Abstract
Purpose
To evaluate the effectiveness and risk of complications of high-dose intravenous pulsed corticosteroids for non-infectious ocular inflammatory diseases.
Methods
Retrospective cohort study. One hundred four eyes of seventy patients who received high-dose intravenous corticosteroids for treatment of active ocular inflammation were identified from five centers. The main outcome measures were control of inflammation and occurrence of ocular or systemic complications within one month after treatment.
Results
Within ≤1 month of starting treatment, 57% of eyes achieved complete control of inflammation (95% confidence interval (CI): 33-83%), improving to 82% when near-complete control was included (95% CI: 61-96%). Most eyes (85%; 95% CI: 70-95%) gained clinically significant improvement in anterior chamber inflammation. One patient developed a colon perforation during treatment. No other major complications were recorded.
Conclusions
Treatment of ocular inflammation with high-dose intravenous corticosteroids resulted in substantial clinical improvement for most cases within one month. Complications of therapy were infrequent.
Keywords: Corticosteroids, Inflammation, Intravenous, Non-infectious, Uveitis
Introduction
Ocular inflammatory disease is an important cause of visual loss and ocular morbidity worldwide1-3. In more severe cases, or cases unlikely to respond to topical corticosteroids, systemic corticosteroids frequently are used to achieve disease control4.
“Pulsed” (high dose) intravenous corticosteroids have been recommended as initial treatment for severe ocular inflammatory diseases, based on the theory that the high dose of anti-inflammatory medication delivered via this technique should result in rapid disease control, allowing more rapid tapering of corticosteroids thereafter and thus minimizing corticosteroid-induced complications5-6. Pulsed intravenous corticosteroids long have been utilized as a treatment for a variety of rheumatologic and autoimmune conditions with or without ocular manifestations7-10. Intravenous corticosteroid courses subsequently have been applied for a variety of ocular inflammatory conditions, including optic neuritis5, Vogt-Koyanagi-Harada disease11, Behçet’s Disease-associated uveitis12, serpiginous choroiditis13, and others. However, use of high-dose intravenous corticosteroids has not been examined in a large-scale or multi-center fashion. This report evaluates the effectiveness and risk of complications observed among ocular inflammation patients treated with high-dose intravenous pulsed corticosteroids in a relatively large, multi-center retrospective cohort study.
Materials and Methods
Patients
The Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study is a large retrospective cohort study of patients with ocular inflammation managed at tertiary ocular inflammation centers between 1979-200714. This cohort includes all individuals examined from the inception of four ocular immunology research centers until the time of the study, and an approximate 40% random sample from a fifth center. Random sampling at the fifth center was conducted for logistical reasons, due to an especially large number of patients at that center. Data collection methods for the SITE Cohort Study are detailed elsewhere14. The study was conducted with the approval of the governing institutional review boards (IRBs) at each participating center.
All patients given high-dose intravenous corticosteroids (500 mg of methylprednisolone per day or more) as a treatment for ocular inflammation were identified from the SITE Cohort Study database. These patients had received treatment with doses ranging from a single dose of 500 mg of methylprednisolone to 1000 mg given on three successive days. Patients subsequently received additional anti-inflammatory treatment according to best medical judgment, typically following published guidelines regarding the use of oral corticosteroids for ocular inflammation4. Eyes with inactive inflammation at the time of intravenous pulse corticosteroid treatment were excluded based on the presumption that the reason for treatment was not ocular disease control (e.g. pre-emptive anti-inflammatory therapy prior to ocular surgery), and because the effect of such treatment on inflammation could not be evaluated. Eyes also were excluded if they underwent ocular surgery within two weeks of initiation of intravenous treatment, since ocular surgery would complicate interpretation of the response. Individuals with non-uveitic ocular inflammatory conditions (such as mucous membrane pemphigoid (MMP) and scleritis) were excluded from the anterior chamber and vitreous inflammation analyses, on grounds that these are not the sites of ocular inflammation for those conditions, but were included in analysis of overall control of inflammation.
Data Collection
Information on all patients with noninfectious ocular inflammatory disease was entered into a customized database (Access, Microsoft Corporation, Redmond, WA) by trained, expert reviewers. Data on each patient and each eye were recorded for every visit. Data utilized in this analysis included demographic information, diagnosis, visual acuity, level of inflammation, ocular examination features, ocular surgeries, and ocular or systemic complications.
For eyes with a diagnosis of uveitis, anterior chamber cells, vitreous cells, and vitreous haze grades also were recorded (when available). These parameters had been recorded using an ordinal scale; grading of anterior chamber cells and vitreous haze at participating centers had used an approach identical to and similar to (respectively) the system later adopted by the Standardization of Uveitis Nomenclature Working Group (SUN)15. Overall inflammatory activity was graded as ‘active’, ‘slightly active’, or ‘inactive’ by the treating provider. ‘Slightly active’ indicated minimal inflammation as described by terms such as “trace” or “slight” activity, whereas ‘inactive’ indicated the complete absence of any inflammatory signs (e.g., “no cells” or “no activity”). ‘Inactive’ inflammation referred to the absence of all inflammatory signs; following the SUN Working Group15, a rare cell would be included in this category for cases of uveitis. ‘Active’ inflammation referred to activity higher than that indicated by ‘slightly active’, e.g. 1+ or higher anterior chamber cells in uveitis cases.
Outcome Measures
The primary outcome measure was overall control of inflammation. For overall inflammatory activity, ‘complete control’ was defined as a clinical improvement from ‘active’ or ‘slightly active’ inflammation at baseline to ‘inactive’ disease by the specified time point. ‘Near complete control’ was defined as an improvement in disease activity from ‘active’ to either a ‘slightly active’ or an ‘inactive’ state.
Secondary outcome measures included clinically significant improvement in intraocular inflammation for uveitis cases and the occurrence of ocular or systemic complications. For anterior chamber cells, vitreous cells, and vitreous haze, ‘clinically significant improvement’ was defined following SUN recommendations as a two-step or greater improvement in scale of inflammation (i.e. 4+ to 2+ or 2+ to 0.5+) or achievement of complete control (grade 0)15. All ocular and systemic complications that occurred in association with intravenous corticosteroid administration were tabulated.
Statistical Methods
Frequencies of variables at enrollment and outcomes were tabulated for the study population. Among eyes or patients at risk of each event, Kaplan-Meier analyses of time-to-first control of inflammation were performed to obtain the cumulative probability of overall control of inflammation by specific time points (e.g., 1 month). The comparison of the time-to-control of inflammation between types of ocular inflammation were performed using a Cox proportional hazards model, accounting for excess correlation between paired eyes of participants for analyses involving eyes16. All data analyses were performed using SAS version 9.1 (SAS, Cary, NC).
Results
Seventy patients (133 eyes with an ocular inflammatory diagnosis) received treatment with high dose intravenous corticosteroids, after excluding patients with no activity in either eye and those receiving intravenous corticosteroids perioperatively. The initial demographic and clinical characteristics of participants and eyes are given in Table 1. Twenty-nine contralateral eyes of these individuals were not actively inflamed at baseline and therefore could not be included in the analysis of time-to-control of inflammation, leaving 104 eyes of 70 patients for analysis of response to high dose intravenous corticosteroids. The median age of individuals treated was 39.8 years; approximately two-thirds each of participants were female and Caucasian. Panuveitis (27.1%) constituted the most frequent indication for intravenous pulse corticosteroid treatment in this group of patients. The most common ocular complications of inflammation present prior to treatment were exudative retinal detachment (13.3%) and cystoid macular edema (8.9%).
Table 1.
Demographics of the study population
| n | % | |
|---|---|---|
| Patient-specific characteristics | 70 | |
| Median age (y) at treatment (range) | 39.8 | 11-82 |
| Gender [% (n)] | ||
| Male | 27 | 38.6% |
| Female | 43 | 61.4% |
| Race [% (n)] | ||
| Caucasian | 44 | 62.9% |
| African-American | 26 | 37.1% |
| Diagnosis [% (n)] | ||
| Anterior Uveitis | 10 | 14.3% |
| Intermediate Uveitis | 8 | 11.4% |
| Posterior Uveitis | 10 | 14.3% |
| Panuveitis | 19 | 27.1% |
| Scleritis | 12 | 17.1% |
| Othera | 11 | 15.7% |
| Bilateral disease [% (n)] | 63 | 90.0% |
| Median visual acuity at visit preceding treatment (IQ range)b | ||
| Better eye | 20/40 | (20/25 - 20/160) |
| Worse eye | 20/250 | (20/50 - count fingers) |
| Eye-specific characteristics | 133 | 100% |
| Median visual acuity at time preceding treatment (IQ range)b | 20/80 | (20/40 - 20/400) |
| Inflammatory Activity | ||
| Overall inflammatory activity | ||
| Active | 104 | 78.2% |
| Slightly active | 4 | 3.0% |
| Inactive | 21 | 15.8% |
| Not given | 4 | 3.0% |
| Anterior chamber cells, grade, %c | ||
| 0 | 27 | 30.0% |
| 0.5+ | 10 | 11.1% |
| 1+ | 11 | 12.2% |
| 2+ | 17 | 18.9% |
| 3+ | 15 | 16.7% |
| 4+ | 7 | 7.8% |
| Not given | 3 | 3.3% |
| Vitreous cells, grade, %c | ||
| 0 | 26 | 28.9% |
| 0.5+ | 10 | 11.1% |
| 1+ | 14 | 15.6% |
| 2+ | 13 | 14.4% |
| 3+ | 13 | 14.4% |
| 4+ | 1 | 1.1% |
| Not given | 13 | 14.4% |
| Vitreous haze, grade, %c | ||
| 0 | 44 | 48.9% |
| 0.5+ | 5 | 5.6% |
| 1+ | 9 | 10.0% |
| 2+ | 6 | 6.7% |
| 3+ | 1 | 1.1% |
| 4+ | 0 | 0% |
| Not given | 25 | 27.8% |
Other diagnoses (n): MMP (n=5), peripheral ulcerative keratitis (n=2), idiopathic orbital pseudotumor (n=2), optic nerve inflammation with associated vitreous cells (n=1), autoimmune optic neuropathy (n=1).
Excludes two patients, four eyes (data not given); not all patients have two eyes with ocular inflammation, so the better eye may not be diseased.
Only among patients with a diagnosis of uveitis
Kaplan-Meier curves showing the time-to complete control of inflammation and time-to-near complete control of inflammation are given as Figures 1 and 2, respectively. There is some controversy as to whether every minimal sign of inflammation must be extinguished to consider treatment a success, so both perspectives are reported. The same Kaplan-Meier curves broken down by type of inflammation (uveitis vs. other forms of inflammation) are given as Figures 3 and 4. For all inflammatory diseases, the Kaplan-Meier estimate of the proportion of eyes with complete control was 11% (95% CI: 5.6-21%) at fourteen days after initiation of treatment, which climbed to 57% (95% CI: 33-83%) by one month. When including eyes that achieved near complete control, in addition to those with complete control, the Kaplan-Meier estimate of the proportion was greater: 30% (95% CI: 21-42%) by fourteen days and 82% (95% CI: 61-96%) by one month. Among the subset of eyes with uveitis, 55% (95% CI: 30-83%) obtained complete control and 85% (95% CI: 66-97%) for near complete control or better by one month. For non-uveitic eyes (MMP, scleritis, etc.), where ascertainment of very low grades of activity may be less easy than with uveitis, 52% (95% CI: 26-84%) gained complete control and 53% (95% CI: 29-82%) attained near complete control or better within one month.
Figure 1.
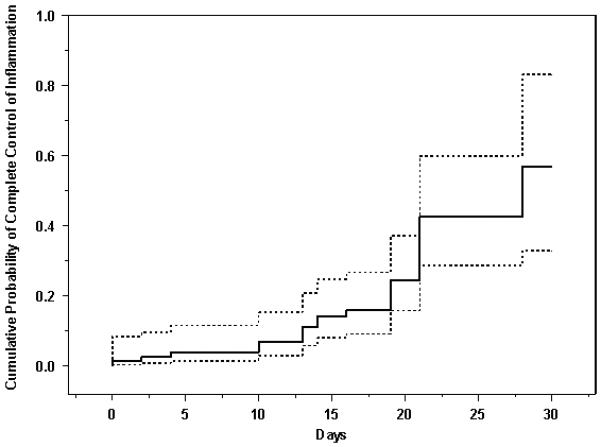
Cumulative probability of complete control of inflammation among eyes with active ocular inflammation treated with high-dose intravenous corticosteroids. Dotted lines give the 95% confidence limits.
Figure 2.
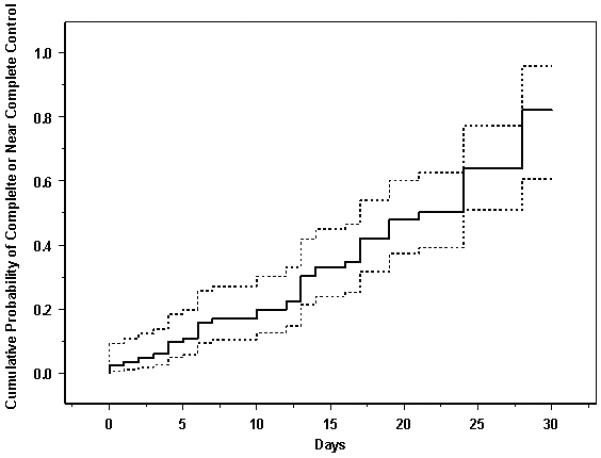
Cumulative probability of achieving complete or near complete control (improvement from “active” inflammation to either a “slightly active” or an “inactive” inflammatory state) following treatment with high-dose intravenous corticosteroids, eyes with active ocular inflammation. Dotted lines give the 95% confidence limits
Figure 3.
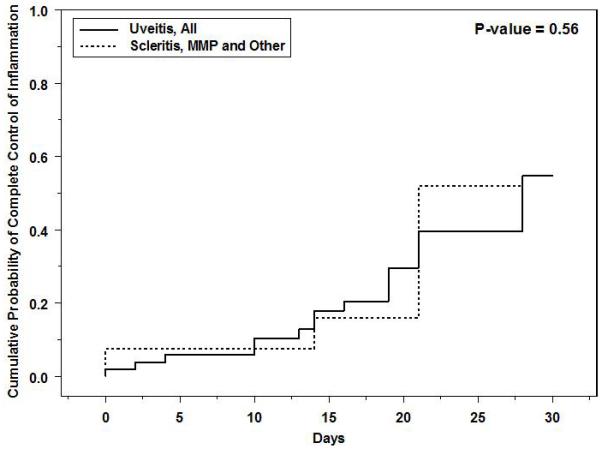
Cumulative probability of complete control of inflammation among eyes with active ocular inflammation treated with high-dose intravenous corticosteroids, by type of ocular inflammation. MMP = mucous membrane pemphigoid.
Figure 4.
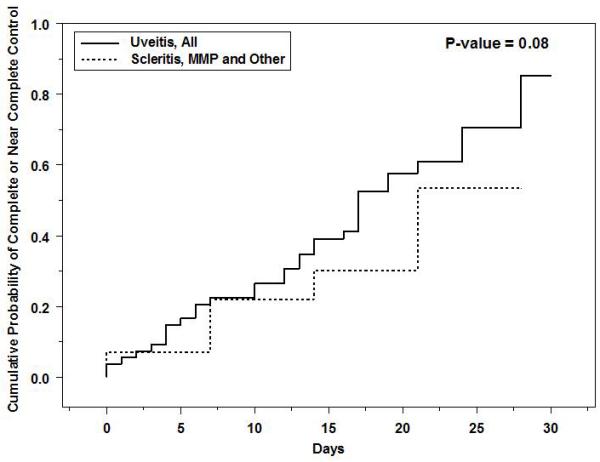
Cumulative probability of achieving complete or near complete control (improvement from “active” inflammation to either a “slightly active” or an “inactive” inflammatory state) following treatment with high-dose intravenous corticosteroids, eyes with active ocular inflammation, by type of ocular inflammation. MMP = mucous membrane pemphigoid.
Multivariate analysis (Table 2) indicated that factors predictive of achieving near-complete or complete control inflammation were white race (adjusted relative risk (RR) with respect to African-American race= 2.82, 95% CI: 1.31-6.1) and uveitis as opposed to scleritis, mucous membrane pemphigoid, or ‘other’ forms of inflammation (adjusted RR = 3.80, 95% CI: 1.51 - 9.5). Other factors assessed (age, sex, bilateral disease, and use of 1 gram of methylprednisolone daily for three days vs. lesser doses) were not associated with significant differences. None of the factors were significantly associated with achievement of complete control of inflammation, a less frequent outcome (with correspondingly less statistical power).
Table 2.
Factors associated with the complete or near complete control of inflammation
| Factors | #of eyes |
Univariate Cox Model |
Multivariate Cox Model† |
Final Multivariate Cox Model* |
|||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P-value | RR(95% CI) | P-value | RR (95% CI) | P-value | ||
| Age | |||||||
| <40 | 55 | 1.00 | 1.00 | ||||
| ≥40 | 49 | 0.59 (0.29 – 1.23) | 0.16 | 0.92 (0.35 – 2.38) | 0.85 | ||
| Sex | |||||||
| Male | 39 | 1.00 | 1.00 | ||||
| Female | 65 | 0.82 (0.44 – 1.53) | 0.62 | 0.89 (0.41 – 1.92) | 0.76 | ||
| Race | |||||||
| Black | 36 | 1.00 | 1.00 | 1.00 | |||
| White | 68 | 1.60 (0.79 – 3.28) | 0.19 | 3.25 (1.47 – 7.2) | 0.004 | 2.82 (1.31 – 6.1) | 0.008 |
| Uveitis Group | |||||||
| Other** | 35 | 1.00 | 1.00 | 1.00 | |||
| Uveitis | 69 | 2.34 (1.00 – 5.51) | 0.051 | 3.54 (0.93 – 13.5) | 0.06 | 3.80 (1.51- 9.5) | 0.004 |
| Bilateral | |||||||
| No | 7 | 1.00 | 1.00 | ||||
| Yes | 97 | 1.85 (0.61 – 5.56) | 0.28 | 2.67 (0.52 – 13.7) | 0.24 | ||
| Full dose *** | |||||||
| No | 42 | 1.00 | 1.00 | ||||
| Yes | 62 | 1.67 (0.74 – 3.79) | 0.22 | 1.12 (0.39 – 3.25) | 0.84 | ||
All the listed risk factors are in the same multivariate Cox model.
All the variables in the univariate analysis were included in the multivariate model, and multivariate model went through a backward selection process by only keeping the statistically significant factors in the final multivariate model.
Other forms of inflammation included scleritis (n=17), the cicatrizing conjunctivitis occurring with Mucous Membrane Pemphigoid (n=10), peripheral ulcerative keratitis (n=3), idiopathic orbital pseudotumor (n=2), optic nerve inflammation with associated vitreous cells (n=2), and autoimmune optic neuropathy (n=1)
Full dose=1 gram of methylprednisolone given intravenously daily for three days; lesser doses were those given for less than three days and/or lower amount given with each dose, the lowest dose included being a single intravenous infusion of 500 mg of methylprednisolone.
The cumulative probability of clinically significant improvement in anterior chamber cells, vitreous cells, and vitreous haze for active uveitis cases following intravenous corticosteroid therapy is given as Figure 5. Improvement in each of the three categories was seen for all these intraocular inflammatory signs: by one month, anterior chamber cells had improved in 84.5% (95% CI: 70.0-94.5%), whereas vitreous cells had improved in 48.5% (95% CI: 32.4-67.4%) and vitreous haze—for which less complete data were available—had improved in 19.1% (95% CI: 7.5-43.7%). With regards to subtypes of uveitis, 100% of eyes with anterior, intermediate, or posterior uveitis achieved significant improvement in anterior chamber cells by one month, and only 62% (95% CI: 40.1-84.8%) of eyes with panuveitis reached this same control milestone by one month. However, the times-to-improvement among these four uveitis subsets did not significantly differ (p=0.10).
Figure 5.
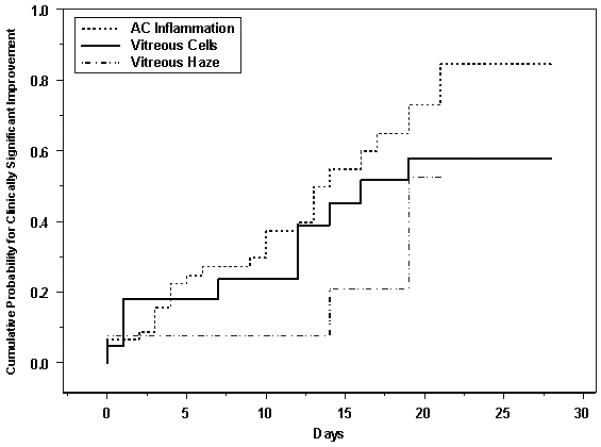
Cumulative probability for clinically significant improvement (improvement by at least two grades or to grade 0)[15], for anterior chamber cells, vitreous cells and vitreous haze—eyes with uveitis treated with high-dose intravenous corticosteroids. AC = anterior chamber.
Thirty-nine (55.7%) patients were using immunosuppressive drugs (antimetabolites, T-cell inhibitors, alkylating agents, or biologics) at the time they first received the intravenous pulse corticosteroid treatment. Use of immunosuppressive drugs along with intravenous corticosteroids was not associated with differences in either the time-to-complete control of inflammation (p=0.40), nor the time-to near-complete or complete control of inflammation (p=0.94). Statistical adjustment for the use of immunosuppressive drugs at the outset of intravenous corticosteroid therapy did not significantly or substantially modify the results described above (data not shown).
With regard to ocular inflammatory complications, there were no statistically significant differences between the day of initiation of treatment to one month thereafter in the proportion with ocular hypertension, hypotony, exudative retinal detachment, cystoid macular edema, or inflammatory lesions (Table 3), all of which were sufficiently rare that a very large change would have been required in order to observe a statistically significant difference. The percentage of eyes with visual acuity of 20/40 or better improved from 35% to 48% by one month following treatment (p=0.06).
Table 3.
Reversible Ocular Complications at the Initial Visit, 1 week and 1 montha
| Reversible Ocular Complications | Baseline | 1 week | 1 month | |||||
|---|---|---|---|---|---|---|---|---|
| # of eyes (N) | n (%) | N | n (%) | P-valueb | N | n (%) | P-valueb | |
| IOP ≥ 21 mmHg | 133 | 10 (7.5) | 83 | 7 (8.4) | 0.81 | 73 | 6 (8.2) | 0.86 |
| IOP ≥ 30 mmHg | 133 | 3 (2.3) | 83 | 1 (1.2) | 0.44 | 73 | 2 (2.7) | 0.81 |
| IOP < 5 mmHg | 133 | 3 (2.3) | 83 | 1 (1.2) | 0.46 | 73 | 1 (1.4) | 0.54 |
|
Active inflammatory chorioretinal
lesions c |
90 | 9 (10.0) | 54 | 1 (1.9) | 0.04 | 48 | 1 (2.1) | 0.1 |
| Snowbanking or snowballs c | 90 | 2 (2.2) | 54 | 5 (9.3) | 0.052 | 48 | 1 (2.1) | 0.64 |
| Cystoid macular edema c | 90 | 8 (8.9) | 54 | 3 (5.6) | 0.44 | 48 | 6 (12.5) | 0.45 |
| Exudative retinal detachment c | 90 | 12 (13.3) | 54 | 5 (9.3) | 0.41 | 48 | 4 (8.3) | 0.39 |
| 20/50 or worse | 133 | 86 (64.7) | 83 | 51 (62.0) | 0.51 | 73 | 38 (52.0) | 0.06 |
Not all cases had visits at the one week and one month time points, so the number “at risk” is given for each
As compared to the initial visit.
Restricted those eyes with uveitis.
One individual (1.4%, 95% Binomial Exact CI: 0.04%-7.7%) developed a colon perforation during the period of intravenous corticosteroid treatment (1000 mg of methylprednisolone daily, intended to be for three days); no other patients were observed to develop serious systemic complications of therapy during or shortly after treatment (expected fluctuations in blood glucose were not considered a serious complication). The colonic perforation required hospitalization, but resolved without requiring surgery.
Discussion
The results of this study suggest that high-dose intravenous “pulsed” corticosteroids typically result in improvement of ocular inflammation. Over half of actively inflamed eyes treated with this therapy were completely controlled (with complete abolition of inflammatory signs) within one month after initiation, and 82% improved to a very low level of inflammation or better. Although uveitis cases tended to respond most favorably, treatment response was favorable for all types of ocular inflammation studied, suggesting that treatment with intravenous corticosteroids is broadly effective.
In uveitic eyes, anterior chamber cells showed improvement by at least two grades—or to grade zero—in nearly all instances. Vitreous cells improved by at least two grades in approximately half of inflamed eyes, and vitreous haze—which was graded less often in our retrospective study—appeared to respond less briskly to intravenous corticosteroid treatment. These results are consistent with the hypothesis that clearance of inflammatory signs from the vitreous gel may take longer or require more treatment than clearance of inflammation from the aqueous in the anterior segment. The fact that the percentage of eyes with anterior uveitis showing such improvement in anterior chamber cells is greater than the percentage of those eyes with “complete control” reflects the fact that anterior chamber cells may substantially improve but not completely disappear with treatment, and that posterior segment inflammatory signs did not resolve as readily.
Intravenous corticosteroid dosages applied in this study did vary, with about two-thirds of eyes receiving less than the traditional dose suggested for active inflammatory disease (1000 mg of methylprednisolone per day for three consecutive days)8. When the results for eyes that received this dose were compared to eyes that received lower dosages (as low as a single dose of 500 mg of methylprednisolone), no substantial difference in outcomes was noted. It is unclear whether this observation reflects a selection bias (i.e., more aggressive diseases may have been selected for the higher dosages by the treating physician) or whether higher dosages are not necessary for treatment of ocular inflammation. A randomized study would be required to distinguish between the two alternative explanations.
The results of the study did not change when the data were adjusted for the use of concurrent immunosuppressive medications at the outset of treatment. Given that immunosuppressive therapy often takes months to contribute to control of inflammation17-21 it is not surprising that concurrent immunosuppressive use did not significantly alter patient outcomes. Furthermore, it is unlikely that in the first 30 days other therapies would have had greater benefits than intravenous corticosteroids, given that lesser corticosteroid treatments and non-steroidal anti-inflammatory drugs are less potent. While subsequent treatments may impact the outcome of therapy, they are part of the high dose intravenous corticosteroid therapy treatment strategy, and our goal was to study the strategy. Subsequent maintenance therapy is required to avoid rapid recurrence of inflammation after initial treatment.
With regard to ocular complications, the proportion of eyes with adverse findings was reduced across all categories with respect to baseline, though without reaching statistical significance. The tendency toward improvement is consistent with the observed benefits of therapy for ocular inflammation, but without statistically significant differences our data do not prove this conclusion; the number of eyes with complications at the outset of therapy was small, limiting the power of our analysis to address this question. However, it is noteworthy that a trend towards ocular hypertension as a potential corticosteroid-related response to systemic treatment was not observed.
One major systemic complication was recorded during the treatment period. Colonic perforation has been reported as a rare complication of corticosteroid therapy22. Other gastrointestinal side effects have been attributed to corticosteroids in earlier studies18 and were seen in the Optic Neuritis Treatment Trial (ONTT)23, though larger meta-analyses suggest that apparent increases in peptic ulcer disease with high dose corticosteroids in fact reflect confounding from concurrent nonsteroidal anti-inflammatory medication24. Albeit uncommon, the risk of serious gastrointestinal side effects and related complications is a concern with the use of corticosteroid therapy for ocular inflammation, and theoretically could be more frequent with the “pulse” intravenous approach than with usual dose oral corticosteroid therapy. Hyperglycemia also is a well-recognized side effect of systemic corticosteroid administration25. While it is important to remember the risk of hyperglycemia in selecting cases, it may be that a relatively quick burst of corticosteroids has less impact on blood sugar elevation in persons at risk6, which was the rationale for the clinician’s choice of intravenous corticosteroid therapy for select diabetic patients. Several additional potential complications of corticosteroid therapy were not observed in our group of patients, including those seen in the ONTT (acute pancreatitis and depression requiring psychotropic medication). While newer local therapies for non-infectious uveitis now are available that may lessen systemic side effects, our results suggest that systemic side effects of the intravenous corticosteroid treatment approach are infrequent. Local treatments (e.g. implants) have not been proposed for non-uveitic ocular inflammation, and in uveitis cases it may be undesirable to place implants until the inflammation has been quieted, for which intravenous corticosteroids may be required in severe cases.
Limitations of this study arise from its retrospective design. For instance, it is likely that only the most severe cases of ocular inflammation were treated using high-dose, intravenous corticosteroids, and therefore the benefits reported here may be underestimated; had less severe cases been treated, more impressive outcomes may have been observed. Also, it is likely that only patients for whom the treatment was judged to be safe would have been selected for therapy; a broader group of recipients (e.g., diabetic persons) potentially could have a higher risk of systemic complications of therapy. Third, the study analyzed response of a heterogeneous group of ocular inflammatory diseases. While sensitivity analysis of the subset with uveitis suggested that results tended to be better than the other groups, it is possible that the less common ocular inflammatory subsets could vary in their responsiveness to treatment by a degree not detectable with the available study power. Relative strengths of the study include a substantially larger sample size than previously has been available, providing reasonable precision for estimates of the effectiveness and risks of the treatment approach. The study was not able to compare the effect of “pulsed” intravenous corticosteroids to oral corticosteroids due to potential indications-for-treatment bias; such a comparison would be made ideally using a randomized clinical trial, a methodology that logistically would be very difficult to implement.
In summary, the strategy of treating ocular inflammation with high dose “pulsed” intravenous corticosteroids (e.g., 500-1000 mg of methylprednisolone for 1-3 days) followed by ongoing anti-inflammatory therapy as indicated resulted in substantial improvement for the large majority of cases with active ocular inflammation. Ocular complications of therapy were not observed and systemic complications were rare. These results provide evidence supporting the use of high dose intravenous corticosteroid treatment for severe ocular inflammation, which previously has been studied only in small cohorts or specific rare disease entities. Further experience or a randomized study—if it could be conducted—would be valuable to more completely investigate the benefits and risks of high-dose intravenous corticosteroids for ocular inflammation.
Acknowledgements
a) Funding/Support:
This study was supported primarily by National Eye Institute (Bethesda, MD) Grant EY014943 (Dr. Kempen). Additional support was provided by Research to Prevent Blindness (New York, NY) and the Paul and Evanina Mackall Foundation (New York, NY). Dr Kempen is a Research to Prevent Blindness James S. Adams Special Scholar Award recipient. Drs. Jabs and Rosenbaum were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the conduct of the study. Dr. Thorne is a Research to Prevent Blindness Harrington Special Scholar Award recipient. Dr. Levy-Clarke was previously supported by and Dr. Nussenblatt continues to be supported by intramural funds of the National Eye Institute. Dr. Suhler also received support from the Unites States Veterans’ Administration. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; nor in the preparation, review, and approval of this manuscript.
b) Financial Disclosures:
C. Stephen Foster: (O) Eyegate, (C, L) Allergan; (C, L) Bausch & Lomb; (C) Sirion; (L) Alcon; (L) Inspire; (L) Ista; (L) Centocor;
Douglas A. Jabs: (C) Abbot Laboratories; (C) Alcon; (C) Roche; (C) Genzyme Corporation; (C) Novartis; (C) Allergan; (C) GlaxoSmithKline; (C) Applied Genetic Technologies Corporation; (C) The Johns Hopkins Dana Center for Preventive Ophthalmology;
John H. Kempen: (C) Alcon; (C) Lux Biosciences; (C) Sanofi-Pasteur
James Rosenbaum: (O) Amgen, (C) Abbott; (C) ESBATech, (C) Lux Biosciences, (C) Centocor, (C) Genentech.
Footnotes
Meeting Presentation: The Association for Research and Vision in Ophthalmology Annual Meeting, May 2010.
c) Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
d) This research has been approved by the Institutional Review Boards of all participating centers.
References
- 1.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004 Jul-Aug;218(4):223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990 Oct;14(5-6):303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 3.ten Doesschate J. Causes of blindness in The Netherlands. Doc Ophthalmol. 1982 Jan 29;52(3-4):279–285. doi: 10.1007/BF01675857. [DOI] [PubMed] [Google Scholar]
- 4.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000 Oct;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 5.Wakefield D. Methylprednisolone pulse therapy in severe anterior uveitis. Aust N Z J Ophthalmol. 1985 Nov;13(4):411–415. doi: 10.1111/j.1442-9071.1985.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 6.Wakefield D, McCluskey P, Penny R. Intravenous pulse methylprednisolone therapy in severe inflammatory eye disease. Arch Ophthalmol. 1986 Jun;104(6):847–851. doi: 10.1001/archopht.1986.01050180081035. [DOI] [PubMed] [Google Scholar]
- 7.Werth VP. Treatment of pemphigus vulgaris with brief, high-dose intravenous glucocorticoids. Arch Dermatol. 1996 Dec;132(12):1435–1439. [PubMed] [Google Scholar]
- 8.Roujeau JC. Pulse glucocorticoid therapy. The ‘big shot’ revisited. Arch Dermatol. 1996 Dec;132(12):1499–1502. [PubMed] [Google Scholar]
- 9.Danao T, Segal AM. Pulsed suppressive treatment in rheumatoid arthritis: intravenous methylprednisolone and nitrogen mustard. J Rheumatol. 1990 Jul;17(7):893–899. [PubMed] [Google Scholar]
- 10.Buttgereit F, Straub RH, Wehling M, Burmester GR. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum. 2004 Nov;50(11):3408–3417. doi: 10.1002/art.20583. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka E, Ohguro N, Yamamoto S, Nakagawa Y, Imoto Y, Tano Y. Evaluation of pulse corticosteroid therapy for vogt-koyanagi-harada disease assessed by optical coherence tomography. Am J Ophthalmol. 2002 Sep;134(3):454–456. doi: 10.1016/s0002-9394(02)01575-1. [DOI] [PubMed] [Google Scholar]
- 12.Reed JB, Morse LS, Schwab IR. High-dose intravenous pulse methylprednisolone hemisuccinate in acute Behcet retinitis. Am J Ophthalmol. 1998 Mar;125(3):409–411. doi: 10.1016/s0002-9394(99)80163-9. [DOI] [PubMed] [Google Scholar]
- 13.Markomichelakis NN, Halkiadakis I, Papaeythymiou-Orchan S, Giannakopoulos N, Ekonomopoulos N, Kouris T. Intravenous pulse methylprednisolone therapy for acute treatment of serpiginous choroiditis. Ocul Immunol Inflamm. 2006 Feb;14(1):29–33. doi: 10.1080/09273940500227192. [DOI] [PubMed] [Google Scholar]
- 14.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008 Jan-Feb;15(1):47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 15.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005 Sep;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84(408):1065–1073. [Google Scholar]
- 17.Pujari SS, Kempen JH, Newcomb CW, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010 Feb;117(2):356–365. doi: 10.1016/j.ophtha.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kacmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010 Mar;117(3):576–584. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel E, Thorne JE, Newcomb CW, et al. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010 Mar;149(3):423–432. e421–422. doi: 10.1016/j.ajo.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009 Nov;116(11):2188–2198. e2181. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009 Oct;148(4):500–509. e502. doi: 10.1016/j.ajo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warshaw AL, Welch JP, Ottinger LW. Acute perforation of the colon associated with chronic corticosteroid therapy. Am J Surg. 1976 Apr;131(4):442–446. doi: 10.1016/0002-9610(76)90154-9. [DOI] [PubMed] [Google Scholar]
- 23.Beck RW, Cleary PA, Anderson MM, Jr., et al. The Optic Neuritis Study Group A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992 Feb 27;326(9):581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 24.Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991 May 1;114(9):735–740. doi: 10.7326/0003-4819-114-9-735. [DOI] [PubMed] [Google Scholar]
- 25.Chan JC, Cockram CS, Critchley JA. Drug-induced disorders of glucose metabolism. Mechanisms and management. Drug Saf. 1996 Aug;15(2):135–157. doi: 10.2165/00002018-199615020-00005. [DOI] [PubMed] [Google Scholar]


