Abstract
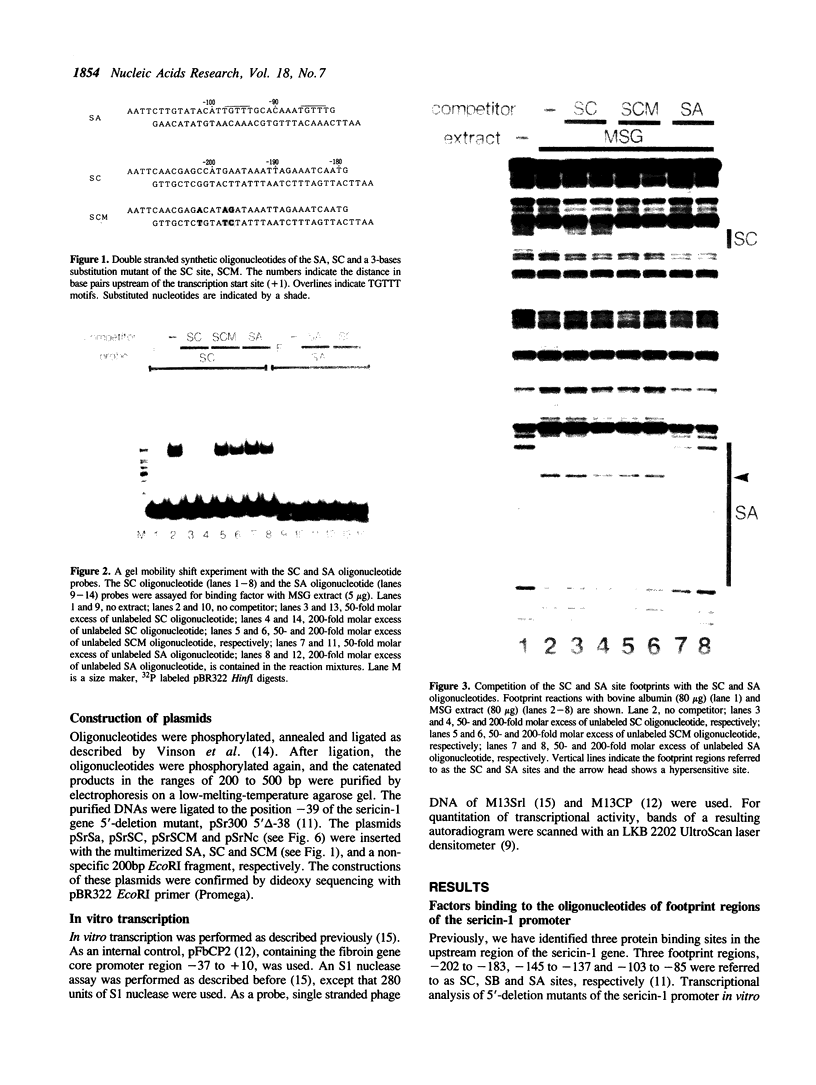
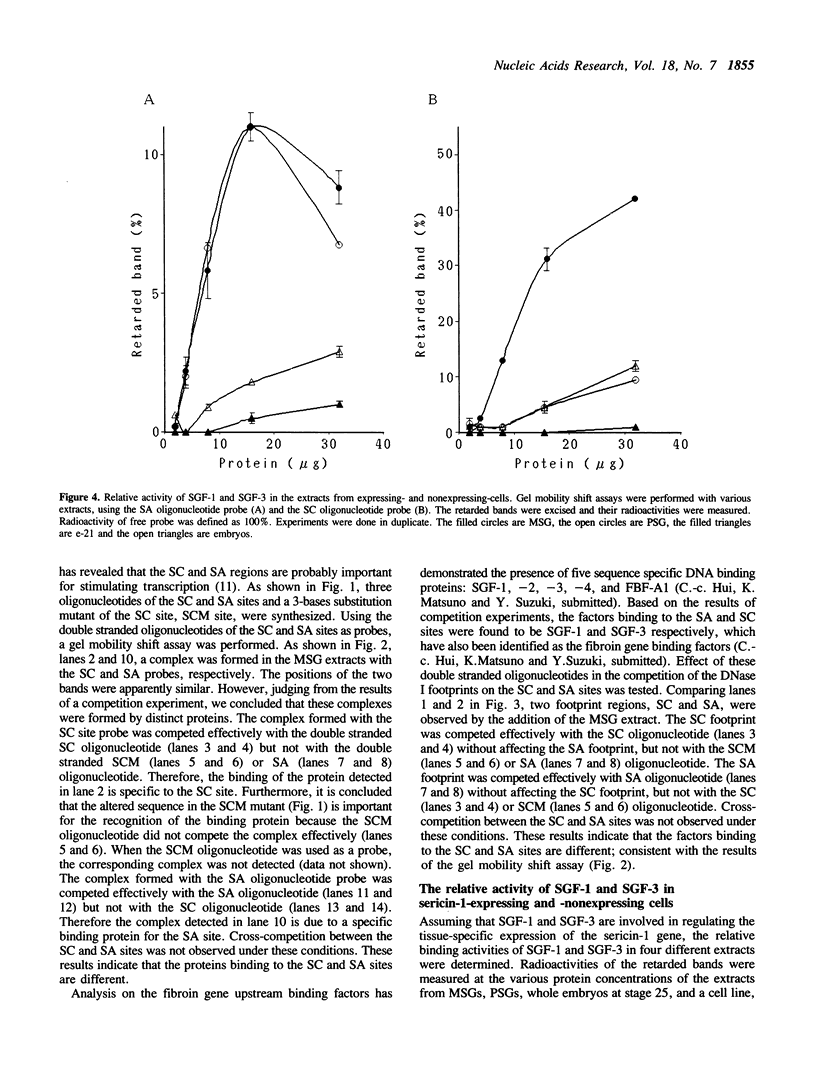
Three protein binding sites have been identified in the upstream region of the sericin-1 gene. Two of them, SA and SC sites, have been known as putative cis-acting elements. Using synthetic oligonucleotides of these binding sites, it was found that silk gland factor-1 (SGF-1) binds to the SA site, and silk gland factor-3 (SGF-3) binds to the SC site but not to a mutated SC site, SCM. Tissue distribution of the two factors was different. SGF-3 is present abundantly in the middle silk gland (MSG) where the sericin-1 gene is transcribed specifically but is also present in other cell types, though in a much less concentration. SGF-1 is observed very abundantly in the two parts of silk gland, MSG and posterior silk gland (PSG), but not in other cells. Templates containing multimerized SA or SC sites at -39 of the sericin-1 gene promoter were tested in MSG nuclear extracts. The SC multimer strongly activated transcription, while the mutant SCM multimer did not. The SA multimer also gave a slight stimulation of transcription. These results suggest that SGF-3 stimulates transcription through an interaction with the SC site, and SGF-1 does so weakly through the SA site.
Full text
PDF
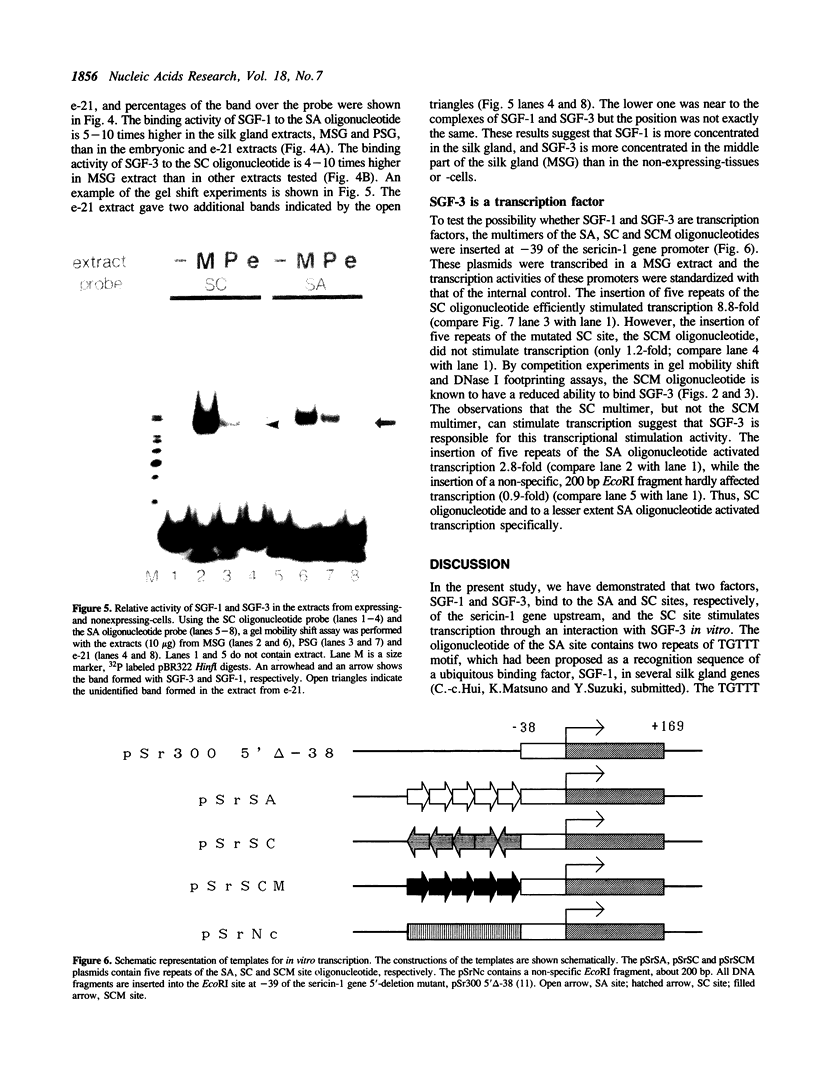


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couble P., Michaille J. J., Garel A., Couble M. L., Prudhomme J. C. Developmental switches of sericin mRNA splicing in individual cells of Bombyx mori silkgland. Dev Biol. 1987 Dec;124(2):431–440. doi: 10.1016/0012-1606(87)90496-9. [DOI] [PubMed] [Google Scholar]
- Hamada Y., Yamashita O., Suzuki Y. Haemolymph control of sericin gene expression studied by organ transplantation. Cell Differ. 1987 Jan;20(1):65–76. doi: 10.1016/0045-6039(87)90466-0. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Hirose S., Tsuda M., Suzuki Y. Enhanced transcription of fibroin gene in vitro on covalently closed circular templates. J Biol Chem. 1985 Sep 5;260(19):10557–10562. [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K., Hui C. C., Takiya S., Suzuki T., Ueno K., Suzuki Y. Transcription signals and protein binding sites for sericin gene transcription in vitro. J Biol Chem. 1989 Nov 5;264(31):18707–18713. [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Obara T., Suzuki Y. Temporal and spatial control of silk gene transcription analyzed by nuclear run-on assays. Dev Biol. 1988 Jun;127(2):384–391. doi: 10.1016/0012-1606(88)90325-9. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Ishikawa E., Suzuki Y. Structural analysis of sericin genes. Homologies with fibroin gene in the 5' flanking nucleotide sequences. J Biol Chem. 1982 Dec 25;257(24):15192–15199. [PubMed] [Google Scholar]
- Robertson M. Homoeo boxes, POU proteins and the limits to promiscuity. Nature. 1988 Dec 8;336(6199):522–524. doi: 10.1038/336522a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Suzuki Y. Interaction of composite protein complex with the fibroin enhancer sequence. J Biol Chem. 1988 Apr 25;263(12):5979–5986. [PubMed] [Google Scholar]
- Suzuki Y., Tsuda M., Takiya S., Hirose S., Suzuki E., Kameda M., Ninaki O. Tissue-specific transcription enhancement of the fibroin gene characterized by cell-free systems. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9522–9526. doi: 10.1073/pnas.83.24.9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Suzuki Y. Faithful transcription initiation of fibroin gene in a homologous cell-free system reveals an enhancing effect of 5' flanking sequence far upstream. Cell. 1981 Nov;27(1 Pt 2):175–182. doi: 10.1016/0092-8674(81)90371-8. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]