Abstract
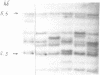
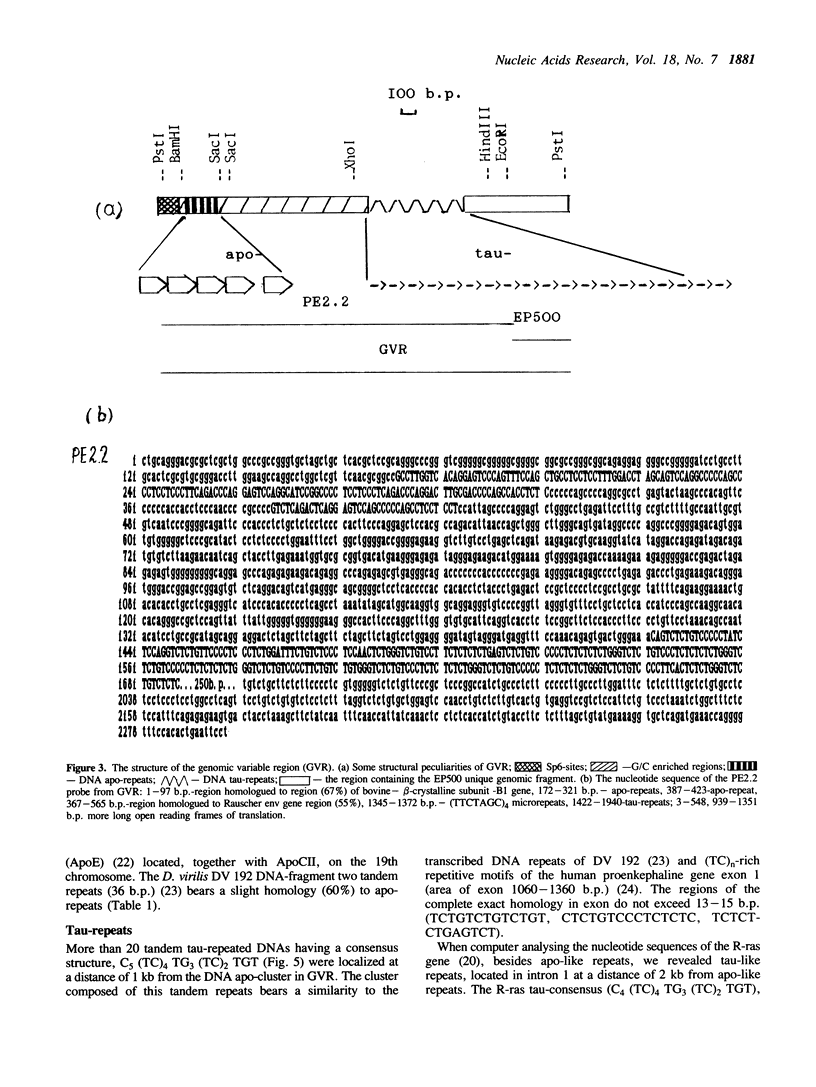
Earlier we found a human hypervariable genomic region (GVR). The DNA hybridization probe isolated from this region detects multiple hypervariability of restriction DNA fragments from genomic loci. The sequencing data suggest that the genomic instability and variability are associated with tandem DNA repeats. The DNA hybridization probe contains two families of simple DNA repeats designated as 'apo' and 'tau'. The (TC)n-rich family of DNA 'tau'-repeats bears some similarity to the simple transcribed repeats of Drosophila virilis, simple repetitive motifs of the human proenkephaline gene exon 1, and short sites of retroviral LTR ends. Apo-repeats show an unusual similarity to Rauscher viral env gene site. Besides GVR, apo- and tau-like repeats are localized in other genomic loci and can form separate tandem clusters and terminal repeats flanking certain copies of retroposons (Alu-SINES).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Selby M. J., Rutter W. J. The highly polymorphic region near the human insulin gene is composed of simple tandemly repeating sequences. Nature. 1982 Jan 7;295(5844):31–35. doi: 10.1038/295031a0. [DOI] [PubMed] [Google Scholar]
- Bestwick R. K., Boswell B. A., Kabat D. Molecular cloning of biologically active Rauscher spleen focus-forming virus and the sequences of its env gene and long terminal repeat. J Virol. 1984 Sep;51(3):695–705. doi: 10.1128/jvi.51.3.695-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Cooke H. Repeated sequence specific to human males. Nature. 1976 Jul 15;262(5565):182–186. doi: 10.1038/262182a0. [DOI] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo S. S., Law S. W., Brewer H. B., Jr The human preproapolipoprotein C-II gene. Complete nucleic acid sequence and genomic organization. FEBS Lett. 1987 Mar 9;213(1):221–226. doi: 10.1016/0014-5793(87)81495-3. [DOI] [PubMed] [Google Scholar]
- Georges M., Lequarré A. S., Castelli M., Hanset R., Vassart G. DNA fingerprinting in domestic animals using four different minisatellite probes. Cytogenet Cell Genet. 1988;47(3):127–131. doi: 10.1159/000132529. [DOI] [PubMed] [Google Scholar]
- Hattori M., Kuhara S., Takenaka O., Sakaki Y. L1 family of repetitive DNA sequences in primates may be derived from a sequence encoding a reverse transcriptase-related protein. Nature. 1986 Jun 5;321(6070):625–628. doi: 10.1038/321625a0. [DOI] [PubMed] [Google Scholar]
- Hong G. F. A systemic DNA sequencing strategy. J Mol Biol. 1982 Jul 5;158(3):539–549. doi: 10.1016/0022-2836(82)90213-3. [DOI] [PubMed] [Google Scholar]
- Horikawa S., Takai T., Toyosato M., Takahashi H., Noda M., Kakidani H., Kubo T., Hirose T., Inayama S., Hayashida H. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983 Dec 8;306(5943):611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Nucleotide sequence of mouse satellite DNA. Nucleic Acids Res. 1981 Feb 11;9(3):683–696. doi: 10.1093/nar/9.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman A. P., Nicholls R. D., Weatherall D. J., Clegg J. B., Higgs D. R. Molecular characterisation of a hypervariable region downstream of the human alpha-globin gene cluster. EMBO J. 1986 Aug;5(8):1857–1863. doi: 10.1002/j.1460-2075.1986.tb04437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Morton D. B. DNA fingerprints of dogs and cats. Anim Genet. 1987;18(1):1–15. doi: 10.1111/j.1365-2052.1987.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Wallis S. C., Pease R. J., Powell L. M., Scott J. A hypervariable region 3' to the human apolipoprotein B gene. Nucleic Acids Res. 1986 Nov 25;14(22):9215–9216. doi: 10.1093/nar/14.22.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Boone L. R., Yang W. K. A novel sequence segment and other nucleotide structural features in the long terminal repeat of a BALB/c mouse genomic leukemia virus-related DNA clone. Nucleic Acids Res. 1983 Aug 25;11(16):5603–5620. doi: 10.1093/nar/11.16.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaev E. I., Shapiro Iu A. Vysokii mezhindividual'nyi polimorfizm po dline i kopiinosti restriknykh fragmentov TURI-semeistva umerennykh povtorov DNK cheloveka. Biull Eksp Biol Med. 1987 Jan;103(1):57–58. [PubMed] [Google Scholar]
- Rogaev E. I. Two novel human DNA tandem repeat families from the hypervariable DNA probe homologous to human apolipoprotein CII-gene intron and D. virilis satellite. Nucleic Acids Res. 1989 Feb 11;17(3):1246–1246. doi: 10.1093/nar/17.3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle N. J., Clarkson R. E., Wong Z., Jeffreys A. J. Clustering of hypervariable minisatellites in the proterminal regions of human autosomes. Genomics. 1988 Nov;3(4):352–360. doi: 10.1016/0888-7543(88)90127-9. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Dzhincharadze A. G., Prosnik M. I., Ivanov P. L., Limborskaia S. A. Genomnaia "daktiloskopiia" organizmov razlichnykh taksonomicheskikh grupp: ispol'zovanie v kachestve gibridizatsionnoi proby DNK faga M13. Genetika. 1988 Feb;24(2):227–238. [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Cheah K. S., Griffin J. R., Pope F. M., Solomon E. A highly polymorphic region 3' to the human type II collagen gene. Nucleic Acids Res. 1985 Jul 11;13(13):4613–4622. doi: 10.1093/nar/13.13.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Nomiyama H., Miyoshi J., Shimada K., Takagi Y. DNA sequences of the integration sites and inverted repeated structure of transposon Tn3. Nucleic Acids Res. 1979;6(5):1831–1841. doi: 10.1093/nar/6.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. Simple DNA sequences of Drosophila virilis isolated by screening with RNA. J Mol Biol. 1984 Jan 15;172(2):229–235. doi: 10.1016/s0022-2836(84)80041-8. [DOI] [PubMed] [Google Scholar]
- Toh H., Ono M., Miyata T. Retroviral gag and DNA endonuclease coding sequences in IgE-binding factor gene. 1985 Nov 28-Dec 4Nature. 318(6044):388–389. doi: 10.1038/318388a0. [DOI] [PubMed] [Google Scholar]
- Vassart G., Georges M., Monsieur R., Brocas H., Lequarre A. S., Christophe D. A sequence in M13 phage detects hypervariable minisatellites in human and animal DNA. Science. 1987 Feb 6;235(4789):683–684. doi: 10.1126/science.2880398. [DOI] [PubMed] [Google Scholar]
- Wirth T., Glöggler K., Baumruker T., Schmidt M., Horak I. Family of middle repetitive DNA sequences in the mouse genome with structural features of solitary retroviral long terminal repeats. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3327–3330. doi: 10.1073/pnas.80.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev I. Z., Rogaev E. I. Strukturnyi analiz al'foidnoi DNK primatov. I. Geterogennost' nukleotidnykh posledovatel'nostei al'foidnykh povtorov DNK cheloveka. Mol Biol (Mosk) 1986 May-Jun;20(3):663–673. [PubMed] [Google Scholar]
- Zaitsev I. Z., Rogaev E. I. Strukturnyi analiz al'foidnoi DNK primatov. II. Evoliutsiia i vozmozhnoe proiskhozhdenie al'foidnoi DNK primatov. Mol Biol (Mosk) 1986 May-Jun;20(3):674–682. [PubMed] [Google Scholar]