Abstract
Besides extracellular matrix production, fibroblasts are able to produce various cytokines. Their ubiquitous position makes fibroblasts appropriate cells for sensing various noxious stimuli and for attracting immune cells in the affected area. In the present study the effect of lipopolysaccharide (LPS) and cobalt chloride (CoCl2) on the above fibroblasts functions were evaluated in primary human skin fibroblasts cultures. Collagen, matrix metalloproteinase-1, tissue inhibitor of metalloproteinases-1, transforming growth factor-β1, interleukin-8 (IL-8) and macrophage chemoattractant protein-1 (MCP-1) were measured in fibroblasts culture supernatants. Fibroblasts proliferation and viability were assessed as well. Hypoxia inducible factor-1α and the phosphorylated p65 portion of NF-κB were assessed in fibroblasts protein extracts. LPS and CoCl2 had a minor effect on fibrosis related factors in human primary fibroblasts, possibly due to the absence of interplay with other cell types in the used experimental system. On the contrary both LPS and CoCl2 increased significantly IL-8. LPS also increased considerably MCP-1, but CoCl2 decreased it. Thus LPS and CoCl2 induce a sentinel, nevertheless not identical, phenotype in primary human fibroblasts. The last disparity could result in different body response to infectious or hypoxic noxious stimuli.
Keywords: fibroblast, hypoxia, infection, fibrosis, chemokines, lipopolysaccharide, cobalt chloride, monocyte chemoattractant protein-1, interlukin-8
Fibroblasts are ubiquitous and their major function is to maintain organ integrity and remodeling by producing, degrading and reproducing extracellular matrix (ECM) components1, 2. Besides the above main function, under certain conditions fibroblasts are able to produce various cytokines and thus to interfere with immune system function3.
From a teleological point of view, their ubiquitous position makes fibroblasts appropriate cells for sensing various noxious stimuli and for attracting immune cells in the affected area contributing to the initiation of the inflammatory response that is invariably developed in any case of tissue damage. In order to accomplish this sentinel function fibroblasts are armored with receptors that recognize both pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs)4-6.
During the inflammatory process fibroblasts produce matrix metalloproteinases (MMPs), which among other functions degrade ECM thus making the invasion of immune cells to the damaged area easier. The above process is tightly regulated by the production of tissue inhibitors of MMPs (TIMPs) that bind to the catalytic site of activated MMPs and inhibit their action7. After the elimination of the noxious agent by the immune cells, fibroblasts produce the ECM that is necessary for tissue repair, which in mammals includes various degree of fibrosis1,2.
In the present study the effect of two different and common in clinical practice noxious stimuli on the above described fibroblasts functions were evaluated in primary human dermal fibroblasts cultures. Lipopolysaccharide (LPS) was used for examining the effect of infection8, whereas cobalt chloride (CoCl2) for imitating hypoxia9, 10. The effect of the above factors on fibroblast viability and proliferation was assessed. Collagen, MMP-1/ collagenase-1 and its inhibitor TIMP-1 and transforming growth factor-β1 (TGF-β1), a key inducer of ECM production1,2,11,12, were measured for evaluating the effect of the above stimuli on ECM turnover. The chemokines interleukin-8 (IL-8)/CXCL8 and macrophage chemoattractant protein-1 (MCP-1)/CCL2, which attract mainly neutrophils and monocytes/macrophages respectively13- 19, were measured in order to examine the effect of infection and hypoxia on the sentinel function of the fibroblasts. The transcription factors hypoxia inducible factor-1α (HIF-1α) and the phosphorylated p65 portion of nuclear factor kappa-light-chain-enhancer of activated B cells (p-NF-κB) were assessed in order to confirm the reliability of the performed experiments20-24. Finally, because of the pivotal role that TGF-β1 plays in fibrosis process1,2,11,12, this cytokine was also measured in peripheral blood mononuclear cell (PBMC) culture supernatants, since monocyte derived macrophages are known potent TGF-β1 producers12,25.
Methods
Fibroblasts cell culture conditions
Primary human dermal fibroblasts derived from a 34 years old healthy female volunteer were purchased by American Type Culture Collection, (Manassas, VA). Fibroblasts were cultured in fibroblast basal medium (American Type Culture Collection) supplemented with fibroblast growth-kit-serum free (American Type Culture Collection) for achieving a final concentration of L-glutamine 7.5 mM, hydrocortisone 1 μg/ml, hemisuccinate 1μg/ml, linoleic acid 0.6μM, lecithin 0.6 μg/ml, recombinant (rh) fibroblast growth factor-β (FGF-β) 5 ng/ml, rh epidermal growth factor (EGF) 5 ng/ml, rh TGF-β1 30pg/ ml, rh insulin 5 μg/ml, and ascorbic acid 50 μg/ml. Streptomycin, penicillin and amphotericin B at concentrations of 100 μg/ml, 100 U/ml and 0.25 μg/ml respectively were added for preventing contamination (antibiotic antimycotic solution, Sigma-Aldrich, St. Louis, MO). Fibroblasts were cultured under sterile conditions at 370C in a humidified atmosphere containing 5% CO2. After two passages fibroblasts were detached with a trypsin-EDTA solution (Sigma-Aldrich), washed and counted by optical microscopy on a Neubauer plaque. Cell viability was assessed by trypan blue assay (Sigma-Aldrich).
Assessment of fibroblast proliferation and viability
For assessing fibroblast proliferation and viability, fibroblasts were plated in 96-well plates, at a number of 10000 cells per well and incubated for 72 h with or without LPS (Sigma-Aldrich) at a concentration of 200 ng/ ml or CoCl2 (Sigma-Aldrich) at a concentration of 100 μM. The above concentrations of LPS and CoCl2 were chosen in accordance to the concentrations used in the literature8-10.
Fibroblast proliferation and viability were assessed by measuring colorimetrically the cleavage of the XTT, a yellow tetrazolium salt, to the orange formazan dye via the succinate-tetrazolium reductase system in the mitochondria of metabolically active cells. For this propose the TACS XTT cell proliferation assay kit (Trevigen, Gaithersburg, MD) was used according to the manufacturer's instructions.
All these experiments were performed in triplicates and the results refer to the mean of the three measurements.
Assessment of collagen, MMP-1, TIMP-1, TGF-β1, IL-8 and MCP-1 in fibroblasts culture supernatant
In order to assess various substances in the cell culture supernatant, fibroblasts were plated in 6-well plates, at a number of 200000 cells per well and incubated for 72 h with or without LPS (Sigma-Aldrich) at a concentration of 200 ng/ml or CoCl2 (Sigma-Aldrich) at a concentration of 100 μM. The above concentrations of LPS and CoCl2 were chosen in accordance to the concentrations used in the literature8-10. Fibroblasts supernatants were stored at -800C.
Collagen was measured in fibroblasts culture supernatant colorimetrically with a collagen assay kit (Quick- Zyme BioSciences, Leiden, The Netherlands). This collagen assay is based on the binding of the dye sirius red to collagen. Following binding of the dye, the collagen-dye complex precipitates, resulting in a colored pellet. This color can be released in an alkaline solution and measured. Human TGF-β1, pro-MMP-1, TIMP-1and MCP-1 were measured in fibroblasts culture supernatant by means of Quantikine ELISA kits (R&D Systems, Minneapolis, MN). Human IL-8 was also measured in fibroblasts culture supernatant by means of ELISA (Diaclone, Besancon, France).
All these experiments were performed in triplicates and the results refer to the mean of the three measurements.
Assessment of HIF-1α and p-NF-κB in fibroblasts
For assessing the transcription factors HIF-1α and p-NF-κB, fibroblasts were plated in 6-well plates, at a number of 200000 cells per well and incubated for 72 h with or without LPS (Sigma-Aldrich) at a concentration of 200 ng/ml or CoCl2 (Sigma-Aldrich) at a concentration of 100 μM. The above concentrations of LPS and CoCl2 were chosen in accordance to the concentrations used in the literature8-10.
Proteins were extracted from fibroblasts pellet by using a denaturing cell extraction buffer (Invitrogen, Camarillo, CA) supplemented with a protease inhibitor cocktail containing aprotinin, leupeptin, ethylenediaminetetraacetic acid disodium salt, 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, bestatin hydrochloride and N-(transepoxysuccinyl)- L-leucine 4-guanidinobutylamide (Sigma- Aldrich) as well as 1mM phenyl-methyl-sulfonyl-fluoride (Sigma-Aldrich) and 1mM of the phosphatase inhibitor sodium orthovanadate (Sigma-Aldrich). After assessment of protein concentration with the Bradford assay (Sigma- Aldrich), samples were stored at -800C.
HIF-1α was measured quantitatively in the fibroblasts protein extracts by means of the Surveyor intracellular human/ mouse total HIF-1α immunoassay ELISA kit (R&D Systems), whereas p-NF-κB was measured semi-quantitatively by means of the PathScan phospho-NF-κB p65 (Ser536) sandwich ELISA Kit (CellSignalling Technology, Danvers, MA). The results were normalized to total protein concentration obtained by the Bradford assay.
All these experiments were performed in triplicates and the results refer to the mean of the three measurements.
Assessment of TGF-β1 in PBMC culture supernatants
Because of the pivotal role that TGF-β1 plays in fibrosis process, this cytokine was also measured in PBMC culture supernatants, since monocyte derived macrophages have a major contribution to TGF-β1 production at the site of inflammation12,25. Blood was drawn from 10 healthy volunteers (5 males, mean age 40.3±9.36 years). An informed consent was obtained from each individual enrolled into the study and the hospital ethics committee gave its approval to the study protocol. PBMC were obtained by a Ficoll-Hypaque (Histopaque-1077, Sigma- Aldrich) gradient density centrifugation. The interface was collected, washed with RPMI-1640 (Gibco, Grand Island, NY) and PBMC number was counted by optical microscopy on a Neubauer plaque. Cell viability was assessed by trypan blue assay (Sigma-Aldrich).
PBMC were plated in 24 well plates, at a number of 1x106 cells per well. The used culture media was RPMI- 1640 (Gibco) supplemented with 10% fetal bovine serum (Gibco) and antibiotics (penicillin 100 U/ml and streptomycin 100 μg/ml). Cultures were incubated for 48 hours at 370C in a humidified atmosphere containing 5% CO2, with or without LPS at a concentration of 200 ng/ ml. PBMC supernatants were stored at -800C. Human TGF-β1was measured by means of a Quantikine ELISA kit (R&D Systems).
The normal distribution of the variable (TGF-β1) was evaluated and finally confirmed using the one-sample Kolmogorov-Smirnov test. Paired t-test was used for comparison of means. Results were expressed as mean±SD and were considered as statistically significant when p<0.05.
Results
Fibroblast proliferation and viability
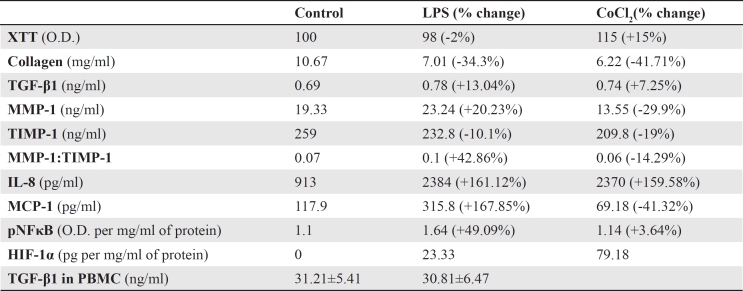
XTT cell proliferation assay showed that neither LPS nor CoCl2 affected significantly fibroblasts proliferation and viability. Compared to untreated fibroblasts, LPS decreased proliferation and viability only by 2%, whereas CoCl2 increased it only by 15% (Table 1).
Table 1. The effect of lipopolysaccharide and cobalt chloride on the evaluated factors.
Collagen, MMP-1, TIMP-1, TGF-β1, IL-8 and MCP-1 in fibroblasts culture supernatant
Collagen production was considerably decreased by both LPS and CoCl2. Compared to untreated fibroblasts, LPS decreased collagen production by 34.3%, from 10.67 mg/ml to 7.01 mg/ml. Similarly, CoCl2 decreased collagen concentration by 41.71%, to 6.22 mg/ml (Table 1).
Compared to untreated fibroblasts, LPS increased MMP-1 by 20.23%, from 19.33 ng/ml to 23.24 ng/ml. On the contrary, CoCl2 decreased MMP-1 by 29.9%, to 13.55 ng/ml. Although not to a great extend, both agents decreased TIMP-1. LPS decreased TIMP-1 by 10.1%, from 259 ng/ml in untreated fibroblasts to 232.8 ng/ml. CoCl2 decreased TIMP-1 by 19%, to 209.8 ng/ml. Regarding the MMP-1 to TIMP-1 ratio, CoCl2 had a minor effect since it decreased it only by 14.29%, from 0.07 to 0.06. The effect of LPS on MMP-1 to TIMP-1 ratio was noteworthy since LPS increased it by 42.86%, to 0.1 (Table 1).
Both LPS and CoCl2 had a minor effect on TGF-β1 roduction. LPS increased TGF-β1 only by 13.04%, from 0.69 ng/ml in untreated fibroblast to 0.78 ng/ml. Similarly, CoCl2 increased TGF-β1 concentration only by 7.25%, to 0.74 ng/ml (Table 1).
Both LPS and CoCl2 induced IL-8 production significantly. LPS increased IL-8 concentration by 161.12%, from 913 pg/ml in untreated fibroblasts to 2384 pg/ml. Similarly, CoCl2 increased IL-8 production by 159.58%, to 2370 pg/ml (Table 1).
Regarding the other evaluated chemokine, LPS and CoCl2 affected MCP-1 production considerably but in the opposite direction. LPS increased MCP-1 production by 167.85%, from 117.9 pg/ml in untreated fibroblasts to 315.8 pg/ml. On the contrary, CoCl2 decreased MCP-1 concentration by 41.32%, to 69.18 pg/ml (Table 1).
HIF-1α and p-NF-κB in fibroblasts protein extracts
HIF-1α was undetected in untreated fibroblasts. In LPS treated fibroblasts, HIF-1α was 23.33 pg per mg/ml of total protein. CoCl2 further increased HIF-1α to 79.18 pg per mg/ml of total protein (Table 1).
Regarding p-NF-κB, semi-quantitative measurement revealed that CoCl2 did not affect its levels since it increased optical density at 450 nm absorption only by 3.64%, from 1.1 per mg/ml of total protein in untreated fibroblasts to 1.14 per mg/ml of total protein. On the contrary, LPS increased p-NF-κB by 49.09%, to an optical density of 1.64 per mg/ml of total protein (Table 1).
TGF-β1 in PBMC culture supernatants
TGF-β1 concentration was high in the supernatant of untreated PBMC, and LPS did not alter significantly its levels. In untreated PBMC TGF-β1 concentration was 31.21±5.41 ng/ml, whereas in LPS treated PBMC was 30.81±6.47 ng/ml (p=0.565, paired t-test). Importantly, compared toTGF-β1 concentration in fibroblasts culture supernatants, TGF-β1 concentration in PBMC culture supernatants was higher by almost 45 times (Table 1).
Discussion
In the present study the effect of LPS, which is bacterial derived and a potent stimulator of the PAMP receptor TLR4 8, as well as of CoCl2, which imitates hypoxia9, 10, on both the classical related to ECM production and the hypothesized sentinel function of the fibroblasts was evaluated in primary human dermal fibroblasts culture. Fibroblasts are ubiquitous and equipped with receptors for both PAMPs and DAPMs and consequently are ideal cells for playing a sentinel role throughout the body4-6. If they could recognize the presence of noxious agents in the area of their residency then it would be quite useful to produce various chemokines for attracting immune cells in order to eliminate the noxious agents.
We evaluated the levels of the transcription factors p-NF-κB and HIF-1α in order to confirm the reliability of the performed experiments. CoCl2 did not affect p-NF-κB levels, which were considerably elevated in the case of fibroblasts treatment with LPS. This confirmed that regarding to the LPS treatment our experimental system worked properly, since phosphorylation of the p65 portion of the transcription factor NF-κB is a downstream event of the TLR4 signal transduction pathway23,24. Interestingly, it is known that NF-κB activation is required for MMP-1, IL-8 and MCP-1 production by human synovial fibroblasts, but not for TIMP-1 production26-29.
In untreated fibroblasts HIF-1α, was undetectable. On the contrary, CoCl2 increased its levels considerably, which confirmed that the performed experiments worked properly, because HIF-1α is known to be stabilized under hypoxic conditions20-22. LPS also increased HIF-1α levels but to a much lesser extent. This increase in HIF- 11α levels in LPS treated fibroblasts could be attributed to the interaction between the two transcription factors30. For example it is established that NF-κB increases HIF- 1α gene transcription31,32. Interestingly, it is known that HIF-11α induces IL-8 and MMP-1 production in human synovial fibroblast33,34. On the contrary, hypoxia inhibits TIMP-1 expression by the above cells34. There is controversy about the effect of hypoxia on MCP-1 production by human fibroblasts, some support that it increases it35 and others support the opposite36.
As shown by the XTT cell proliferation and viability assay, both LPS and CoCl2 did not affect significantly the number of metabolically active primary fibroblasts after 72 h of culture. Thus it seems that these cells are quite resistant to the above noxious stimuli, which can induce apoptosis37,38. This makes fibroblasts available and thus suitable for orchestrating the tissue response to injury, i.e. for affecting both the inflammatory and the repair processes.
Both LPS and CoCl2 decreased collagen levels almost to the same degree. Because collagen is the major ECM component produced by the fibroblasts1,2, it could be assumed that both agents are able to diminish this classical function of the fibroblasts, at least in the absence of the interplay with other cell types, like in the cell culture system used. The MMP-1 to TIMP-1 ratio was slightly decreased in the CoCl2 treated cells, which means that the reduced levels of collagen in this case were due to decreased production. On the contrary the MMP-1 to TIMP-1 ratio was significantly increased in LPS treated fibroblasts, which means that in this case the reduced collagen levels could be the result of decreased production and of increased degradation as well7,39. Regarding the inflammation process, increased degradation of collagen induced by the LPS makes the invasion of the immune cells to the area easier.
The fact that TGF-β1 production was not increased either by LPS or by CoCl2 was unexpected, but it could contribute to the relatively low levels of collagen found in our experiments, since this cytokine is the major driving force for collagen production by the fibroblast1,2,11,12. TGF-β1 levels are generally high in the areas of tissue injury, for example in a wound, contributing in the tissue repair process12,40. Also TGF-β1 can induce its own production by the fibroblast forming a positive feedback loop2,25,41. The absence of the interplay between fibroblast and other cell types could explain our results. TGF-β1 concentration in fibroblasts cell culture supernatants was low, and much lesser than the concentrations used in in vitro studies for induction of collagen production by fibroblasts42. It seems that higher concentrations of TGF-β1 are required for the beginning of the previous described positive feedback loop. In real life such rich TGF-β1 sources could be other cell types, like the monocyte derived macrophages. These cells invade to the injured area and are known potent TGF-β1 producers12,25.
In order to check this hypothesis, PBMC were isolated from 10 healthy volunteers and cultured under basal conditions or LPS stimulation. Interestingly, LPS did not induce further TGF-β1 production by PBMC, which is in accordance with other studies43,44. However, the TGF-β1 concentrations were high in both instances, almost by 45 times higher when compared with the concentrations found in fibroblast culture supernatants. Thus, macrophages attracted to the injured area could offer the trigger for additional TGF-β1production by the fibroblast and for the beginning of the tissue repair process, which in mammals inevitably includes various degrees of fibrosis1,2.
But did the resident fibroblasts play a role in the immune cells attraction to the injured area? Our results confirmed that both LPS and CoCl2 are strong inducers of IL-8 production by the fibroblasts. This chemokine is a potent attractor for neutrophils13-15. Additionally, IL-8 activates the neutrophils45. Thus under both infectious and hypoxic conditions fibroblasts can serve as sentinel cells by attracting neutrophils in the affected area. However, regarding the MCP-1 chemokine, which is a potent attractor for monocytes/macrophages and to a lesser extend for T-lymphocytes and mast cells16-19, LPS and CoCl2 had significant but opposite effect. LPS increased MCP-1 production by the fibroblast, whereas CoCl2 decreased MCP-1 production by the fibroblasts. One could assume that in case of an infectious injury regional fibroblasts attract macrophages in the area, which in turn contribute to the elimination of the noxious agent, and to the repair process through the offer of large quantities of TGF-β1 that induces ECM production by the fibroblast. On the contrary, in case of a hypoxic injury, the attraction of the macrophages to the affected area is less potent, a fact that disturbs the clearance of the necrotic due to hypoxia resident cells by the macrophages and the tissue repair process, while it increases the susceptibility to infections. Interestingly, treatment with neutralizing anti- MCP-1 antibodies reduces the number of macrophages at wound site19, and wound healing is impaired in MCP-1 knock-out mice46, which are in accordance with our hypothesis. Also the derived from the present study data, could explain to some extend the known from the clinical practice impaired resolution of inflammation and tissue repair in areas of hypoxia, like in lower extremity ulcers due to peripheral artery disease or diabetic microvascular dysfunction47,48.
In conclusion, the present study showed that LPS and CoCl2 have a minor effect on fibrosis related factors in human primary fibroblasts, possibly due to the absence of interplay with other cell types in the used experimental system. On the contrary both LPS and CoCl2 induce a sentinel, nevertheless not identical, phenotype in primary human fibroblasts. The last disparity could result in different body response to infectious or hypoxic noxious stimuli.
References
- 1.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 2.Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc. 2008;5:338–342. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordana M, Sarnstrand B, Sime PJ, Ramis I. Immune-inflammatory functions of fibroblasts. Eur Respir J. 1994;7:2212–2222. doi: 10.1183/09031936.94.07122212. [DOI] [PubMed] [Google Scholar]
- 4.Kurt-Jones EA, Sandor F, Ortiz Y, Bowen GN, Counter SL, Wang TC, et al. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J Endotoxin Res. 2004;10:419–424. doi: 10.1179/096805104225006516. [DOI] [PubMed] [Google Scholar]
- 5.Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang PL, Azuma Y, Shinohara M, Ohura K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochem Biophys Res Commun. 2000;273:1161–1167. doi: 10.1006/bbrc.2000.3060. [DOI] [PubMed] [Google Scholar]
- 7.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 8.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 9.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 10.Poulios E, Trougakos IP, Gonos ES. Comparative effects of hypoxia on normal and immortalized human diploid fibroblasts. Anticancer Res. 2006;26:2165–2168. [PubMed] [Google Scholar]
- 11.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 12.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, et al. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rennekampff HO, Hansbrough JF, Kiessig V, Dore C, Sticherling M, Schroder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res. 2000;93:41–54. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]
- 16.Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 17.Strieter RM, Koch AE, Antony VB, Fick RB, Jr, Standiford TJ, Kunkel SL. The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemoattractant protein-1. J Lab Clin Med. 1994;123:183–197. [PubMed] [Google Scholar]
- 18.DiPietro LA, Polverini PJ, Rahbe SM, Kovacs EJ. Modulation of JE/MCP-1 expression in dermal wound repair. Am J Pathol. 1995;146:868–875. [PMC free article] [PubMed] [Google Scholar]
- 19.Dipietro LA, Reintjes MG, Low QE, Levi B, Gamelli RL. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen. 2001;1:28–33. doi: 10.1046/j.1524-475x.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 21.Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71:642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 24.Baccala R, Gonzalez-Quintial R, Lawson BR, Stern ME, Kono DH, Beutler B, et al. Sensors of the innate immune system: their mode of action. Nat Rev Rheumatol. 2009;5:448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 25.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Distler JH, Jungel A, Huber LC, Seemayer CA, Reich CF, 3rd, Gay RE, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci U S A. 2005;102:2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuchiya A, Imai K, Asamitsu K, Waguri-Nagaya Y, Otsuka T, Okamoto T. Inhibition of inflammatory cytokine production from rheumatoid synovial fibroblasts by a novel IkappaB kinase inhibitor. J Pharmacol Exp Ther. 333:236, 243. doi: 10.1124/jpet.109.158899. [DOI] [PubMed] [Google Scholar]
- 28.Wen D, Nong Y, Morgan JG, Gangurde P, Bielecki A, Dasilva J, et al. A selective small molecule IkappaB. Kinase beta inhibitor blocks nuclear factor kappaB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther. 2006;317:989–1001. doi: 10.1124/jpet.105.097584. [DOI] [PubMed] [Google Scholar]
- 29.Feldmann M, Andreakos E, Smith C, Bondeson J, Yoshimura S, Kiriakidis S, et al. Is NF-kappaB a useful therapeutic target in rheumatoid arthritis? Ann Rheum Dis. 2002;61(12):ii13–18. doi: 10.1136/ard.61.suppl_2.ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van UdenP, Kenneth NS, Rocha S. Regulation of hypoxiainducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn JK, Koh EM, Cha HS, Lee YS, Kim J, Bae EK, et al. Role of hypoxia-inducible factor-1alpha in hypoxia-induced expressions of IL-8, MMP-1 and MMP-3 in rheumatoid fibroblast-like synoviocytes. Rheumatology (Oxford) 2008;47:834–839. doi: 10.1093/rheumatology/ken086. [DOI] [PubMed] [Google Scholar]
- 34.Cha HS, Ahn KS, Jeon CH, Kim J, Song YW, Koh EM. Influence of hypoxia on the expression of matrix metalloproteinase-1, -3 and tissue inhibitor of metalloproteinase-1 in rheumatoid synovial fibroblasts. Clin Exp Rheumatol. 2003;21:593–598. [PubMed] [Google Scholar]
- 35.Galindo M, Santiago B, Alcami J, Rivero M, Martin-Serrano J, Pablos JL. Hypoxia induces expression of the chemokines monocyte chemoattractant protein-1 (MCP-1) and IL-8 in human dermal fibroblasts. Clin Exp Immunol. 2001;123:36–41. doi: 10.1046/j.1365-2249.2001.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safronova O, Nakahama K, Onodera M, Muneta T, Morita I. Effect of hypoxia on monocyte chemotactic protein-1 (MCP-1) gene expression induced by Interleukin-1beta in human synovial fibroblasts. Inflamm Res. 2003;52:480–486. doi: 10.1007/s00011-003-1205-5. [DOI] [PubMed] [Google Scholar]
- 37.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L899–914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- 38.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saarialho-Kere UK. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res. 1998;290(Suppl):S47–54. doi: 10.1007/pl00007453. [DOI] [PubMed] [Google Scholar]
- 40.Schmid P, Cox D, Bilbe G, McMaster G, Morrison C, Stahelin H, et al. TGF-beta s and TGF-beta type II receptor in human epidermis: differential expression in acute and chronic skin wounds. J Pathol. 1993;171:191–197. doi: 10.1002/path.1711710307. [DOI] [PubMed] [Google Scholar]
- 41.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- 42.Xu Q, Norman JT, Shrivastav S, Lucio-Cazana J, Kopp JB. In vitro models of TGF-beta-induced fibrosis suitable for highthroughput screening of antifibrotic agents. Am J Physiol Renal Physiol. 2007;293:F631–640. doi: 10.1152/ajprenal.00379.2006. [DOI] [PubMed] [Google Scholar]
- 43.Bryn T, Yaqub S, Mahic M, Henjum K, Aandahl EM, Tasken K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. Int Immunol. 2008;20:235–245. doi: 10.1093/intimm/dxm134. [DOI] [PubMed] [Google Scholar]
- 44.Boeuf P, Vigan-Womas I, Jublot D, Loizon S, Barale JC, Akanmori BD, et al. CyProQuant-PCR: a real time RT-PCR technique for profiling human cytokines, based on external RNA standards, readily automatable for clinical use. BMC Immunol. 2005;6:5–18. doi: 10.1186/1471-2172-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, et al. Wound healing in MIP-1alpha(-/- ) and MCP-1(-/-) mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lumsden AB, Davies MG, Peden EK. Medical and endovascular management of critical limb ischemia. J Endovasc Ther. 2009;16:1131–1162. doi: 10.1583/08-2657.1. [DOI] [PubMed] [Google Scholar]
- 48.Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604–614. doi: 10.1002/dmrr.1004. [DOI] [PubMed] [Google Scholar]