Abstract
Purpose
To report the management of Vogt-Koyanagi-Harada (VKH) disease based on indocyanine green angiography (ICGA).
Methods
VKH patients with acute episodes of inflammation (inaugural or recurrent) who had received standard ICGA-guided care were studied retrospectively. Standard of care included high dose systemic corticosteroids at presentation and close ICGA follow-up with addition of immunosuppressive agents and/or intensification of ongoing therapy when recurrent choroidal lesions were detected by ICGA. Visual acuity, number of subclinical recurrences, type and duration of therapy, proportion of quiescent patients after therapy, and ICGA findings were recorded.
Results
Nine patients including 8 female and one male subject were studied. Five patients had inaugural disease and 4 presented with recurrent acute episodes. Visual acuity increased from 0.86±0.36 to 1.14±0.34 in the right eyes, and from 0.77±0.34 to 1.05±0.33 in the left eyes. The number of ICGA-detected occult choroidal recurrences amounted to 13. Mean duration of treatment was 30.1±34.6 months leading to recurrence-free status after discontinuation of therapy in 6 cases with mean duration of 29.5 months.
Conclusion
Continuous monitoring and aggressive therapy guided by ICGA in VKH disease prolongs treatment as compared to textbook guidelines but offers the prospect of reaching inflammation-free status after discontinuation of therapy. Zero tolerance to subclinical choroidal inflammation avoids irremediable evolution towards sunset glow fundus in patients treated early after the initial acute inflammatory attack.
Keywords: Vogt-Koyanagi-Harada Disease, Indocyanine Green Angiography, Therapy
INTRODUCTION
Vogt-Koyanagi-Harada (VKH) disease is a bilateral granulomatous choroiditis that invariably starts in the choroidal stroma and is therefore called a primary stromal choroiditis.1 The inflammatory process may evolve silently in the choroid without being clinically detected. When the condition becomes clinically apparent, it initially presents as panuveitis or more frequently as a predominantly posterior uveitis with papillitis and exudative retinal detachments. The disease is an autoimmune T-helper 1 type reaction directed against melanocytes in the choroidal stroma which explains its site of origin.2,3
The inflammatory reaction in VKH can sometimes be mild and remain subclinical, making its diagnosis difficult without sensitive investigational tests. Most of the time, however, the inflammatory reaction is hyperacute involving the retina, optic nerve, vitreous and anterior chamber. Although these structures are involved only as a consequence of primary choroidal inflammation, they are accessible to clinical examination and will render the disease clinically apparent by demonstrating classical signs of multiple exudative retinal detachments and papillitis. These clinical signs respond to inflammation suppressive therapy (IST) including high dose systemic corticosteroids, which is necessary for control of hyperacute disease.4–6 After initial aggressive IST, clinical disease is rapidly brought under control within 6 to 16 weeks and treatment is then progressively tapered with a total treatment duration of 6 to 9 months according to textbooks.7,8
Development of sunset glow fundus is reported in almost 100% of VKH cases, even when the disease seems to be under control clinically.9–11 Development of sunset glow fundus in the absence of clinically manifest disease suggests silently progressive choroidal inflammation and indicates that the post-acute treatment regimen may be insufficient.12
Before the development of indocyanine green angiography (ICGA), there was no sensitive method to detect subtle choroidal inflammation, and ultrasonography was the only imaging modality used for VKH disease by providing only gross information in pronounced disease. In contrast to ultrasonography, ICGA is sensitive enough to detect small inflammatory foci within the choroidal stroma as well as inflammation of choroidal stromal vessels.13 ICGA is currently the most appropriate method for investigating choroidal inflammatory diseases, as it is sensitive enough to demonstrate minimal and often, subclinical lesions of the choroid both in inaugural disease and in insufficiently treated disease.14–17 ICGA signs of acute VKH disease have been clearly standardized with four signs consistently present. These include early choroidal vessel hyperfluorescence, intermediate to late phase fuzziness of choroidal stromal vessels, disc hyperfluorescence, and hypofluorescent dark dots (HDDs).16 The contribution of ICGA is particularly useful in cases of suspected VKH disease without an acute onset which are usually seen at a later stage, when suboptimal therapy has already been initiated, as well as in cases without a complete set of signs in whom diagnosis is more difficult. In such cases, ICGA signs indicating choroidal inflammatory activity include HDDs and fuzziness of stromal vessels; the other two signs (early hyperfluorescent choroidal vessels and disc hyperfluorescence) are indicative of more acute disease.18–22
Choroidal disease in VKH is silently progressive; therefore to avoid late complications such as sunset glow fundus, the inflammation needs to be treated adequately. On the other hand, only ICGA monitoring allows detection of subclinical inflammation and correct orientation of treatment. Herein we report the results of ICGA-guided management of acute episodes (inaugural or recurrent) of VKH disease.
METHODS
Patients with a diagnosis of VKH disease in an acute stage (inaugural episode or acute recurrent disease) who were referred to the uveitis clinic of the Center for Ophthalmic Specialized Care (COS) from 1995 to 2010 were retrospectively studied.
All patients had a complete routine uveitis work-up as applied for patients with posterior uveitis. At each major visit a complete ocular examination was performed including Snellen visual acuity, slit lamp examination, applanation tonometry, dilated fundus examination, laser flare photometry, computerized visual field (VF) testing, optical coherence tomography (OCT) since available, and dual fluorescein angiography (FA) and ICGA. FA and ICGA were performed using a standard protocol as previously described.23 FA and ICGA were usually performed simultaneously. A Topcon 50 IA camera (Topcon, Tokyo, Japan) coupled to the ImageNet (Topcon, Tokyo, Japan) image digitalizing system was used to acquire the images.
To exclude autofluorescence, evidence of pre-injection fluorescence was sought with the highest flash intensity used for ICGA. At the same time, red-free posterior pole frames were taken. After a bolus injection of 500 mg ICG (Cardiogreen; Peaselt, Lorei, Germany) diluted in 7.5 ml of 0.9% salt solution, ICGA frames of the posterior pole were taken up to 2 to 3 minutes (early phase angiogram). ICG background fluorescence was analyzed in detail 12±3 minutes after ICG injection (intermediate phase angiogram) by capturing posterior pole frames and a minimum of 8 frames from the whole periphery over 360°. At the end of the intermediate ICGA phase, FA was performed. Early fluorescein angiographic frames of the posterior pole were taken up to 2 minutes. Between 4 to 7 minutes, 360° panoramic imaging of the periphery was performed (minimum of 8 frames), followed by late fluorescein posterior pole frames at 10 minutes. Late ICG background fluorescence was analyzed at 32±4 minutes after ICG injection (late phase angiogram) in the same fashion as that during the intermediate phase.
Laser flare assessment was performed using a Kowa FM-500 laser flare meter (Kowa Company, Ltd., Electronics and Optics Division, Tokyo, Japan) and OCT was performed using the OTI-Spectral OCT/SLO (OTI Inc., Toronto, Canada). For VF assessment the G1 program of the OCTOPUS 900 (Octopus 900, G Standard; Haag-Streit International, Bern, Switzerland) was used.
All patients underwent the standard of care of our center (Fig. 1) for treatment of acute active episodes of VKH disease (inaugural disease or acute recurrences) with very high dose oral corticosteroids (prednisone, 1.2–1.7 mg/kg) or in case of hyperacute disease, a combination of intravenous methylprednisolone (1000 mg/day) for 3 days followed by high-dose oral corticosteroids (prednisone, 1 mg/kg). ICGA, according to the above mentioned protocol, was performed at presentation before initiating treatment and every month±1 week thereafter until disappearance of HDDs and normalization of choroidal vessels, at least up to 4 months after presentation. During the tapering phase of corticosteroids beyond 4 months, ICGA was performed every 6±2 weeks. In case of subclinical reappearance of HDDs and/or fuzzy choroidal vessels, corticosteroid therapy was increased to 1mg/kg again and in parallel an immunosuppressant agent was added, azathioprine or mycophenolate mofetil usually being the first choice. Progressive tapering was then started again under ICGA control. Therapy was increased again by two steps each time ICGA signs reappeared.
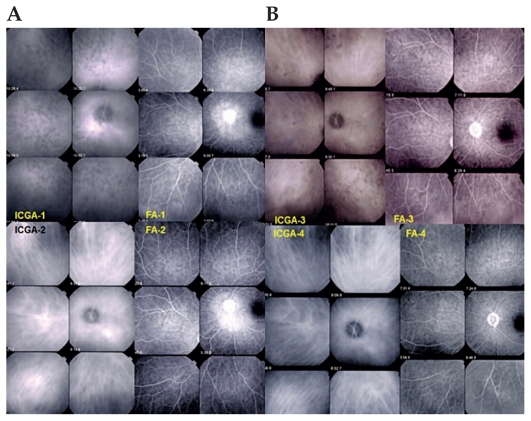
Figure 1.
(A) An example of ICGA-guided management of VKH. A patient with an inaugural episode of VKH disease shows numerous HDDs and complete vessel fuzziness (top left frames, ICGA-1). FA (top right frames, FA-1) shows disc hyperfluorescence and peripapillary exudative retinal detachment. After intravenous methylprednisolone and oral prednisone, there is complete disappearance of HDDs and fuzziness of choroidal vessels, the pattern of which is clearly seen again (bottom left frames, ICGA-2), while there is residual disc and peripapillary hyperfluorescence (bottom right frames, FA-2). (B) After prednisone tapering, subclinical recurrence of choroidal inflammation with reappearance of HDDs and fuzziness of choroidal vessels (top left frames, ICGA-3) are seen, while FA is quasi normal with only residual disc hyperfluorescence (top right frames, FA-3). After increase of oral prednisone and addition of mycophenolate mofetil, the choroidal inflammatory lesions (HDDs) disappeared two months later and choroidal vessels regained a normal appearance (bottom left frames, ICGA-4).
ICGA, indocyanine green angiography
VKH, Vogt-Koyanagi-Harada
HDDs, hypofluorescent dark dots
FA, fluorescein angiography
Visual outcomes, proportion of cases having presented with subclinical recurrence, proportion of cases having needed additional immunosuppressive therapy, type and duration of therapy, quiescence of disease at the end of follow-up, course of fundus appearance, and presence and evolution of ICGA signs (ICGA sign free cases) were the main parameters analyzed in the study.
RESULTS
Out of 1,268 new patients seen at the uveitis clinic at the Center for Ophthalmic Specialized Care (COS), Lausanne, Switzerland from 1995 to 2010, 22 (1.73%) subjects including 15 women and 7 men were diagnosed with VKH disease. Nine patients (1 man and 8 women) with mean age of 33.9±10.6 years had sufficient data to be included in the present study (Table 1). One patient had ICGA-guided follow-up who responded insufficiently to several treatments probably because of lack of compliance; this case was excluded from final analysis.
Table 1.
Demographic data, type of disease, follow-up period and ICGA findings
| Cases (Sex) | Age (Years) | Type of acute episode | F/U (months) | ICGA signs | |||
|---|---|---|---|---|---|---|---|
| Early hyperfluorescent vessels | Disc hyperfluorescence | Fuzzy vessels | HDDs | ||||
| Case 1 (F) | 37 | Inaugural | Under therapy | Yes | No | Yes | Yes |
| Case 2 (F) | 19 | Recurrent | 4 m | Yes | Yes | Yes | Yes |
| Case 3 (F) | 31 | Inaugural | Lost to F/U | Yes | Yes | Yes | Yes |
| Case 4 (F) | 35 | Inaugural | 14 m | Yes | Yes | Yes | Yes |
| Case 5 (F) | 24 | Recurrent | 114 m | Yes | Yes | Yes | Yes |
| Case 6 (M) | 46 | Inaugural | 9 m | Yes | Yes | Yes | Yes |
| Case 7 (F) | 51 | Recurrent | 28 m | Yes | Yes | Yes | Yes |
| Case 8 (F) | 24 | Inaugural | 14 m | No | Unilateral | Yes | Yes |
| Case 9 (F) | 38 | Recurrent | 132 m | Yes | Unilateral | Yes | Yes |
F, Female; M, Male; ICGA, indocyanine green angiography; F/U, follow-up; HDDs, hypofluorescent dark dots
Five subjects were seen for an acute inaugural episode, 3 cases experienced an acute recurrence and had been initially treated at another center, and the ninth patient had uncontrollable disease. Mean duration from onset of symptoms (initial episode or acute recurrent attack) to initiation of therapy was 19.1±13.1 days (excluding the ninth patient). Serous retinal detachment was seen in 7 of 9 cases (Fig. 2); 8 of 9 patients had cerebrospinal fluid (CSF) pleocytosis. The patient with normal CSF was diagnosed 7 days after the onset of symptoms.
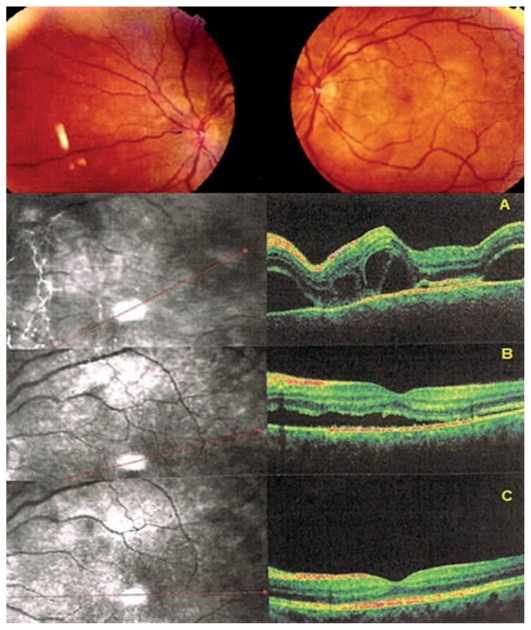
Figure 2.
Fundus and OCT findings in acute VKH. Fundus signs showed bilateral serous retinal detachments in this patient (only the left eye is shown) diagnosed 7 days after the onset of symptoms. OCT illustrates subretinal fluid at presentation (top scan, A). After 3 days of intravenous methylprednisolone, the amount of fluid has decreased substantially (middle scan, B) with quasi normalization after one month of treatment (bottom scan, C).
OCT, optical coherence tomography
VKH, Vogt-Koyanagi-Harada
Mean visual acuity at presentation was 0.86±0.36 and 0.77±0.34 in the right and left eyes respectively, and 1.14±0.34 and 1.05±0.33 respectively at final follow-up.
The mean number of ICGA recurrences was 2.1±1.4 episodes with 3 patients demonstrating no clinical or ICGA signs of recurrence after the initial high-dose treatment was tapered. Excluding these 3 patients, the mean number of recurrences was 3.2±1.7 in the remaining 6 patients. The total number of ICGA recurrences was 19, with 6 recurrences (all in one poorly responsive case) being both clinical and ICGA-based.
All patients were treated using high-dose corticosteroid therapy, this included high-dose intravenous methylprednisolone (500–1000 mg daily) for 3 days in 4 of 9 patients, and oral prednisone (1.2–1.7 mg/kg) in the remaining. In 2 of 9 (22%) patients no immunosuppressive agent was added and no recurrence occurred after slow tapering of steroids. In 7 of 9 patients (78%) however, immunosuppressive therapy was required in addition to prednisone therapy from the beginning. In one patient, azathioprine was added after one month because of corticosteroid intolerance and slow response to therapy based on ICGA findings. This patient experienced no recurrence after tapering treatment. In the 6 remaining patients immunosuppressive agents had to be added because of recurrences during tapering of initial treatment. These included azathioprine (N=5), mycophenolate mofetil (N=4), cyclosporine (N=3), and infliximab, adalimumab and interferon-α (N=1 for each). Two patients required simultaneous use of two immunosuppressive agents and three patients received two immunosuppressants sequentially, i.e. azathioprine was replaced by the less toxic mycophenolate mofetil.
Mean total duration of therapy was 30.1±34.6 (range, 9–114) months. In the patient resistant to therapy, treatment was administered continuously for 132 months. Six patients remained quiescent, developed no ICGA signs and were recurrence-free for a mean duration of 29.5±22.7 months after cessation of treatment. One patient was lost to follow-up while still under treatment and recurrence-free for 6 months and the other patient was still under treatment at the time of this report, 13 months after the acute episode.
Sunset glow fundus sign was present in 4 of 9 patients, all of whom had experienced recurrences including the poorly responsive patient. In 5 other patients there were no sunset glow fundus signs, all of whom were first seen at the time of their inaugural acute episode. None of the patients showed late signs of disease such as poliosis, alopecia or vitiligo.
ICGA signs were identical whether the acute episode was inaugural or recurrent. At presentation, 8 of 9 patients showed early hyperfluorescent choroidal vessels (Fig. 1), 8 of 9 subjects presented with disc hyperfluorescence which was unilateral in two, all cases showed fuzzy choroidal vessels bilaterally (Fig. 3) and bilateral round HDDs (Fig. 4). At the time of occult choroidal recurrence, HDDs and fuzziness of vessels were the only ICGA signs.
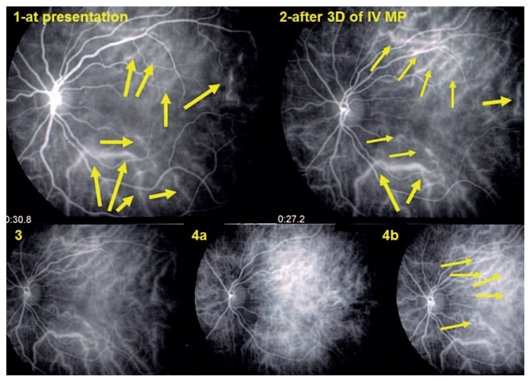
Figure 3.
Early choroidal hyperfluorescent vessels three days after administration of intravenous methylprednisolone (top right) in a patient with an acute inaugural VKH episode at presentation (top left). Normalization of choroidal vessels after 3 months of combined prednisone and mycophenolate mofetil therapy (bottom left, 3). Reappearance of early hyperfluorescent choroidal vessels, together with HDDs 6 weeks later upon discontinuation of prednisone (bottom frames 4a and 4b).
VKH, Vogt-Koyanagi-Harada
HDDs, hypofluorescent dark dots
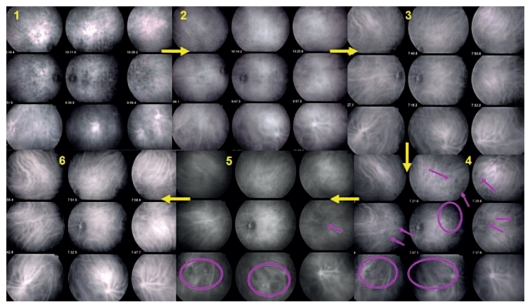
Figure 4.
Numerous HDDs and fuzzy vessels at presentation (1). Decrease in HDDs after one month of prednisone therapy with persistence of fuzzy vessels observed with ICGA-guided follow-up (2). Choroidal inflammation finally responded to combined prednisone and mycophenolate mofetil therapy resulting in resolution of HDDs and restoration of normal pattern of choroidal vessels (3). Six weeks later and after discontinuation of prednisone, recurrence of occult choroidal inflammation was noted with reappearance of HDDs and vessel fuzziness in some areas of the fundus (4, crimson circles and arrows) which slowly responded to the addition of cyclosporine after two months (5) leading to a quasi normal choroidal appearance after another two months (6).
ICGA, indocyanine green angiography
HDDs, hypofluorescent dark dots
DISCUSSION
The initial pathological process in VKH disease starts within the choroidal stroma at the level of melanocytic islets and goes clinically undetected until inflammation affects neighboring structures including the retina and optic disc, usually in an acute or hyperacute fashion.24–26 The standard of care is to start vigorous IST which classically consists of high-dose systemic corticosteroids tapered over a period of 6–9 months.7,8 The disease responds well to such an approach and clinical signs regress satisfactorily.27 Nevertheless, even in patients with apparently inactive disease, close to 100% of cases evolve towards sunset glow fundus.10,28 This evolution towards depigmentation of the fundus despite apparently quiescent disease shows that persistent choroidal disease, albeit without clinical signs, is invariably present after clinical disease has been brought under control.29,30 Subclinical choroidal inflammation in posterior uveitides can only be detected by ICGA.31–38 This is the case for VKH at the onset of disease when no clinical signs are present yet, or after treatment once clinical disease has been mastered.14,22 It has been shown that in seemingly pure anterior recurrences of VKH, ICGA clearly shows subclinical choroidal lesions.17 We have also shown that insufficient therapy in the post-acute phase when clinical signs have resolved, fails to control choroidal lesions.15 The amount of evidence at our disposal on continuing smouldering choroidal inflammation clearly indicates that in order to extinguish VKH disease, subclinical choroidal inflammation has to be treated and the only way to assess and follow choroidal involvement is by using ICGA.
ICGA-guided therapy has been our standard of care since we used ICGA for posterior uveitis back in the early 90′s. The current study showed that in 8 patients, a number of 13 subclinical recurrences were detected by ICGA leading to reinforced and prolonged therapy. Mean duration of treatment in our series was 30 months which is by far longer than indicated in textbooks.7,8 However the absence of sunset glow fundus in patients who had received ICGA-guided therapy since their inaugural episode justifies such an aggressive therapeutic approach. In contrast, all patients included for an acute recurrence developed sunset glow fundus (sometimes already present at inclusion) most certainly due to previous damage caused by ongoing smouldering subclinical choroidal inflammation. Furthermore, visual outcome was excellent for this group of VKH patients receiving ICGA-guided therapy. Such an aggressive and closely monitored attitude is further supported by the good proportion of patients in whom the disease remained quiescent after discontinuation of therapy with mean inflammation-free status exceeding two and a half years. This group of patients with quiescent disease included most of the patients in whom the acute episode had been a recurrence and sunset glow fundus was present. None of our patients developed late signs of VKH disease such as poliosis, vitiligo or alopecia indicating that zero tolerance even to low grade choroidal inflammation prevents evolution to late stage disease including sunset glow fundus in patients taken care of since the inaugural inflammatory episode. We believe that classification criteria39 employing late signs are obsolete because they do not characterize late disease, in fact they reflect insufficiently treated disease.
ICGA signs have been standardized for early acute VKH disease and findings were found to be quite consistent.16 Four main ICGA signs have been suggested to assess choroidal involvement in acute episodes of VKH. These include (1) early hyperfluorescent choroidal vessels indicating acute choroidal vasculitis, (2) disc hyperfluorescence reflecting hyperacute disease, (3) fuzzy choroidal vessels seen in intermediate to early late phase frames denoting choroidal inflammatory vasculopathy, and (4) HDDs indicating foci of choroidal inflammation. All of these signs were present in our group of patients with acute inflammatory attacks, except in one patient who did not demonstrate disc hyperfluorescence and another patient who did not show early hyperfluorescent choroidal vessels. At the time of occult choroidal recurrence, the degree of inflammation is much less severe than the initial acute episode so that disc hyperfluorescence is never observed and early hyperfluorescent choroidal vessels were either absent or not clear-cut. All 13 episodes of occult recurrence were clearly identified by the reappearance of HDDs in parallel with fuzziness of choroidal vessels.
To meaningfully assess posterior uveitis nowadays, it is difficult to imagine not using ICGA when choroidal involvement is suspected. ICGA represents the only modality capable of identifying choroidal inflammation, hidden to other investigational modalities. This peculiarity of choroidal inflammation is characterized by the analogic angiographic concepts including the “iceberg effect” where most of the inflammatory process is not detectable by usual diagnostic means, and the “submarine effect” when only ICGA is positive and no other clue to choroidal inflammation exists. Obviously if choroidal inflammation can only be assessed by ICGA, monitoring of its evolution and adjustment of therapy can also only be performed by ICGA follow-up, as was illustrated in this study.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Bouchenaki N, Herbort CP. Stromal choroiditis. In: Pleyer U, Mondino B, editors. Essentials in Ophthalmology: Uveitis and Immunological Disorders. 1st ed. Berlin, Heidelberg, New York: Springer; 2004. pp. 234–253. [Google Scholar]
- 2.Sugita S, Takase H, Taguchi C, Imai Y, Kamoi K, Kawaguchi T, et al. Ocular infiltrating CD4+ T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Invest Ophthalmol Vis Sci. 2006;47:2547–2554. doi: 10.1167/iovs.05-1547. [DOI] [PubMed] [Google Scholar]
- 3.Damico FM, Cunha-Neto E, Goldberg AC, Iwai LK, Marin ML, Hammer J, et al. T-cell recognition and cytokine profile induced by melanocyte epitopes in patients with HLA-DRB1*0405-positive and -negative Vogt-Koyanagi-Harada uveitis. Invest Ophthalmol Vis Sci. 2005;46:2465–2471. doi: 10.1167/iovs.04-1273. [DOI] [PubMed] [Google Scholar]
- 4.Sonoda S, Nakao K, Ohba N. Extensive chorioretinal atrophy in Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol. 1999;43:113–119. doi: 10.1016/s0021-5155(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 5.Rao NA. Treatment of Vogt-Koyanagi-Harada disease by corticosteroids and immunosuppressive agents. Ocul Immunol Inflamm. 2006;14:71–72. doi: 10.1080/09273940600674368. [DOI] [PubMed] [Google Scholar]
- 6.Bouchenaki N, Morisod L, Herbort CP. Vogt-Koyanagi-Harada syndrome: importance of rapid diagnosis and therapeutic intervention. Klin Monbl Augenheilkd. 2000;216:290–294. doi: 10.1055/s-2000-10987. [DOI] [PubMed] [Google Scholar]
- 7.Nussenblatt RB. Vogt-Koyanagi-Harada syndrome. In: Nussenblatt RB, Whitcup SM, editors. Uveitis: Fundamentals and Clinical Practice. 3rd ed. Philadelphia: Mosby; 2004. pp. 324–338. [Google Scholar]
- 8.Rao NA, Inomata H, Moorthy RS. Vogt-Koyanagi-Harada syndrome. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Ocular Infection and Immunity. 1st ed. St. Louis: Mosby; 1996. pp. 734–753. [Google Scholar]
- 9.Tugal-Tutkun I, Ozyazgan Y, Akova Y, Sullu Y, Akyol N, Soylu M, et al. The spectrum of Vogt-Koyanagi-Harada disease in Turkey: VKH in Turkey. Int Ophthalmol. 2007;27:117–123. doi: 10.1007/s10792-006-9001-1. [DOI] [PubMed] [Google Scholar]
- 10.Keino H, Goto H, Usui M. Sunset glow fundus in Vogt-Koyanagi-Harada disease with or without chronic inflammation. Graefes Arch Clin Exp Ophthalmol. 2002;240:878–882. doi: 10.1007/s00417-002-0538-z. [DOI] [PubMed] [Google Scholar]
- 11.Yang P, Wang H, Zhou H, Huang X, Zhong H, Chen L, et al. Clinical manifestations and diagnosis of Vogt-Koyanagi-Harada syndrome. Zhonghua Yan Ke Za Zhi. 2002;38:736–739. [PubMed] [Google Scholar]
- 12.Foster DJ, Cano MR, Green RL, Rao NA. Echographic features of the Vogt-Koyanagi-Harada Syndrome. Arch Ophthalmol. 1990;108:1421–1426. doi: 10.1001/archopht.1990.01070120069031. [DOI] [PubMed] [Google Scholar]
- 13.Bouchenaki N, Cimino L, Auer C, Tao Tran V, Herbort CP. Assessment and classification of choroidal vasculitis in posterior uveitis using indocyanine green angiography. Klin Monbl Augenheilkd. 2002;219:243–249. doi: 10.1055/s-2002-30661. [DOI] [PubMed] [Google Scholar]
- 14.Herbort CP, Mantovani A, Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: angiographic signs and utility in patient follow-up. Int Ophthalmol. 2007;27:173–182. doi: 10.1007/s10792-007-9060-y. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi T, Horie S, Bouchenaki N, Ohno-Matsui K, Mochizuki M, Herbort CP. Suboptimal therapy controls clinically apparent disease but not subclinical progression of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2010;30:41–50. doi: 10.1007/s10792-008-9288-1. [DOI] [PubMed] [Google Scholar]
- 16.Miyanaga M, Kawaguchi T, Miyata K, Horie S, Mochizuki M, Herbort CP. Indocyanine green angiography findings in initial acute pretreatment Vogt-Koyanagi-Harada disease in Japanese patients. Jpn J Ophthalmol. 2010;54:377–382. doi: 10.1007/s10384-010-0853-6. [DOI] [PubMed] [Google Scholar]
- 17.Bascal K, Wen DS, Chee SP. Concomitant choroidal inflammation during anterior segment recurrence in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2008;145:480–486. doi: 10.1016/j.ajo.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Howe L, Stanford M, Graham E, Marshall J. Indocyanine green angiography in inflammatory eye diseases. Eye. 1998;12:761–767. doi: 10.1038/eye.1998.199. [DOI] [PubMed] [Google Scholar]
- 19.Harada T, Kanbara Y, Takeuchi T, Niwa T, Majima T. Exploration of Vogt-Koyanagi Harada syndrome by infrared choroidal angiography with indocyanine green. Eur J Ophthalmol. 1997;7:163–170. doi: 10.1177/112067219700700208. [DOI] [PubMed] [Google Scholar]
- 20.Oshima Y, Harino S, Hara Y, Tano Y. Indocyanine green angiographic findings in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 1996;122:58–66. doi: 10.1016/s0002-9394(14)71964-6. [DOI] [PubMed] [Google Scholar]
- 21.Okada AA, Mizusawa T, Sakai J, Usui M. Videofunduscopy and videoangiography using the scanning laser ophthalmoscope in Vogt-Koyanagi-Harada syndrome. Br J Ophthalmol. 1998;82:1175–1181. doi: 10.1136/bjo.82.10.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchenaki N, Herbort CP. The contribution of indocyanine green angiography to the appraisal and management of Vogt-Koyanagi-Harada disease. Ophthalmology. 2001;108:54–64. doi: 10.1016/s0161-6420(00)00428-0. [DOI] [PubMed] [Google Scholar]
- 23.Herbort CP, LeHoang P, Guex-Crosier Y. Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard protocol. Ophthalmology. 1998;105:432–440. doi: 10.1016/S0161-6420(98)93024-X. [DOI] [PubMed] [Google Scholar]
- 24.Rubsamen PE, Gass JD. Vogt-Koyanagi-Harada syndrome. Clinical course, therapy, and long-term visual outcome. Arch Ophthalmol. 1991;109:682–687. doi: 10.1001/archopht.1991.01080050096037. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Resta A, Herbort CP, Abu El Asrar A, Kawaguchi T, Mochizuki M, et al. Work-up, diagnosis and management of acute Vogt-Koyanagi-Harada disease: a case of acute myopization with granulomatous uveitis. Int Ophthalmol. 2007;27:100–115. doi: 10.1007/s10792-007-9052-y. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama Y, Kimura Y, Kishi S, Shimizu K. Serous detachment of the ciliary body in Harada disease. Am J Ophthalmol. 1988;125:666–672. doi: 10.1016/s0002-9394(98)00033-6. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka E, Ohguro N, Yamamoto S, Nakagawa Y, Imoto Y, Tano Y. Evaluation of pulse corticosteroid therapy for Vogt-Koyanagi-Harada disease assessed by optical coherence tomography. Am J Ophthalmol. 2002;134:454–456. doi: 10.1016/s0002-9394(02)01575-1. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki S. Quantitative evaluation of “sunset-glow” fundus in Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol. 1999;43:327–333. doi: 10.1016/s0021-5155(99)00016-7. [DOI] [PubMed] [Google Scholar]
- 29.Chee SP, Jap A, Bacsal K. Prognostic factors of Vogt-Koyanagi-Harada disease in Singapore. Am J Ophthalmol. 2009;147:154–161. doi: 10.1016/j.ajo.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Inomata H, Rao NA. Depigmented atrophic lesions in sunset glow fundi of Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2001;131:607–614. doi: 10.1016/s0002-9394(00)00851-5. [DOI] [PubMed] [Google Scholar]
- 31.Papadia M, Herbort CP. Idiopathic choroidal neovascularization as the inaugural sign of multiple evanescent white dot syndrome. Middle East Afr J Ophthalmol. 2010;17:270–274. doi: 10.4103/0974-9233.65490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadia M, Herbort CP. Unilateral papillitis, the tip of the iceberg of bilateral ICGA-detected tuberculous choroiditis. Ocul Immuno Inflamm. 2011;19:124–126. doi: 10.3109/09273948.2010.530872. [DOI] [PubMed] [Google Scholar]
- 33.Bouchenaki N, Herbort CP. Indocyanine green angiography (ICGA) in the assessment and follow-up of choroidal inflammation in active chronically evolving Vogt-Koyanagi-Harada disease. In: Dodds EM, Couto CA, editors. Uveitis in the third millennium. 1st ed. Buenos Aires: Elsevier Science B.V; 2000. pp. 35–38. [Google Scholar]
- 34.Herbort CP, Bodaghi B, Lehoang P. Indocyanine green angiography in ocular inflammatory diseases: principles, schematic interpretation, semiology and clinical value. J Fr Ophthalmol. 2001;24:423–447. [PubMed] [Google Scholar]
- 35.Altan-Yaycioglu R, Akova YA, Akca S, Yilmaz G. Inflammation of the posterior uvea: findings on fundus fluorescein and indocyanine green angiography. Ocul Immunol Inflamm. 2006;14:171–179. doi: 10.1080/09273940600660524. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Yang P, Wen F, Zhou H, Huang X. Fundus fluorescein and indocyanine green angiographic study of Behçet’s disease and Vogt-Koyanagi-Harada syndrome. Zhonghua Yan Ke Za Zhi. 2002;38:210–212. [PubMed] [Google Scholar]
- 37.Kohno T, Miki T, Shiraki K, Kano K, Matsushita M, Hayashi K, et al. Subtraction ICG angiography in Harada’s disease. Br J Ophthalmol. 1999;83:822–833. doi: 10.1136/bjo.83.7.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbort CP. Indocyanine Green Angiography. In: Gupta A, Gupta V, Herbort CP, Khairallah M, editors. Uveitis, Text and Imaging. 1st ed. New Delhi: Jaypee; 2009. pp. 88–143. [Google Scholar]
- 39.Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–652. doi: 10.1016/s0002-9394(01)00925-4. [DOI] [PubMed] [Google Scholar]