactor would be required as the bacteria would need to be immobilized on a support material to prevent them being washed out, in a continuous flow system.
A pilot-scale (10 m3 reactor volume) system in Nochten, eastern Germany, is being used to oxidize ferrous iron and precipitate schwertmannite from acidic (pH 5.3) ground water containing, typically, 350 mg ferrous iron L−1 with a flow rate of 2,500 L h−1 (Janneck et al., 2010). The iron oxidation efficiency is ∼70%, which is partly due to the temperature at which the system operates (ranging from 12 to 18°C). In addition, hydrolysis of ferric iron causes the pH of the oxidized groundwater to fall to 3.0, which results in a considerable proportion of the ferric iron being retained in solution. The Nochten plant was originally inoculated with At. ferrooxidans and L. ferrooxidans, but analysis of the indigenous microbial population several months after the plant was commissioned revealed that these bacteria were not detectable, but that another iron-oxidizing acidophile (“Fv. myxofaciens”) and bacteria related to the neutrophilic iron-oxidizer Gallionella, were the dominant bacteria present (Heinzel et al., 2009). “Fv. myxofaciens” has also been used in laboratory-scale systems to remediate mine waters (Rowe and Johnson, 2008; Hedrich and Johnson, 2012). Besides its fast specific rate of iron oxidation (Table 2), “Fv. myxofaciens” also has the important advantage of producing copious amounts of extracellular polymeric substances, by which it attaches to a variety of materials (including glass, plastics, metals, and minerals) and causing it to grow as long macroscopic streamers in flowing waters. This obviates the need of an inert support material for these bacteria and maximizes the biovolume of an operating reactor. A modular system for remediating acidic iron-rich mine waters using “Fv. myxofaciens” to oxidize ferrous iron and controlled pH adjustment to precipitate schwertmannite has recently been described by Hedrich and Johnson (2012). The system was shown to be highly efficient (an equivalent 10 m3 primary reactor was calculated to be able to process ∼2.7 L pH 2 mine water containing 250 mg L−1 ferrous iron s−1) and produced pure-grade schwertmannite from mine water containing elevated concentrations of copper, zinc, aluminum, and manganese, in addition to iron. Concentrations of soluble iron were lowered from 250 g L−1 in the untreated water to <1 mg L−1 in the processed water.
Technologies utilizing acidophilic iron-reducers
Development of technologies that use iron-reducing acidophiles has lagged well behind those that use iron-oxidizers. However, there has been at least one recent report that has demonstrated the potential for utilizing acidophilic iron-reducing bacteria to extract metals from mineral reserves. Not all metals occur exclusively as oxidized ores. Nickel is found within sulfide minerals (such as pentlandite) in, for example, reduced black shales, but is more abundant in the lithosphere in oxidized lateritic ores (∼73% of estimated global reserves). In the case of limonitic laterites, nickel is intimately associated with ferric oxyhydroxide minerals such as goethite (FeOOH). Using an oxidative dissolution approach to process such ores is obviously not viable, whereas a reductive approach is feasible. By coupling the oxidation of an organic or an inorganic substrate to the reduction of ferric iron within the goethite lattice, the oxidized mineral is destroyed and the associated nickel liberated. When the reaction occurs in an acidic medium, both the iron and nickel that are solubilized remain in solution.
Bridge and Johnson (2000) showed that a variety of ferric iron minerals, including goethite, could be solubilized by the heterotrophic acidophilic iron-reducer Acidiphilium sp. SJH. In terms of an industrial process, however, elemental sulfur has several advantages (including cost) over an organic electron donor, and Hallberg et al. (2011b) elected to use At. ferrooxidans to demonstrate the bioleaching of a nickel limonite by reductive dissolution, using the reaction shown in the equation below:
Over 80% of the nickel present in a test ore was shown to be recovered when processed in this way, in a bioreactor maintained at 30°C and pH 1.8. Currently, similar ores are processed by high pressure acid leaching at elevated temperatures (in excess of 250°C). The bioreductive technology appears to have generic application for oxidized ores (e.g., of manganese, as well as iron) and bioprocessing of nickel laterites using this approach has been integrated into a full cycle operation (the “Ferredox” process; du Plessis et al., 2011). This recent development suggests that harnessing the potential of iron-metabolizing prokaryotes in new and emerging technologies could expand significantly in the near future.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Part of this work was carried out in the frame of ProMinE (European project contract NMP-2008-LARGE-2:# 228559; DBJ and SH acknowledge the financial support given to this project by the European Commission under the Seventh Framework Program for Research and Development. Tadayoshi Kanao would like to thank the Japanese Ministry of Education, Culture, Sports, Science and Technology for providing financial support for his research.
References
- Amouric A., Appia-Ayme C., Yarzabal A., Bonnefoy V. (2009). Regulation of the iron and sulfur oxidation pathways in the acidophilic Acidithiobacillus ferrooxidans. Adv. Mat. Res. 71–73; 163–166. [Google Scholar]
- Amouric A., Brochier-Armanet C., Johnson D. B., Bonnefoy V., Hallberg K. B. (2011). Phylogenetic and genetic variation among Fe(II)-oxidizing acidithiobacilli supports the view that these comprise multiple species with different ferrous iron oxidation pathways. Microbiology 157, 111–122 10.1099/mic.0.044537-0 [DOI] [PubMed] [Google Scholar]
- Baker B. J., Tyson G. W., Webb R. I., Flanagan J., Hugenholtz P., Allen E. E., Banfield J. F. (2006). Lineages of acidophilic archaea revealed by community genomic analysis. Science 314, 1933–1935 10.1126/science.314.5807.1843a [DOI] [PubMed] [Google Scholar]
- Bigham J. M., Schwertmann U., Traina S. J., Winland R. L., Wolf M. (1996). Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta 60, 2111–2121 10.1016/0016-7037(96)00091-9 [DOI] [Google Scholar]
- Bonnefoy V., Holmes D. S. (2011). Genomic insights into microbial iron oxidation and iron uptake strategies in extremely acidic environments. Environ. Microbiol. 10.1111/j.1462-2920.2011.02626.x [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Bridge T. A. M., Johnson D. B. (2000). Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol. J. 17, 193–206 10.1080/01490450050121161 [DOI] [Google Scholar]
- Brock T. D., Gustafson J. (1976). Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl. Environ. Microbiol. 32, 567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland K., Johnson D. B. (2008). Evidence that the potential for dissimilatory ferric iron reduction is widespread among acidophilic heterotrophic bacteria. FEMS Microbiol. Lett. 279, 30–35 10.1111/j.1574-6968.2007.00998.x [DOI] [PubMed] [Google Scholar]
- Denef V. J., Mueller R. S., Banfield J. F. (2010). AMD biofilms: using model communities to study microbial evolution and ecological complexity in nature. ISME J. 4, 599–610 10.1038/ismej.2009.158 [DOI] [PubMed] [Google Scholar]
- Diez Ercilla M., López Pamo E., Sánchez España J. (2009). Photoreduction of Fe(III) in the acidic mine pit lake of San Telmo (Iberian Pyrite Belt): field and experimental work. Aquat. Geochem. 15, 391–419 10.1007/s10498-008-9044-1 [DOI] [Google Scholar]
- Dopson M., Baker-Austin C., Hind A., Bowman J. P., Bond P. L. (2004). Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl. Environ. Microbiol. 70, 2079–2088 10.1128/AEM.70.4.2079-2088.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobner E., Huber H., Stetter K. O. (1990). Thiobacillus ferrooxidans, a facultative hydrogen oxidizer. Appl. Environ. Microbiol. 56, 2922–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis C. A., Slabbert W., Hallberg K. B., Johnson D. B. (2011). Ferredox: a biohydrometallurgical processing concept for limonitic nickel laterites. Hydrometallurgy 109, 221–229 10.1016/j.hydromet.2011.07.005 [DOI] [Google Scholar]
- Geller W., Klapper H., Salomons W. (eds). (1998). Acid Mining Lakes – Acid Mine Drainage, Limnology and Reclamation. Environmental Science Series. Berlin, Springer [Google Scholar]
- Golyshina O. V., Yakimov M. M., Lünsdorf H., Ferrer M., Nimtz M., Timmis K. N., Wray V., Tindall B. J., Golyshin P. N. (2009). Acidiplasma aeolicum gen. nov., sp. nov., a euryarchaeon of the family Ferroplasmaceae isolated from a hydrothermal pool, and transfer of Ferroplasma cupricumulans to Acidiplasma cupricumulans comb. nov. Int. J. Syst. Evol. Microbiol. 59, 2815–2823 10.1099/ijs.0.009639-0 [DOI] [PubMed] [Google Scholar]
- González-Toril E., Llobet-Brossa E., Casamayor E. O., Amman R., Amils R. (2003). Microbial ecology of an extreme acidic environment, the Tinto river. Appl. Environ. Microbiol. 69, 4853–4865 10.1128/AEM.69.11.6959.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross W. (2000). Ecophysiology of algae living in highly acidic environments. Hydrobiology 433, 31–37 10.1023/A:1004054317446 [DOI] [Google Scholar]
- Hallberg K. B., Hedrich S., Johnson D. B. (2011a). Acidiferribacter thiooxydans, gen. nov. sp. nov.; an acidophilic, thermo-tolerant, facultatively anaerobic iron- and sulfur-oxidizer of the family Ectothiorhodospiraceae. Extremophiles 15, 271–279 10.1007/s00792-011-0359-2 [DOI] [PubMed] [Google Scholar]
- Hallberg K. B., Grail B. M., du Plessis C., Johnson D. B. (2011b). Reductive dissolution of ferric iron minerals: a new approach for bioprocessing nickel laterites. Miner. Eng. 24, 620–624 10.1016/j.mineng.2010.09.005 [DOI] [Google Scholar]
- Hallberg K. B., Thomson H. E. C., Boeselt I., Johnson D. B. (2001). “Aerobic and anaerobic sulfur metabolism by acidophilic bacteria,” in Biohydrometallurgy: Fundamentals, Technology and Sustainable Development, Process Metallurgy 11A, eds Ciminelli V. S. T., Garcia O., Jr. (Amsterdam: Elsevier; ), 423–431 [Google Scholar]
- Hedrich S., Johnson D. B. (2012). A modular continuous flow reactor system for the selective bio-oxidation and precipitation of iron in mine-impacted waters. Bioresour. Technol. 106, 44–49 10.1016/j.biortech.2011.11.130 [DOI] [PubMed] [Google Scholar]
- Hedrich S., Schlömann M., Johnson D. B. (2011). The iron-oxidizing proteobacteria. Microbiology 157, 1551–1564 10.1099/mic.0.045344-0 [DOI] [PubMed] [Google Scholar]
- Heinzel E., Hedrich S., Janneck E., Glombitza F., Seifert J., Schlömann M. (2009). Bacterial diversity in a mine water treatment plant. Appl. Environ. Microbiol. 75, 858–861 10.1128/AEM.01045-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janneck E., Arnold I., Koch T., Meyer J., Burghard D., Ehinger S. (2010). “Microbial synthesis of schwertmannite from lignite mine water and its utilization for removal of arsenic from mine waters and for production of iron pigments,” in Proceedings of the International Mine Water Association Symposium 2010: Mine Water and Innovative Thinking, eds Wolkersdorfer C., Freund A. (Sydney: Cape Breton University Press; ), 131–134 [Google Scholar]
- Johnson D. B. (1995). Selective solid media for isolating and enumerating acidophilic bacteria. J. Microbiol. Methods 23, 205–218 10.1016/0167-7012(95)00015-D [DOI] [Google Scholar]
- Johnson D. B. (2010). “The biogeochemistry of biomining,” in Geomicrobiology: Molecular and Environmental Perspective, eds Barton L., Mandl M. (Dordrecht: Springer; ), 401–426 [Google Scholar]
- Johnson D. B., Bridge T. A. M. (2002). Reduction of ferric iron by acidophilic heterotrophic bacteria: evidence for constitutive and inducible enzyme systems in Acidiphilium spp. J. Appl. Microbiol. 92, 315–321 10.1046/j.1365-2672.2002.01535.x [DOI] [PubMed] [Google Scholar]
- Johnson D. B., Hallberg K. B. (2009). Carbon, iron and sulfur metabolism in acidophilic microorganisms. Adv. Microb. Physiol. 54, 202–256 [DOI] [PubMed] [Google Scholar]
- Johnson D. B., McGinness S. (1991). Ferric iron reduction by acidophilic heterotrophic bacteria. Appl. Environ. Microbiol. 57, 207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. B., McGinness S., Ghauri M. A. (1993). Biogeochemical cycling of iron and sulfur in leaching environments. FEMS Microbiol. Rev. 11, 63–70 10.1111/j.1574-6976.1993.tb00268.x [DOI] [Google Scholar]
- Kanao T., Kamimura K., Sugio T. (2007). Identification of a gene encoding a tetrathionate hydrolase in Acidithiobacillus ferrooxidans. J. Biotechnol. 132, 16–22 10.1016/j.jbiotec.2007.08.030 [DOI] [PubMed] [Google Scholar]
- Kelly D. P. (1978). “Bioenergetics of chemolithotrophic bacteria,” in Companion to Microbiology; Selected Topics for Further Discussion, eds Bull A. T., Meadow P. M. (London: Longman; ), 363–386 [Google Scholar]
- Kimura S., Bryan C. G., Hallberg K. B., Johnson D. B. (2011). Biodiversity and geochemistry of an extremely acidic, low temperature subterranean environment sustained by chemolithotrophy. Environ. Microbiol. 13, 2092–2104 [DOI] [PubMed] [Google Scholar]
- Küsel K. (2003). Microbial cycling of iron and sulfur in acidic coal mining lake sediments. Water Air Soil Pollut. 3, 67–90 10.1023/A:1026098305581 [DOI] [Google Scholar]
- Küsel K., Dorsch T., Acker G., Stackebrandt E. (1999). Microbial reduction of Fe(III) in acidic sediments: isolation of Acidiphilium cryptum JF-5 capable of coupling the reduction of Fe(III) to the oxidation of glucose. Appl. Environ. Microbiol. 65, 3633–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küsel K., Roth U., Drake H. (2002). Microbial reduction of Fe(III) in the presence of oxygen under low pH conditions. Environ. Microbiol. 4, 414–421 10.1046/j.1462-2920.2002.00314.x [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. P. (1987). Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53, 1536–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Gischkat S., Reiche M., Akob D. M., Hallberg K. B., Küsel K. (2010). Ecophysiology of Fe-cycling bacteria in acidic sediments. Appl. Environ. Microbiol. 76, 8174–8183 10.1128/AEM.00711-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalady J. L., Jones D. S., Lyon E. H. (2007). Extremely acidic, pendulous cave wall biofilms from the Frasassi cave system, Italy. Environ. Microbiol. 9, 1402–1414 10.1111/j.1462-2920.2007.01256.x [DOI] [PubMed] [Google Scholar]
- Magnuson T. S., Swenson M. W., Paszczynski A. J., Deobald L. A., Kerk D., Cummings D. E. (2010). Proteogenomic and functional analysis of chromate reduction in Acidiphilium cryptum JF-5, an Fe(III)-respiring acidophile. Biometals 23, 1129–1138 10.1007/s10534-010-9360-y [DOI] [PubMed] [Google Scholar]
- Meier J., Babenzien H. D., Wendt-Potthoff K. (2004). Microbial cycling of iron and sulfur in sediments of acidic and pH-neutral mining lakes in Lusatia (Brandenburg, Germany). Biogeochemistry 67, 135–156 10.1023/B:BIOG.0000015278.23470.f7 [DOI] [Google Scholar]
- Monhemius A. J. (1977). Precipitation diagrams for metal hydroxides, sulfides, arsenates and phosphates. Trans. Inst. Min. Metall. 86, C202–C206 [Google Scholar]
- Ňancucheo I., Johnson D. B. (2010). Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl. Environ. Microbiol. 76, 461–467 10.1128/AEM.01832-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ňancucheo I., Johnson D. B. (2011). Safeguarding reactive mine tailings by ecological engineering: the significance of microbial communities and interactions. Appl. Environ. Microbiol. 77, 8201–8208 10.1128/AEM.06155-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle J. L. C., Simmons S., Bathe S., Norris P. R. (2009). Ferrous iron oxidation and rusticyanin in halotolerant, acidophilic “Thiobacillus prosperous.” Microbiology 155, 1302–1309 10.1099/mic.0.023192-0 [DOI] [PubMed] [Google Scholar]
- Ohmura N., Sasaki K., Matsumoto N., Saiki H. (2002). Anaerobic respiration using Fe3+, S0 and H2 in the chemolithotrophic bacterium Acidithiobacillus ferrooxidans. J. Bacteriol. 184, 2081–2087 10.1128/JB.184.8.2081-2087.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okibe N., Johnson D. B. (2004). Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: the significance of microbial interactions. Biotechnol. Bioeng. 87, 574–583 10.1002/bit.20138 [DOI] [PubMed] [Google Scholar]
- Okibe N., Johnson D. B. (2011). A rapid ATP-based method for determining active microbial populations in mineral leach liquors. Hydrometallurgy 108, 195–198 10.1016/j.hydromet.2011.04.008 [DOI] [Google Scholar]
- Pronk J. T., Liem K., Bos P., Kuenen J. G. (1991). Energy transduction by anaerobic ferric iron reduction in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 57, 2063–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D. E. (2002). Heavy metal mining using microbes. Annu. Rev. Microbiol. 56, 65–91 10.1146/annurev.micro.56.012302.161052 [DOI] [PubMed] [Google Scholar]
- Rawlings D. E., Johnson D. B. (eds). (2007a). Biomining. Heidelberg: Springer-Verlag [Google Scholar]
- Rawlings D. E., Johnson D. B. (2007b). The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153, 315–324 10.1099/mic.0.2006/001206-0 [DOI] [PubMed] [Google Scholar]
- Regenspurg S., Brand A., Peiffer S. (2004). Formation and stability of schwertmannite in acidic mining lakes. Geochim. Cosmochim. Acta 68, 1185–1197 10.1016/j.gca.2003.07.015 [DOI] [Google Scholar]
- Rohwerder T., Gehrke T., Kinzler K., Sand W. (2003). Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sufide oxidation. Appl. Microbiol. Biotechnol. 63, 239–248 10.1007/s00253-003-1448-7 [DOI] [PubMed] [Google Scholar]
- Rowe O. F., Johnson D. B. (2008). Comparison of ferric iron generation by different species of acidophilic bacteria immobilised in packed-bed reactors. Syst. Appl. Microbiol. 31, 68–77 10.1016/j.syapm.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Rowe O. F., Sánchez-España J., Hallberg K. B., Johnson D. B. (2007). Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 9, 1761–1771 10.1111/j.1462-2920.2007.01294.x [DOI] [PubMed] [Google Scholar]
- San Martin-Uriz P., Gomez M. J., Arcas A., Bargiela R., Amils R. (2011). Draft genome Sequence of the electricigen Acidiphilium sp. strain PM (DSM 24941). J. Bacteriol. 193, 5585–5586 10.1128/JB.05386-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm W., Morgan J. J. (1981). Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters. New York: Wiley [Google Scholar]
- Welham N. J., Malatt K. A., Vukcevic S. (2000). The effect of solution speciation on iron-sulfur-arsenic-chloride systems at 298 K. Hydrometallurgy 57, 209–223 10.1016/S0304-386X(00)00121-3 [DOI] [Google Scholar]
- Yarzábal A., Appia-Ayme C., Ratouchniak J., Bonnefoy V. (2004). Regulation of the expression of the Acidithiobacillus ferrooxidans rus operon encoding two cytochromes c, a cytochrome oxidase and rusticyanin. Microbiology 150, 2113–2123 10.1099/mic.0.26966-0 [DOI] [PubMed] [Google Scholar]