Abstract
Neurobehavioral disorders are increasingly prevalent in children, however their etiology is not well understood. An association between prenatal cellular telephone use and hyperactivity in children has been postulated, yet the direct effects of radiofrequency radiation exposure on neurodevelopment remain unknown. Here we used a mouse model to demonstrate that in-utero radiofrequency exposure from cellular telephones does affect adult behavior. Mice exposed in-utero were hyperactive and had impaired memory as determined using the object recognition, light/dark box and step-down assays. Whole cell patch clamp recordings of miniature excitatory postsynaptic currents (mEPSCs) revealed that these behavioral changes were due to altered neuronal developmental programming. Exposed mice had dose-responsive impaired glutamatergic synaptic transmission onto layer V pyramidal neurons of the prefrontal cortex. We present the first experimental evidence of neuropathology due to in-utero cellular telephone radiation. Further experiments are needed in humans or non-human primates to determine the risk of exposure during pregnancy.
To date, 3–7% of school-aged children suffer from attention deficit hyperactivity disorder (ADHD)1. Children diagnosed with ADHD are at greater risk for low academic achievement, poor school performance, and delinquent behavior inconsistent with their developmental level2,3. The diagnosis of ADHD has increased at an average rate of 3% per year since 1997, making the condition a growing public health concern1. The behavioral problems in ADHD have been associated with neuropathology localized primarily to the prefrontal cortex. Children with ADHD have a reduction in prefrontal cortex volume, a reduction in gray and white matter, and asymmetry4,5. These children also have a deficit in working memory associated with inattention and controlled by activity of neurons in the prefrontal cortex6. A recent study showed that poor attention and low working memory capacity may be due to the inability to override the involuntary capture of attention by irrelevant information7. This too is controlled by the prefrontal cortex, as the shifting of one's attention voluntarily is driven by “top-down” signals in the prefrontal cortex while the involuntary capture of attention depends on “bottom-up” signals from both subcortical structures and the visual cortex7.
The etiology of ADHD remains unknown and growing evidence suggests that it is not solely due to genetic factors8. Risk factors include family psychiatric history, socioeconomic status, gender, and smoking during pregnancy9,10. A recent epidemiologic study found an association between prenatal cellular telephone exposure and subsequent behavioral problems in the exposed offspring11. This association is important given the increasing number of cellular phone users worldwide, reaching approximately four billion as of December 200812. However, evidence of direct causation is lacking.
The specific absorption rate (SAR) is a measure of tissue radiation exposure. The European Union has set a SAR limit of 2.0 W/kg and in the United States this limit is set at 1.6 W/kg13. The in-utero effects of radiation exposure within this SAR limit on neurodevelopment remain unknown. To determine if prenatal exposure to radiofrequency radiation leads to impaired memory or behavior after birth, we performed behavioral and electrophysiological studies in mice exposed in-utero to 800–1900 Mhz radiofrequency radiation from cellular telephones.
Results
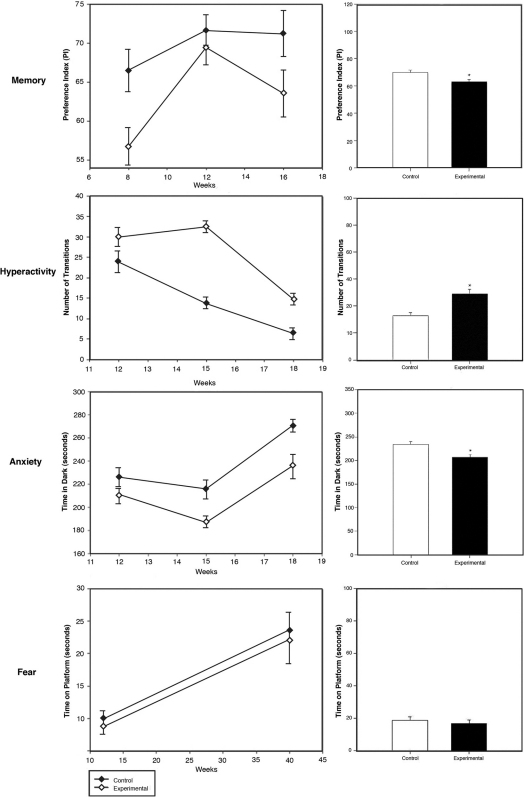
In order to determine if in-utero cell phone radiation exposure affects behavior we chose to conduct a battery of tests that identify impairments in memory, hyperactivity, anxiety, and fear, which are often associated with ADHD. Thirty-three female mice were exposed throughout gestation (days 1–17) to radiation from muted and silenced 800–1900 Mhz cellular phones with a SAR of 1.6 W/kg. The phones were positioned above each cage over the feeding bottle area at a distance of 4.5–22.3 cm from each mouse, depending on the location of the animal within the cage, and placed on an uninterrupted active call for the duration of the trial. A control group of forty-two female mice was kept concurrently under the same conditions, however using a deactivated phone. Parturition was not different between groups and occurred at 19 days ± 1 day. In order to evaluate memory in the exposed and unexposed mice, 161 progeny were given a standard object recognition memory test in three different cohorts at 8, 12, and 16 weeks of age (82 experimental and 79 control mice). The mice were allowed to explore two identical objects for 15 minutes per day for two days and on the third day one object was replaced with a novel object. On day 3 the mice were filmed for 5 minutes exploring the novel and familiar objects. Three observers, blinded to the treatment, viewed the footage and recorded the exploration time for the novel and familiar objects. The preference index was defined as the time spent exploring the new object divided by the time spent exploring both the new and old object, multiplied by one hundred. A decrease in preference index indicates diminished memory. The preference index of the experimental group at 8, 12, and 16 weeks was less than the control and the results were significant at each time point [Figure 1]. The mean preference index in the exposed group was 56.8, 69.4 and 63.5 compared to 66.5, 71.7, and 71.2 in the control group at 8, 12 and 16 weeks, respectively. The experimental group had a cumulative mean preference index of 63.0% and the control group 69.9% (p = 0.003, n = 161, t test). Compared to the control group, the exposed mice had a significantly lower mean preference index suggesting impairment in memory [Figure 1]. In order to ensure that our findings are in fact due to memory deficits and not distractibility or hyperactivity we calculated the percent time spent idle - not exploring either of the objects. The mean idle time in the exposed group was 90.06, 90.53, and 96.48 compared to 92.12, 91.89, and 97.07 in the control group at 8, 12 and 16 weeks, respectively. The control group had a cumulative mean idle time of 90.8% while the experimental group had a cumulative mean idle time of 90.4% and the difference between the two groups was not statistically significant (p = .58).
Figure 1. Behavioral testing in exposed and control mice.
The left column displays the data determined in mice at several ages after exposure. The right column demonstrates the cumulative average. To test memory the Standard object recognition memory test was used and a Preference Index (percent of total exploration time spent exploring the new object) shown at 8, 12, and 16 weeks of age. The cumulative mean preference index of the experimental group was 63.0% and the control group 69.9% (*p = 0.003, n = 161). To test hyperactivity we used the Light/Dark box test and display transitions at 12, 15, and 18 weeks of age. The cumulative mean number of transitions in the experimental group was 24.4 and the control group 16.4 (*p = <0.001, n = 141). To test anxiety we measured time spent in the dark at 12, 15, and 18 weeks of age. The cumulative average time spent in the dark in the experimental group was 207 seconds and in the control was 234 seconds (*p < 0.001, n = 141). To measure fear we used the Step down assay and display the time spent on the platform at 12 weeks and adulthood. The cumulative mean time spent on the platform in the experimental group was 16.7 seconds and in the control was 18.5 seconds (p = 0.59, n = 98).
To explore fearful behavior we performed the light/dark box test measuring hyperactivity/anxiety and the step down assay assessing fear of exploring the environment. The light/dark box test measures anxiety using a rodent's natural aversion to bright light14. The box contained two compartments: one white compartment that was illuminated and one black compartment that remained dark. The number of transitions between the two compartments was used to determine locomotion and in turn hyperactivity15. Anxious behavior is measured by recording the time spent in each compartment15. A total of 141 progeny were given the light/dark box test in three different cohorts at 12, 15, and 18 weeks of age (71 experimental and 70 control mice). Each mouse was placed in the light/dark box for 5 minutes and filmed. Three observers, blinded to the treatment regimen, viewed the footage and recorded the time spent in the dark compartment along with the number of transitions between each compartment. The average number of transitions in the experimental group at 12, 15, and 18 weeks was fewer than in respective controls and the results were significant at each time point [Figure 1]. The average number of transitions in exposed mice was 29.9, 32.5 and 14.8 compared to 23.9, 13.8, and 6.5 in the control group at 12, 15 and 18 weeks, respectively. The experimental group showed a cumulative mean of 24.4 transitions and the control group showed a mean of 16.4 transitions (p <0.001). Compared to the control group, the greater number of transitions between the two compartments in the experimental group suggested hyperactive behavior [Figure 1].
To identify whether anxiety might be a factor contributing to the behavioral phenotype reported in the light/dark box experiment, we first compared the duration of time in the dark versus the time spent in the light. An increased time in the dark indicates anxious behavior15. At 12, 15, and 18 weeks the experimental group spent less time in the dark and the results were significant at each time point [Figure 1]. The duration of time in darkness of the exposed group was 210.8, 187.0 and 235.8 seconds compared to 225.6, 215.5 and 270.6 seconds in the control group at 12, 15 and 18 weeks, respectively. The mice exposed in utero spent a cumulative average of 207 seconds in the dark while the control mice spent an average of 234 seconds in the dark indicating decreased anxiety in the cellular phone exposed mice (p < 0.001) [Figure 1].
The Step Down Assay was performed on 98 mice at 12 weeks and in adulthood to determine fear of exploring the environment (51 control and 47 experimental mice). The test is performed by recording the time spent on a standard platform. A greater period of time on the platform indicates increased fearfulness. Exposed mice showed no significant difference in time spent on the platform when compared to the controls [Figure 1]. The control mice spent an average of 18.5 seconds while the experimental group spent an average of 16.7 seconds (p = 0.59) [Figure 1].
Overall, the mice exposed in-utero to radiation were hyperactive, had decreased memory, and decreased anxiety.
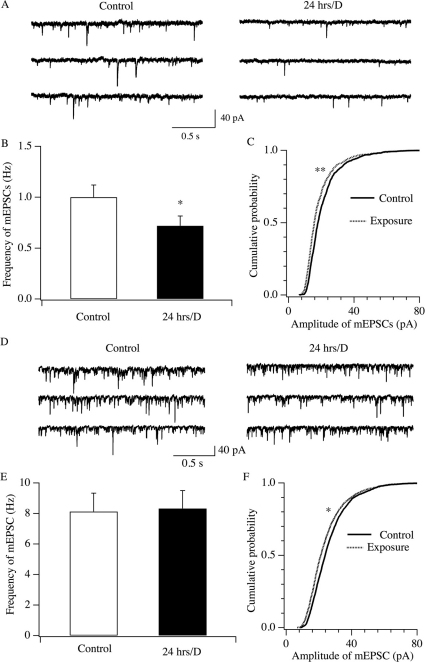
To understand the mechanisms underlying the changes in the memory and hyperactivity in animals exposed to radiation in-utero, we examined whether changes in the neuronal circuitry occurred in brain areas responsible for these compromised behaviors. Specifically, we asked whether changes in the synaptic transmission in CNS neurons are responsible for impaired memory and hyperactivity in radiation-exposed animals. The prefrontal cortex (PFC) is responsible for executive functions by screening distractions and maintaining attention in goal-oriented behaviors. Impairment of the PFC leads to dysregulated behavior/emotion such as ADHD16. The pyramidal neurons, the primary cell type in this structure, regulate attention and behavior through a complex and interconnected network. Whole cell patch clamp recordings of miniature excitatory postsynaptic currents (mEPSCs) were performed in pyramidal neurons of the PFC in control and cell phone-exposed mice. mEPSCs were generated by random vesicle release of glutamate from presynaptic neurons in the absence of stimulation. The measurement of mEPSCs is used to analyze the efficacy of synaptic transmission. Changes in mEPSC frequency are thought to result from modification of the presynaptic component of synaptic transmission, while amplitude changes indicate alterations in the postsynaptic component17,18. Coronal prefrontal cortex slices (300 μm) were prepared from 3–4 week old mice. mEPSCs were recorded in layer V pyramidal neurons in the prefrontal cortex in mice exposed to in-utero radiation for 9, 15 and 24 hours/day throughout gestation; the detection and analysis of mEPSC frequency and amplitude were performed as we described previously18. In animals exposed to in-utero radiation for 24 hours/day, a decrease in the frequency of mEPSCs was seen (control: 1.00 ± 0.12 Hz, n = 40; 24 hours/day: 0.72 ± 0.06 Hz, n = 43, p<0.05, t test, Figure 2A and B). The cumulative probability curves for the amplitude of mEPSC events recorded from the in utero cell phone-exposed mice (24 hours/day) shifted significantly to the left relative to those recorded from the controls (P<0.01, Kolmogorov-Smirnov test; control: 2765 events, cell phone exposure: 2224 events), indicating that the amplitude of mEPSCs was decreased [Figure 2C]. In a subset of experiments, we examined whether the reduction of mEPSC frequency depended on dosages of exposure in mice prenatally exposed 0, 9, 15 and 24 hours per day [Figure 3]. The trend of the dose-dependent decrease in the frequency of mEPSCs (0 hour/day: 1.37±0.41, n = 9; 9 hours/day: 1.27 ± 0.21 Hz, n = 9; 15 hours/day: 1.04 ± 0.20 Hz, n = 10; 24 hours/day: 0.72± 0.13, n = 11) was statistically significant (linear correlation: Correlation Coef = −0.97, Unadjusted r2 = 0.94, P<0.05).
Figure 2. Synaptic efficacy of glutamatergic synapses is decreased in brain neurons of mice after prenatal exposure to cell phone radiation.
A–C, mEPSCs were recorded in layer V pyramidal neurons of the prefrontal cortex. Representative traces of mEPSCs from control and cell phone exposure groups are shown in A. mEPSC frequency and cumulative probability of mEPSC amplitude from both groups are shown in B (*, P<0.05, t test) and C (**, P<0.01, Kolmogorov-Smirnov test; controls, 2225 events; Exposed, 2766 events). D–F, representative traces, frequency and amplitude of mEPSCs recorded in neurons in the VMH are shown. *, P<0.05, Kolmogorov-Smirnov test; Control: 2161 events, Cell phone group: 2261 events.
Figure 3. A dose-dependent attenuation in the frequency of mEPSCs in layer V pyramidal neurons in mice.
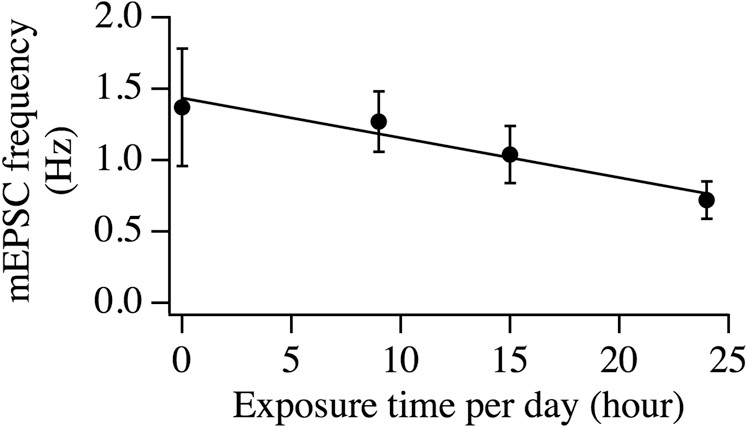
The frequency of mEPSCs recorded in mice prenatally exposed to cell phone radiation at of dose of 0, 9, 15 and 24 hrs per day are shown. Error bars are SEM. The dose responsive relationship is determined using regression analysis (Correlation coefficient, −0.97; r2, 0.94; P<0.05).
Altogether, these results indicate that synaptic efficacy of glutamatergic transmission decreases at both pre- and postsynaptic sites in layer V pyramidal neurons. Thus, we demonstrate impairment in glutamatergic transmission (release from nerve terminals and glutamate receptor response) onto pyramidal neurons in the PFC after in-utero exposure to radiation from cellular telephones.
In a parallel experiment we examined whether in-utero radiation exposure led to changes in synaptic transmission in another brain area. mEPSCs were recorded in neurons in the ventral medial hypothalamus (VMH), a brain area implicated in the regulation of energy homeostasis19,20. Our results indicated that in mice exposed to radiation for 24 hours/day, the frequency of mEPSCs (control: 8.13±1.20 Hz, n = 14; cell phone radiation: 8.32±1.17 Hz, n = 14) was not significantly different from that in control mice (P>0.05, t test, Figure 2D and E). However, the cumulative probability of mEPSC amplitude recorded in radiation-exposed mice significantly shifted to the left (P<0.05, Kolmogorov-Smirnov test; control: 2161 events, cell phone group: 2261 events; Figure 2F), suggesting that the amplitude of mEPSCs is smaller in the cell-phone exposed group than in controls. This result implies that an impairment of glutamatergic transmission occurs at the postsynaptic site. In summary, our results suggest that the effects of prenatal exposure to the cell phone radiation were not limited to the cortex.
Maternal stress can alter fetal development by increasing offspring exposure to corticosterone, causing cognitive deficits, hyperactivity, and alterations of the hypothalamo-pituitary-adrenal axis21. In order to exclude the possibility that impaired memory and behavior in exposed mice was caused by stress resulting from experimental manipulation, we measured serum corticosterone levels on day twelve of gestation using an ELISA assay. The mean corticosterone level in the exposed female mice (69.91 ng/ml, n = 6) was not significantly different from that in the control females (69.94 ng/ml, n = 6) [Figure 4], eliminating stress as a source of the observed behavioral and electrophysiologic differences.
Figure 4. Corticosterone levels during pregnancy were unaltered by exposure.
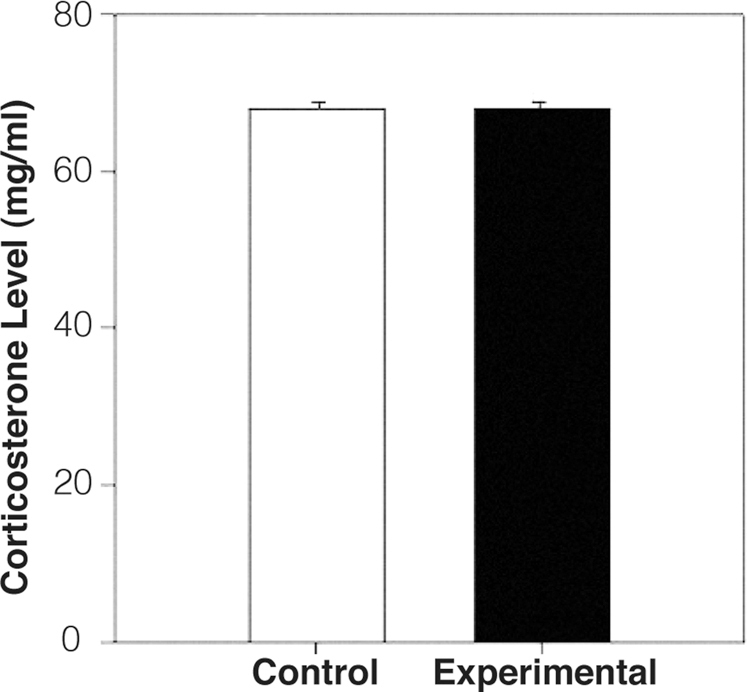
The mean corticosterone level in the pregnant control females was 69.94 ng/ml and in the exposed female mice was 69.91 ng/ml.
Discussion
Here we demonstrate that fetal exposure to 800–1900 Mhz-rated radiofrequency radiation from cellular telephones leads to behavioral and neurophysiological alterations that persist into adulthood. Mice exposed during pregnancy had impaired memory, were hyperactive, and had decreased anxiety, indicating that in-utero exposure to radiofrequency is a potential cause of neurobehavioral disorders. We further demonstrated impairment of glutamatergic synaptic transmission onto pyramidal cells in the prefrontal cortex associated with these behavioral changes, suggesting a mechanism by which in-utero cellular telephone radiation exposure may lead to the increased prevalence of neurobehavioral disorders.
This is the first study to specifically identify effects of radiofrequency exposure on the mouse fetus. During critical windows in neurogenesis the brain is susceptible to numerous environmental insults; common medically relevant exposures include ionizing radiation, alcohol, tobacco, drugs and stress. The effects of these agents are dependent on dose and timing of exposure. Even small exposures during periods of neurogenesis have a more profound effect than exposure as an adult. Alcohol affects cerebral neurogenesis, patterning of brain development and subsequent behavior. Maternal smoking also affects fetal development; fetal tobacco exposure results in a higher incidence of behavioral and cognitive impairment including ADHD. Similarly, prenatal exposure to cocaine can lead to behavioral disorders. Even prenatal maternal stress can lower intelligence and language abilities in offspring. As demonstrated by these examples, environmental exposures occurring in fetal life can lead to persistent neurological deficits. Exposure to these insults as an adult does not carry the same consequences. It is therefore not surprising that studies exposing adult animals to radiofrequency radiation failed to find similar significant defects in behavior. The exposure to cellular telephones in pregnancy may have a comparable effect on the fetus and similar implications for society as do exposures to other common neurodevelopmental toxicants. While this data demonstrates a clear association between fetal EMR exposure and neurodevelopment, it is important to recognize that the extrapolation of this animal model to humans is limited; the exposures used here are not identical to those experienced by the human fetus.
The molecular and cellular effects of radiofrequency exposure are not yet fully characterized. Multiple targets have been identified in vitro. Electromagnetic frequency exposure has been demonstrated to affect cell division and proliferation, both by inducing apoptosis and altering the cell cycle22. Electromagnetic radiation may promote the formation of reactive oxygen species (ROS) causing cell damage23. One study specifically analyzing the effects of radiofrequency radiation on glioma cells demonstrated altered oxidative stress, a potential mediator of the alterations caused by electromagnetic radiation24. Electromagnetic frequency radiation has also been found to activate ERK and p38 MAPK signaling25. Although the precise molecular mechanisms that led to altered glutamatergic synaptic transmission in the prefrontal cortex identified in this study are not yet fully known, here we provide the first evidence that links changes in neuronal circuitry centered on layer V pyramidal neurons in the PFC with impaired memory and cognitive behaviors in animals exposed to radiation from cellular phone use. Our results indicate that the release of glutamate from the nerve terminals on PFC neurons and response of PFC neurons to glutamate are impaired in mice prenatally exposed to cell phone radiation. These results are consistent with previous reports that compromised glutamatergic transmission onto PFC neurons underlies impaired memory and cognitive functions in animals26,27. Our results also imply that the effects of prenatal exposure to radiation on the brain might be global, since glutamatergic transmission onto neurons in another area of the brain (i.e., the VMH) was decreased as well. The effects of prenatal exposure to cell phone radiation may have more profound effects on brain functions than reported in this study. However, the effect was not identical; there are likely to be cell type specific or regional variations in susceptibility. Alternatively, the depth of the VMH may have shielded this region from maximal exposure.
Given the recent advancements in the technology of cellular telephones (i.e. smart phones), they are now used in a capacity beyond that of a basic telephone. For many, cellular telephones are used as a bedside alarm clock and personal organizer. Cellular telephone usage can reach 24 hours/day, leaving users increasingly exposed to the potentially harmful effects of radiofrequency radiation exposure. Our findings indicated significant electrophysiological and behavioral changes in mice exposed in-utero to radiation. The significant trend between the groups treated for 0, 9, 15, and 24 hours/day demonstrates that the effects are directly proportional to usage time, and suggests that safety limits, particularly for pregnant women, can be established. Though it is difficult to translate these findings to human risks and vulnerability, we identify a novel potential contribution to the increased prevalence in hyperactive children, one that is easily prevented. However, it is important to note that hyperactivity and anxiety are closely related and my confound one another.
In this study we used cellular telephones as a source of EMR to mimic human exposure. However there are several limitations to this study that include lack of a defined exposure from a traditional EMF generator. Further we did not measure the level of exposure and the distance to the source was not fixed; mice were free to move within the confines of the cage. Power density measurements with respect to orientation, polarization, reflection, and interference were not considered. In order to determine the maximal effects and potential risks associated with exposure, the mice were exposed from conception to birth, however mouse brain development is incomplete at birth and distinct from that of humans. While neurological effects were found here, future studies should focus on a more narrow gestational age of exposure, use EMF generators to more precisely define exposure, and limit variation in the distance from the source. Definitive studies in humans are required prior to extrapolating these behavioral findings to humans.
In summary, we demonstrate that fetal radiofrequency radiation exposure led to neurobehavioral disorders in mice. We anticipate these findings will improve our understanding of the etiology of neurobehavioral disorders. The rise in behavioral disorders in developed countries may be, at least in part, due to a contribution from fetal cellular telephone radiation exposure. Further testing is warranted in humans and non-human primates to determine if the risks are similar and to establish safe exposure limits during pregnancy.
Methods
Exposure and Behavioral Tests
Over five separate experiments, a total of 27 breeding cages were set-up each containing 3 CD-1 female mice and 1 CD-1 male mouse (13 experimental cages and 14 control cages). Each experimental cage was equipped with a muted and silenced 800–1900 Mhz cellular phone with a SAR of 1.6 W/kg placed over the feeding bottle area at a distance of 4.5–22.3 cm from the mice. The cellular phones were then placed on an active call for 24 hours per day and the 33 experimental female mice were exposed throughout gestation (days 1–17). An additional six females were exposed to an active phone for either 9 or 15 hours per day. Each control cage was equipped with a deactivated phone and was kept under the same conditions. To assure equal exposure time independent of the variable length of gestation (18–20 days), at the end of day 17 all phones were removed. On day 18 all female mice were separated and placed in their own cage yielding a total of 39 exposed pregnant females and 42 unexposed pregnant females. Throughout the experiment, both the control and experimental mice were fed and given water ad libitum. The mice were maintained on a 12 hour light/dark cycle (07:00 on) and all procedures were approved by the Yale University Animal Care and Use Committee.
Memory was evaluated using a standard object recognition memory test. A total of 161 pups were tested (82 experimental mice and 79 control mice) at 8, 12, and 16 weeks. The test consisted of two learning days (Day 1 and 2) and one test day (Day 3). On Day 1 four opaque exploration chambers were set-up in the exam room at a luminosity of 420–440 Lux. Prior to conducting each test, the mice were placed in the testing room and allowed 1 hour to acclimate to the light. Two identical objects were then placed in each of the four chambers and a single mouse was placed in each chamber to explore the two identical objects for 15 minutes. Before repeating the experiment, the objects and the chambers were cleaned thoroughly with a detergent solution to remove any scents or odors. On Day 3 a video camera was placed over all 4 chambers and the objects were rearranged so that each chamber had one familiar object and one novel object. The mice were then allowed to explore both objects and were filmed for 5 minutes. Upon completing the experiment, 3 observers, blinded to the treatment regimen, viewed the first 2 minutes of footage to determine the time spent exploring the novel object. Exploration of the new object was defined as sniffing at less than 1 cm. A preference index was then calculated by dividing the time spent exploring the new object by the total exploration time multiplied by one hundred. The percent time spent idle - not exploring either of the objects was also calculated in order to ensure that our findings are in fact due to memory deficits and not distractibility or hyperactivity.
The light-dark box test was conducted using a light-dark box, constructed of black and white Plexiglass (45×27×27 cm). The dark compartment (18×27 cm) was made of black Plexiglass with a black Plexiglass cover and the light compartment (27×27 cm) was made of white Plexiglass and remained open. The light compartment was kept at a luminosity of 420–440 Lux. An opening (7.5×7.5 cm) was located in the wall between the two chambers allowing free access between the light and dark compartments. A video camera was then placed over the box for filming. Prior to conducting each test, the mice were placed in the testing room and allowed 1 hour to acclimate to the light. A single mouse was then placed in the light chamber and was allowed to explore the box for 5 minutes while being filmed. Before repeating the experiment, the chambers were cleaned thoroughly with a detergent solution to remove any scents or odors. Three observers, blinded to the treatment regimen, then viewed the footage and recorded the total time spent in the dark as well as the total number of transitions. This data was then interpreted as described in the text to analyze anxiety and hyperactivity.
The Step Down Assay was performed to determine fearful behavior by placing a mouse gently on a platform (96 well plate) and recording the time on the platform. The timer was stopped once the mouse stepped off the platform with all four paws. Before repeating the experiment, the platform was cleaned thoroughly with a detergent solution to remove any scents or odors.
Corticosterone Measurement
Gestational stress was analyzed by collecting serum on Day 12 of gestation from 6 exposed and 6 unexposed pregnant females. Serum samples were tested for corticosterone levels using an enzyme immunoassay kit (Assay Designs, Ann Arbor, MI) as recommended by the manufacturer.
Electrophysiology
Mice from control and cell phone-exposed groups were anesthetized with ether and then decapitated. The brains were rapidly removed and immersed in an oxygenated cutting solution at 4°C containing (in mM): sucrose 220, KCl 2.5, CaCl2 1, MgCl2 6, NaH2PO4 1.25, NaHCO3 26, and glucose 10, and adjusted to pH 7.3 with NaOH. Coronal cortical slices (300 µm thick) were prepared from the prefrontal area of the brain and the ventral medial hypothalamus (VMH) using a vibratome. After preparation, slices were maintained in a holding chamber with artificial cerebrospinal fluid (ACSF) (bubbled with 5% CO2 and 95% O2) containing (in mM): NaCl 124, KCl 3, CaCl2 2, MgCl2 2, NaH2PO4 1.23, NaHCO3 26, glucose 10, pH 7.4 with NaOH, and were transferred to a recording chamber constantly perfused with bath solution (33°C) at 2 ml/min after at least a 1 hr recovery.
Whole-cell voltage clamp (at −60 mV) was performed to observe miniature excitatory postsynaptic currents (mEPSCs) in layer V cortical neurons with a Multiclamp 700 A amplifier (Molecular devices, CA). The patch pipettes (tip resistance = 4–6 MΩ) were made of borosilicate glass (World Precision Instruments) with a pipette puller (Sutter P-97) and back filled with a pipette solution containing (in mM): K-gluconate 135, MgCl2 2, HEPES 10, EGTA 1.1, Mg-ATP 2, Na2-phosphocreatine 10, and Na2-GTP 0.3, pH 7.3 with KOH. mEPSCs were recorded in pyramidal neurons under voltage clamp (at −60 mV) in the presence of tetrodotoxin (TTX, 0.5μM,) and a GABA-A receptor antagonist picrotoxin (50 μM). Both input resistance and series resistance were monitored constantly during experiments. The series resistance (between 20 and 40 MΩ) was partially compensated by the amplifier and only recordings with stable series and input resistance throughout experiments were accepted. All data were sampled at 3–10 kHz and filtered at 1–3 kHz with an Apple Macintosh computer using Axograph X (AxoGraph Scientific). mEPSC events were detected and analyzed with AxoGraph X and plotted with Igor Pro software (WaveMetrics, Lake Oswego, OR) as described previously by Rao, et al (2007). Linear correlation was performed with the software GB-STAT (Dynamic Microsystems, Inc, ilver Spring, MD).
Author Contributions
TSA treated the mice, performed the behavioral studies, analyzed the data and wrote the manuscript. GG and XBG performed and analyzed the electrophysiology studies. HST designed the experiment, analyzed the data and edited the manuscript.
Acknowledgments
The authors thank Arie Kaffman and Richard Hochberg for critical reading of the manuscript and thank Neil Odem, Michael Lee and Yuzhe Feng for their technical assistance and analysis of behavioral test results. Supported by grants from EHHI and NICHD (HD052668).
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders (2000) Fourth Edition, Text Revision. Washington, DC, American Psychiatric Association.
- Barkley R. Behavioral Inhibition, Sustained Attention, and Executive Functions: Constructing a Unifying Theory of ADHD. Psychol Bull 121, 65–94 (1997). [DOI] [PubMed] [Google Scholar]
- Rappley M. Attention deficit-hyperactivity disorder. N Engl J Med 352, 165–73 (2005). [DOI] [PubMed] [Google Scholar]
- Sowell E. R. Cortical abnormalities in children and adolescent with attention-deficit hyperactivity disorder. Lancet 362, 1699–707 (2003). [DOI] [PubMed] [Google Scholar]
- Castellanos F. X. Development trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288, 1740–48 (2002). [DOI] [PubMed] [Google Scholar]
- Castellanos F. X. & Tannock R. Neuroscience of attention deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 3, 617–28 (2002). [DOI] [PubMed] [Google Scholar]
- Fukuda K. & Vogel E. K. Human variation in overriding attentional capture. J Neurosci 29, 8726–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I. Beyond polemics: science and ethics of ADHD. Nat Rev Neurosci 9, 957–64 (2008). [DOI] [PubMed] [Google Scholar]
- Brassett-Harknett A. & Butler N. Attention-deficit/hyperactivity disorder: an overview of the etiology and a review of the literature relating to the correlates and lifecourse outcomes for men and women. Clin Psychol Rev. 27,188–210 (2007). [DOI] [PubMed] [Google Scholar]
- Biederman J. & Faraone S. V. Attention-deficit hyperactivity disorder. Lancet 366, 237–248 (2005). [DOI] [PubMed] [Google Scholar]
- Divan H. Kheifets L. Obel C. & Olsen J. Prenatal and postnatal exposure to cell phone use and behavioral problems in children. Epidemiology 19, 523–529 (2008). [DOI] [PubMed] [Google Scholar]
- Measuring the Information Society: The ICT Development Index. International Telecommunication Union. p108. (2009).
- Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys. 74, 494–522 (1998). [PubMed] [Google Scholar]
- Bourin M. & Hascoet M. The mouse light/dark box test. Eur J Pharamocol. 463, 55–65 (2003). [DOI] [PubMed] [Google Scholar]
- Corbetta S. et al. Hyperactivity and novelty-induced hyperactivity in mice lacking Rac3. Behav Brain Res 186, 246–255 (2008). [DOI] [PubMed] [Google Scholar]
- Arnsten A. F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10, 410–22 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless M. A., Whistler J. L., Malenka R. C. et al. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 411, 583–587 (2001). [DOI] [PubMed] [Google Scholar]
- Rao Y. et al. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 117, 4022–33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- M. López et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. .Nat Med. 161001–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. et al. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 1288–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M. Lemaire V. Le Moal M. & Abrous D. N. Age-dependent effect of prenatal stress on hippocampal cell proliferation in female rats. Eur J Neurosci. 29 635–40 (2009). [DOI] [PubMed] [Google Scholar]
- Panagopoulos D. J. Chavdoula E. D. Nezis I. P. & Margaritis L. H. Cell death induced by GSM 900-MHz and DCS 1800-MHz mobile telephony radiation. Mutat Res. 626, 69–78 (2007). [DOI] [PubMed] [Google Scholar]
- Zmyślony M. et al. Acute exposure to 930 MHz CW electromagnetic radiation in vitro affects reactive oxygen species level in rat lymphocytes treated by iron ions. Bioelectromagnetics. 25, 324–8 (2004). [DOI] [PubMed] [Google Scholar]
- Cao Y. et al. 900-MHz microwave radiation enhances gamma-ray adverse effects on SHG44 cells. J Toxicol Environ Health A. 72, 727–32 (2009). [DOI] [PubMed] [Google Scholar]
- French P. W., Penny R. Laurence J. A. & McKenzie D. R. Mobile phones, heat shock proteins and cancer. Differentiation 67, 93–97 (2001). [DOI] [PubMed] [Google Scholar]
- Jentsch J. D. et al. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 34, 2601–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T. et al. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res. 15, 291–302 (2009). [DOI] [PubMed] [Google Scholar]