Abstract
Diabetes is a major cause of chronic kidney disease, and oral antidiabetic drugs are the mainstay of therapy for most patients with Type 2 diabetes. Here we evaluated their role on renal outcomes by using a national Veterans Administration database to assemble a retrospective cohort of 93,577 diabetic patients who filled an incident oral antidiabetic drug prescription for metformin, sulfonylurea, or rosiglitazone, and had an estimated glomerular filtration rate (eGFR) of 60 ml/min or better. The primary composite outcome was a persistent decline in eGFR from baseline of 25% or more (eGFR event) or a diagnosis of end-stage renal disease (ESRD). The secondary outcome was an eGFR event, ESRD, or death. Sensitivity analyses included using a more stringent definition of the eGFR event requiring an eGFR <60 ml/min per 1.73 m2 in addition to the 25% or more decline; controlling for baseline proteinuria thereby restricting data to 15,065 patients; and not requiring persistent treatment with the initial oral antidiabetic drug. Compared to patients using metformin, sulfonylurea users had an increased risk for both the primary and the secondary outcome, each with an adjusted hazard ratio of 1.20. Results of sensitivity analyses were consistent with the main findings. The risk associated with rosiglitazone was similar to metformin for both outcomes. Thus, compared to metformin, oral antidiabetic drug treatment with sulfonylureas increased the risk of a decline in eGFR, ESRD, or death.
Keywords: chronic kidney disease, diabetes, diabetic nephropathy
Chronic kidney disease (CKD) is a major public health problem. CKD prevalence is increasing worldwide,1 partly related to the epidemic of obesity and Type 2 diabetes mellitus (DM). In the United States, diabetes accounts for 45% of incident end-stage renal disease (ESRD).2 In 2006, the US federal government estimated cost was $23 billion for ESRD treatment, and the corresponding CKD treatment cost was $49 billion.2 Patients with CKD have an increased risk of premature death,3, 4 which is further increased by the presence of DM.
A number of randomized clinical trials have shown that control of hyperglycemia,5, 6, 7, 8 blood pressure, and blockade of the renin–angiotensin–aldosterone system9, 10 can slow the progression of diabetic kidney disease. Most previous studies focused on the effect of achieving glycemic targets5, 6, 7, 8 on CKD progression regardless of the oral antidiabetic drug (OAD) used, or on the effect of OADs on proteinuria (an early marker of the development of diabetic kidney disease).11, 12 Few studies have compared the effects of individual OADs on kidney function decline. A recent systematic review on the ‘Comparative Effectiveness and Safety of Oral Diabetes Medications for Adults with Type 2 Diabetes', sponsored by the Agency for Healthcare Research and Quality, concluded that there was insufficient and low-quality evidence on the effectiveness of individual OADs on the development or progression of nephropathy.13
The aim of this study was to determine whether initial treatment with different OAD monotherapies was associated with differential declines in kidney function. Using regional Veterans Health Administration (VHA) data, we recently reported that sulfonylurea users had worse kidney outcomes than metformin users.14 The aim of the present study was to evaluate a larger national VHA cohort of OAD initiators, to increase the precision of our estimates, and to extend our assessment to include rosiglitazone.
RESULTS
Study cohort and analytic population
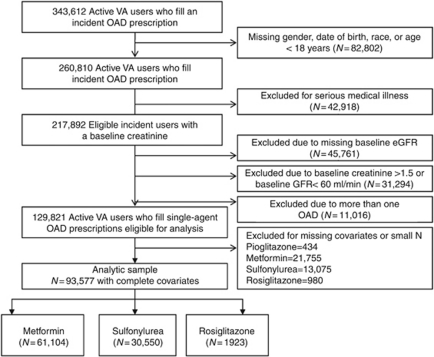
Of the 343,612 Type 2 DM veterans identified as incident users of OADs (Figure 1), we excluded 24% for missing race, 13% with severe medical conditions, 13% for missing baseline estimated glomerular filtration rate (eGFR)/creatinine, 9% because of an eGFR<60 or a creatinine >1.5 mg/dl, and 3% who were not on OAD monotherapy. Of the 129,821 patients who met the eligibility criteria, 72% (93,577) had complete baseline covariates and were included in the final analyses. Of those patients, 65% (61,104) initiated metformin, 33% (30,550) initiated sulfonylureas, and 2% (1923) initiated rosiglitazone. The characteristics of the patients excluded from the analytical data set owing to missing covariates were similar to the 72% included in the analysis (Supplementary Table 1 online).
Figure 1.
Flowchart of eligible patients. eGFR, estimated glomerular filtration rate; OAD, oral antidiabetic drug; VA, Veterans Affairs.
Patient characteristics
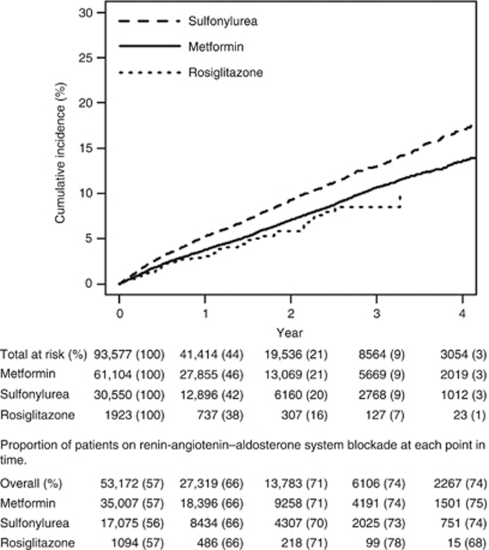
Baseline characteristics were similar across OAD groups (Table 1). Overall, 78% were white, and 96% were men, and median serum creatinine was 1.0 mg/dl (interquartile range (IQR)=0.9, 1.1). Metformin initiators had the highest body mass index median (32.3 kg/m2; IQR=29, 37) compared with rosiglitazone (31 kg/m2; IQR=28, 35) and sulfonylurea (31 kg/m2; 27, 35) initiators. Median HbA1c was lowest among rosiglitazone (6.8; IQR=6.2, 7.6) compared with sulfonylurea (7.3; IQR=6.6, 8.4) and metformin (7.1; IQR=6.5, 7.9) initiators. The median length of follow-up by exposure group was 0.9 years for metformin (range=0.25–5.5 years/IQR=0.5, 1.8), 0.8 years for sulfonylureas (range=0.25–5.5 years/IQR=0.4–1.7), and 0.7 for rosiglitazone (range=0.25–5.3/IQR=0.3, 1.5). The percentage of patients on angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) increased over time and was similar across groups at baseline and during follow-up (Figure 2).
Table 1. Baseline characteristics of the study cohort.
| Characteristics | Metformin (N=61,104) | Sulfonylurea (N=30,550) | Rosiglitazone (N=1923) |
|---|---|---|---|
| Age, median (IQR, year) | 60 (55, 69) | 62 (56, 72) | 64 (57,72) |
| Male, % | 95 | 97 | 97 |
| Race, % | |||
| White | 79 | 76 | 72 |
| Black | 16 | 18 | 16 |
| Hispanic | 4 | 5 | 11 |
| Other | 1 | 1 | 1 |
| Baseline creatinine, mg/dl, median (IQR) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) |
| Glomerular filtration rate, ml/min | 81 (72, 93) | 80 (70, 93) | 79 (69, 91) |
| Urine microalbumin–creatinine ratio test available, % | 18 | 22 | 19 |
| Microalbuminuria present, % | 3 | 3 | 4 |
| HbA1c, median (IQR) | 7.1 (6.5, 7.9) | 7.3 (6.6, 8.4) | 6.8 (6.2, 7.6) |
| Systolic blood pressure, median (IQR) | 134 (124, 144) | 135 (124, 146) | 133 (122, 143) |
| Diastolic blood pressure, median (IQR) | 77 (70, 84) | 76 (69, 84) | 74 (67, 81) |
| Body mass index (kg/m2), median (IQR) | 32.3 (28.8, 36.7) | 30.7 (27.3, 34.7) | 30.9 (27.5, 34.7) |
| Coronary artery disease, % | 21 | 23 | 23 |
| Cerebrovascular disease, % | 9 | 11 | 8 |
| Peripheral vascular disease, % | 3 | 3 | 3 |
| Smoking, % | 12 | 11 | 8 |
| ACEI or ARBs, % | 57 | 56 | 57 |
| Thiazides, % | 33 | 30 | 28 |
| Loop diuretics, % | 8 | 12 | 10 |
| Statins, % | 62 | 55 | 59 |
| Number of outpatient medications, median (IQR) | 5 (3, 8) | 5 (3, 8) | 5 (3, 7) |
| Number of outpatient visits, median (IQR) | 5 (3, 8) | 5 (3, 8) | 4 (2, 7.5) |
| Hospitalized in the prior year, % | 8 | 10 | 8 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; IQR, interquartile range.
Figure 2.
Crude cumulative incidence of the composite outcome (persistent reduction of baseline estimated glomerular filtration rate of 25% or end-stage renal disease by oral antidiabetic drug exposure group).
Primary and secondary outcomes
The annual rate of the primary composite end point of a persistent 25% decline in eGFR or ESRD was 3.8%, 5.0%, and 3.2% in metformin, sulfonylurea, and rosiglitazone initiators, respectively. eGFR events accounted for 97% of the primary composite end points (97%, 97%, and 95% in the metformin, sulfonylurea, and rosiglitazone initiators groups, respectively). The annual rate of the secondary composite end point, which included all-cause mortality, was 4.1, 5.5, and 3.4% in the respective three OAD groups (Table 2).
Table 2. Event rates and adjusted hazard ratios for primary and secondary outcomes among incident OAD users.
| Metformin (N=61,104) | Sulfonylurea (N=30,550) | Rosiglitazone (N=1923) | |
|---|---|---|---|
| Primary analysis–Persistent exposure required (PER)a | |||
| Person time (years) | 77,420 | 36,592 | 2014 |
| Number of GFR or ESRD events | 2926 | 1841 | 64 |
| Rate/1000 person-years | 3.8 | 5 | 3.2 |
| aHRb (95% CI) for the primary outcome | Reference | 1.20 (1.13, 1.28) | 0.92 (0.71, 1.18) |
| Number of GFR, ESRD or death events | 3149 | 2027 | 68 |
| Rate/100 person-years | 4.1 | 5.5 | 3.4 |
| aHRb (95% CI) for the secondary outcome | Reference | 1.20 (1.13, 1.28) | 0.89 (0.69, 1.12) |
| Sensitivity analyses | |||
| PER with a more stringent definition of GFR eventc | |||
| Person time (years) | 79,197 | 37,508 | 20,44 |
| Number of GFR or ESRD events | 1390 | 908 | 34 |
| Rate/100 person-years | 1.76 | 2.42 | 1.66 |
| aHRb (95% CI) for the primary outcome | Reference | 1.17 (1.07, 1.27) | 0.90 (0.64, 1.27) |
| Number of GFR, ESRD or death events | 1622 | 1102 | 38 |
| Rate/1000 person-years | 2.05 | 2.94 | 1.86 |
| aHRb (95% CI) for the secondary outcome | Reference | 1.16 (1.08, 1.27) | 0.83 (0.60, 1.15) |
| PER adjusted for ACR | 10,293 | 4366 | 401 |
| Person Time (years) | 12291 | 4959 | 415 |
| Number of GFR or ESRD events | 461 | 245 | 11 |
| Rate/100 person-years | 3.8 | 4.9 | 2.7 |
| aHRb (95% CI) for the primary outcome | Reference | 1.22 (1.03, 1.44) | 0.67 (0.37, 1.25) |
| Number of GFR, ESRD, or death events | 493 | 263 | 12 |
| Rate/1000 person-years | 4.0 | 5.3 | 2.9 |
| aHRb (95% CI) for the secondary outcome | Reference | 1.20 (1.02, 1.41) | 0.70 (0.39, 1.24) |
| Persistent exposure not required (PENR)d | |||
| Person time (years) | 139,773 | 76,244 | 4492 |
| Number of GFR or ESRD events | 5752 | 3787 | 170 |
| Rate/100 person-years | 4.1 | 5.0 | 3.8 |
| aHRb (95% CI) for the primary outcome | Reference | 1.11 (1.06, 1.16) | 1.01 (0.87, 1.18) |
| Number of GFR, ESRD, or death events | 6403 | 4405 | 188 |
| Rate/100 person-years | 4.6 | 5.8 | 4.2 |
| aHRb (95% CI) for the secondary outcome | Reference | 1.13 (1.08, 1.18) | 0.97 (0.84, 1.12) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ACR, albumin to creatinine ratio; aHR, adjusted hazard ratio; ARB, angiotensin receptor blocker; BMI, body mass index; CI, confidence interval; ESRD, end-stage renal disease; GFR, glomerular filtration rate; LDL, low-density lipoprotein; OAD, oral antidiabetic drug.
PER: considers patients persistent on their incident regimen until they have a gap in use of medications that reaches 90 days, or have added or switched to a different OAD or insulin, have a study outcome, have left the Veterans Affairs (VA), reached the end of the study or reached a creatinine of 1.5 mg/dl or higher.
Cox proportional hazards model for time to renal disease. Adjusted hazard ratio is for each exposure compared with metformin as reference. All models were adjusted for age, sex, race, fiscal year of cohort entry, number of medications, number of outpatient visits, history of hospitalization, baseline HbA1c, BMI, serum creatinine, LDL cholesterol, use of medications (ACEI or ARBs, thiazide or loop diuretics, or statins), smoking-related illness, myocardial infarction; obstructive coronary disease, or prescription for a long acting nitrate; stroke/transient ischemic attack; atrial fibrillation/flutter; mitral/aortic or rheumatic heart disease; asthma/obstructive pulmonary disease; procedures for carotid/peripheral artery revascularization or bypass or lower extremity amputation. All continuous variables were modeled as third degree polynomials.
GFR event defined as a persistent decline of 25% of baseline GFR plus reaching a GFR<60 ml/min.
PENR: patients remain in their initial OAD exposure group, regardless of their persistence on drug therapy, until a study outcome, or end of the study patients remain in their original exposure group regardless of changes in therapy after cohort entry (akin to an intent to treat analysis).
Compared with metformin, sulfonylurea use was associated with an increased risk of reaching the primary composite end point (adjusted hazard ratio (aHR)=1.20 (95% confidence interval (CI): 1.13, 1.28)), as well as the secondary outcome (aHR=1.20 (95% CI: 1.13, 1.28)). Compared with metformin, rosiglitazone use was not significantly associated with the primary or secondary outcome (Table 2). We also made a direct comparison of rosiglitazone and sulfonylurea use. Compared with sulfonylureas, rosiglitazone use was associated with a decreased risk for both the primary (aHR=0.76 (95% CI: 0.59, 0.99)) and secondary outcome (aHR=0.73 (95% CI: 0.57, 0.94)). Unadjusted cumulative incidence curves are shown in Figure 2. The percentage of patients who remained at risk at each time interval was similar across all exposure groups. In addition, the percentage of patients who remained on ACE inhibitors or ARBs was also similar.
Propensity score–matched analyses yielded similar results. Compared with metformin, sulfonylurea use (1 to 1 greedy matching n=57,904) was associated with an increased risk for the primary outcome (aHR=1.23 (95% CI: 1.15, 1.31); P<0.0001); compared with metformin, rosiglitazone use (1 to 3 greedy matching n=7648) was not statistically associated with a higher or lower risk for the primary outcome (aHR=0.92 (95% CI: 0.67, 1.25); P=0.59)).
Sensitivity analysis and subgroup analyses
Results were consistent with the primary analysis across all sensitivity and subgroup analyses.
eGFR event defined as persistent 25% decline from baseline eGFR and incident CKD (eGFR<60 ml/min)
When the requirement of achieving a GFR<60 ml/min was added to the 25% GFR decline, the number of events was substantially reduced (from 4831 to 2332). However, results of this sensitivity analysis were similar to those from the main analyses. Sulfonylurea use was associated with a higher risk for both the primary outcome (aHR=1.17 (95% CI: 1.07, 1.27)) and the secondary outcome (aHR=1.17 (95% CI: 1.10, 1.25); P<0.0001) compared with metformin. There were no differences between rosiglitazone and metformin for either the primary or secondary outcome.
The subset with baseline albumin to creatinine ratio (ACR) measurement
Among the 15,065 patients with a baseline ACR measurement, OAD-specific rates of both primary and secondary end points were similar to those of the entire cohort. Compared with metformin, sulfonylurea use was associated with an increased risk of both the primary (aHR=1.22 (95% CI: 1.03, 1.44)) and secondary (aHR=1.20 (95% CI: 1.02, 1.481)) outcomes. There was no significant difference observed between rosiglitazone use and metformin use for either the primary or the secondary outcome (Table 2).
Persistent exposure not required
In this analysis, each individual remained in their incident OAD regimen exposure group throughout follow-up regardless of changes to their regimen (similar to an intention-to-treat analysis). The strength of the observed associations was attenuated but consistent with the primary analysis such that sulfonylurea use was associated with a higher risk of reaching the primary outcome (aHR=1.11 (95% CI: 1.06, 1.16)) and secondary outcome (aHR=1.13 (95% CI: 1.08, 1.18; P<0.0001)) compared with metformin. There were no differences between the rosiglitazone therapy and metformin for either the primary or the secondary outcome in this analysis (Table 2).
Subgroup analyses
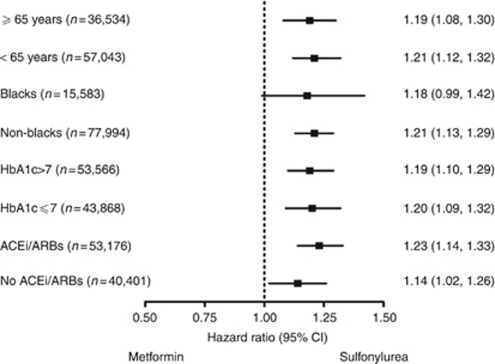
Prespecified assessments were performed and no significant interactions were identified between OAD categories and the a priori subgroups: age, race, use of ACE inhibitors/ARBs, and HbA1c levels (Figure 3).
Figure 3.
Adjusted hazard ratios for the composite outcome of glomerular filtration rate event or end-stage renal disease among age, race, HbA1c, and renin–angiotensin–aldosterone system blockade subgroups. Hazard ratios greater than 1 demonstrate an increased risk for composite outcome with sulfonylurea compared with metformin. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
DISCUSSION
DM is the most common cause of CKD and progression to ESRD in the United States.2 Patients with CKD and ESRD experience an increased risk of premature death even at early disease stages.3, 4 In addition to the human costs, treatment costs pose an enormous burden on the health-care system. Although the efficacy of OADs on glucose control is well established,13 their effects on long-term kidney function outcomes are less clear.13, 15 Furthermore, whether individual OADs have different effects on kidney function is unknown.
In this large national retrospective cohort study of 93,577 diabetic veterans initiating OAD monotherapy, initiation of sulfonylureas compared with metformin was associated with a 20% increased risk of the composite outcome of an eGFR event or ESRD. This association was consistently observed across all planned sensitivity analyses, including the use of a more stringent GFR event definition, a subgroup analysis of patients with baseline urine protein measurement, and an analysis in which persistent exposure was not required. Supplemental analyses using a propensity score–matched design yielded almost identical results. These results are also consistent with our previous findings in a smaller regional VHA cohort from the southeast United States.14
A number of renoprotective properties of metformin have been recently recognized. Patients with CKD have metabolic disturbances, including insulin resistance,16, 17, 18, 19, 20, 21 oxidative stress,22 and chronic inflammation,23, 24, 25, 26 all of which have been proposed to have a significant role in CKD progression.18, 27 Recent data indicate that metformin has important antioxidant features28 in addition to its known insulin-sensitizing properties. Both of these properties are relevant in early stages of diabetic kidney disease, when beneficial effects can be attained before the development of irreversible glomerular damage. It is also possible that different OADs confer a differential risk of acute renal injury (AKI). Morales et al.28 demonstrated in animal models that metformin prevented gentamicin-induced AKI by normalizing oxidative stress and restoring mitochondrial functional integrity. The contribution of AKI to CKD progression and ESRD has recently been highlighted.29, 30 Thus, metformin could slow CKD progression if it prevented or reduced the severity of AKI.
Another possible explanation for the differences observed is weight gain associated with sulfonylureas vs. metformin. Excess weight has been recognized as a significant risk factor for CKD progression.31, 32, 33, 34, 35, 36 Multiple epidemiological and mechanistic studies have confirmed the adverse effects of obesity on kidney function. The so-called ‘obesity glomerulomegaly' is primarily a model of hyper-filtration associated with the development of proteinuria. Sulfonylureas are well known to promote weight gain.15, 32, 34 In a systematic review, the weighted mean absolute difference in body weight between sulfonylureas and placebo in clinical trials was 3.8 kg (3.6–4.0 kg).15 In a previous observational study in a VHA regional cohort, the mean adjusted weight difference between sulfonylurea users and metformin users at 12 months following initiation of treatment was 3.18 kg.37 Whether differences in weight gain among metformin compared with sulfonylurea users could contribute to differences observed in CKD and death requires further scrutiny.
Recently, the United Kingdom Prospective Diabetes Study (UKPDS)38 10-year posttrial follow-up reported that significant risk reductions (=0.79) persisted for any diabetes-related end point (95% CI=0.83–0.99) for metformin compared with the dietary restriction group, but no difference was observed in risk of microvascular disease (including plasma creatinine or ACR). It is important to highlight that in UKPDS patients were assigned to metformin only if they were more than 120% of ideal body weight. Of 342 individuals randomized to metformin, only 136 (40%) completed the posttrial monitoring. In the sulfonylurea vs. dietary restriction arm, there was a 24% risk reduction for any diabetes-related end point (P=0.001), including renal microvascular outcomes. Unlike UKPDS, we had sufficient power to directly compare metformin with sulfonylurea therapy.
Our study found that rosiglitazone was associated with a similar risk of kidney decline as metformin and lower risk than sulfonylureas. Both metformin and rosiglitazone are insulin sensitizers.39 Previous studies found that rosiglitazone decreased albuminuria in patients with DM.11, 12, 40, 41 Our results are similar to those from the ADOPT clinical trial, which reported a hazard ratio of 0.91 (95% CI: 0.67, 1.23) for developing eGFR <60 cc/min comparing rosiglitazone with glyburide.42 When using this same outcome, the aHR for the same comparison in our cohort was 0.77 (95% CI: 0.55, 1.10; data not shown). With the more sensitive definition requiring only a persistent decline of GFR of 25% from baseline, the difference in outcome was statistically significant. Although our study suggests that rosiglitazone does not differ from metformin and is associated with slower decline in kidney function compared with sulfonylureas, the sample size for the rosiglitazone group was small, which limits the precision of these estimates.
Our comparative effectiveness study has noteworthy strengths. We assembled a large national cohort of diabetic patients who allowed detection of small differences in risk. This study applied a new-user design43 and strict criteria to minimize misclassification of exposures, outcomes, and covariates. Our analyses accounted for available laboratory and physiological measurements that complemented administrative data, reducing concerns about residual confounding. Our previous studies evaluating the associations between the choice of first OAD and intermediate outcomes, using a regional VHA cohort, showed results consistent with those from a recent systematic review on comparative effectiveness and safety of medications for Type 2 diabetes.13 Our group estimated that after 1 year, compared with sulfonylurea use, metformin initiators had 3.2 kg lower weight; a nonsignificant 5 mg/dl lower low-density lipoprotein; 8.7 mg/dl lower triglycerides; and no difference in HbA1c.37, 44 The consistency of our findings in the primary, secondary, and sensitivity analyses, as well as with the small amount of informative data from randomized clinical trials42 and our previous study using data from the southeastern region of the United States,14 lends further credibility to these results and suggests a lack of systematic bias in the present cohort study.
Although an intention-to-treat analysis is considered the gold standard in clinical trials, trials are designed to minimize non-adherence, crossover, and differential use of co-therapies. We required persistent exposure on the OADs of interest to best address the effect of these drugs. To avoid confusion with clinical trials, we termed the analysis that kept patients in their initial exposure group as persistent exposure not required. In this analysis, the associations we observed were similar to those of the primary analysis but attenuated suggestive of increasing exposure misclassification.
If the observed more rapid decline in renal function with sulfonylureas compared with metformin is indeed causal, then our observed hazard ratio would mean that, in clinical practice, for every 1000 patients begun on sulfonylureas rather than metformin therapy, an excess of about 8 persons annually will experience a 25% or more decline in GFR.
Our study is not without limitations. First, although all individuals had a GFR ⩾60 ml/min, there were no major differences in measured characteristics between exposure groups at baseline, and patients were censored after reaching a serum creatinine ⩾1.5 mg/dl; confounding by indication is still of concern if patients who initiated sulfonylurea or metformin were systematically different in ways that make them more likely to be diagnosed with kidney disease. Second, our cohort consisted mainly of male veterans, and findings should be generalized to other populations with caution. Third, only a minority of the cohort had data on proteinuria, a key predictor of CKD progression. The subgroup analysis restricted to individuals with a baseline measurement of urine protein strengthens our findings. Fourth, in this retrospective study, some individuals with reversible AKI may have been misclassified as having CKD progression. We attempted to minimize such misclassification by requiring a confirmatory eGFR measurement 3–12 months after the first measurement.
Given the known limitations of using creatinine alone for estimating kidney function,45 we used eGFR (Modification of Diet in Renal Disease four-component equation) as the measurement of kidney function, although it is less accurate in a range of values >60 ml/min. Nevertheless, we performed a sensitivity analysis with a more stringent definition of eGFR event, requiring not only a persistent 25% drop of baseline eGFR but also reaching an eGFR ⩽60 ml/min, which should mitigate these concerns. Finally, we used refill data as a proxy for medication exposure. Although exposure misclassification cannot be ruled out, our group has previously demonstrated that prescription fills are a good proxy for medication use, and we anticipate that this potential misclassification would be non-differential with regard to exposure group, and thus would tend to bias our estimates toward the null hypothesis.37, 44
In summary, in this large national retrospective cohort study, initiation of sulfonylureas was associated with an increased risk of a clinically significant decline in kidney function or death compared with initiation of metformin. Our data support the current recommendations by the American Diabetes Association and the International Diabetes Federation in their recommendation of metformin as the first-line therapy for DM, in patients with earlier stages of kidney disease. More data are needed on risks and benefits of specific OADs in more advanced CKD.
MATERIALS AND METHODS
Study design and data sources
We conducted a retrospective cohort study of diabetic patients seen within the VHA system between 1 October 2001 and 30 September 2008. The cohort was constructed using national VHA databases from the Decision Support Services, which contain prescriptions data and laboratory results. The primary source of the prescription data was the Veterans Health Information System and Technology Architecture (VistA) and included inpatient and outpatient prescriptions dispensed by a VHA Pharmacy or a Consolidated Mail Outpatient Pharmacy.
The VHA national medical data sets contain electronically captured patient demographics and diagnostic and procedure information from inpatient and outpatient encounters, coded according to the International Classification of Diseases, Ninth Revision; Clinical Modification (ICD9-CM). Data on vital signs (blood pressure, weight, and height) and vital status were obtained from the VHA Corporate Data Warehouse and Vital Status master files, respectively. The institutional review boards of Vanderbilt University Medical Center and the VHA Tennessee Valley Healthcare System (VHA TVHS) and the Research and Development Committee of the VHA TVHS approved this study.
Study population
The study population included veterans ⩾18 years old, who received regular care in the VHA health-care system and filled an incident OAD prescription during the study period. Incident prescriptions were defined as the first OAD prescription filled after 365 days of active use of the VHA pharmacy services without any antidiabetic drug filled.43 Incident OAD users with known birth date and gender, and at least 365 days of baseline data (for ascertainment of other selection criteria and covariates), were included in our cohort. Approximately 86% of our cohort members had a diabetes-related ICD9-CM code within the 365 days preceding or at the time of OAD initiation. We excluded patients with a baseline diagnosis for serious medical conditions (congestive heart failure, HIV/AIDS, cancer (except for non-melanoma skin cancer), transplant, end-stage kidney or liver disease, or respiratory failure) or cocaine use, as these conditions may influence the selection of antidiabetic medications and affect the frequency of study outcomes. We also excluded patients who had a baseline serum creatinine >1.5 mg/dl or with an eGFR <60 ml/min, as these patients would be less likely to be started on metformin. We excluded all patients missing race information, as this is necessary for the calculation of eGFR using the Modification of Diet in Renal Disease equation.
Follow-up
Patients were followed up from the date of incident OAD prescription fill until development of the study outcome or a censoring event. Censoring events were as follows: (1) leaving the VHA system, defined as 181 days of no contact with the VHA system; (2) the end of the study (30 September 2008); (3) non-persistence on the incident OAD regimen defined as 90 days with no drug supply; switching or adding another antidiabetic drug; and (4) the day after reaching a creatinine value ⩾1.5 mg/dl. At these serum creatinine values, metformin is no longer recommended,46 and thus patients become less likely to continue metformin treatment. Such selective dropout would create a bias favoring metformin in these comparisons.
Exposure
The incident OAD monotherapy categories were metformin, sulfonylurea, and rosiglitazone. Combination therapies and insulin users during the baseline period were excluded because they often represent regimen intensification or persons with higher baseline HbA1c. Pioglitazone was not in the Veterans Affairs formulary for most of the study years, and hence the number of users was small and excluded from further analyses.
Using pharmacy information, we calculated ‘days supply in hand'. Given that patients may ‘stockpile' medications, we estimated how many pills a patient had on each follow-up day. For example, if a patient filled a 90-day supply of metformin and refilled it on day 80, then on day 80 the patient had 100 days supply in hand (90 from the new fill plus 10 leftover from initial fill). Days supply in hand was reset to 0 with a change in OAD dose. Initial dosing for the different OADs is provided in the Supplementary Tables.
Outcomes
All eGFRs were estimated using the Modification of Diet in Renal Disease four-component equation.37 High eGFR values were truncated at 150 ml/min. Serum creatinine levels <0.4 mg/dl were considered implausible and were excluded from the analysis.
The primary end point was a composite of a GFR event or reaching ESRD. The secondary end point was a composite of GFR event, ESRD, or all-cause mortality.
A GFR event was defined as a persistent 25% or greater decline from the baseline eGFR. This cutoff is clinically significant and similar to the one chosen by other studies that included this higher range of eGFR values (incident or early CKD).47, 48, 49 Because the GFR event/ESRD definition was meant to capture CKD progression, and not episodes of reversible AKI, we required that the GFR event criteria also be met 3–12 months later. Persons who were censored the day after a creatinine of 1.5 mg/dl or greater would be counted as having an outcome of interest if they met criteria for a GFR event.
ESRD was defined as reaching one of the following: an eGFR <15 ml/min per 1.73 m2 or the first inpatient or outpatient code for dialysis or related procedures or renal transplantation (see Supplementary Information). We also required that ESRD criteria (by eGFR or code) be met at 3–12 months to prevent capturing reversible AKI episodes.
All-cause mortality was determined by a date of death in the Veterans Affairs Vital Status Master file. Information from multiple sources including Medicare, VHA utilization, Social Security, and VHA compensation and pension benefits is used to determine this date and has been shown to be highly accurate when compared with the National Death Index.50
Covariates
Important comorbidities were selected a priori and identified using ICD9-CM–coded health-care encounters or prescriptions for specific medications in the 365-day baseline period. The study covariates included age, sex, race/ethnicity (white, black, Hispanic), year of cohort entry, physiologic variables collected closest to cohort entry (systolic and diastolic blood pressure, serum creatinine, HbA1c, and body mass index), cardiovascular disease (coronary artery disease, cardiovascular disease, and peripheral vascular disease), smoking, chronic obstructive pulmonary disease, use of medications known to affect creatinine values (ACE inhibitors or ARBs, loop and thiazide diuretics), measures of health-care utilization including the number of outpatient visits, hospitalization during the baseline period (yes, no), and the unique number of prescription medications on the index date, and Veterans Affairs site of incident care.
Information on baseline proteinuria was available in the form of micro-ACR in a subset of patients (n=15,065). Patients were considered to have microalbuminuria if their ACR was ⩾30 mg/g. Baseline creatinine was obtained exclusively from outpatient values.
Sensitivity and subgroup analyses
Sensitivity analyses included the following: (1) using a more stringent definition of eGFR event, which required a GFR<60 ml/min in addition to 25% or more decline; (2) controlling for baseline proteinuria, restricted to those with this measurement (n=15,065); and (3) not requiring persistent exposure to the initial OAD during follow-up. This analysis is similar to an intention-to-treat analysis in randomized trials.
Because of known differential effects of age, race, renin–angiotensin system blockade, and HbA1C on CKD progression, we assessed for effect modification through stratified analyses for age:<65 years or ⩾65 years, race: African American: yes/no, ACE inhibitors/ARBs: yes/no, and HbA1c=<7 or >7. Age and HbA1c were dichotomized for the subgroup analyses but used as continuous variables in the main analysis.
Statistical analysis
The primary analysis was time to the renal composite end point among those who persisted on their initial OAD. We evaluated time to the first of eGFR event or ESRD as the primary outcome, and an eGFR event, ESRD, or death as the secondary outcome. In a supplemental analysis, persistent exposure on the incident regiment was not required. In addition, we performed propensity score–matched analyses for the primary outcome. The propensity score models for sulfonylurea vs. metformin and rosiglitazone vs. metformin yielded c statistics of 0.67 and 0.69, respectively, reflecting that imbalance in baseline covariates was mild. Sulfonylurea vs. metformin used a 1 to 1 greedy matching (N=57,904) and rosiglitazone vs. metformin a 1 to 3 greedy matching (N=7648). Cox proportional hazards regression models were used to analyze the association between OAD regimen and time to the study outcomes adjusting for the study covariates, and using metformin as the reference for all comparisons. hazard ratio and 95% CI were calculated. The proportional hazards assumption for the drug groups was verified graphically and by testing for interactions with drug group and time. There was no evidence of departure from model assumptions. All continuous covariates were modeled with a third-degree polynomial term to account for nonlinear effects. Statistical analyses were conducted using R Statistical Program (R Foundation, available at http://www.r-project.org) and SAS for Windows 11.0 (SAS Institute, Cary, NC).
Acknowledgments
Role of the Funding Source and Human Subjects Protection: This research was supported by the Agency for Healthcare Research and Quality and the Veterans Affairs Tennessee Valley Healthcare System (TVHS) Clinical Center of Excellence, Nashville, TN, Geriatric Research Education and Clinical Center, Nashville, TN. The funding sources did not participate in the planning, collection, analysis, or interpretation of data, or in the decision to submit for publication. The investigators had full access to the data and were responsible for the study protocol, statistical analysis plan, progress of the study, analysis, reporting of the study, and the decision to publish. Agency for Healthcare Research and Quality and the US Department of Veterans Affairs had the opportunity to comment on this manuscript before submission. This project was funded under Contract No. 290-05-0042 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The work of AMH and CLR was supported in full by the Career Development Program from the Department of Veterans Affairs, Health Administration, Office of Research and Development, for AMH CDA (2-031-09S) from CSR&D and for CLR (04-342-2) from HSR&D.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Table S1. Baseline characteristics of the patients who were eligible to enter in the study but were excluded owing to missing covariates.
Table S2. Definitions for severe medical conditions.
Table S3. The definition of the composite outcome (reaching the first of GFR event or ESRD).
Table S4. Definition of comorbidities and medications.
Table S5. Average dose per day for the incident prescription.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- Collins AJ, Foley RN, Herzog C, et al. Excerpts from the US renal data system 2009 annual data report Am J Kidney Dis 201055S1–420.A426–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ. 2006;333:1047. doi: 10.1136/bmj.39001.657755.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocco E, Velussi M, Cernigoi AM, et al. Evidence of a threshold value of glycated hemoglobin to improve the course of renal function in type 2 diabetes with typical diabetic glomerulopathy. J Nephrol. 2001;14:461–471. [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- Levin SR, Coburn JW, Abraira C, et al. Effect of intensive glycemic control on microalbuminuria in type 2 diabetes. veterans affairs cooperative study on glycemic control and complications in type 2 diabetes feasibility trial investigators. Diabetes Care. 2000;23:1478–1485. doi: 10.2337/diacare.23.10.1478. [DOI] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Bakris G, Viberti G, Weston WM, et al. Rosiglitazone reduces urinary albumin excretion in type II diabetes. J Hum Hypertens. 2003;17:7–12. doi: 10.1038/sj.jhh.1001444. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Ruilope LM, McMorn SO, et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens. 2006;24:2047–2055. doi: 10.1097/01.hjh.0000244955.39491.88. [DOI] [PubMed] [Google Scholar]
- Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AM, Roumie CL, Greevy RA, et al. Comparative effectiveness of incident oral antidiabetic drugs on kidney function in veterans with type 2 diabetes mellitus Am Soc NephrolMeeting 2010 Poster. [DOI] [PMC free article] [PubMed]
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147:386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- Chen J, Muntner P, Hamm LL, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. 2003;14:469–477. doi: 10.1097/01.asn.0000046029.53933.09. [DOI] [PubMed] [Google Scholar]
- Fliser D, Pacini G, Engelleiter R, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53:1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Maesato K, Moriya H, et al. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45:275–280. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Nerpin E, Riserus U, Ingelsson E, et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care. 2008;31:1550–1555. doi: 10.2337/dc08-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Nishizawa Y. Chronic kidney disease as a metabolic syndrome with malnutrition--need for strict control of risk factors. Intern Med. 2005;44:179–187. doi: 10.2169/internalmedicine.44.179. [DOI] [PubMed] [Google Scholar]
- Trirogoff ML, Shintani A, Himmelfarb J, et al. Body mass index and fat mass are the primary correlates of insulin resistance in nondiabetic stage 3-4 chronic kidney disease patients. Am J Clin Nutr. 2007;86:1642–1648. doi: 10.1093/ajcn/86.5.1642. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Stenvinkel P, Ikizler TA, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–892. [PubMed] [Google Scholar]
- Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Katz R, Cushman M, et al. Cystatin-C and inflammatory markers in the ambulatory elderly. Am J Med. 2005;118:1416. doi: 10.1016/j.amjmed.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Sacks F, Pfeffer M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- Caravaca F, Cerezo I, Macias R, et al. Insulin resistance in chronic kidney disease: its clinical characteristics and prognosis significance. Nefrologia. 2010;30:661–668. doi: 10.3265/Nefrologia.pre2010.Aug.10491. [DOI] [PubMed] [Google Scholar]
- Morales AI, Detaille D, Prieto M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–869. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusa MD, Chertow GM, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol. 2009;4:520–522. doi: 10.2215/CJN.06711208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- Juhaeri J, Stevens J, Chambless LE, et al. Associations between weight gain and incident hypertension in a bi-ethnic cohort: the atherosclerosis risk in communities study. Int J Obes Relat Metab Disord. 2002;26:58–64. doi: 10.1038/sj.ijo.0801846. [DOI] [PubMed] [Google Scholar]
- Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the american diabetes association and the european association for the study of diabetes. Diabetologia. 2009;52:17–30. doi: 10.1007/s00125-008-1157-y. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17:S213–S217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- Huizinga MM, Roumie CL, Greevy RA, et al. Glycemic and weight changes after persistent use of incident oral diabetes therapy: a veterans administration retrospective cohort study. Pharmacoepidemiol Drug Saf. 2010;19:1108–1112. doi: 10.1002/pds.2035. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Lachin JM, Zinman B, et al. Effects of rosiglitazone, glyburide, and metformin on {beta}-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60:1552–1560. doi: 10.2337/db10-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imano E, Kanda T, Nakatani Y, et al. Effect of troglitazone on microalbuminuria in patients with incipient diabetic nephropathy. Diabetes Care. 1998;21:2135–2139. doi: 10.2337/diacare.21.12.2135. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ushiyama C, Osada S, et al. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism. 2001;50:1193–1196. doi: 10.1053/meta.2001.26703. [DOI] [PubMed] [Google Scholar]
- Lachin JM, Viberti G, Zinman B, et al. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin J Am Soc Nephrol. 2011;6:1032–1040. doi: 10.2215/CJN.09291010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- Roumie CL, Huizinga MM, Liu X, et al. The effect of incident antidiabetic regimens on lipid profiles in veterans with type 2 diabetes: a retrospective cohort. Pharmacoepidemiol Drug Saf. 2010;20:36–44. doi: 10.1002/pds.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992–999. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- Bash LD, Coresh J, Kottgen A, et al. Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol. 2009;170:414–424. doi: 10.1093/aje/kwp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck GJ, Berg RL, Coggins CH, et al. Design and statistical issues of the modification of diet in renal disease trial. the modification of diet in renal disease study group. Control Clin Trials. 1991;12:566–586. doi: 10.1016/0197-2456(91)90069-x. [DOI] [PubMed] [Google Scholar]
- Fried LF, Duckworth W, Zhang JH, et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D) Clin J Am Soc Nephrol. 2009;4:361–368. doi: 10.2215/CJN.03350708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold N, Sohn M, Maynard C, et al. VIReC technical report 2: VA NDI mortality data merge projectIn: Hines IL, Edward Hines Jr VA (eds).Hospital VA Information Resource Center 2006. Available at http://www.virec.research.va.gov/References/ TechnicalReports/VIReCTechnicalReports.htm .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.