Abstract
The information generated by cone photoreceptors in the retina is compressed and transferred to higher processing centers through three distinct types of ganglion cells known as magno, parvo and konio cells. These ganglion cells, which travel from the retina to the lateral geniculate nucleus (LGN) and then to the primary visual cortex, have different structural and functional characteristics, and are organized in distinct layers in the LGN and the primary visual cortex. Magno cells are large, have thick axons and usually collect input from many retinal cells. Parvo cells are smaller, with fine axons and less myelin than mango cells. Konio cells are diverse small cells with wide fields of input consisting of different cells types. The three cellular pathways also differ in function. Magno cells respond rapidly to changing stimuli, while parvo cells need time to respond. The distinct patterns of structure and function in these cells have provided an opportunity for clinical assessment of their function. Functional assessment of these cells is currently used in the field of ophthalmology where frequency-doubling technology perimetry selectively assesses the function of magno cells. Evidence has accrued that the three pathways show characteristic patterns of malfunctions in multiple sclerosis, schizophrenia, Parkinson’s and Alzheimer’s diseases, and several other disorders. The combination of behavioral assessment with other techniques, such as event related potentials and functional magnetic resonance imaging, seems to bear promising future clinical applications.
Keywords: Retinal Ganglion Cells, Magno, Parvo, Konio
INTRODUCTION
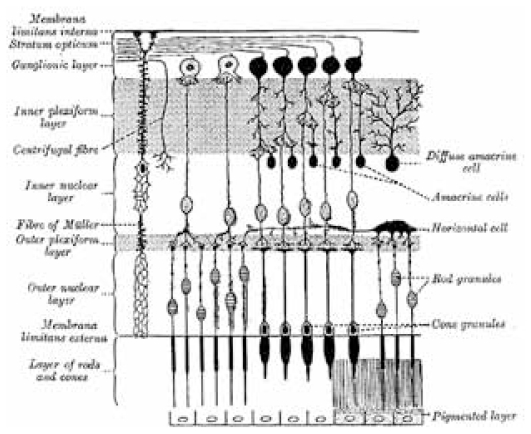
Data processing in the visual system starts in the eye itself, distinguishing it from any other organ in the body. While the eye itself is not considered as a part of the central nervous system, the multilayered structure of the retina enables early processing of retinal cone responses. Cone photoreceptors consist of three cell types known as long, medium, and short (L, M, and S), are located in the outermost layer of the retina, and are connected to the ganglion cells by bipolar cells. A complex interconnected network is created with horizontal cells located between cones and bipolar cells, and amacrine cells in between bipolar and ganglion cells (Fig. 1).
Figure 1.
Schematic representation of the microscopic structure of the retina.
RETINAL GANGLION CELLS
Ganglion cell axons travel from the retina to the lateral geniculate nucleus (LGN) and then to different parts of the visual cortex. Several physiologically and morphologically distinct types of ganglion cells exist. One, known as the magno cell, responds rapidly to stimulation, has thick axons with more myelin and large receptive fields (i.e., collects information from several cones). Another type known as the parvo cell, has thin axons with less myelin, responds slowly to stimuli and has smaller receptive fields. In the LGN, distinct layers of magno and parvo cells can be identified using several staining methods. Magno cells collect information from all types of cones; hence they detect “luminance” and can signal motion, stereopsis and depth. The parvo system is mostly used for detection of “chromatic” modulation, and thus the form and material of an object. Their processing pathways also differ: magno cell information is processed through the “where” pathway to the parieto-occipital cortex, while information from parvo cells is mostly processed through the “what” pathway in the inferior temporo-occipital cortex.1
One other major type of ganglion cells is known as the konio cell. Konio cells are much less in number than the other two types and form three tiny separate layers in between the magno and parvo cells in the LGN. Structurally, they are smaller than parvo cells. The physiological response of konio cells is not as well studied as the other two cell types, while they may play a role in seasonal mood changes2 and color constancy mechanism3,4. Seasonal mood changes might as well be affected by the recently-found ganglion cells known as melanopsin containing retinal ganglion cells (mRGC).5
Evidence has accumulated that these three pathways show characteristic patterns of malfunction in multiple sclerosis (MS)6,7, schizophrenia8,9, Parksinson’s10 and Alzheimer’s disease (AD)11,12, and several other disorders13,14. The combination of behavioral assessment and other techniques, such as event related potentials15 and functional magnetic resonance imaging (fMRI)16,17, has been shown to bear promising future clinical applications.
It should be noted that about 7 to 12 other types of ganglion cells, including a photosensitive ganglion cell5, have been reported.18 However, these cell types are much less frequent and do not create distinct layers in the LGN.
RECEPTIVE FIELDS
Bipolar cells collect, compare, and relay information from cone photoreceptors to ganglion cells. Complex networks of horizontal and amacrine cells assist in the collection and comparison of information received from cones. Cone photoreceptors that provide input to a ganglion cell through bipolar cells are called the receptive field of that particular ganglion cell.
Two types of bipolar cells have been identified. The first polarizes in the same way that cones polarize, i.e., hyperpolarization in reaction to light; these are called “Off” cells. The other type, the “On” cells, polarizes in the reverse direction. This difference arises from different glutamate receptors on the surface of these cells. In the fovea, where visual acuity is highest, most cones connect with one “Off” and one “On” bipolar cell.18,19 In the periphery, the number of On/Off cells increases. Overall, such a structure creates a receptive field, which, for example, can detect an edge or create a center/ surround organization.20
TRANSFER OF INFORMATION FROM THE RETINA TO THE VISUAL CORTEX
The distinctive structural, morphological and physiological features of the three types of ganglion cells have made them a good candidate for early diagnosis and treatment of several diseases. For example, evidence exists that the demyelination process in multiple sclerosis affects magno and parvo pathways, in a specific order.1 In addition to distinct features of the three pathways, information compression which occurs in the optic nerve facilitates the detection of any ganglion cell pathology. The optic nerve, with its 1.6 million fibers, carries information from about 4 million cones.21 Therefore, data compression occurs at the retinal level. In a model of L, M, and S cone responses in natural environments, a mathematically optimized solution for data compression matches the physiological response of the three types of ganglion cells.22 A mathematical model frequently used in the engineering field, known as “principal component analysis”, can extract the principal components of data. With linear transformation of data, the axes rotate to match the principal components such that the smallest number of “codes” is required to transfer information between two points. The principal components of a model of LMS cone responses match the function of magno, parvo, and konio cells, i.e., the first principal component detects only luminance (similar to magno cells), the second detects a comparison of L and M cones (similar to parvo cells), and the third matches a comparison between S and the average of L and M cone responses (similar to konio cells).
A similar mathematical model, known as independent component analysis, yields filters (or small templates) similar to simple cells in the visual cortex, where the ganglion cells synapse.23 The extraction of filters in the cortex relies on the structure of the receptive fields in early layers, including ganglion cells.
“Data decompression” seems to take place in the cortex, since the 1.5 million ganglion cells connect to about 120 million neurons in the visual cortex.24 Therefore, lesions in each of the three compressed pathways cause considerable functional loss as compared to lesions in other areas of the central nervous system. This fact has been used in clinical practice since long ago; for detection of tumors that may compress the optic nerve and lead to signs such as reduced visual acuity, visual field defects, and optic atrophy or edema. Advances in technology have enabled researchers to detect subtle changes in a variety of other disorders, in addition to optic nerve tumors.
METHODS OF ASSESSMENT OF GANGLION CELL FUNCTION
Most of the available information on retinal ganglion cells originates from animal models. In animals, electrophysiology has been the main method for assessing ganglion cell function. Recording electrical activity can be performed at the intracellular level, the extracellular level, or using multiple electrodes with tens or even hundreds of concurrent activity recordings. Most data comes from studies on pigeons, owls, cats, and monkeys, particularly macaque and rhesus monkeys which are assumed to be very similar to humans. The ability of monkeys to be trained has provided extensive insight by combining electrophysiological methods with behavioral, imaging, and histological methods.
Noninvasive methods used in humans include behavioral and psychophysical techniques combined with functional imaging, such as fMRI, PET (positron emission tomography) scan and NIRS (near infrared spectroscopy). Noninvasive electrophysiological data from humans using electroencephalography (EEG) or EEG during presentation of stimuli, known as event-related potentials (ERP), have been valuable for evaluation of ganglion cell function. Therapeutic interventions for epilepsy and Parkinson’s disease have sometimes provided the opportunity to record invasively from humans.25
Behavioral techniques include simple tasks, such as contrast sensitivity measurement, designed to distinctively stimulate magno, parvo, or konio cells. Stimuli containing only luminance information (i.e., with no chromatic modulation) selectively stimulate magno cells, while chromatic modulation without any luminance changes elicits a response from parvo and konio cells. Since parvo cells mainly receive input from L and M cones in an excitatory/ inhibitory combination, a combination of chromatic stimuli that selectively excite L and M cones can excite parvo cells. Such stimuli may consist of two colors close to dark red and dark green with the same luminance levels (isoluminant). Konio cells, on the other hand, seem to compare inputs from S cones with either L or M cone inputs. Therefore, an isoluminant stimulus combining violet/blue with greenish yellow would selectively provoke a response from konio cells. More complex tasks have been used to determine the function of each of the pathways in more detail. For example, by decomposition of natural images to information that selectively excites magno, parvo, and konio cells, it has been shown that the structure of natural scenes plays a major role in ganglion cell function, and this role is much more prominent for parvo cells as compared to magno cells.4
Behavioral techniques have been used in combination with EEG recordings as well as functional imaging methods. For example, during visual evoked potential (VEP, the event related potential generated by visual stimuli), a C1 component was identified. C1 is a positive signal recorded in the central parieto-occipital area, beginning 40 to 60 ms after the stimuli is shown and reaching its peak at around 70 to 100 ms when it resolves into a P1. C1 has been reported to be present when only parvo cells are excited (chromatic isoluminant stimuli), but not with luminance-only stimuli (magno cells).26 It has been possible to extract other meaningful information from ERP as well. In an interesting study, the timing of display of a target stimulus among some non-target stimuli could be identified by a single trial.27
Numerous studies have used retinotopic maps in combination with fMRI to locate the cortical area responsible for processing chromatic or luminance information. One study has reported that the superior colliculus, which receives a tiny part of information from the retina, was mainly activated by luminance stimuli only and not by other chromatically modulated stimuli.16 The low temporal resolution of functional imaging methods has been a major obstacle for their use in combination with behavioral tasks.
CLINICAL FINDINGS
Glaucoma
In ophthalmology, two recent perimetry methods are based on evaluation of selective cone/ ganglion cell functions using psychophysical tasks. Frequency doubling technology (FDT) perimetry was launched in 1997 and since then, several studies have shown its sensitivity for detection of early glaucoma and other disorders.28–30 The device is based on the perception of an illusion requiring intact function of the magnocellular pathway. In this illusion, when an achromatic sinusoidal low-spatial frequency grating is flickered in counter-phase at frequencies above 7 Hz, the spatial frequency seems to be doubled. Since only magno cells can respond rapidly to flickering stimuli, inability to elicit this illusion implies magno cell dysfunction, a situation that occurs in early glaucoma, ocular hypertension31, and even HIV infection32. Shortwave automated perimetry (SWAP) utilizes a bright yellow background with superimposed blue stimuli that specifically evaluates the function of S cones, and hence konio cells.
A recent comparison of visual evoked potentials of cone photoreceptors demonstrated that the S cone response in patients with open angle glaucoma had a different slope for the first positive wave as compared to age-matched healthy subjects. However, no difference in L or M cone responses were observed.33
Multiple Sclerosis
Multiple sclerosis has been a subject of intense research in the past decade. While uveitis and periphlebitis have already been recognized as ophthalmologic findings in MS, other findings such as nerve fiber layer changes have recently been under scrutiny. Optical coherence tomography demonstrated thinning of the retinal nerve fiber layer in multiple sclerosis which surprisingly did not correlate with optic neuritis.34–36 A postmortem analysis of 82 MS patients has shown cone and ganglion cell loss in 79% of cases and a loss in amacrine and bipolar cells in 40%.37 In another study, 8 postmortem MS patients showed a significant loss of parvo cells in the LGN.38 The results of psychophysical tests have been controversial. In MS patients with optic neuritis, parvo cells seem to be affected based on tests including luminance-modulated39 and chromatic-modulated40 contrast sensitivity, visual evoked potentials41 and temporal frequency discrimination42. In early MS cases with no optic neuritis, patients are almost equally divided into three groups: damage limited to magno cells, parvo cells, or both.6 Contrast sensitivity impairment at high spatial frequencies was suggested to be indicative of parvo cell damage, while low spatial frequency impairments were associated with magno cell damage. In the same study, N1 and P1 VEP responses were correlated with parvo and magno cell responses, respectively. Psychophysical data were supported by impairment in VEP responses which occurred in addition to spatial frequency contrast sensitivity impairments. Murav’eva et al6 suggested that MS patients who develop visual symptoms have parvo cell damage, while those with magnocellular layer damage are usually asymptomatic.
Alzheimer’s Disease
Evidence suggests rapid deterioration of the magnocellular pathway in Alzheimer’s disease. AD patients tend to underreport their visual problems in comparison with healthy age matched controls, therefore their visual problems are left unnoticed. Eight out of ten AD patients were reported to have extensive axonal degeneration in the optic nerve43 and these findings were supported by follow-up studies44,45, though other studies disagree with these findings.46,47 This is in contrast to the widespread belief that AD does not involve sensory perception. Moreover, postmortem biopsies of the LGN demonstrated plaques in both parvo and magno layers; interestingly parvo layers showed a higher density of plaques.48
Psychophysical tasks of motion coherence and flicker detection, which evaluate the function of magno cells, show significant functional loss in AD patients which increases with the severity of the disease.49,50 VEP tasks using chromatic and achromatic modulation have revealed delays with achromatic stimuli only, confirming magnocellular pathway involvement in AD.12
Schizophrenia
Most of the deficits found in schizophrenia have been reported to involve magno cells, however parvo cells seem to be affected as well. Abnormalities in motion perception and sensitivity to low contrast achromatic stimuli have been reported.51 However, chromatic stimuli that selectively elicit a response from parvo cells have also shown deficits in schizophrenic patients.52 VEP responses of schizophrenic patients have shown a lower amplitude compared to normal subjects for luminance modulated stimuli (magno cells), but not for chromatic modulated stimuli (parvo cells).53,54
Other Disorders
Severe magno cell deficits have been reported in patients with Parkinson’s disease and are correlated with the stage of the disease. Parvo cells seem to be affected as well, but not in correlation with disease severity and independent of magno cell damage. Konio cells have been reported to be intact in Parkinson’s disease.10
Tests for magno and parvo cell function have been reported to be impaired in early chiasmal tumors.14 Magno cell deficits have also been suggested to be involved in migraine.55 Dyslexia is another disorder for which the magno cells seem to be more involved than the other two pathways.
CONCLUSION
Recent advances in techniques of selective stimulation of the three different ganglion cell pathways have revealed specific deficits in various disorders. Assessment of each of these three pathways can therefore be used to diagnose or monitor these impairments. The combination of behavioral techniques with electrophysiological and imaging methods has been able to delineate specific damage in each pathway.
The compression of data that occurs during transfer of data from the retina to the cortex makes the pathways very sensitive to various types of damage and a good candidate from a diagnostic point of view. Recent advances have shown important clinical utilities for such tests which need to be thoroughly examined. Easy access to the eyes, simple and independent excitation of each of these pathways, and the possibility of combination with other techniques, promises clinical applications for such tests.
Several common disorders in ophthalmology, such as ocular hypertension and glaucoma, show distinctive patterns of damage to retinal ganglion pathways. Neurological disorders that affect retinal ganglionic pathways include MS, Alzheimer’s disease, and Parkinson’s disease. Some psychiatric disorders such as schizophrenia and depression have been shown to affect retinal ganglion pathways in a similar way.
A potential diagnostic device that would evaluate all three pathways with simple functional tests and combine behavioral tests with simultaneous EEG recordings, could be an essential part of many clinical settings in the near future.
Acknowledgements
We thank Dr Hamid Ahmadieh for his helpful comments.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Callaway EM. Structure and function of parallel pathways in the primate early visual system. J Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs) Biol Psychiatry. 2006;59:502–507. doi: 10.1016/j.biopsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi Q, Spehar B, DeBonet J. Color constancy in variegated scenes: role of low-level mechanisms in discounting illumination changes. J Opt Soc Am A Opt Image Sci Vis. 1997;14:2608–2621. doi: 10.1364/josaa.14.002608. [DOI] [PubMed] [Google Scholar]
- 4.Yoonessi A, Kingdom FA. Comparison of sensitivity to color changes in natural and phase-scrambled scenes. J Opt Soc Am A Opt Image Sci Vis. 2008;25:676–684. doi: 10.1364/josaa.25.000676. [DOI] [PubMed] [Google Scholar]
- 5.Paul KN, Saafir TB, Tosini G. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev Endocr Metab Disord. 2009;10:271–278. doi: 10.1007/s11154-009-9120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murav’eva SV, Deshkovich AA, Shelepin YE. The human magno and parvo systems and selective impairments of their functions. Neurosci Behav Physiol. 2009;39:535–543. doi: 10.1007/s11055-009-9161-3. [DOI] [PubMed] [Google Scholar]
- 7.Thurtell MJ, Bala E, Yaniglos SS, Rucker JC, Peachey NS, Leigh RJ. Evaluation of optic neuropathy in multiple sclerosis using low-contrast visual evoked potentials. Neurology. 2009;73:1849–1857. doi: 10.1212/WNL.0b013e3181c3fd43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delord S, Ducato MG, Pins D, Devinck F, Thomas P, Boucart M, et al. Psychophysical assessment of magno- and parvocellular function in schizophrenia. Vis Neurosci. 2006;23:645–650. doi: 10.1017/S0952523806233017. [DOI] [PubMed] [Google Scholar]
- 9.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva MF, Faria P, Regateiro FS, Forjaz V, Januário C, Freire A, et al. Independent patterns of damage within magno-, parvo- and koniocellular pathways in Parkinson’s disease. Brain. 2005;128:2260–2271. doi: 10.1093/brain/awh581. [DOI] [PubMed] [Google Scholar]
- 11.Guo L, Duggan J, Cordeiro MF. Alzheimer’s disease and retinal neurodegeneration. Curr Alzheimer Res. 2010;7:3–14. doi: 10.2174/156720510790274491. [DOI] [PubMed] [Google Scholar]
- 12.Sartucci F, Borghetti D, Bocci T, Murri L, Orsini P, Porciatti V, et al. Dysfunction of the magnocellular stream in Alzheimer’s disease evaluated by pattern electroretinograms and visual evoked potentials. Brain Res Bull. 2010;82:169–176. doi: 10.1016/j.brainresbull.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papia MF, Burke MW, Zangenehpour S, Palmour RM, Ervin FR, Ptito M. Reduced soma size of the M-neurons in the lateral geniculate nucleus following foetal alcohol exposure in non-human primates. Exp Brain Res. 2010;205:263–271. doi: 10.1007/s00221-010-2364-6. [DOI] [PubMed] [Google Scholar]
- 14.Gutowski NJ, Heron JR, Scase MO. Early impairment of foveal magno- and parvocellular pathways in juxta chiasmal tumours. Vision Res. 1997;37:1401–1408. doi: 10.1016/s0042-6989(96)00306-9. [DOI] [PubMed] [Google Scholar]
- 15.Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grueschow M, Rieger J, Stadler J, Tempelmann C, Heinze H, Speck O, et al. Topography of responses to colour and luminance in human subcortical visual pathways as revealed by high-resolution fMRI at 7T. J Vis. 2008;8:959. [Google Scholar]
- 17.Lu K, Perthen JE, Duncan RO, Zangwill LM, Liu TT. Noninvasive measurement of the cerebral blood flow response in human lateral geniculate nucleus with arterial spin labeling fMRI. Hum Brain Mapp. 2008;29:1207–1214. doi: 10.1002/hbm.20459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 19.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 20.Dacey D, Packer OS, Diller L, Brainard D, Peterson B, Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Res. 2000;40:1801–1811. doi: 10.1016/s0042-6989(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 21.Rodieck RW. The First Steps in Seeing. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- 22.Ruderman DL. The statistics of natural images. Network: Computation in Neural Systems. 1994;5:517–548. [Google Scholar]
- 23.van Hateren JH, Ruderman DL. Independent component analysis of natural image sequences yields spatio-temporal filters similar to simple cells in primary visual cortex. Proc Biol Sci. 1998;265:2315–2320. doi: 10.1098/rspb.1998.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- 25.Engel AK, Moll CK, Fried I, Ojemann GA. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci. 2005;6:35–47. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- 26.Foxe JJ, Strugstad EC, Sehatpour P, Molholm S, Pasieka W, Schroeder CE, et al. Parvocellular and magnocellular contributions to the initial generators of the visual evoked potential: high-density electrical mapping of the “C1” component. Brain Topogr. 2008;21:11–21. doi: 10.1007/s10548-008-0063-4. [DOI] [PubMed] [Google Scholar]
- 27.Luo A, Sajda P. Using single-trial EEG to estimate the timing of target onset during rapid serial visual presentation. Conf Proc IEEE Eng Med Biol Soc. 2006;1:79–82. doi: 10.1109/IEMBS.2006.259464. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol. 2004;137:863–871. doi: 10.1016/j.ajo.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Cello KE, Nelson-Quigg JM, Johnson CA. Frequency doubling technology perimetry for detection of glaucomatous visual field loss. Am J Ophthalmol. 2000;129:314–322. doi: 10.1016/s0002-9394(99)00414-6. [DOI] [PubMed] [Google Scholar]
- 30.Anderson AJ, Johnson CA. Frequency-doubling technology perimetry. Ophthalmol Clin North Am. 2003;16:213–225. doi: 10.1016/s0896-1549(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 31.Centofanti M, Fogagnolo P, Oddone F, Orzalesi N, Vetrugno M, Manni G, et al. Learning effect of humphrey matrix frequency doubling technology perimetry in patients with ocular hypertension. J Glaucoma. 2008;17:436–441. doi: 10.1097/IJG.0b013e31815f531d. [DOI] [PubMed] [Google Scholar]
- 32.Faria E, Arantes TE, Garcia CR, Mello PA, Muccioli C. Structural and functional assessment in HIV-infected patients using optical coherence tomography and frequency-doubling technology perimetry. Am J Ophthalmol. 2010;149:571–576. doi: 10.1016/j.ajo.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Bessler P, Klee S, Kellner U, Haueisen J. Silent substitution stimulation of S-cone pathway and L- and M-cone pathway in glaucoma. Invest Ophthalmol Vis Sci. 2010;51:319–326. doi: 10.1167/iovs.09-3467. [DOI] [PubMed] [Google Scholar]
- 34.Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–391. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 35.Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, et al. A preliminary longitudinal study of the retinal nerve fiber layer in progressive multiple sclerosis. J Neurol. 2010;257:1083–1091. doi: 10.1007/s00415-010-5467-x. [DOI] [PubMed] [Google Scholar]
- 36.Costello F, Hodge W, Pan YI, Metz L, Kardon RH. Retinal nerve fiber layer and future risk of multiple sclerosis. Can J Neurol Sci. 2008;35:482–487. doi: 10.1017/s031716710000915x. [DOI] [PubMed] [Google Scholar]
- 37.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain. 2001;124:1813–1820. doi: 10.1093/brain/124.9.1813. [DOI] [PubMed] [Google Scholar]
- 39.Plant GT, Hess RF. Regional threshold contrast sensitivity within the central visual field in optic neuritis. Brain. 1987;110:489–515. doi: 10.1093/brain/110.2.489. [DOI] [PubMed] [Google Scholar]
- 40.Mullen KT, Plant GT. Colour and luminance vision in human optic neuritis. Brain. 1986;109:1–13. doi: 10.1093/brain/109.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Plant GT. Transient visually evoked potentials to sinusoidal gratings in optic neuritis. J Neurol Neurosurg Psychiatry. 1983;46:1125–1133. doi: 10.1136/jnnp.46.12.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plant GT, Hess RF. Temporal frequency discrimination in optic neuritis and multiple sclerosis. Brain. 1985;108:647–676. doi: 10.1093/brain/108.3.647. [DOI] [PubMed] [Google Scholar]
- 43.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 44.Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 1989;501:364–372. doi: 10.1016/0006-8993(89)90653-7. [DOI] [PubMed] [Google Scholar]
- 45.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–384. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 46.Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Ann Neurol. 1993;33:248–257. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- 47.Davies DC, McCoubrie P, McDonald B, Jobst KA. Myelinated axon number in the optic nerve is unaffected by Alzheimer’s disease. Br J Ophthalmol. 1995;79:596–600. doi: 10.1136/bjo.79.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 49.Gilmore GC, Whitehouse PJ. Contrast sensitivity in Alzheimer’s disease: a 1-year longitudinal analysis. Optom Vis Sci. 1995;72:83–91. doi: 10.1097/00006324-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Gilmore GC, Wenk HE, Naylor LA, Koss E. Motion perception and Alzheimer’s disease. J Gerontol. 1994;49:P52–P57. doi: 10.1093/geronj/49.2.p52. [DOI] [PubMed] [Google Scholar]
- 51.Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 52.Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- 53.Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophr Res. 2005;76:55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Benedek K, Tajti J, Janáky M, Vécsei L, Benedek G. Spatial contrast sensitivity of migraine patients without aura. Cephalalgia. 2002;22:142–145. doi: 10.1046/j.1468-2982.2002.00351.x. [DOI] [PubMed] [Google Scholar]