Abstract
Background:
Despite thousands of papers, the value of quality of life (QoL) in curing disease remains uncertain. Until now, we lacked tools for the diagnosis and specific treatment of diseased QoL. We approached this problem stepwise by theory building, modelling, an exploratory trial and now a definitive randomised controlled trial (RCT) in breast cancer, whose results we report here.
Methods:
In all, 200 representative Bavarian primary breast cancer patients were recruited by five hospitals and treated by 146 care professionals. Patients were randomised to either (1) a novel care pathway including diagnosis of ‘diseased’ QoL (any QoL measure below 50 points) using a QoL profile and expert report sent to the patient's coordinating practitioner, who arranged QoL therapy consisting of up to five standardised treatments for specific QoL defects or (2) standard postoperative care adhering to the German national guideline for breast cancer. The primary end point was the proportion of patients in each group with diseased QoL 6 months after surgery. Patients were blinded to their allocated group.
Results:
At 0 and 3 months after surgery, diseased QoL was diagnosed in 70% of patients. The QoL pathway reduced rates of diseased QoL to 56% at 6 months, especially in emotion and coping, compared with 71% in controls (P=0.048). Relative risk reduction was 21% (95% confidence interval (CI): 0–37), absolute risk reduction 15% (95% CI: 0.3–29), number needed to treat (NNT)=7 (95% CI: 3–37). When QoL therapy finished after successful treatment, diseased QoL often returned again, indicating good responsiveness of the QoL pathway.
Conclusion:
A three-component outcome system including clinician-derived objective, patient-reported subjective end points and qualitative analysis of clinical relevance was developed in the last 10 years for cancer as a complex intervention. A separate QoL pathway was implemented for the diagnosis and treatment of diseased QoL and its effectiveness tested in a community-based, pragmatic, definitive RCT. While the pathway was active, it was effective with an NNT of 7.
Keywords: quality of life (QoL), breast cancer, definitive RCT, complex intervention, effectiveness of the QoL system
Introduction and theoretical underpinnings of the study
Thousands of papers have been published on quality of life (QoL) following the conceptual and methodological work of Ware (1976) and the first measurement of QoL in a randomised clinical trial (Troidl et al, 1979). However, whether this construct has relevance for the individual patient remains unclear (Lorenz et al, 1999). Significant measurement, clinical practice and management problems have persisted for almost 20 years (Lohr, 1992). Some fragments of the puzzle were successfully completed in a few randomised trials (Rubenstein et al, 1995; Detmar et al, 2002; Velikova et al, 2004) and systematic reviews. Measuring QoL and providing the results to physicians as the mainstream of research improves understanding of a patient's functional problems by enabling physician–patient and patient–physician communication (Deyo and Patrick, 1989; Rubenstein et al, 1995; Greenhalgh and Meadows, 1999; Espallargues et al, 2000; Detmar et al, 2002; Symonds et al, 2002; Velikova et al, 2004; Sloan et al, 2006).
However, powerful barriers against the routine clinical use of QoL have persisted, with doubts about the validity of self-perceived symptoms presented to clinicians, in busy ambulatory settings, by non-medical professionals, using unfamiliar formats (Wasson et al, 1992; Wright et al, 2003) and unclear formatting of patient data in longitudinal studies (Wright et al, 1998).
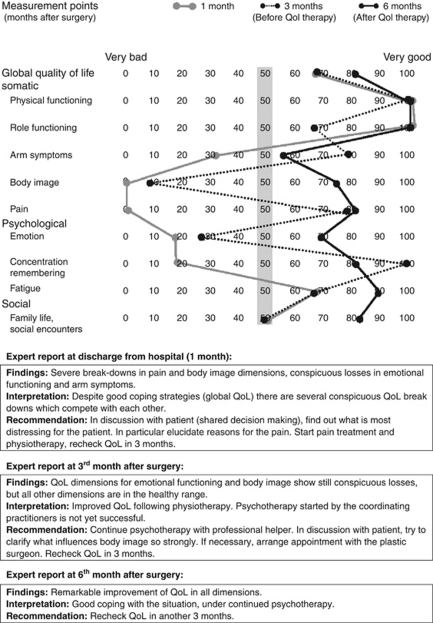
Up till now, several essentials for managing diseased QoL have been missing: (1) Guidance about how to define and use diseased QoL as an integral part of cancer treatment, for example, in patients with good prognostic stage (UICC 0 and I) but persistently low QoL in several dimensions (Klinkhammer-Schalke et al, 2008a) requesting the doctor's help. Figure 1 demonstrates a prototype of such a patient. (2) Specific therapy for patients with such diseased QoL using defined procedures and allied health professionals, for example, pain relief delivered by a certified pain unit, arm symptoms treated by physiotherapists controlled in a quality circle (Figure 1). (3) Confidence in the effectiveness of such QoL therapies (Greenhalgh and Meadows, 1999), also shown as a positive example in Figure 1.
Figure 1.
QoL profiles and expert consensus reports of a specific intervention group patient with effective QoL diagnosis and therapy. Female, with primary breast cancer, randomly assigned to QoL diagnosis and treatment group; 50 years, married, one child. Prognostic classification pT1c, SN0, G2, ER+, PR+, HER2neu−. BCT with sentinel node excision followed by radiation and anti-oestrogen treatment in months 3 and 6 with Tamoxifen. Cutoff for diseased/healthy QoL 50 points (grey bar). Global QoL assessed by clinician (1 month) and CP (3 and 6 months): 100, 70, 85 points obtained by the physicians and the values of the patient in the figure, showed good doctor–patient agreement.
To achieve these aims, an integrated quality of life diagnosis and therapy pathway (QoL pathway) was designed, implemented and evaluated, guided by the UK Medical Research Council framework for developing and testing complex interventions (Campbell et al, 2000), including:
Theory development
This consisted of defining disease-related QoL (Koller and Lorenz, 2002; Lorenz and Koller, 2002) in a three-component outcome model (Lorenz et al, 1999) with both traditionally objective and patient-reported subjective end points (Table 1).
Table 1. Concept and terminology of disease-related QoL and diseased QoL used in outcome assessment of complex interventions for breast cancer.
| Disease-related QoL | Health, disease and QoL are key concepts in medicine (Wulff, 2002), but their definitions should be derived from empirically testable and clinically relevant attributes (Lorenz et al, 1999; Lorenz and Koller, 2002; Wulff, 2002; Velikova et al, 2004). Health is neither absence of disease nor an aggregate amount of disease states. Disease as the vehicle of clinical experience (Wulff, 2002) has its own, characteristic (molecular, clinical, physical and psychosocial) specificity (Lorenz et al, 1999; Bowling, 2001). QoL as part of the outcome construct is again specifically related to this disease concept. For example, in sepsis, emotional distress (depression, sickness behaviour) is associated with cytokine expression in specific brain areas (Bauhofer et al, 2004). Clinically measured QoL is related to the total concept of disease. Hence, QoL – is assessed in a specifically diagnosed disease, including the critical model of Wulff (Lorenz et al, 1999; Wulff, 2002) – entails self-perception and self-report in three domains: somatic, psychological and social – includes health- and therapy-related expectations and coping – is influenced by basic psychosocial variables such as negative affect – is part of a three-component outcome model (Lorenz et al, 1999; Koller and Lorenz, 2002; Lorenz and Koller, 2002) For each attribute in this definition, at least one empirical (prospective) trial is available and usable |
| Diseased QoL | As part of the disease concept, QoL is not just a measurement, but has a medical/clinical value. ‘Diseased’ characterises QoL as negative medical value, ‘healed’ QoL is the corresponding positive result of therapeutic improvement. The cutoff point between disease and health for any dimension in the QoL profile is <50 points in scales of 0–100 (worst–optimal QoL) – It is not yet defined by a gold standard, but by face validity using 4-point Likert scales (Koller and Lorenz, 2002) – by psychological theories on adaptation level and social comparison (Koller and Lorenz, 2002), – by asking patients at which value in a range of 0–100 they would approach their doctor (iatrotropy; Koller and Lorenz, 2002) and – by a change in global QoL as a general dimension if QoL in a specific dimension (in 1/10 in the QoL profile) declined below a value of 50 as the lowest in the range of the normal German population (Klinkhammer-Schalke et al, 2008b) |
Abbreviation: QoL=quality of life.
Methodology: Definitions in medicine according to Popper et al (1998): combine attributes, but only from empirical studies. Presentation of attributes: use meta-levels according to Reichenbach (1947, 1951).
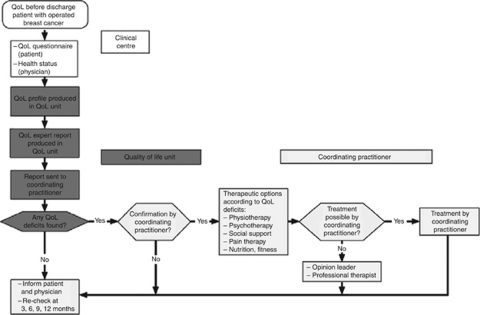
Modelling
This included a care algorithm (Figure 2) defining the QoL pathway (Klinkhammer-Schalke et al, 2008a). Implementation used a multifaceted strategy, according to the Leeds-Castle Conference on guideline implementation (Gross et al, 2000), emphasising especially outreach visits.
Figure 2.
Care pathway for QoL diagnosis and therapy – QoL pathway – tailored to breast cancer patients.
Exploratory trial
This demonstrated feasibility (Klinkhammer-Schalke et al, 2005, 2008b) in breast cancer patients, though the approach is entirely generic. Patients experiencing the QoL pathway were compared with standard follow-up and treatment according to German Cancer Society breast cancer guideline. Quality of life was measured in both groups, but could not be used for decision making or therapy in the controls, since neither QoL profile nor expert report were transmitted to coordinating practitioners (CPs).
Definitive randomised controlled trial
Following pre-publication of the study protocol for judgment by external referees (Koller et al, 2006), then creation of the patient record file (PRF) and approval by the university ethic committee (8 July 2004), and registration by Clinical Trials gov (protocol ID: TUZ-QL-RS-04), we now describe the definitive randomised controlled trial (RCT), again in breast cancer, aimed at quantifying the effectiveness of the pathway.
The trial does not stand by itself, but is part of a long lasting (1998–2011), and completely empirical programme of work (Koller and Lorenz, 2002) to build a database of evidence for this complex intervention (MRC framework, 2000). Additional systematic reviews (Greenhalgh et al, 2005; Frost et al, 2007; Valderas et al, 2008; Luckett et al, 2009) and a longitudinal analysis of patient–physician communication (Takeuchi et al, 2011) published since the start of our RCT did not interfere with our project.
Materials and Methods
Objectives and hypothesis
The trial was designed as a definitive pragmatic RCT on the clinical effectiveness of the QoL pathway, not its efficacy (Campbell et al, 2000). The results should be analysed not only globally, but also in concrete detail using the 10 dimensions of the QoL profile. The influence or even the cause of the traditional medical therapy on QoL deficits classified as diseased should only be analysed in an exploratory way as secondary end points (Appendix A). Typical conditions for care including patients from both urban and rural areas were selected to fit into an existing health care system. That was necessary for external validity.
The null hypothesis was: The sophisticated QoL pathway (Figure 2) including a new QoL profile and defined diagnostic and therapeutic options did not influence QoL in breast cancer patients 6 months after surgery, compared with standard postoperative care.
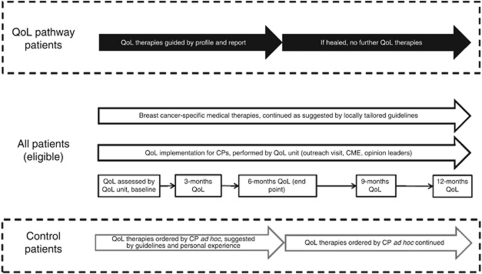
Design of complex intervention
This is demonstrated in Figure 3. Each patient's QoL response was transformed into a profile (Figure 1) using a computerised QoL visualisation programme (Klinkhammer-Schalke et al, 2008a, 2008b). This was handed out to five experts in the QoL unit who independently formulated their QoL diagnosis and treatment recommendations. The QoL profile, health status form and individual expert decisions were discussed weekly at consensus meetings of the five experts, resulting in an expert consensus report. This was sent immediately to the CP of intervention patients (Figure 2).
Figure 3.
Diagnosis and therapy of diseased QoL in breast cancer patients: design of a complex intervention.
Therapeutic options for QoL deficits
Five therapeutic options for diseased QoL were identified in the modelling paper (Figures 1 and 2), but were only used while the relevant score in the QoL profile was below 50 points (Figure 1). No maintenance therapy was used once the score rose to ⩾50. Each low QoL dimension, up to a maximum of five, was treated with the corresponding therapeutic option. If there were more than five low QoL dimensions, therapy was focused on those the patient preferred (shared decision making).
Standardisation and effectiveness of the therapies were achieved by several procedures: (1) Professional therapists were recruited from the trial region using strict aptitude criteria. (2) Quality circles (Klinkhammer-Schalke et al, 2008a) for each therapy provided continued medical education (CME). Classical PDCA cycles (Klinkhammer-Schalke et al, 2008a) produced audit check lists and selection criteria, followed by the address of all professionals who met those criteria. (3) To ensure that each chosen therapy was effective, evidence-based methods were used to select them (Table 2).
Table 2. QoL therapy procedures showing the best possible effectiveness for the five therapeutic options to cure diseased QoL in the context of the care pathway.
| Procedure | 1. Physiotherapy | 2. Psychotherapy | 3. Social support | 4. Pain therapy | 5. Nutrition and fitness |
|---|---|---|---|---|---|
| Definition of therapeutic option and methodsa | Physical decongestive therapy of upper limb by manual lymph drainage with sequential compression therapy | Individual, experiential psychotherapy and cognitive behavioural interventions for adjustment disorders | Network nodes for social counselling (financial security, well-being, partnership, children, homecare, profession) organised in quality circle | Combination of drugs (WHO analgesic ladder) with non-drug measures, for example, psychotherapy and disease-modifying therapies | Combination of self-help groups: counselling for diet and ambulatory sports training programmes, all enrolled in quality circles |
| Level of evidence for an effective outcome measureb | 1 Questionable effective (Preston et al, 2009) | 1 Effective for QoL (Newell et al, 2002) | 4 GCP Effectiveness not tested | 1 Effective for pain (Hanks et al, 2001) | 4 GCP Effectiveness not tested (Beliveau and Gingras, 2005) |
| Methodological standard achieved in local consensus of recommendationsc | Formal consensus, Nominal Group Process (Lorenz et al, 2001) | Informal consensus, decisions, cases analysed in quality circle | Informal consensus within quality circle | Informal consensus, locally tailored guidelines, in quality circle | Informal consensus, developed only within QoL unit |
Abbreviations: GCP=good clinical practice; QoL=quality of life.
General sources of information (Lorenz et al, 2001).
German national guideline for breast cancer (http://www.AWMF.de) in 2009.
Scottish Intercollegiate Guidelines Network (SIGN) grading system in 1999.
German guideline manual in 2001 (Lorenz et al, 2001).
Study type
Two-arm randomised controlled, single-blind clinical trial of a complex intervention recruiting hospital and general practice patients, with equal allocation ratio and balanced randomisation (Koller et al, 2006).
Selection of patients
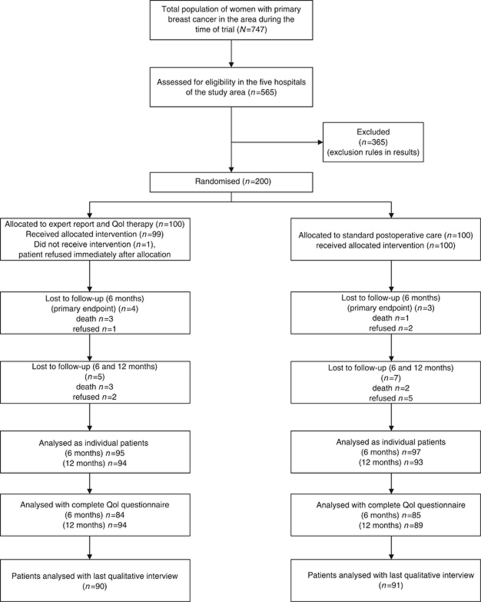
To ensure high external validity for this community-based trial, only inhabitants of two defined areas (urban county of Regensburg, rural county of Amberg-Sulzbach, Central Bavaria) were included (Klinkhammer-Schalke et al, 2008a). In all, 747 patients with primary, newly diagnosed, female breast cancer were registered in these areas by an extended clinical cancer registry (Regensburg Tumor Center) (Hofstaedter and Klinkhammer-Schalke, 2011) during the recruitment period (1 September 2004 to 1 August 2006) (Figure 4). The crude incidence rate for breast cancer in that time was 157/100.000 female inhabitants but 160 for the whole of Bavaria (epidemiological cancer registry Bavaria). To ensure that the study design was as pragmatic as possible, patients with all prognostic stages (UICC I–IV, ICD 10, C 50) and carcinoma in situ (UICC 0, ICD 10, D05), all ages above 18 and of all national and ethnic origins were included. All exclusion criteria as demonstrated in results were prospectively defined in the PRF and were found reasonable by both referees (Koller et al, 2006) and the ethical committee.
Figure 4.
Trial profile: flow of patients through each stage of the study. Complete=questionnaire answering the primary end point of the study. In Figure 6, only 83 instead of 85 patients could be analysed in the control group because no data were given for 1/10 QoL dimensions. Since, however, the two patients showed breakdowns in more than one dimension, the primary end point could be analysed and counted in them. Flowchart was constructed according to Altman (1996).
All five hospitals in the two areas (three in Regensburg, two in Amberg-Sulzbach) served as primary recruitment sites (Figure 4). Two QoL physicians from each clinic screened theatre lists every weekday to identify eligible patients (n=565). From these subjects, recruitment documents were prepared that included (1) the QoL questionnaire (EORTC QLQ-C30, version 3.0, plus breast cancer module QLQ-BR23, Fayers et al, 2001), filled out by the patient alone, away from any person who might interfere with her decisions. (2) A health status form concurrently prepared by the physician that included diagnostic, therapeutic and social information (for details see Klinkhammer-Schalke et al, 2008a). This document also included the clinician's judgment of the patient's global QoL using the same scale as the EORTC questionnaire. (3) A fax document confirming the intention of the clinician to recruit the patient for the study. The study patients were finally selected by two coordinators in the QoL unit using the exact arrival time of faxed recruitment documents from the five hospitals for consecutive enrolment (study number). ‘Exact’ means ‘per minute’ since the maximum of fax documents was 6 per day.
Patients were allocated randomly by study personnel in the QoL unit to the QoL pathway or control group written on a paper card in 1/200 opaque-sealed envelopes. For concealed sequence generation, the envelopes were prepared by an external assistant 2 months before starting the trial on 16 November 2004. Recruitment was completed on 2 July 2006. Follow-up ended on 28 August 2007 and the last qualitative interview was on 9 October 2007.
Selection of care professionals
To ensure high external validity, professionals were defined and selected by systematic procedures, facilitated by standardised documentation in the Tumor Center. At the start of the trial performance, there were 67 physicians in the study area classified as general practitioners or gynaecologists. In the German care system, the latter are mostly responsible for breast cancer diagnosis and treatment. The CPs in the trial were taken from this group.
They were defined as those who cared for breast cancer patients before and after hospital stay. They were eligible for the project if they gave their informed consent, had at least reasonable experience in caring for breast cancer patients (at least three patients annually in the years 1999–2001), and agreed to participate in the multifaceted implementation procedure including outreach visits, opinion leaders and continuing medical education in a quality circle. In that way, they had to agree with QoL therapy procedures worked out carefully and systematically in Table 2. For further details see Klinkhammer-Schalke et al (2008a, 2008b).
Professional therapists were those who provided QoL enhancing therapies such as pain therapy, psychotherapy, physiotherapy and lymphatic drainage, sports activities and nutrition counselling or social counselling (Table 2). Their professional background required specific training in their area of expertise. Professional therapists participated in quality circles of the Tumor Center Regensburg, providing regular training using PDCA cycles (PDCA=plan, do, check and act). The same professional therapists also looked after control patients.
Study groups
The ‘positive expectation’ of this trial was an increased rate of individual patients with healed QoL caused by the care pathway as complex intervention (Figure 2). Central in the concept of this experimental comparison is the CP. He was well trained by the QoL unit and supported by five groups of also trained professional therapists. However, if one of the study groups completely followed the pathway, whereas the other did not, there were many steps in the communication, decision making and action during the performance of the experiment, which had to be controlled for contamination, for example, Hawthorne effects and carry-over effects (Torgerson, 2001). Cluster randomisation using general practices or similar health service structures was not considered the best solution. It would too much emphasise the effect of implementation compared with the healing effect of the pathway. The problem of conceptual vs operational (practical) variables for comparison is well known in social psychology (West and Wicklund, 1980).
In daily routine, patients filled out their first QoL questionnaire the day before hospital discharge following surgery. After final confirmation of eligibility by the QoL unit, they were randomised to either QoL pathway or standard care (Prasser et al, 2002) (Figure 4).
Outcomes
A clinically based hierarchy of outcomes was designed to avoid arbitrary statistical approaches such as Bonferroni adjustment (Schulz and Grimes, 2005). The first outcome level (primary end point) was the proportion of patients with diseased QoL 6 months postoperatively. The second outcome level contained several confirmatory end points enhancing the credibility of the primary end point, such as the rate of breakdown in emotional functioning or global QoL – similar to the example given by Schulz and Grimes (2005) for wound infection and fever. The third outcome level included secondary end points, such as baseline patient characteristics, and risk factors for diseased QoL, obtained from a planned, exploratory subgroup analysis. Comorbidity was assessed using the ASA classification (ASA=American Society of Anesthesiologists: 1=no comorbidity, 2–4=comorbidity). Cofatality was defined as a family disaster for the patient, in addition to her cancer (examples in results).
Diseased QoL as defined in Table 1 was a drop below 50 points in any of the 10 major QoL dimensions on a scale from 100 to 0 points (worst QoL). Healed QoL was a shift to 50 points or more (Table 1). The primary end point used meaningful changes in QoL in each patient as an immediate, personally relevant treatment goal, not mean values in the overall patient group. The effect size was calculated as the number needed to treat (NNT) to raise all QoL subscales above 49 in one patient at 6 months.
The time point of 6 months after surgery was chosen since QoL diagnosis and therapy was expected to have its maximal impact then. At hospital discharge, neither was yet started (Figure 2). Furthermore, not only surgical treatment but also various combinations of therapy started in the hospital before discharge, that is, surgery, radiotherapy or chemotherapy plus tamoxifen. Hence patients were exposed to surgery in combination with several other therapies at study baseline (Appendix A). At 3 months, diseased QoL was diagnosed and specific QoL therapies had just been ordered by the CP, but had not yet started. Hence, QoL at this time was not used for outcome calculations. At 6 months, QoL therapy was either in progress or completed, so could be effective. Quality of life therapy was discontinued once QoL was healed, so QoL measurements made at 9 and 12 months reflected the cessation of maintenance therapy rather than effectiveness.
Sample size for the primary end point was calculated according to Friedman et al (1985), with an assumption that a halving of the rate of diseased QoL at 6 months would justify the cost and complexity of the new QoL pathway. With a probability from our pilot studies of diseased QoL at 6 months of 0.50 in the control group and 0.25 in the intervention group (Klinkhammer-Schalke et al, 2008b), two groups of 80 patients were needed with 2α set at 0.05 and 1–β at 0.9. Adding 20 patients to each group to compensate for loss to follow-up, missing data and contamination (Torgerson, 2001) gave a total sample size of 200 patients.
Randomisation
Balanced randomisation was used with random permuted blocks of 20 patients followed by a sequence of 10 blocks for n=200 with a second, simple randomisation. Five digit random numbers from the RAND Corporation (1955) were used for both steps.
Allocation concealment and blinding
Patients were informed verbally and in writing that they would be kept blind throughout the study about their allocated group (single-blind design) and gave their written consent. The CP selected by the patient was informed by the study coordinators 1 week after allocation to which group the patient was assigned, but was strictly obliged not to share this knowledge with the patient. In control patients, the CP received neither the QoL profile nor the expert report, but could still order specific QoL therapies delivered by the same trained professionals participating in the same PDCA cycles if they wished, and often did (Figure 3).
Data management and statistical analysis
Data from each patient were collected centrally in the QoL unit and copied from a handwritten PRF into a Microsoft ACCESS (version 2003, Microsoft Corporation, Redmond, WA, USA) database. Descriptive statistical analyses used 95% confidence intervals (95% CIs) (Altman, 1998; Daly, 1998). Inferential analyses used Mann–Whitney U or χ2-tests or Fisher's exact test if the smallest expected cell value was <5 (Cochran, 1977). All significance tests were two-sided. P-values (2α) <0.05 were considered statistically significant. All analyses were performed using SPSS for Windows, version 15.0 (IBM Corp., Armonk, NY, USA). Confidence intervals for NNT were calculated using an asymptotic bootstrapping algorithm implemented in R.
Results
Effectiveness
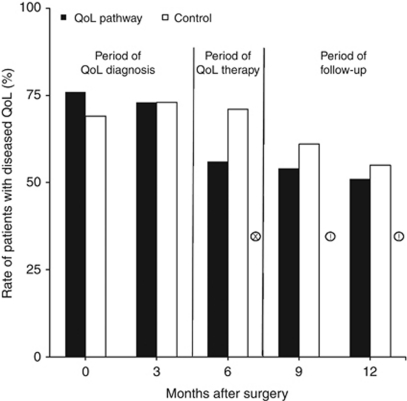
At the prospectively defined and published 6 months end point (Koller et al, 2006), 60/85 (95% CI: 51–68) patients in the control group or 71% showed diseased QoL in at least one dimension (Figure 5). In the treatment group, this occurred in 47/84 (95% CI: 38–56) patients or 56% (χ2-test P=0.048). This corresponded to a 21% relative risk reduction (95% CI: 0–37), 15% absolute risk reduction (95% CI: 0.3–29) and an NNT of 7 (95% CI: 3–37).
Figure 5.
Proportion of patients with diseased QoL following breast cancer surgery and adjuvant therapies: comparison of QoL diagnosis and therapy (QoL pathway) with guideline consistent, traditional postoperative care (control) over 1 year. χ2-test: !=P<0.1, x=P<0.05.
These positive results were supported by two additional confirmatory end points:
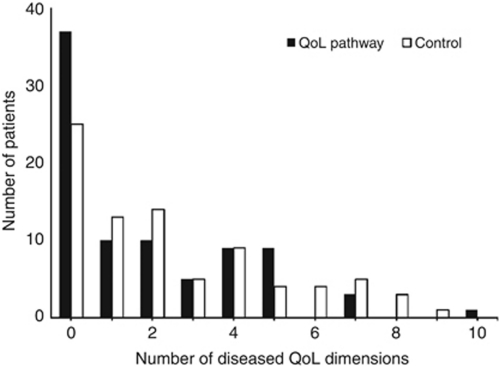
The number of diseased QoL dimensions per patient was lower in the QoL pathway group (Figure 6) than in the control group at 6 months, with more than five dimensions in 4/84 (CI: 1–10) or 5% of the treatment group compared with 13/83 (CI: 7–21) or 16% of the control group (χ2-test P=0.035, Fisher's exact test P=0.042). This difference was not observed at the other four measurement occasions. The critical number of five QoL dimensions was chosen because it corresponded to the maximum of five QoL therapeutic options for QoL deficits in the QoL pathway (Figure 2).
The number of patients with zero QoL in at least one dimension at 6 months was 13/84 (CI: 7–21) patients or 15% in the treatment group and 21/84 (CI: 14–30) or 25% in the control group (χ2-test P=0.124).
Figure 6.
Number of diseased QoL dimensions per patient 6 months after surgery: Comparison of QoL diagnosis and therapy pathway (dark bars, n=84) with that of standard postoperative care (light bars, n=83).
Impact of omitting maintenance therapy
Specific therapies were discontinued once a patient no longer showed diseased QoL in the targeted dimensions in 25 patients with one specific therapy and in six patients with two low dimensions, mostly psychotherapy and pain therapy. This had negative consequences for effectiveness, but was a very valuable criterion for good measurement of QoL called responsiveness. That is a must in most pharmacological studies (Wood-Dauphinee and Troidl, 1989). The rate of diseased QoL fell 15% in the control group from 71% to 56% from month 6 to month 12, but the drop in the intervention group was only 5%, from 56% to 51% (Figure 5). Even with QoL therapy, about half the patients still had diseased QoL 1 year after breast cancer surgery.
Zero QoL in at least one dimension occurred in 13% of the control group (11/88) vs 10% (9/93) of the intervention group (at 6 months). This very small difference was related almost exclusively to pain. Zero QoL was measured in 7% (6/85) of the control group vs 1% (1/93) in the intervention group (χ2-test P=0.049, Fisher's exact test P=0.062). For fatigue, the rate of zero QoL was 6% (5/87) in the control group vs 1% (1/93) in the intervention group (χ2-test P=0.092, Fisher's exact test P=0.116).
Confirmatory primary end points
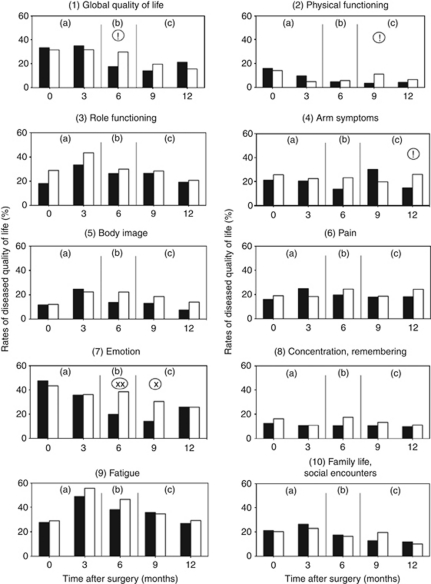
The effectiveness of QoL diagnosis and therapy 6 months after surgery and the effect of deliberately omitting maintenance therapy after this were confirmed by analysing the rates of patients with diseased QoL in each dimension of the profile (Figure 7).
Figure 7.
Proportions of patients with diseased global QoL and single dimensions of QoL: comparison of QoL pathway (▪) with traditional postoperative care (□). χ2-test: !=P<0.1, x=P<0.05, xx=P<0.01. (a) Period of QoL diagnosis; (b) period of QoL therapy; (c) period of follow-up (maintenance omitted if QoL ⩾50 points).
The high rates in the control group (white columns) 3 months after hospital admission emphasise the severity and clinical relevance of reduced QoL in patients with breast cancer. At this time, most patients were at home with many social and psychological problems, were receiving or had just stopped radiotherapy and chemotherapy, were taking anti-oestrogen treatment and fully recognised their situation. This was reflected in lowest measured scores for role functioning, body image, fatigue and the whole social domain and near lowest for global QoL (coping), emotion and pain, in total 7/10 dimensions. There was little change in the control group at 6 months. At 12 months, fatigue (>30% of patients), low role functioning, arm symptoms, emotion and pain were all present in >20%. These control figures help us to estimate the volume of specific QoL therapies needed in such a patient group.
The effectiveness of QoL therapies is shown by the fewer patients in the treatment group (black columns) suffering from diseased QoL in each dimension 6 months after surgery. Almost all were reduced except for the social domain. The extent of the drop differed markedly: a significant drop only for emotion, almost significant for global QoL (coping). It seems that, in this patient group and despite regular PDCA quality circles, physiotherapy, pain therapy, physical training and social counselling were largely ineffective.
The rebound after omitting maintenance therapy beyond 6 months provides further evidence of effectiveness (Figure 7). At 9 and 12 months, there were still some effects, but the benefits of therapy disappeared completely for emotion and global QoL.
Exclusion rules and external validity
Since the 200 patients in the RCT (Figure 4) were diagnosed and treated – except for the QoL pathway – quite uniformly in agreement with the German National breast cancer guideline (Table 2), the sample was reasonably representative of the designated parent population (n=747) obtained by an extended clinical cancer registry in the centre of Bavaria.
However, two parameters used for comparison between study sample and regional population in the preceding exploratory trial (Klinkhammer-Schalke et al, 2008b) made us cautious: age and prognostic stage (UICC). In order to avoid excluding women ⩾70 years, no upper limit for age was stated. But at the end of the RCT, 28.2% of patients ⩾70 years were found in the parent population and only 15.6% in the study sample, the same result as in the exploratory trial.
A similar finding was obtained for prognostic status, which was also not an exclusion criterion: UICC status 0 I II III IV formed 5%, 37%, 32%, 15% and 11%, respectively, of the parent population, but 2%, 45%, 29%, 23% and 2% of the study sample, again quite different. Age and prognostic status both predicted worse objective outcome. Their influence on the external validity of QoL assessment cannot be excluded.
There were 365 exclusions, mostly already in the hospital and for the following reasons: (1) CP chosen by the patient was not trained in the QoL system and thus did not meet inclusion criteria n=150; (2) no QoL physician (neither clinician nor CP) available for the patient, due to illness, holiday or refusal of physician to participate n=20; (3) patient misclassified in the operation schedule (not primary operation or no breast tumour) n=34; (4) patient from district outside the study region n=80; (5) patient unable to fill out the questionnaire for physical, language or psychological reasons n=32; (6) patient with severe comorbidity n=2; (7) patient pregnant n=2; (8) patient refused to participate in the study n=45.
The selection criteria for the CPs, more or less those for successful implementation, were responsible for the largest number of patient exclusions (n=150). Only 39 CPs in the study region fulfilled all of them, and the patient had a free choice of medical practitioner. Since one of the suitable CPs then refused to participate, 38 physicians started the trial as CPs. During the study, 19 additional practitioners were included when they fulfilled entry criteria, but three were also subsequently excluded due to retirement and illness. Hence, 54 CPs completed the trial. Similarly, five experts in the QoL unit, 12 local opinion leaders and 75 professional therapists were chosen (Figure 2). For more detailed selection criteria, see reference (Klinkhammer-Schalke et al, 2008a, 2008b).
The low percentage of older women was probably caused by an increasing rate of demented people at the age of >80 years who could not fill out the EORTC questionnaire. The low percentage of women with poor prognosis was possibly elicited by refusals, people feeling more sick than others. Fortunately, neither of these two baseline characteristics differed between the two treatment groups (Appendix A) thus impairing internal validity.
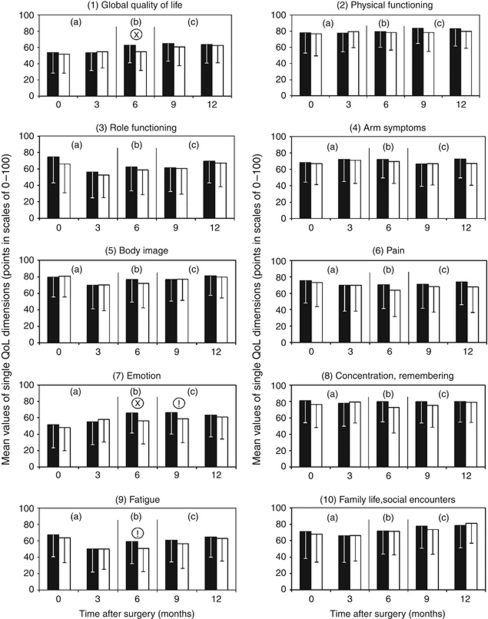
Baseline values for QoL are shown as mean values±s.d. to allow comparison with other QoL studies (Figure 8). In our care pathway, these did not represent primary end points but they are valuable for exploring our primary end point. Mean scores showed considerable variation across the 10 QoL dimensions, for example, for emotion (X̄=48) and physical functioning and cognition (X̄=77 and 76) as the three extremes.
Figure 8.
Mean values±s.d. for the 10 dimensions of the QoL profile: comparison of QoL pathway (▪) with traditional postoperative care (□). Mann–Whitney U-test !=P<0.10, x=P<0.05. Global QoL P=0.018, Emotion P=0.030 and P=0.060, respectively, Fatigue P=0.051. (a) Period of QoL diagnosis; (b) period of QoL therapy; (c) period of follow-up (maintenance omitted if QoL ⩾50 points).
Factors influencing the primary end point
Analysis of >30 baseline factors demonstrated reasonable matching of the patient groups (Appendix A). One combination of breast conserving therapy, chemotherapy and radiotherapy was significantly different at 12 months, when many patients changed therapy combination. This mismatch cannot have influenced intervention effectiveness or measurements made at 6 months. The planned subgroup analysis identified various combinations of classical cancer treatments as strongly influencing effectiveness (Table 3). At 6 months, chemotherapy significantly worsened QoL in combination with the three other treatments, but not at 12 months. At 12 months, the opposite was found for mastectomy vs breast conserving therapy.
Table 3. Influence of treatment combinations on QoL outcome at 6 and 12 months.
|
Treatment combinations
|
Rates of diseased QoL/total patients in each treatment combination
|
|||
|---|---|---|---|---|
| Description | Combination | Rates | % | χ2 test |
| At 6 months | ||||
| Chemo | BCT+CT+RT+ET vs BCT+no CT+RT+ET | 26/35 vs 18/40 | 74% vs 45% | P=0.01* |
| Operation | BCT+CT+RT+ET vs ME+CT+RT+ET | 26/35 vs 4/9 | 74% vs 44% | P=0.09 |
| At 12 months | ||||
| Chemo | BCT+CT+RT+ET vs BCT+no CT+RT+ET | 41/87 vs 16/32 | 47% vs 50% | P=0.78 |
| Operation | BCT+CT+RT+ET vs ME+CT+RT+ET | 41/87 vs 19/25 | 47% vs 76% | P=0.01* |
Abbreviations: BCT=breast conserving therapy; ME=mastectomy; CT=chemotherapy; RT=radiotherapy; ET=endocrine therapy (e.g., Tamoxifen); QoL=quality of life.
Bold letters emphasise subgroups different in treatment.
*P<0.05.
Comorbidity assessed using preoperative ASA classification was high, as expected in surgical populations. Illness in close relatives (cofatalities) deserves more detail: the six cases in the QoL pathway group at 6 months were severe dementia of husband, suicide of husband and mother-in-law, death of a daughter, cerebral tumour of a grandson, husband needing care for severe chronic disease, twin sisters developed breast cancer. Similar family illness rates were found in the control group. Finally, at 0 and 3 months (before initiation of QoL therapy), no mismatch occurred in any QoL dimension (Figure 7).
An additional factor that might influence the primary end point in this study on complex interventions (Campbell et al, 2000) were the CPs, with their many different attitudes and behaviours (Table 4). To retain good external validity, this variability was not restricted. One question is, ‘Is there any evidence that patients in the control group received less empathy, in terms of fewer QoL therapies?’ At 3 months, at least one therapeutic option for QoL was ordered for 42% of the patients in the QoL pathway group vs 35% in the control group (n.s.). At 6 months, the position reversed with 35% in QoL pathway vs 39% in the control group (n.s.). Thus, differences in the overall CP empathy were not a confounding factor for effectiveness.
Table 4. Activities and communications among members of the care pathway relevant for group comparison and mutual exclusion.
| Activities and communications | Intervention group (QoL pathway) | Control group (standard postoperative care) |
|---|---|---|
| CP and professional therapists trained in treatment options and trial protocol | ✓ | ✓ |
| CP able to order one or more QoL therapeutic options also in standard care (e.g., physiotherapy) | ✓ | ✓ |
| Timing and extent of adjuvant therapies with negative influence on QoL (chemotherapy, radiation, endocrine therapy) captured | ✓ | ✓ |
| CP receives QoL profile and expert report | ✓ | − |
| Onset and duration of any QoL therapies are prompted by profile and expert report | ✓ | − |
| CP is able to communicate with professional therapists and opinion leaders about the meaning of the profile and what should be done | ✓ | − |
| QoL unit calls CP by telephone, 4 weeks after patient's regular follow-up date, asking if anything was done in response to QoL diagnosis | ✓ | − |
Abbreviations: CP=coordinating practitioner; QoL=quality of life; ✓=present; −=absent.
Activities are present in both groups, contamination occurs and reduces effectiveness.
More interesting was the CP's behaviour in cases of QoL deficit for the three most common QoL deficits: global QoL (coping), emotional functioning and arm symptoms (Table 5). At 3 months, coping strategies including counselling were applied more often but not significantly more in the QoL pathway group than the control group (P<0.055). Significantly more psychotherapy was given to women in the QoL pathway group (P<0.005), but just the opposite for physiotherapy in the control group. At 6 months, the results were much more similar in the intervention and control groups, the largest difference in therapeutic options now observed was in physiotherapy for arm symptoms. We conclude that different rates of ordering therapeutic options themselves were not responsible for the effectiveness of the QoL pathway. Its effectiveness instead stems from helping CPs to match QoL therapy to QoL diagnosis using the QoL profile and expert report, so improving the appropriateness with which therapies were ordered.
Table 5. Rates of therapeutic options in QoL pathway group compared with those in the control group.
|
Rates of therapeutic options in each group (n=92–99)
|
||||
|---|---|---|---|---|
|
3 months
|
6 months
|
|||
| QoL pathway | Control | QoL pathway | Control | |
| Any QoL therapy given? | 42 | 35 | 35 | 39 |
| Coping strategies and counselling | 21 | 12* | 19 | 10 |
| Psychotherapy | 10 | 1** | 3 | 3 |
| Physiotherapy | 18 | 25 | 16 | 30*** |
Abbreviation: QoL=quality of life.
Comparison of two time intervals (3 and 6 months) and the most important QoL dimensions (global QoL, emotion and arm symptoms).
Source: Interview of coordinating practitioners by study coordinator 4 weeks after sending the expert report in the QoL pathway group or letter without information for control group patient (for details see Klinkhammer-Schalke et al (2008a)).
χ2-test *P<0.055; **P<0.050; ***P<0.020.
Interpretations and conclusion
We demonstrate that an effective, replicable and scalable pathway for QoL diagnosis and treatment can be established within a tumour centre and delivered by a geographically widespread community of more than a hundred CPs and professional therapists. A complex intervention (Campbell et al, 2000) for QoL measurement and treatment was implemented using a QoL profile, transmitted to physicians in a format similar to an ECG (Figure 1). By interpreting this in a QoL unit (Klinkhammer-Schalke et al, 2008a), we can satisfy most health care stakeholders: patients and their carers, CPs and allied health professionals, care managers in health insurance companies and also the general public.
For designing and evaluation of this complex intervention across several phases, we used the methodological framework of the MRC (Campbell et al, 2000). These phases are not common in traditional RCTs comparing drugs. In addition, we published the study protocol before carrying out the trial (Koller et al, 2006) in order to seek the necessary discussion and also formal consensus about basal conditions, study design and outcomes in the scientific community (Chalmers and Altman, 1999). Changes of the protocol, qualitative analyses, also recommended by the MRC guidelines, and the definitive, primary end point were then included in the final PRF.
This enterprise, which consumed so much effort in management (grants and papers), time (about 13 years) and staff problems (number of participants and changes), has, however, successfully been completed twice before: for induction of anaesthesia and perioperative cardio-respiratory complications (Lorenz et al, 1994) and for sepsis following surgery for colorectal cancer (Bauhofer et al, 2007). In both cases, the publication and critical appraisal of the final study protocol were welcomed by leaders of the Cochrane Collaboration (Chalmers and Altman, 1999). Hence, as the third example, we have now applied the full process once again, on postoperative QoL diagnosis and therapy in breast cancer. All three series of studies confirm that serious, common problems (diseases) can be successfully solved by such strategies.
We consider the external validity of this randomised trial to be high, because it was carefully designed to reflect routine practice. The study was run within a population- and community-based tumour centre covering a well-defined part of Bavaria from which two areas were selected, with urban and rural populations. All breast cancer patients from this area were screened for trial eligibility (Figure 4). The QoL pathway was defined and implemented in a replicable way, with the first patient entering the pilot pathway in December 2002, 2 years before the randomised trial (Klinkhammer-Schalke et al, 2008a). Hence, the results of this trial can be confidently transferred to other tumour centre patients, certainly to those who are members of the German association of cancer registries.
One problem, however, has probably to be settled before further testing of our QoL pathway in other places: implementation using modern methods derived from clinical guidelines (Snyder and Aaronson, 2009). The exclusion criteria for CPs were too small number of patients seen per year and not participation in the implementation of QoL in clinical practice, especially time consuming outreach visits. The large number of care givers, however, in the clinical pathway (Figure 2) reflected exactly the clinical routine in our country.
Conclusion
A three-component outcome system including traditionally objective and patient-reported subjective end points with qualitative analysis of clinical relevance was developed over the last 10 years. This was implemented with a separate QoL pathway for diagnosis and treatment of diseased QoL for cancer patients as a complex intervention. It was evaluated stepwise according to the MRC framework and finally now with a community-based, pragmatic, definitive RCT. Diseased QoL was specifically treated by a maximum of five therapeutic options per patient: evidence and guideline supported physiotherapy, psychotherapy, social support, pain therapy or nutrition/fitness counselling. While it was active, the QoL pathway was effective with NNT=7 (CI: 3–35).
Acknowledgments
Bavarian Ministry of Health, programme ‘Bayern aktiv’ Grant No. 710 302 and EKFS (Else Kröner-Fresenius-Stiftung). Thanks to our study participants and to Dr Tom Nicholls, Warwick University for calculating the confidence intervals on NNT.
APPENDIX A
Table 6. Patient characteristics (baseline values) with potential influence on primary end point: distribution in the two experimental groups.
| Characteristics | QoL pathway (n=99) | Control (n=100) |
|---|---|---|
| (1) Classical medical outcome oriented | ||
| Patients included in each of the five QoL measurements (n) | 99/96/95/95/94 | 100/98/97/96/94 |
| Age at recruitment (years, median (range)) | 58 (31–85) | 57 (28–85) |
| BCT/mastectomy (n) | 74/25 | 76/24 |
| Prognostic stage group (UICC in %) | ||
| UICC 0 | 2 | 2 |
| UICC I | 45 | 44 |
| UICC II (II a and b combined) | 26 | 32 |
| UICC III (III a, b, c combined) | 25 | 20 |
| UICC IV | 2 | 2 |
| Receptor status positive (in %) | ||
| Oestrogen | 86 | 83 |
| Progesterone | 82 | 82 |
| HER2neu | 20 | 21 |
| Mortality rate (%) up to 6 months | 3 | 1 |
| Up to 12 months | 3 | 2 |
| Significant comorbidity up to 6 months (%) | 70 | 63 |
| (2) QoL outcome oriented (%) | ||
| Married, widowed, divorced, other | 78/13/8/1 | 80/7/7/6 |
| Children (0/ 1–2/ 3 or more) | 13/71/15 | 18/64/18 |
| House wife or pensioner | 48 | 48 |
| Working outside home | 52 | 52 |
| Cofatality, up to 6 months | 6 | 5 |
| Up to 12 months | 9 | 7 |
| Daily living in | ||
| Urban area (Regensburg) | 76 | 82 |
| Rural area (Amberg-Sulzbach) | 24 | 18 |
| Rates of patients with diseased QoL at 0 and 3 months (single dimension) | Figure 7 | Figure 7 |
| (3) Classical treatment oriented | ||
| (Most frequent combinations up to 6 months) (n) | ||
| BCT + RT + ET | 21 | 20 |
| BCT + CT + RT + ET | 16 | 19 |
| BCT + CT + RT | 12 | 19 |
| BCT + CT | 12 | 9 |
| ME + ET | 8 | 4 |
| BCT alone | 6 | 4 |
| ME + CT + RT + ET | 6 | 4 |
| Less frequent combinations | 18 | 21 |
| (Most frequent combinations up to 12 months) (n) | ||
| BCT + CT +RT + ET | 48 | 42 |
| BCT + RT + ET | 16 | 17 |
| ME + CT + RT + ET | 13 | 13 |
| BCT + CT + RT | 3 | 12* |
| ME + ET | 7 | 4 |
| Less frequent combinations | 12 | 12 |
| Treatment completed/ongoing at 6 months (n) | ||
| Chemotherapy | 50/5 | 58/9 |
| Radiotherapy | 51/12 | 60/18 |
| Endocrine therapy | 3/59 | 1/54 |
Abbreviations: BCT=breast conserving therapy; CT=chemotherapy; ET=endocrine therapy (e.g., Tamoxifen); ME=mastectomy; RT=radiotherapy; QoL=quality of life.
Not frequent=⩽10 per whole trial sample. *χ2-test P=0.017.
APPENDIX B
Regensburg QoL Study Group, Germany
Allgäuer M, Andreesen R, Bawidamann G, Beha M, Berg-Wurms U, Berberich W, Breunig M, Biehler FX, Brauner M, Brey R, Dengler R, Dietmaier M, Dittmann K, Djukic M, Dodenhöft JD, Eichenseer H, Ernst B, Franken P, Frühwirth O, Gerken M, Gerl G, Göritz M, Götz H, Götz M, Grandel H, Hanauer E, Hannig K, Häusler R, Hausmann W, Hettenbach R, Höglsperger H, Hofstädter A, Keicher C, Kölbl O, Köppl A, Kotowitz D, Krämer B, Kreuser ED, Kreutzmann L, Krüger A, Kurkowski A, Lenz A, Liebl G, Manna S, Mergner-Gradl AL, Meyringer S, Misler K, Mögele A, Münch B, Ortmann O, Pawlik M, Popowa S, Porsch P, Prahl R, Prößl R, Pyrkocz S, Reiff RW, Riederer M, Rösler H, Rohn H, Rost U, Rücker C, Rümler H, Salih-Ali B, Sanders R, Scharl A, Scharpf B, Schatz R, Schlegel G, Schleicher B, Schneider W, Seelbach-Göbel B, Seiler H, Stadler R, Stadtmüller S, Strik D, Strobel S, Sudheimer U, Ulrich G, Vietoris S, Wille N, Weidinger-Köppen S, Wilczek-Engelmann T, Wolf M, Zorzi E.
References
- Altman D (1996) Better reporting of randomized controlled trials: the Consort statement. BMJ 313: 570–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D (1998) Confidence intervals for the number needed to treat. BMJ 317: 1309–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauhofer A, Plaul U, Torossian A, Koller M, Stinner B, Celik I, Sitter H, Greger B, Middeke M, Schein M, Wyatt J, Nyström P, Hartung T, Rothmund M, Lorenz W (2007) Perioperative prophylaxis with granulocyte colony-stimulating factor (G-CSF) in high-risk colorectal cancer patients for an improved recovery: a randomized controlled trial. Surgery 141: 501–510 [DOI] [PubMed] [Google Scholar]
- Bauhofer A, Schwarting R, Köster M, Schmitt A, Lorenz W, Pawlak C (2004) Sickness behavior of rats with abdominal sepsis can be improved by antibiotic and G-CSF prophylaxis in clinic modeling randomized trials. Inflamm Res 53: 697–705 [DOI] [PubMed] [Google Scholar]
- Beliveau R, Gingras D (2005) Les Aliments Contre le Cancer. Editions du Trecarre Quebecor Media Inc: Outremont, QC Canada [Google Scholar]
- Bowling A (2001) Measuring disease. Health-Related Quality of Life: Conceptual Meaning Use and Measurement. Open University Press: Buckingham [Google Scholar]
- Campbell M, Fitzpatrick R, Haines A, Kinmonth A, Sandercock P, Spiegelhalter D, Tyrer P (2000) Framework for design and evaluation of complex interventions to improve health. BMJ 321: 694–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers I, Altman D (1999) How can medical journals help prevent poor medical research? Some opportunities presented by electronic publishing. Lancet 353: 490–493 [DOI] [PubMed] [Google Scholar]
- Cochran W (1977) Some Methods for Strengthening the Common × 2-Tests. Biometrics 1954, 10: 417--451 cited by P Armitage: Statistical methods in medical research Fourth Printing Blackwell Scientific publications: Oxford London. 134–138 [Google Scholar]
- Daly L (1998) Confidence limits made easy: interval estimation using a substitution method. Am J Epidemiol 47: 783–790 [DOI] [PubMed] [Google Scholar]
- Detmar S, Muller M, Schornagel J, Wever L, Aaronson N (2002) Health-related quality-of-life assessments and patient-physician communication. A randomized controlled trial. JAMA 288(23): 3027–3034 [DOI] [PubMed] [Google Scholar]
- Deyo R, Patrick D (1989) Barriers to the use of health status measures in clinical investigation patient care and policy research. Med Care 27: 254–268 [DOI] [PubMed] [Google Scholar]
- Espallargues M, Valderas J, Alonso J (2000) Provision of feedback on perceived health status to health care professionals: a systematic review of its impact. Med Care 38: 175–186 [DOI] [PubMed] [Google Scholar]
- Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomly A (2001) European Organization for Research and Treatment of Cancer QLQ-C30 Scoring Manual, 3rd ed. EORTC: Brussels [Google Scholar]
- Friedman L, Furberg C, Demets D (1985) Fundamentals of Clinical Trials. PSG Publishing Company: Littleton, MA, USA, pp 83–108 [Google Scholar]
- Frost M, Bonomi A, Cappelleri J, Schünemann H, Moynihan T, Aaronson N (2007) Applying quality-of-life data formally and systematically into clinical practice. Mayo Clin Proc 82(10): 1214–1228 [DOI] [PubMed] [Google Scholar]
- Greenhalgh J, Long A, Flynn R (2005) The use of reported outcome measures in routine clinical practice: lack of impact or lack of theory? Soc Sci Med 60: 833–843 [DOI] [PubMed] [Google Scholar]
- Greenhalgh J, Meadows K (1999) The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcome of patient care: a literature review. J Evaluation Clin Prac 5: 401–416 [DOI] [PubMed] [Google Scholar]
- Gross P, Greenfield S, Cretin S, Ferguson J, Grimshaw J, Grol R, Klazinga N, Lorenz W, Meyer G, Riccobono C, Schoenbaum S, Schyve P, Shaw C (2000) Optimal methods for guideline implementation. Conclusions from Leeds Castle meeting. Med Care 39: 85–92 [PubMed] [Google Scholar]
- Hanks G, Conno de F, Cherny N, Hanna M, Kalso E, McQuay H, Mercandante S, Meynadier J, Poulain P, Ripamonti C, Radbruch L, Roca I, Casas J, Sawe J, Twycross R, Ventafridda V (2001) Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 84: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstaedter F, Klinkhammer-Schalke M (2011) Aufgaben und Strukturen für die klinische Krebsregistrierung. Onkologe 17: 121–125 [Google Scholar]
- Klinkhammer-Schalke M, Koller M, Steinger B, Ehret C, Ernst B, Wyatt J, Hofstaedter F, Lorenz W (2008b) Implementing a system of quality of life diagnosis and therapy for breast cancer patients: results of an exploratory trial as prerequisite for a subsequent RCT. Br J Cancer 99: 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkhammer-Schalke M, Koller M, Steinger B, Jakob C, Ernst B, Hofstaedter F, Lorenz W (2005) Implementing PDCA quality management for five quality of life specific therapies in breast cancer patients within a regional Tumor Centre. Qual Life Res 14(9): P185 [Google Scholar]
- Klinkhammer-Schalke M, Koller M, Wyatt J, Steinger B, Ehret C, Ernst B, Hofstaedter F, Lorenz W (2008a) Quality of life diagnosis and therapy as complex intervention for improvement of health in breast cancer patients: delineating the conceptual methodological and logistic requirements (modeling). Langenbecks Arch Surg 393(1): 1–12 [DOI] [PubMed] [Google Scholar]
- Koller M, Klinkhammer-Schalke M, Ehret C, Steinger B, Ernst B, Hofstaedter F, Lorenz W (2006) Diagnosis and therapy of illness-related quality of life in breast cancer patients. Protocol of a randomized clinical trial at the Regensburg Tumor Center. ZEFQ 100: 175–182 [PubMed] [Google Scholar]
- Koller M, Lorenz W (2002) Quality of life: a deconstruction for clinicians. J R Soc Med 95: 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K (1992) Applications of health status assessment measures in clinical practice. Overview of the third conference on advances in health status assessment. Med Care 30(Suppl): MS1–M14 [DOI] [PubMed] [Google Scholar]
- Lorenz W, Duda D, Dick W, Sitter H, Doenicke A, Black A, Weber D, Menke H, Stinner B, Rothmund M, Ohmann C, Healy MJR (1994) Incidence and clinical importance of perioperative histamine release: randomised study of volume loading and antihistamines after induction of anaesthesia. Lancet 343: 933–940 [DOI] [PubMed] [Google Scholar]
- Lorenz W, Koller M (2002) Empirically-based concepts of outcome and quality of life in medicine. In: Gimmler A, Lenk C, Aumüller G (eds.). Health and Quality of Life. Philosophical Medical and Cultural Aspects. LIT-Verlag: Münster, pp 129–141 [Google Scholar]
- Lorenz W, Ollenschläger G, Geraedts M, Gerlach F, Gandjour A, Helou A Kirchner H, Koller M, Lauterbach K, Reinauer H, Sitter H, Thomeczek C (2001) Das Leitlinien Manual. ZEFQ 95(Suppl I): 1–84 [Google Scholar]
- Lorenz W, Troidl H, Solomkin JS, Nies C, Sitter H, Koller M, Krack W, Roizen M (1999) Second step: Testing -- outcome measurements. World J Surg 23: 768–780 [DOI] [PubMed] [Google Scholar]
- Luckett T, Butow PN, King MT (2009) Improving patient outcomes through the routine use of patient-reported data in cancer clinics: future directions. Psychooncology 18: 1129–1138 [DOI] [PubMed] [Google Scholar]
- Newell S, Sanson-Fisher RW, Savolainen N (2002) Systematic review of psychological therapies for cancer patients: overview and recommendations for future research. JNCI 94: 558–584 [DOI] [PubMed] [Google Scholar]
- Popper K, Troidl H, McKneally M (1998) Toward a definition of surgical research. In: Troidl H, McKneally M, Mulder D, Wechsler A, McPeek B, Spitzer W (eds.). Surgical Research. Springer: Berlin, Heidelberg, New York [Google Scholar]
- Prasser U, Dengler R, Hofstaedter A (2002) Projektgruppe Mammakarzinom. Mammakarzinom. In: Tumorzentrum Regensburg Empfehlungen der Projektgruppen. Studio Druck: Regensburg, pp 241–296 [Google Scholar]
- Preston N, Seers K, Mortimer P (2009) Physical therapies for reducing and controlling lymphedema of the limbs (review). The Cochrane Collaboration (2) [DOI] [PubMed]
- RAND Corporation (1955) A Million Random Digits with 100 000 Normal Deviates. Free Press: Glenco III [Google Scholar]
- Reichenbach H (1947) Elements of Symbolic Logic. Macmillan Company: New York, pp 8–16 [Google Scholar]
- Reichenbach H (1951) The Rise of Scientific Philosophy. University of California: Berkely and Los Angeles, pp 215–249 [Google Scholar]
- Rubenstein L, McCoy J, Cope D, Barrett P, Hirsch S, Messer K, Young R (1995) Improving patient quality of life with feedback to physicians about functional status. J Gen Intern Med 10: 607–614 [DOI] [PubMed] [Google Scholar]
- Schulz K, Grimes D (2005) Multiplicity in randomised trials I: endpoints and treatments. Lancet 365: 1591–1595 [DOI] [PubMed] [Google Scholar]
- Sloan J, Frost M, Berzon R, Dueck A, Guyatt G, Moinpour C, Sprangers M, Ferrans C, Cella D (2006) The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer 14(10): 988–998 [DOI] [PubMed] [Google Scholar]
- Snyder C, Aaronson N (2009) Use of patient-reported outcomes in clinical practice. Lancet 374: 369–370 [DOI] [PubMed] [Google Scholar]
- Symonds T, Berzon R, Margins P, Rummans T (2002) The clinical significance of quality of life results: practical considerations for specific audiences. Mayo Clin Proc 77: 572–583 [DOI] [PubMed] [Google Scholar]
- Takeuchi EE, Keding A, Awad N, Hofmann U, Campbell LJ, Selby PJ, Brown JM, Velikova G (2011) Impact of patient-reported outcomes in oncology: a longitudinal analysis of patient-physician-communication. JCO 29(21): 2910–2917 [DOI] [PubMed] [Google Scholar]
- Torgerson D (2001) Contamination in trials: is cluster randomisation the answer? BMJ 322: 355–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troidl H, Menge K, Lorenz W, Vestweber K, Barth H, Hamelmann H (1979) Quality of life and stomach replacement. In: Herfarth C, Schlag P (eds.). Gastric Cancer. Springer Verlag: Berlin Heidelberg, pp 312–317 [Google Scholar]
- Valderas J, Kotzeva A, Espallargues M, Guyatt G, Ferrans C, Halyard M, Revicki D, Symonds T, Parada A, Alonso J (2008) The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res 17: 179–193 [DOI] [PubMed] [Google Scholar]
- Velikova G, Booth L, Smith A (2004) Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 22: 714–724 [DOI] [PubMed] [Google Scholar]
- Ware J (1976) Scales for measuring general health perceptions. Health Serv Res 11: 396–415 [PMC free article] [PubMed] [Google Scholar]
- Wasson J, Keller A, Rubenstein L, Hays R, Nelson E, Johnson D (1992) Benefits and obstacles of health status assessment in ambulatory settings. The clinicians point of view. Med Care 30: MS42–MS49 [DOI] [PubMed] [Google Scholar]
- West S, Wicklund R (1980) A Primer of Social Psychological Theory. Brooks: Monterey, pp 279–283 [Google Scholar]
- Wood-Dauphinee S, Troidl H (1989) Assessing quality of life in surgical studies. Theor Surg 4: 35–44 [Google Scholar]
- Wright P, Jansen C, Wyatt J (1998) How to limit clinical errors in interpretation of data. Lancet 352: 1539–1543 [DOI] [PubMed] [Google Scholar]
- Wright P, Selby P, Crawford M, Gillibrand A, Johnston C, Perren T, Rush R, Smith G, Velikova G, Watson K, Gould A, Cull A (2003) Feasibility and compliance of automated measurement of quality of life in oncology practice. J Clin Oncol 21: 374–382 [DOI] [PubMed] [Google Scholar]
- Wulff H (2002) The relationship between health disease and quality of life. In: Gimmler A, Lenk C, Aumüller G (eds.). Health and Quality of Life. Philosophical Medical and Cultural Aspects LIT-Verlag: Münster [Google Scholar]