In a Letter to the Editor by Miller et al., arguments are made concerning the localization of HYAL-2 in chondrocytes and other cells. The authors present historical as well as new evidence that the hyaluronidase, HYAL-2 is present on the surface of cells, in contradiction to the recent work of Chow et al.1 The letter also provides commentary as to why the results published by Chow et al. are inconclusive and thus, conclude that their view is the only correct possibility. Unfortunately the arguments in the Letter to the Editor are incorrect or speculative in nature and the data presented is, itself, inconclusive.
The primary data supplied by the authors of the Letter-to-the-Editor show that a human chondrosarcoma cell line, CH1.2 exhibits the capacity to support the entry of jaagsiekte sheep retrovirus, a process dependent on the expression of cell surface, GPI-linked HYAL-2. Even though this cell line expresses some phenotypic properties of a differentiated human chondrocyte, it cannot be considered a chondrocyte, nor should it be labeled as one. Numerous phenotypic alterations have occurred in this cell line and one cannot be sure whether any of these have altered the post-translational processing of the HYAL-2. The original CH1 line was derived from an aggressive human chondrosarcoma2. The line exhibited a doubling time of 2 days and expressed abundant mRNA for TGF-beta. Although the line synthesizes aggrecan mRNA, multiple splicing isoforms are expressed—isoforms that are not exhibited in normal cartilage or benign cartilaginous tumors3. The study by Chow et al. utilized primary cultures of human articular chondrocytes derived from normal donors with no known history of disease. While no in vitro model system is perfect, the use of such primary cells represents the best approach to address whether HYAL-2 is present in chondrocytes under physiological conditions.
The Letter by Miller et al. also comments on the work of Chow et al. concerning the various epitope-tagged constructs. The authors make a valid point that by constructing HYAL-2 with a carboxy-terminal V5 tag, this tagged sequence would be removed upon transfer to a GPI linkage, assuming that is, that the transfer occurs in these cells. Nonetheless, Chow et al., demonstrate V5-tagged protein bands of approximately 55 kD (the size of full-length HYAL-2) and further, demonstrate that the expression of these bands is blocked with HYAL-2 antisense morpholino oligonucleotides. So, even granting that a transfer to GPI occurs to a fraction of the HYAL-2 synthesized, the presence of HYAL-2 protein with a V5-tag still attached implies the existence of second pool of HYAL-2 that is not GPI linked. A similar argument is made for constructs with a myc epitope tag added to the amino-terminal of HYAL-2. The contention by Miller et al. is that the myc tag would be removed along with the signal leader peptide following passage into the endoplasmic reticulum. This however is incorrect. The myc-tagged HYAL-2 was cloned into a pSecTaq2 that contains not only a start codon but also an Ig kappa signal peptide leader sequence. It is this signal peptide that is cleaved by the signal peptidase leaving the myc-tag intact and still attached to the HYAL-2. It is for this reason that Chow et al. again obtained full-length myc-tagged protein at 55 kD. In a preliminary experiment, the Chow et al. authors incorporated the myc-tag into the amino-terminal of a HYAL-2 devoid of its native leader sequence. Again, using the pSecTaq2 vector leader sequence to direct insertion of the protein into the endoplasmic reticulum, a full-length myc-tagged HYAL-2 protein was obtained. Additionally, as with the previous work, this myc-tagged HYAL-2 could only be detected intracellular as it required permeabilization of the cell membrane for detection.
A second criticism in the Letter by Miller et al. is that the peptide-specific antibodies generated by Chow et al., are not recognizing native protein. While this is always a possibility, there is no direct evidence to support the suggestion. Such statements could be made concerning the use of all peptide-specific polyclonal antibodies. Nonetheless, Chow et al., did demonstrate in Fig. 8B of their manuscript that an increase in HYAL-2 protein, detected by the antibody by western blotting, is matched by an increase in hyaluronidase activity (at the same molecular size) as measured using hyaluronan zymography. Thus, while it is still possible that antigen and the hyaluronidase activity represent separate pools of HYAL-2, the most straightforward explanation is that the antibody is recognizing the native functional enzyme. Further, looking at the immunofluorescence localization provided by Chow et al., it is unclear why there would be such a defined localization of the antigen to vesicles throughout the cytoplasm if the antibody were recognizing denatured protein. The work by Miller et al. in the Letter only supports the contention that there is HYAL-2 present at the cell surface of this chondrosarcoma cell line. There are no data given as to whether there remains within the cell, a larger pool of HYAL-2, such as within lysosomes. It is also not clear in the studies presented in the Letter as to how much HYAL-2 is necessary to support viral entry. Is it possible that HYAL-2 is present in a GPI linkage but below the level detected by immunofluorescence?
Along these same lines, the Letter by Miller et al., discuss another contradiction concerning the susceptibility of HEK293 and HeLa cells to jaagsiekte sheep retrovirus. In the Letter, by Miller et al., state that HeLa cells are not susceptible to infection by virus. However, Chow et al. demonstrate that HYAL-2 mRNA and full-length HYAL-2 protein are present in both HEK293 and HeLa cells. Miller et al. speculate that perhaps some defect in the endogenous HYAL-2 prevents this protein from being expressed in HeLa cells. Again, a simpler explanation is that the HYAL-2 in both of these cell lines is primarily expressed intracellularly and that in some cells, a fraction of the total can also be expressed extracellularly after transfer to a GPI linkage.
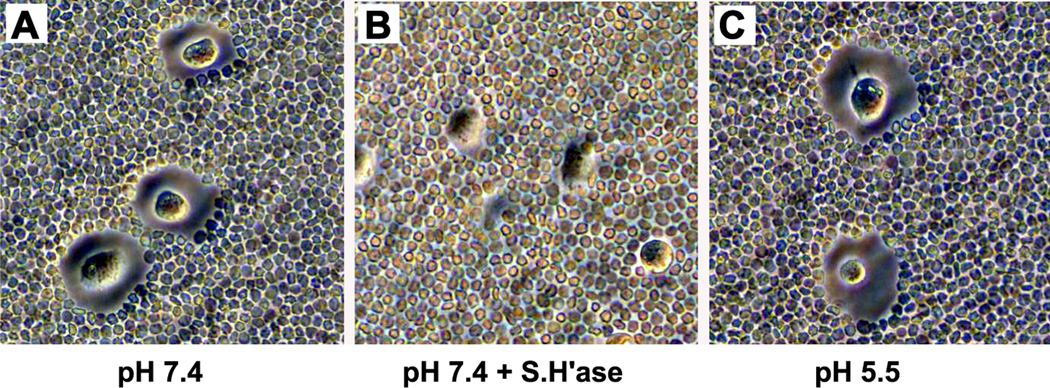
The issues presented in the Letter, also sidestep the major question that was being addressed by Chow et al. The real issue is not whether there is a sub-threshold level of HYAL-2 present at the surface of cells that can support the entry of jaagsiekte sheep retrovirus. The major question is whether there is hyaluronidase activity present at the cell surface that could participate in the catabolism of hyaluronan. It has been argued that if HYAL-2 were present on the surface of cells such as chondrocytes, at high enough levels, certain environmental conditions might exist under which the enzymatic processing of hyaluronan could take place extracellularly. Both HYAL-1 and HYAL-2 have well-documented pH 3.7 optima for their hyaluronidase activity. Even Dr. Miller in his previous studies 4 could not detect any hyaluronidase activity in lysates or conditioned media of NIH 3T3 or Hela cells at pH 7.5. Nonetheless, it is sometimes argued that perhaps some unique lipid environment of the plasma membrane may alter this pH requirement allowing HYAL-2 to be active at higher pH conditions. We attempted to address this issue by testing the capacity of chondrocytes to degrade their hyaluronan-rich pericellular matrix under reduced pH conditions. We have shown previously that the pericellular matrices observed on chondrocytes in vitro represent primarily aggrecan attached to a limited scaffolding of hyaluronan5–7. These matrices remain tightly bound to the chondrocyte cell surface (Fig. 1A) but are highly sensitive to even dilute concentrations of Streptomyces or testicular hyaluronidase (Fig. 1B). Using this model, the pH of the culture medium of separate cultures of bovine articular chondrocytes was lowered by increments of 0.5 units. Below pH 5.0 the cells lift off the dish and could not be analyzed (data not shown). However, as shown in Fig. 1C, chondrocytes incubated at pH 5.5 exhibit no change in the size of the cell-associated matrix, even after 6 hours under these conditions. It is unlikely that chondrocytes in cartilage ever reach this pH. However, this demonstrates that, if the cellular environment did reach this pH, there still would be no effect on the matrix structure or content of extracellular hyaluronan. This figure was not included in the final version of the manuscript by Chow et al., published in Osteoarthritis and Cartilage. In retrospect, it would have been useful to include these data. In the discussion of this figure we argued that perhaps our analytical approaches were not sensitive enough or appropriate to detect minute levels of membrane-associated HYAL-2 or, perhaps there was a different hyaluronidase besides HYAL-2 was present on the surface of these cells. The functional assay shown herein in Fig. 1 suggests that even if HYAL-2 or some other hyaluronidase were present, it is unlikely to be a major contributor to the physiological turnover of hyaluronan.
Fig 1.
Endogenous pericellular matrices are sensitive to Streptomyces hyaluronidase but stable at pH 5.5. Fixed erythrocytes were applied as a uniform-sized particle suspension to low density cultures of bovine articular chondrocytes to reveal the pericellular matrix surrounding living cells as described previously5–7. Panel A: pH 7.4 conditions; panel B: pH 7.4 in the presence of 2 units/ml Streptomyces hyaluronidase and; panel C: pH 5.5 conditions.
In summary, the arguments made by Miller and colleagues in their Letter to the Editor are in some cases incorrect, in other cases speculative and the data they present, inconclusive. The myc tag was not cleaved from the amino terminal of the HYAL-2 upon insertion into the endoplasmic reticulum. Thus, the fact that the myc epitope was intracellularly-localized in the study by Chow et al. suggests that the HYAL-2 is either not GPI-linked or, that a portion of the HYAL-2 is transferred to a GPI linkages but is not exported to the cell surface. Given that full-length HYAL-2 was obtained containing an epitope tag on either the carboxy- or amino-terminus, suggests that a significant pool of HYAL-2 exists within the cell that has not been enzymatically processed and transferred to a GPI linkage. That the polyclonal antibody staining matches the immunostaining of the recombinant HYAL-2 with a myc epitope tag, suggest that the native endogenous HYAL-2 expressed by chondrocytes is also present predominately as a intracellular protein.
In conclusion, there will always be possible counter arguments to all published studies. However, this is probably best evaluated by experiment rather than speculation. Nonetheless, future studies may more conclusively demonstrate that a level of HYAL-2 exists on the surface of most cells including chondrocytes. However, it is more likely that the membrane-bound HYAL-2 levels will vary from one cell type to another. In some cells this level will be undetectable by standard immunological approaches and some will be so low that they will not support the entry of HYAL-2 dependent retroviruses. But in all cells HYAL-2 and HYAL-1 will always be predominate intracellularly, contributing as low pH optima hydrolases to the intracellular degradation of hyaluronan.
References
- 1.Chow G, Knudson CB, Knudson W. Expression and cellular localization of human hyaluronidase-2 in articular cartilage and cultured cell lines. Osteoarthritis Cartilage. 2006;14:849–858. doi: 10.1016/j.joca.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chansky H, Robbins JR, Cha S, Raskind WH, Conrad EU, Sandell LJ. Expression of cartilage extracellular matrix and potential regulatory genes in a new human chondrosarcoma cell line. J Orthop Res. 1998;16:521–530. doi: 10.1002/jor.1100160502. [DOI] [PubMed] [Google Scholar]
- 3.Matsui Y, Araki N, Tsuboi H, Tsumaki N, Nakata K, Kawabata H, et al. Differential expression of aggrecan mRNA isoforms by chondrosarcoma cells. Anticancer Res. 2002;22:4169–4172. [PubMed] [Google Scholar]
- 4.Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci U S A. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudson W, Bartnik E, Knudson CB. Assembly of pericellular matrices by COS-7 cells transfected with CD44 homing receptor genes. Proc Natl Acad Sci U S A. 1993;90:4003–4007. doi: 10.1073/pnas.90.9.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudson W, Aguiar DJ, Hua Q, Knudson CB. CD44- anchored hyaluronan-rich pericellular matrices: an ultrastructural and biochemical analysis. Exp Cell Res. 1996;228:216–228. doi: 10.1006/excr.1996.0320. [DOI] [PubMed] [Google Scholar]