Abstract
Background
Glucocorticoid-induced TNF receptor-related protein ligand (GITRL), a ligand for the T cell co-stimulatory molecule GITR, is expressed by keratinocytes and involved in chemokine production. The expression of GITRL in skin inflammation is unknown.
Objectives
This study investigated cytokine regulation of keratinocyte GITRL expression.
Methods
GITRL expression was evaluated in cytokine treated human epidermal keratinocytes (HEK)s, murine PAM 212 cell line, murine and human skin explants by real time PCR, flow cytometry and immunostaining. Functional responses to GITR fusion protein were examined by real time PCR and ELISA. GITRL expression in AD and psoriasis was studied by immunohistochemistry.
Results
Skin biopsies from STAT6VT transgenic mice, which develop spontaneous atopic skin inflammation, were found by immunofluoresence, to have increased keratinocyte GITRL expression. Exposure to Th2 cytokines augmented GITRL mRNA expression in the murine PAM 212 keratinocytic cell line and murine skin explants. In contrast, GITRL mRNA and protein expression was only increased in HEKs and human skin explants in the presence of the combination of TNFα and Th2 cytokines. A synergistic effect of Th2 cytokines and GITR fusion protein on production of CCL17, the Th2 chemokine, by murine keratinocytes was demonstrated. Immunohistochemical staining showed that acute AD lesions have increased expression of GITRL compared with normal skin, chronic AD lesions and psoriatic plaques.
Conclusions and Clinical Relevance
Our studies demonstrate that GITRL expression is augmented by Th2 cytokines and TNFα in keratinocytes. Increased GITRL expession in acute AD skin lesions is shown. This data suggests a link between cytokine regulated keratinocyte GITRL expression and its role in inflammatory responses in AD.
Keywords: Skin, Th2 cytokines, atopic dermatitis
INTRODUCTION
Inflammatory dermatoses are associated with differential proinflammatory cytokine environments. Acute AD skin lesions show increased expression of IL4 and IL13 mRNA. In contrast, chronic AD skin has high levels of IFNγ producing cells [1]. Psoriasis is characterized by elevated TNFα and IFNγ production in the skin [2].
We have recently demonstrated expression of glucocorticoid-induced tumor necrosis factor (TNF) receptor-related ligand (GITRL) on both murine and human keratinocytes [3]. GITRL is a member of the TNF superfamily, originally identified on professional antigen-presenting cells including macrophages, B cells, and dendritic cells [4–6]. GITRL ligates its receptor, GITR, which is expressed at a low level on naïve T cells [5–8], with increased expression on CD4+CD25+ regulatory T cells (nTreg cells) [9,10] and activated effector T cells [7]. Similar to other members of the TNF superfamily [11], the GITR/GITRL couplet can transduce signals through both the receptor and ligand (reverse signalling) [12]. Reverse signaling through GITRL induces production and release of CCL17 and CCL27 by keratinocytes [3]. Both of these agents are important chemoattractants for the cutaneous influx of activated T cells associated with inflammatory dermatoses including atopic dermatitis (AD) [13, 14] and psoriasis [15–17].
The expression of GITRL in inflammatory dermatoses is undefined and the regulatory effect of cytokines is poorly understood. Multiple studies emphasized modulation of GITRL expression [18–21]. Stephens et al. showed that surface expression of GITRL on murine splenic B cells can be temporarily increased in response to LPS, CPG and IL4 + CD40 [22]. Retinal pigment epithelial cells have been shown to increase GITRL expression in vitro in the presence of IL-1α, TNFα and IFNγ [23].
We hypothesized that upregulation of GITRL expression by keratinocytes would be associated with skin inflammation and regulated by the local cytokine milieu. In this study we demonstrate that upregulation of GITRL expression in keratinocytes is dependent on Th2 cytokines +/− TNFα and selectively associated with acute AD.
MATERIALS AND METHODS
Reagents
Recombinant human and murine IL4, IL13, IFNγ and TNFα were purchased from R&D Systems (Minneapolis, MN). Rabbit anti-mouse GITRL Ab (M-173) and goat anti-human GITRL Ab (C-18) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-human GITRL monoclonal Ab (clone 109114) and mouse IgG were purchased from R&D Systems (Minneapolis, MN). Cy3-conjugated goat anti-rabbit, and FITC-conjugated goat anti-mouse secondary Abs were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Biotinylated rabbit anti-goat Ab was purchased from Dako (Carpinteria, CA). GITR (murine): Fc (human) (recombinant) fusion protein (GITR: Fc FP) (ALX-522-024) and control: Fc (human) (reconbinant) fusion protein (control: Fc FP) (ALX-203-004-C050) were purchased from Alexis Biochemicals (San Diego, CA).
Human subjects
Subjects included six healthy control subjects, six patients with psoriasis, eight patients with chronic AD lesions and nine patients with acute AD lesions. Acute and chronic AD skin lesions were defined as reported by us earlier [24]. Skin lesions that appeared on the patient’s skin within two days of the skin biopsy were considered acute, lesions that were persistent beyond two weeks were considered chronic. The study was approved by the Institutional Review Board at National Jewish Health (Denver, CO). All subjects gave written consent prior to participation. Up to six 2 mm punch biopsies were collected from each individual and either immediately placed in 10% buffered formalin for immunohistochemistry or placed in RPMI 10% FCS to be used in ex vivo experiments.
Mice
Heterozygous STAT6VT and STAT6VT+IL4−/− transgenic mice were previously described [25,26]. In our animal facility the STAT6VT mice were bred with C57BL/6 mice to maintain a population of transgenic mice and wild type controls. STAT6VT+ IL4−/− transgenic mice were backcrossed with STAT6VT- IL4−/− littermates to maintain IL4 deficiency. The experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at National Jewish Health (Denver, CO).
Cell culture
PAM 212 mouse cells were kindly supplied by Dr. Ansel’s lab at University of Colorado Denver (Denver, CO). They were cultured in the RPMI 1640 medium (Cellgro, Manassas, CA) containing 10% heat-activated fetal calf serum (Gemini, West Sacramento, CA), 40 mmol/l L-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. The cells were plated overnight in 48-well tissue culture plates (5×104 cells/ml) before the addition of IL4, IL13, and TNFα for 24h. For CCL17 assays, the cells were stimulated with GITR: Fc FP for additional 24h before cell culture supernatants were collected for analysis by ELISA.
Human epidermal keratinocytes (HEK)s were purchased from Cascade Biologics (Invitrogen, Carlsbad, CA) and cultured in serum-free media with gentamicin and amphotericin B (0.25 μg/ml). Keratinocytes were cultured in medium with low calcium (0.06mM CaCl2) and were referred to as undifferentiated keratinocytes [27]. For keratinocyte differentiation the cells were cultured for seven days in the medium with high calcium (1.3mM CaCl2) [27]. Cells were plated on matrix treated 24-well plates (1×105 in 1 ml) or 48-well plates (5×104 in 0.5 ml) for 24h, followed by stimulation with cytokines (TNFα (20ng/ml), IL4 (50ng/ml), IL13 (50ng/ml), IFNγ (100U/ml)).
Ex vivo experiments
Three month old male C57BL/6 mice were shaven along their backs. Further hair removal was achieved with Nair (Church & Dwight, Princeton, NJ). Three days later, the mice underwent isoflurane euthanasia. The shaved skin was dissected from the bodies and tape-stripped twice. Four mm punch biopsies were then taken along the skin. There was no visible inflammation evident at the time the biopsies were collected. The biopsies were incubated in 200 μl of RPMI 10% fetal calf serum with IL4 (50ng/ml) and IL13 (50ng/ml) for 24h.
Two mm human skin biopsies were placed in 200 μl of RPMI 10% fetal calf serum with or without TNFα (20ng/ml), IL4 (50ng/ml) and IL13 (50ng/ml) or all three cytokines for 24h.
Immunofluorescence studies
Four mm punch skin biopsies were obtained from three STAT6VT transgenic mice, three STAT6VT+IL4−/− transgenic mice and three wild type (C57BL/6) mice following isoflurane euthanasia. Hair removal was performed three days earlier as described for ex vivo experiments. Skin samples were fixed in 10% phosphate-buffered saline (PBS)-buffered formalin, embedded in paraffin, and processed for histology. The samples were blocked for 30 min using SuperBlock (Skytec, Logan, UT) and incubated overnight at 4°C with a primary ab against GITRL. A Cy3 conjugated secondary Ab was added for 1h at room temperature along with 4′–6-diamidino-2-phenylindole, dihydrochloride (DAPI) (Sigma, St Louis, MO) before the slides were mounted in ProLong® Gold Antifade Reagent (Invitrogen). All slides were analyzed by fluorescent microscopy (Leica Microsystems, Wetzlar, Germany) with imaging software Slidebook (Intelligent Imaging Innovations, Denver, CO).
Immunohistochemistry
Sections were deparaffinated in xylene, dehydrated in ethanol, and washed in PBS. Successive permeabilization steps (with Triton 0.2% in PBS) and hydrogen peroxide (5% in PBS) were done for 30 min each. The sections were then washed three times for 5 min in PBS before incubation with a universal blocking solution (DakoCytomation, MN) for 30 min. Tissue sections were immunostained with goat anti human GITRL (200μg/ml). The secondary Ab, biotinylated rabbit anti goat was applied at concentration of 1/100. To confirm specificity sections were stained with a normal goat IgG-isotype control Ab and anti-GITRL Ab preadsorbed with a five-fold mass excess of immunizing peptide for GITRL (Santa Cruz Biotechnology Santa Cruz, CA). The immunostains were developed using StreptAB Complex/HRP (Vector Laboratories (Burlingame, CA) and diaminobenzidine substrate (DAB) as per the manufacturer’s instructions. All slides were coded prior to analysis and read blindly. The intensity of the immunostaining was graded using a scoring system 0–8 where 0 is no staining, and 8 the most intense staining.
Flow cytometry
HEKs were detached from 24-well tissue culture plates using HyQtase (Hyclone, Logan, UT). The supernatants were removed and 350μl of HyQtase was added per well for 10 min at 37°C. When cell detachment was confirmed under inverted microscope, the cell suspension was removed and spun down at 1,000 rpm for five min at 4°C. The cells were fixed in 1% paraformaldehyde and washed twice in PBS, followed by incubation with SuperBlock solution. The cells were stained overnight with mouse anti-human GITRL Ab or mouse IgG followed by FITC-conjugated secondary Ab. All samples were analyzed by flow cytometry (FACScan Becton Dickinson Cytometer, Franklin Lakes, NJ) and CellQuest Pro software (Becton Dickinson).
Real-time PCR
Total cellular RNA was isolated by the acid guanidinium thiocyanate–phenol–chloroform method, according to the manufacturer’s guidelines (Qiagen, Valencia, CA), transcribed into cDNA, and analyzed by real-time PCR using the dual-labeled fluorigenic probe method on an ABI Prism 7000 Sequence detector (Perkin-Elmer Applied Biosystems, Foster City, CA). Primers and probes for murine and human GITRL and 18S RNA were purchased from Applied Biosystems. Standard curves were generated using the fluorescent data from two-fold serial dilutions of total cDNA of the highest expression sample. Quantities of target gene expression in test samples were normalized to the corresponding 18S RNA transcript.
ELISA
Supernatants were harvested and frozen at −80°C. Analysis with a CCL17 Quantikine Elisa kit (R&D Systems) was according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was conducted using Graph Pad Prism, version 4.03 (San Diego, CA). Statistical differences between groups were determined using an unpaired t test with significant differences conferred when P<0.05. In cases where multiple groups were compared with a control, data were analyzed by a one-way analysis of variance (ANOVA), and significant differences were determined by a Bonferroni test.
RESULTS
GITRL expression is upregulated in the skin of STAT6VT transgenic mice
Based on our previous observations that GITRL is expressed on keratinocytes [3], the purpose of this work was to explore the expression of GITRL in skin inflammation. In this first set of experiments we examined the expression of GITRL in the skin of STAT6VT transgenic mice, which express a mutant STAT6 (STAT6VT) in T cells that is constitutively active in the absence of IL4 [28]. These mice are prone to the development of spontaneous atopic skin inflammation. IL4 and IL13 expression is increased in their skin [26].
Skin biopsies were collected from three STAT6VT transgenic mice, three STAT6VT+IL4−/− transgenic mice and three wild type controls (C57BL/6). The formaldehyde preserved biopsies were stained with fluorescent Abs against GITRL. A significant increase in the expression of GITRL, by the keratinocyte layer of the biopsies, was observed in STAT6VT mice compared with both WT controls and STAT6VT mice lacking endogenous IL4 (P<0.01) (Fig 1a,1b). There was also a significant difference in expression between control mice and STAT6VT mice lacking IL4 (P<0.01), suggesting that IL4 was not the only mediator of increased GITRL expression (Fig 1a,1b). This experiment indicates increased keratinocyte expression of GITRL in STAT6VT mice and suggests that this increase may be cytokine regulated, specifically by Th2 cytokines.
Figure 1.
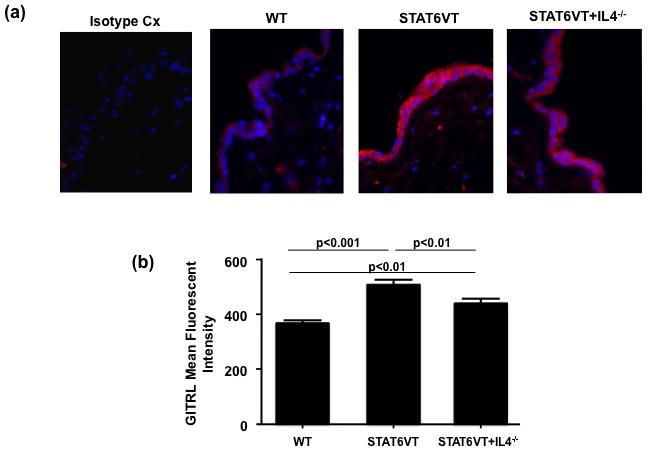
Th2 cytokines upregulate murine keratinocyte GITRL mRNA expression
In light of this data we sought to define the effect of Th2 cytokines on GITRL in a murine keratinocyte cell line, PAM 212, in which we have previously confirmed endogenous expression of GITRL [3]. Keratinocytes were incubated in the presence of IL4, IL13 or both cytokines for 24h. The cells were then harvested and analyzed for GITRL mRNA expression by RT-PCR. After 24h of treatment with Th2 cytokines, GITRL mRNA levels were significantly (P<0.05) increased in Th2 treated PAM 212 cells compared with untreated cells ([mean±SD]: 0.13±0.03 [IL4], 0.18±0.02 [IL13] and 0.27±0.07 [IL4/IL13] vs. 0.04±0.01 [untreated cells] ng GITRL per ng 18S RNA,). Incubation with IL4 and IL13 increased GITRL levels significantly more than with IL4 alone (P<0.05), but not more than IL13 alone (Fig. 2a). A detailed time course study determined that GITRL mRNA induction by Th2 cytokines was the highest at 24h (data not shown).
Figure 2.
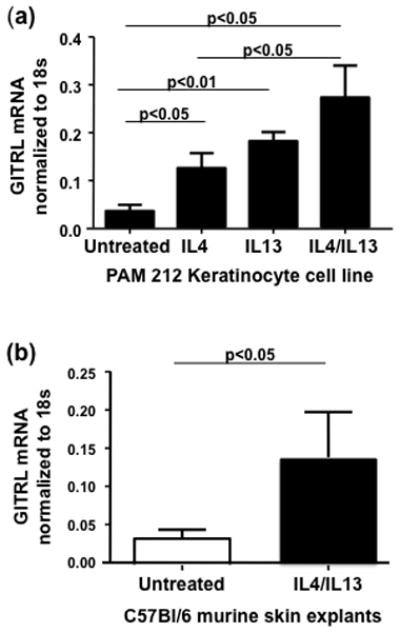
To further confirm the effect of IL4 and IL13 on murine GITRL expression, skin biopsies from male C57BL/6 mice were incubated with Th2 cytokines for 24h and then harvested and analyzed for GITRL mRNA expression. A significant increase in GITRL mRNA expression was found after incubation with IL4 and IL13 compared with untreated skin biopsies (0.14±0.060 vs 0.034±0.01 ng GITRL per ng 18S RNA, P<0.05) (Fig. 2b).
Th2 cytokines and GITR Fusion protein synergize to increase CCL17 production by murine keratinocytes
CCL17 is the primary chemoattractant of Th2 producing, skin homing T cells and its expression correlates with severity of AD skin disease. We have previously reported that ligation of GITRL on the surface of murine keratinocytes, using GITR Fusion Protein (GITR: Fc FP), induces production of CCL17 [3]. We now sought to understand the effect, on CCL17 production, of ligating murine GITRL in a Th2 cytokine milieu. PAM 212 murine keratinocytes were incubated with IL4, IL13 as described above, for 24h before addition of murine GITR: Fc FP or control: Fc FP for additional 24h. Supernatants were then collected and analyzed by ELISA for CCL17 content. Both Th2 cytokines and GITR: Fc FP induced CCL17 production. GITR: Fc FP induced significantly greater CCL17 production in Th2 pretreated kertainocytes than either trigger alone (P<0.05) (Fig 3a). Control fusion protein did not affect CC17 production by murine keratinocytes (40±12 pg/ml CCL17 was detected in the kertainocyte culture media after 24h of stimulation with 10μg/ml of the control: Fc FP; and 95±20pg/ml CCL17 was detected in the keratinocyte culture media after stimulation of the Th2 pretreated PAM 212 cells with control: Fc FP).
Figure 3.
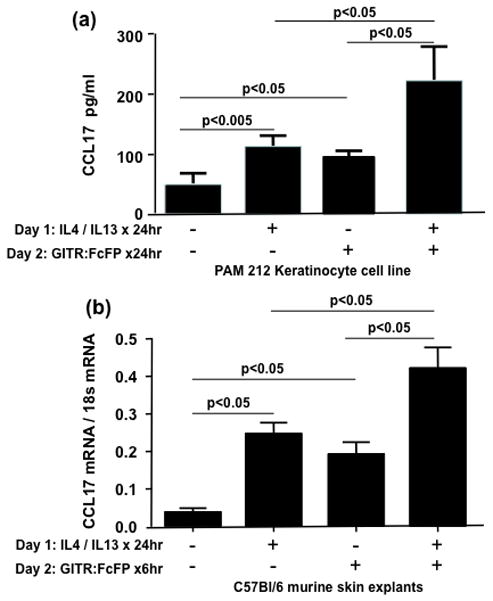
To further explore the synergistic effect of IL4 and IL13 and GITR:Fc FP on murine CCL17 production, skin biopsies from male C57BL/6 mice were incubated with or without IL4 and IL13 for 24h and then murine GITR: Fc FP for 6h before being harvested and analyzed for CCL17 mRNA expression. There was a significant increase in CCL17 mRNA both in the presence of Th2 cytokines or GITR: Fc FP alone. However, the two triggers together induced significantly greater expression of CCL17 mRNA as compared to either one alone (0.42±0.06 [both stimuli] vs 0.25±0.02 [1L4/1L13] and 0.19±0.03 [GITR:FcFP] ng GITRL per ng 18S RNA, P<0.05) (Fig 3b). Control fusion protein did not alter CC17 production by murine skin explants (0.07±0.01 and 0.18±0.03 ng GITRL per ng 18S RNA for the control: Fc FP protein treated keratinocytes after the cells were preincubated in media only or with TH2 cytokines for 24h, respectively).
In the presence of IL4 and IL13, TNFα induces an increase in human keratinocyte GITRL expression
In the following experiments, we extended our study on cytokine regulation of keratinocyte GITRL expression to human keratinocytes. We have previously confirmed expression of GITRL mRNA and surface protein in human epidermal keratinocytes (HEKs) and human skin explants [3]. Undifferentiated HEKs were incubated in the presence of recombinant human IL4, IL13 (both 50ng/ml), TNFα (20ng/ml) or IFNγ (100U/ml) or these cytokine in combination for 24h. The range of cytokines studied was broadened to include TNFα and IFNγ as they are important players in inflammatory dermatological conditions including psoriasis and chronic atopic dermatitis. After incubation with cytokines, the cells were harvested and analyzed for GITRL mRNA expression by real-time PCR. Incubation with TNFα or IFNγ alone had no effect on GITRL mRNA levels. After treatment with IL4 and IL13, mRNA levels for GITRL were significantly increased compared with untreated cells ([mean±SD]: 0.83±0.04 vs 0.24±0.03 ng GITRL per ng 18S RNA, P<0.001). However, addition of TNFα along with IL4 and IL13, significantly increased expression of GITRL mRNA compared to the Th2 cytokines alone (1.53±0.10 vs 0.83±0.04 ng GITRL per ng 18S RNA, P<0.01). Addition of IFNγ in conjunction with IL4 and IL13 inhibited the increase in GITRL mRNA (Fig 4a). ). As in in vitro experiments with murine PAM 212 kertainocyte cell line a detailed time course study determined that GITRL mRNA induction by Th2 cytokines/TNFα was the highest at 24h (data not shown).
Figure 4.
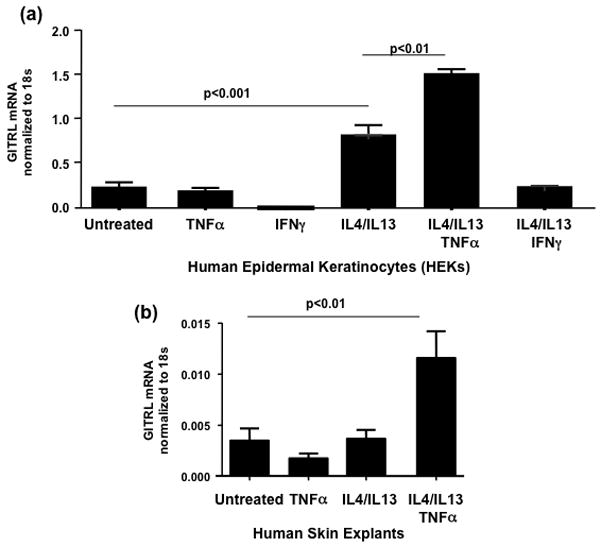
Subsequently we performed similar experiments in keratinocytes differentiated for seven days in high calcium (1.3mM CaCl2) media. Only addition of IL4/IL13/TNFα significantly increased GITRL expression above untreated cells (0.055±0.013 vs 0.009±0.002 ng GITRL per ng 18S RNA, P<0.01). Th2 cytokines alone did not alter GITRL mRNA expression in differentiated keratinocytes (data not shown).
To further explore cytokine regulation of GITRL in human keratinocytes, 2 mm human skin punch biopsies were incubated with IL4 and IL13 or TNFα, or all three cytokines for 24h and then harvested and analyzed for GITRL mRNA expression. Neither TNFα nor IL4 and IL13 alone had a significant effect on GITRL mRNA levels. However, there was a significant increase in GITRL mRNA expression after incubation with all three cytokines together compared with untreated controls (0.012±0.003 vs 0.004±0.001 ng GITRL per ng 18S RNA, P<0.01) (Fig 4b).
Surface expression of GITRL on human keratinocytes is increased in the collective presence of IL4, IL13 and TNFα
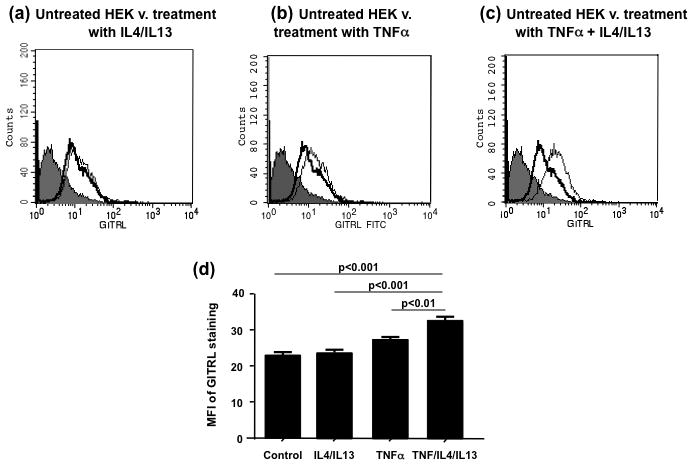
In the next set of experiments, we evaluated the expression of GITRL on the surface of undifferentiated HEKs by flow cytometry after exposure to IL4 and IL13 or TNFα or all three recombinant cytokines for 24h. There was no significant difference between the geometric mean fluorescence for GITRL in untreated cells and in cells stimulated with either TNFα or IL4 and IL13. However the geometric mean fluorescence for GITRL in HEKs stimulated with all three cytokines, IL4, IL13 and TNFα, was significantly increased compared with untreated cells and cells incubated with IL4/IL13 or TNFα alone ([mean±SD]: 32.50±1.2 vs 22.9±1.1 or 23.7±0.9 or 27.2±0.7 respectively P<0.01) (Fig.5a–e). The data demonstrates that in human keratinocytes augmentation of GITRL expression requires TNFα along with addition of Th2 cytokines.
Figure 5.
GITRL expression is upregulated in keratinocytes from acute AD lesions
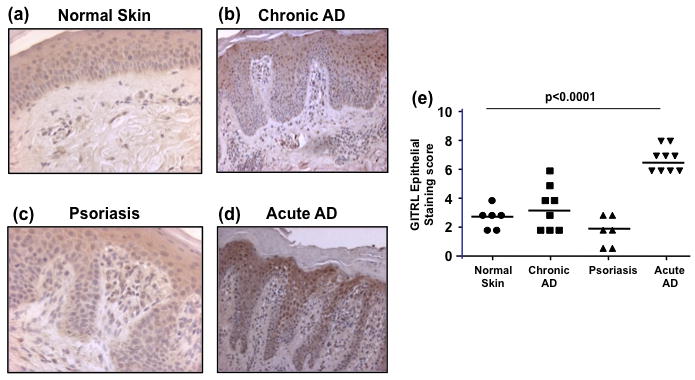
There is now much data demonstrating the differential role of cytokines in the pathogenesis of inflammatory dermatoses. Acute AD is associated with Th2 cytokines and also TNFα. In contrast chronic AD exhibits expression of IFNγ [1]. Psoriasis is associated with increased cutaneous production of IFNγ and TNFα [2]. Th2 cytokines were found here to increase GITRL expression in keratinocytes. Thus we proposed that GITRL expression would be upregulated in acute AD lesions. To explore this hypothesis, 2 mm punch skin biopsies were excised from normal individuals and patients with either acute and chronic AD lesions or psoriatic lesions and formalin preserved before staining with anti human GITRL Abs (Fig. 6a–d). Skin samples from all study groups demonstrated presence of GITRL positive cells in dermis (Suppl. Fig. 1); as well, GITRL expression was observed in epidermal keratinocyte layers. Keratinocyte GITRL expression in the study groups was evaluated further. It was found that there was no significant difference between keratinocyte GITRL expression in normal skin and either chronic AD lesions or psoriatic lesions. However, there was a significant increase in keratinocyte GITRL expression in acute AD lesions compared with all other conditions and normal skin (P<0.0001) (Fig 6e). GITRL staining was observed in keratinocytes both in the upper and lower layers of epidermis (Suppl. Fig. 2).
Figure 6.
DISCUSSION
It has been demonstrated here, uniquely, that keratinocyte expression of GITRL is differentially regulated by inflammatory cytokines. At the outset we demonstrated increased expression of GITRL protein in the skin of STAT6VT mice. This is the first time GITRL expression has been detailed in these mice which have previously been shown to have increased IL4 and IL13 expression in their skin [25, 26]. GITRL protein levels were reduced significantly in mice that had both constitutively active STAT6 and an inability to produce IL4 compared with IL4 sufficient STAT6VT mice suggesting that GITRL expression was partially regulated by IL4 in a STAT-dependent manner. Subsequently, we demonstrated in both a murine cell line and explants that both IL4 and IL13 independently increase GITRL mRNA.
Cytokine regulation of GITRL was further studied in undifferentiated HEKs. Pretreatment with Th2 cytokines increased GITRL mRNA expression but TNFα alone had no effect. However, its addition to cultures containing Th2 cytokines, augmented expression beyond that of IL4 and IL13 alone. Furthermore, TNFα, along with IL4 and IL13 was essential for upregulation of GITRL mRNA expression in human skin explants. In contrast, IFNγ inhibited augmentation of GITRL expression by Th2 cytokines. Differences between the murine and human GITRL have previously been identified. Human and mouse orthologs of GITRL share about 50% amino acid sequence identity which is similar to other TNF/TNFR family members [5], but mouse GITRL does not recognize the human receptor, and vice versa [29]. Thus, differential regulation of GITRL in murine and human keratinocytes appears to coexist with differential structure.
We have previously demonstrated that ligation of GITRL on keratinocytes, stimulates CCL17 production [3]. Th2 cytokines also induce keratinocyte production of CCL17 [30, 31]. Here we explored the effect of both stimuli. It was demonstrated that GITR: FC FP and Th2 cytokines collectively increased CCL17 mRNA levels and protein release more than either stimulus alone. This synergy does not appear to reduce the threshold for GITRL signaling, as a ten fold smaller dose of GITR: FC FP (1μg/ml) which does not induce CCL17 alone also had no effect on CCL17 induced by Th2 cytokines (data not shown).
Finally, we demonstrated that acute AD lesions have increased keratinocyte GITRL expression compared with normal skin or inflamed lesions from chronic AD sites or acute psoriatic plaques. This is consistent with upregulation by IL4/IL13 and TNFα. TNFα is not generally considered a primary cytokine in acute AD lesions. However, it has been shown that in the presence of additional triggers, cutaneous TNFα can be generated. TNFα can be produced by keratinocytes in response to staphylococcal enterotoxin A, B, C, (SEA), (SEB), (SEC) [32] or in response to dust mites [33]. A synergistic effect of Th2 cytokines and TNFα has previously been demonstrated in the induction by keratinocytes of Thymic Stromal Lymphopoietin (TSLP) [34] which is central to the pathogenesis of AD. Synergistic responses are not always seen when keratinocytes are triggered by Th2 cytokines and TNFα simultaneously [35]. IL4 and IL13 can inhibit the TNFα and IFNγ mediated induction of both Human {beta}-Defensin 3 (HBD-3) and CCL22 [36]. Chronic patches of AD and psoriatic lesions were not found to express GITRL significantly above baseline levels. Thus, the increase in GITRL was not a generalized finding in skin inflammation.
Baumgartner-Nielsen et al. [37] previously demonstrated the expression of GITRL in the dermis of AD lesions. In our experiments GITRL positive cells were seen both in the dermis and epidermis in skin biopsy samples. The current paper focused on GITRL function in keratinocytes. Baumgartner-Nielsen et al. [37] did not report the presence of GITRL staining in keratinocytes. We suspect that different tissue processing effects binding of the GITRL antibody in skin sections. In our study we examined GITRL expressing in the skin sections using sections from paraffin embedded skin biopsies. Skin sections were deparaffinized prior to the staining. The specificity of the staining was confirmed with the five-fold mass excess of the immunizing peptide. Baumgartner-Nielsen et al. [37] examined GITRL expression in cryopreserved skin biopsies.
Wang et al. [38] and Kamimura et al. [39] recently reported GITR expression by human keratinocytes. In the current study we did not examine GITR expression by murine or human keratinocytes. Our data does not exclude the possibility that GITR: Fc FP protein while stimulating GITRL may inhibit the activation of GITR signaling in keratinocytes.
In summary, we have demonstrated for the first time that epidermal expression of GITRL is increased by Th2 cytokines and TNFα. We have also revealed a novel collaborative induction of cutaneous CCL17 production via engagement of keratinocyte GITRL in the presence of Th2 cytokines. Most importantly, GITRL was found to be selectively upregulated in the keratinocytes of acute AD patients. This is the first data to link GITRL to the pathogenesis of AD.
Supplementary Material
Acknowledgments
The project described was supported in part by Colorado CTSA grant 1 UL1 RR025780 from NCRR/NIH, by NIH Grant AR41256 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and U19 AI070448 from the National Institute of Allergy and Infectious Diseases.
We thank Shih-Yun Lyman for her assistance in the preparation of this paper.
ABBREVIATIONS
- AD
Atopic Dermatitis
- CLA
Cutaneous lymphocyte-associated antigen
- CCL17
Chemokine (C-C motif) ligand 17
- CCL22
Chemokine (C-C motif) ligand 22
- CCL27
Chemokine (C-C motif) ligand 27
- FLG
Filaggrin
- GITR: Fc FP
GITR fusion protein
- GITRL
Glucocorticoid-induced TNF receptor-related protein ligand
- HEKs
Human Epidermal Keratinocytes
- HBD3
Human {beta}-Defensin 3
- IL4
Interleukin 4
- IL13
Interleukin 13
- IFNγ
Interferon gamma
- nTreg cell
Naturally occurring CD4+CD25+ regulatory T cell
- PBS
Phosphate-buffered saline
- SEA, SEB, SEC
Staphylococcal enterotoxin A, B, C
- TNF
Tumor necrosis factor
- TSLP
Thymic Stromal Lymphopoietin
Footnotes
The authors declare no conflict of interests.
References
- 1.Fiset PO, Leung DY, Hamid Q. Immunopathology of atopic dermatitis. J Allergy Clin Immunol. 2006;118:287–90. doi: 10.1016/j.jaci.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC, Morel F. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J Immunol. 2010;184:5263–70. doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- 3.Byrne AM, Goleva E, Leung DY. Identification of glucocorticoid-induced TNF receptor-related protein ligand on keratinocytes: ligation by GITR induces keratinocyte chemokine production and augments T-cell proliferation. J Invest Dermatol. 2009;129:2784–94. doi: 10.1038/jid.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurney AL, Marsters SA, Huang RM, Pitti RM, Mark DT, Baldwin DT, Gray AM, Dowd AD, Brush AD, Heldens AD, Schow AD, Goddard AD, Wood WI, Baker KP, Godowski PJ, Ashkenazi A. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr Biol. 1999;9:215–8. doi: 10.1016/s0960-9822(99)80093-1. [DOI] [PubMed] [Google Scholar]
- 5.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci U S A. 2003;100:15059–64. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv Exp Med Biol. 2009;647:156–73. doi: 10.1007/978-0-387-89520-8_11. [DOI] [PubMed] [Google Scholar]
- 7.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–22. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 8.Azuma M. Role of the glucocorticoid-induced TNFR-related protein (GITR)-GITR ligand pathway in innate and adaptive immunity. Crit Rev Immunol. 2010;30:547–57. doi: 10.1615/critrevimmunol.v30.i6.40. [DOI] [PubMed] [Google Scholar]
- 9.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 11.Chen NJ, Huang MW, Hsieh SL. Enhanced secretion of IFN-gamma by activated Th1 cells occurs via reverse signaling through TNF-related activation-induced cytokine. J Immunol. 2001;166:270–6. doi: 10.4049/jimmunol.166.1.270. [DOI] [PubMed] [Google Scholar]
- 12.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–86. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 13.Ogg G. Role of T cells in the pathogenesis of atopic dermatitis. Clin Exp Allergy. 2009;39:310–6. doi: 10.1111/j.1365-2222.2008.03146.x. [DOI] [PubMed] [Google Scholar]
- 14.Hon KL, Leung TF, Ma KC, Li AM, Wong Y, Fok TF. Serum levels of cutaneous T-cell attracting chemokine (CTACK) as a laboratory marker of the severity of atopic dermatitis in children. Clin Exp Dermatol. 2004;29:293–6. doi: 10.1111/j.1365-2230.2004.01501.x. [DOI] [PubMed] [Google Scholar]
- 15.Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3–9. doi: 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A. Psoriasis--as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494–501. doi: 10.1016/j.it.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Campanati A, Goteri G, Simonetti O, Ganzetti G, Giuliodori K, Stramazzotti D, Morichetti D, Bernardini ML, Mannello B, Fabris G, Offidani A. CTACK /CCL27 expression in psoriatic skin and its modification after administration of etanercept. Br J Dermatol. 2007;157:1155–60. doi: 10.1111/j.1365-2133.2007.08200.x. [DOI] [PubMed] [Google Scholar]
- 18.Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C. GITR- GITRL system, a novel player in shock and inflammation. ScientificWorldJournal. 2007;7:533–66. doi: 10.1100/tsw.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanabuchi S, Watanabe N, Wang YH, Ito T, Shaw J, Cao W, Qin FX, Liu YJ. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107:3617–23. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 20.Mahesh SP, Li Z, Liu B, Fariss RN, Nussenblatt RB. Expression of GITR ligand abrogates immunosuppressive function of ocular tissue and differentially modulates inflammatory cytokines and chemokines. Eur J Immunol. 2006;36:2128–38. doi: 10.1002/eji.200635893. [DOI] [PubMed] [Google Scholar]
- 21.Nardelli B, Zaritskaya L, McAuliffe W, Ni Y, Lincoln C, Cho YH, Birse CE, Halpern W, Ullrich S, Moore PA. Osteostat/tumor necrosis factor superfamily 18 inhibits osteoclastogenesis and is selectively expressed by vascular endothelial cells. Endocrinology. 2006;147:70–8. doi: 10.1210/en.2005-0518. [DOI] [PubMed] [Google Scholar]
- 22.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 23.Kim BJ, Li Z, Fariss RN, Shen DF, Mahesh SP, Egwuagu C, Yu CR, Nagineni CN, Chan CC, Nussenblatt RB. Constitutive and cytokine-induced GITR ligand expression on human retinal pigment epithelium and photoreceptors. Invest Ophthalmol Vis Sci. 2004;45:3170–6. doi: 10.1167/iovs.03-0919. [DOI] [PubMed] [Google Scholar]
- 24.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruns HA, Schindler U, Kaplan MH. Expression of a constitutively active Stat6 in vivo alters lymphocyte homeostasis with distinct effects in T and B cells. J Immunol. 2003;170:3478–87. doi: 10.4049/jimmunol.170.7.3478. [DOI] [PubMed] [Google Scholar]
- 26.Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, Travers JB, Kaplan MH. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–90. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, Hansen KC, Leung DY. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–58. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 28.Daniel C, Salvekar A, Schindler U. A gain-of-function mutation in STAT6. J Biol Chem. 2000;275:14255–9. doi: 10.1074/jbc.c000129200. [DOI] [PubMed] [Google Scholar]
- 29.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–71. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 30.Wirnsberger G, Hebenstreit D, Posselt G, Horejs-Hoeck J, Duschl A. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur J Immunol. 2006;36:1882–91. doi: 10.1002/eji.200635972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liddiard K, Welch JS, Lozach J, Heinz S, Glass CK, Greaves DR. Interleukin-4 induction of the CC chemokine TARC (CCL17) in murine macrophages is mediated by multiple STAT6 sites in the TARC gene promoter. BMC Mol Biol. 2006;7:45. doi: 10.1186/1471-2199-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsunaga T, Katayama I, Yokozeki H, Nishioka K. Superantigen-induced cytokine expression in organ-cultured human skin. J Dermatol Sci. 1996;11:104–10. doi: 10.1016/0923-1811(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 33.Maeda S, Maeda S, Shibata S, Chimura N, Fukata T. House dust mite major allergen Der f 1 enhances proinflammatory cytokine and chemokine gene expression in a cell line of canine epidermal keratinocytes. Vet Immunol Immunopathol. 2009;131:298–302. doi: 10.1016/j.vetimm.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–7. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 35.Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pita O, Leung DY, Howell MD. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–92. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 36.Xiao T, Kagami S, Saeki H, Sugaya M, Kakinuma T, Fujita H, Yano S, Mitsui H, Torii H, Komine M, Asahina A, Nakamura K, Tamaki K. Both IL-4 and IL-13 inhibit the TNF-alpha and IFN-gamma enhanced MDC production in a human keratinocyte cell line, HaCaT cells. J Dermatol Sci. 2003;31:111–117. doi: 10.1016/s0923-1811(02)00149-4. [DOI] [PubMed] [Google Scholar]
- 37.Baumgartner-Nielsen J, Vestergaard C, Thestrup-Pedersen K, Deleuran M, Deleuran B. Glucocorticoid-induced tumour necrosis factor receptor (GITR) and its ligand (GITRL) in atopic dermatitis. Acta Derm Venereol. 2006;86:393–8. doi: 10.2340/00015555-0118. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Devgan V, Corrado M, Prabhu NS, El-Deiry WS, Riccardi C, Pandolfi PP, Missero C, Dotto GP. Glucocorticoid-induced tumor necrosis factor receptor is a p21Cip1/WAF1 transcriptional target conferring resistance of keratinocytes to UV light-induced apoptosis. J Biol Chem. 2005;280:37725–31. doi: 10.1074/jbc.M507976200. [DOI] [PubMed] [Google Scholar]
- 39.Kamimura Y, Iwai H, Piao J, Hashiguchi M, Azuma M. The glucocorticoid-induced TNF receptor-related protein (GITR)-GITR ligand pathway acts as a mediator of cutaneous dendritic cell migration and promotes T cell-mediated acquired immunity. J Immunol. 2009;182:2708–16. doi: 10.4049/jimmunol.0803704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


