Summary
Malignant gliomas are hypervascular tumors that are highly resistant to all the currently available multimodal treatments. Therefore, anti-angiogenic therapies targeting VEGF or VEGF receptors (VEGFRs) were designed and thought to be an effective tool for controlling the growth of malignant gliomas. However, recent results of early clinical trials using humanized monoclonal antibodies against VEGF (Bevacizumab), as well as small-molecule tyrosine kinase inhibitors that target different VEGF receptors (VEGFRs) (Vetalanib, Vandetanib, Sunitinib, Sorafenib, etc) alone or in combination with other therapeutic agents demonstrated differing outcomes, with the majority of reports indicating that glioma developed resistance to the employed anti-angiogenic treatments. It has been noted that continued anti-angiogenic therapy targeting only the VEGF-VEGFR system might affect pro-angiogenic factors other than VEGF, such as basic fibroblast growth factor (bFGF), stromal derived factor 1 (SDF-1) and Tie-2. These factors may in turn stimulate angiogenesis by mobilizing bone marrow derived precursor cells, such as endothelial progenitor cells (EPCs), which are known to promote angiogenesis and vasculogenesis. In this short review, the current antiangiogenic treatments, possible mechanisms of activation of alternative pathways of angiogenesis, and possible involvement of bone marrow derived progenitor cells in the failure of anti-angiogenic treatments are discussed.
Keywords: Glioblastoma, anti-angiogenic treatments, Chimeric mouse model, Optical imaging, Stem cell tracking
Introduction
Anti-angiogenic treatments are being utilized to control different malignant tumors including glioblastoma (GBM) as a second line of defense when traditional treatments using chemotherapy, radiation therapy or surgery fail to control the primary as well as recurrent tumors. Most of the anti-angiogenic agents that are under clinical trails target VEGF-VEGFR interactions. However, recent findings indicate that the effects of antiangiogenic treatments are transient and the tumors become refractory and more aggressive following such treatments. The hypothesis behind the refractoriness of the tumors is that the tumors initiate the alternative pathways of angiogenesis and may initiate the mobilization of bone marrow derived progenitor cells that are involved in making blood vessels by vasculogenesis. In this short review, the current antiangiogenic treatments, possible mechanisms of activation of alternative pathways of angiogenesis, and possible involvement of bone marrow derived progenitor cells in the failure of antiangiogenic treatments are discussed.
Challenges in glioma treatment
Glioblastoma (GBM) are hypervascular tumors that are highly resistant to all the currently available multimodal treatments. GBM are among the most devastating tumors, with survival of only one to three years after diagnosis even with the best of treatments (Remer and Murphy, 2004;Dhermain et al., 2005). Surgery and radiation therapy (followed by adjuvant chemotherapy), which form the standard practice, very often fail because of uncertainty in delineating the margin of the tumor (Dhermain et al., 2005). Although standard therapies can prolong a patient’s duration of survival, the median survival time for patients with recurrent GBM is usually less than 1 year (Chang et al., 2006). Animal studies have indicated that angiogenesis and increased vascular permeability are essential for the survival and proliferation of glioma cells (Goldbrunner et al., 2004). On the other hand, GBM is characterized by the release of vascular endothelial growth factor (VEGF), an important regulator and promoter of angiogenesis (Norden et al., 2008c) and vascular permeability. Therefore, anti-angiogenic therapies targeting VEGF or VEGF receptors (VEGFRs) were designed and thought to be an effective tool for controlling the growth of malignant gliomas. However, recent results of early clinical trials using humanized monoclonal antibodies against VEGF (Bevacizumab), as well as small-molecule tyrosine kinase inhibitors that target different VEGF receptors (VEGFRs) (Vetalanib, Vandetanib, Sunitinib, Sorafenib, etc) alone or in combination with other therapeutic agents (Los et al., 2007;Dietrich et al., 2008;Norden et al., 2008b,c) demonstrated differing outcomes, with the majority of reports indicating that glioma developed resistance to the employed anti-angiogenic treatments (Verhoeff et al., 2009;Yamanaka and Saya, 2009;Ahluwalia and Gladson, 2010;Sierra et al., 2010). It has been noted that continued anti-angiogenic therapy targeting only the VEGF-VEGFR system might affect pro-angiogenic factors other than VEGF, such as basic fibroblast growth factor (bFGF), stromal derived factor 1 (SDF-1) and Tie-2 (Norden et al., 2008b). But these factors may in turn stimulate angiogenesis by mobilizing bone marrow derived precursor cells- such as endothelial progenitor cells (EPCs)- which are known to promote angiogenesis and vasculogenesis (Batchelor et al., 2007;Kerbel, 2008;Norden et al., 2008b;Kioi et al., 2010).
Mechanism of neovascularization
The formation of blood vessels occurs by two mechanisms: vasculogenesis and angiogenesis. Vasculogenesis is the process where blood vessels are formed de novo by in situ differentiation of the primitive progenitors- i.e. angioblasts- into mature endothelial cells, which was thought to only take place during embryonic development (Risau and Flamme, 1995). In contrast, angiogenesis occurs both during the embryonic development and the postnatal life and is defined as a process that gives rise to new blood vessels by proliferation and migration of preexisting, differentiated endothelial cells (Folkman and Shing, 1992;Folkman, 1995). It was generally considered that blood vessel formation during postnatal life is restricted to angiogenesis only, and for decades tumor vascularization was thought to be the exclusive result of the sprouting of new vessels from the preexisting ones. However, recent studies demonstrated the existence of additional angiogenic and vasculogenic mechanisms associated with tumor growth, such as intussusceptive angiogenesis, vessel cooption, vasculogenic mimicry, lymphangiogenesis, and the recruitment of endothelial progenitor cells (Dome et al., 2007;Hillen and Griffioen, 2007;Folkins et al., 2009;El Hallani et al., 2010a;Patenaude et al., 2010;Yu et al., 2010). In most cases, these mechanisms take place concomitantly and are the potential targets for novel antiangiogenic/antitumor therapeutic strategies.
The idea of tumor cell derived vascular channel formation was first put forward by Maniotis and his group (Maniotis et al., 1999). The investigators have proved that vascular mimicry is wide spread and can be found in different tumor types, such as melanoma, hepatocellular carcinoma, and GBM (Folberg et al., 2000;Folberg and Maniotis, 2004;Guzman et al., 2007;El Hallani et al., 2010a). The investigators demonstrated that GBM stem-cell-like cells expressed pro-vascular molecules and allowing them to form blood vessels de novo (El Hallani et al., 2010b). Very recently, Soda and his colleagues (Soda et al., 2011) demonstrated that tumor derived endothelial cells originated from the transplanted GBM but not from the fusion of host endothelial cells. The investigators suggested that hypoxia is the important factor for the transdifferentiation of GBM to endothelial cells and the process was independent of VEGF (Soda et al., 2011). Now the question is how can we target these transdifferntiated endothelial cells derived from GBM, as these cells are devoid of VEGF receptors, and receptor tyrosin kinase inhibitors may not have effect during anti-angiogenic treatments (Hormigo et al., 2011;Soda et al., 2011)?
Correlation among angiogenesis/vasculogenesis, angiogenic factors and EPCs
Growth and metastasis of tumors usually depend on angiogenesis, the formation of new blood vessels. Chemokines released by tumor cells promote activation, proliferation and migration of endothelial cells (ECs) to the tumor tissue (Samejima and Yamazaki, 1988;Shi et al., 1998;Ellis et al., 2001;Liekens et al., 2001), allowing for rapid formation of functional neovasculatures. Circulating endothelial cells contributing to tumor angiogenesis can originate from the sprouting and co-option of neighboring pre-existing vessels (Hotfilder et al., 1997;Tomanek and Schatteman, 2000;Zhang et al., 2002). Additionally, tumor angiogenesis may also be supported by the mobilization and functional incorporation of bone-marrow derived EPCs - supporting the growth of xenografted lymphoma, lung cancer and other tumors (Asahara et al., 1997;Lyden et al., 2001;Jiang et al., 2002;Rafii et al., 2002;Reyes et al., 2002). EPCs have been detected in the circulation of cancer patients and lymphoma-bearing mice. When infused into immune compromised mice, EPCs were incorporated into the vasculature of xenotransplanted tumors and were correlated to tumor volume and production of vascular endothelial growth factor (VEGF) (Mancuso et al., 2003;Beerepoot et al., 2004). As reported by our group and other investigators, hypoxia induced SDF-1 has been detected as one of the potent chemo-attractants for the migration and incorporation of bone marrow derived EPCs due to the presence of CXCR4 receptors in these cells (Ceradini et al., 2004;Jin et al., 2006;Arbab et al., 2008). Moreover, we have also reported the role of inflammatory cytokine RANTES, which also act as chemo attractant for these EPCs (Silverman et al., 2007). In a very recent article, Kioi et al (Kioi et al., 2010) have shown the importance of vasculogenesis in the survival of glioma cells following radiation in an orthotopic glioma model. The authors pointed out the involvement of SDF-1-CXCR4 interaction for the vasculogenesis.
Anti-angiogenic treatment and current challenges
Because of the hypervascular nature of glioblastoma and active angiogenesis, investigators have added anti-angiogenic treatment as an adjuvant to normalize blood vessels and control abnormal angiogenesis (Los et al., 2007;Dietrich et al., 2008;Norden et al., 2008b,c). Different targets have been selected to control abnormal angiogenesis. Cilingitide has been used to target α(v)β(3) and α(v)β(5) integrins, which are both highly expressed in some glioma cells and on the endothelial lining of blood vessels (Reardon et al., 2008). Results of early clinical trials are promising. Bevacizumab, a humanized monoclonal antibody against VEGF, is in the process of a clinical trial. Bevacizumab is commonly combined with cytotoxic chemotherapy and results in dramatic improvement on radiographic images, prolongation of progression free survival, and less need for corticosteroids (Norden et al., 2008c). However, prolonged use of bevacizumab or stopping anti-angiogenic treatment resulted in deteriorated clinical outcome for the treatments of glioma patients (Dietrich et al., 2008;Norden et al., 2008c). Agents that interfere the VEGF-VEGFR signal transduction pathway, such as vetanalib, cediranib, sunitinib, etc., have been used in clinical trails with varying degrees of success (Norden et al., 2008a, b). Evidence of relapse to progressive tumor growth following treatment reflects development of resistance to antiangiogenic therapies (Bergers and Hanahan, 2008). These receptors blockers were originally used for colorectal carcinoma and then later used in glioma patients as an adjuvant to the established treatments. Initial clinical trials showed remarkable improvement on MRI images with respect to tumor size and vascular permeability (Norden et al., 2008b). However, there are reports showing extension of abnormal high signal intensity areas on T2-weighted images away from the tumor mass and it is thought to be due to invasive tumor mass (cells) (Norden et al., 2008b, a, c;Verhoeff et al., 2009;Gerstner et al., 2010;Wong and Brem, 2011).
Recent studies suggest that inhibition of angiogenesis is even a driving force for tumor conversion to a greater malignancy, reflected in heightened invasion and dissemination into surrounding tissues and, in some cases, increased lymphatic and metastatic activities (Saidi et al., 2008). In GBM, antiangiogenic therapy induced a phenotypic change from single-cell infiltration to migration of cell clusters along normal blood vessels (Pàez-Ribes et al., 2009;de Groot et al., 2010;Rahman et al., 2010). This suggests that the invasive growth program induced in response to antiangiogenic therapy may be qualitatively different than the pathway used in the usual tumor progression. Thus, an elucidation of the mechanisms of resistance to antiangiogenic therapy is of critical importance to the use of this potentially useful therapy in order to prevent its failure.
Prolonged treatment with these receptor blockers also impacted negatively on the outcome of the treatment. Antiangiogenic therapy disturbs tumor vasculature, leading to marked hypoxia. One possible mechanism for resistance to antiangiogenic therapy might be the activation of alternative angiogenesis signaling pathways, such as basic fibroblast growth factor (bFGF), Tie-2, stromal-cell derived factor-1α (SDF-1α), and increased VEGF production leading to increased invasiveness of the tumor cells (Batchelor et al., 2007;Kerbel, 2008;Norden et al., 2008b). A second additional and distinct potential mechanism of resistance might be the recruitment of endothelial progenitor cells (EPCs) and pro-angiogenic monocytes from the bone marrow. Hypoxia creates conditions permissive for the recruitment of a heterogeneous population of bone marrow-derived monocytic cells that promotes angiogenesis and growth. Thus, in many cases, the inhibitory therapy targeting VEGF and/or VEGFRs may end up enhancing a paradoxical and unwanted angiogenic and pro-growth response. In GBM, hypoxia leads to up-regulation of hypoxia inducible factor-1α (HIF-1α). HIF-1α up-regulates SDF-1α, which in turn may recruit various pro-angiogenic bone marrow-derived cells. Activation of this pathway provides a mechanistic rationale for how hypoxia can promote tumor resistance to anti-VEGF therapy. SDF-1α is one of the potent chemoattractants for bone marrow derived EPCs due to the presence of CXCR4 receptors in these cells (Jin et al., 2006;Arbab et al., 2008). Any therapy that invites more EPCs might promote neovascularization and pro growth, a paradoxical effect of antiangiogenic therapy.
Our recent work in rat orthotopic human glioma model showed paradoxically increased production of VEGF at the peripheral part of tumors, as well as the elevated expression of HIF-1α and SDF-1, and a significant increase in the number of dilated blood vessels in animals that underwent two weeks of PTK787 (small molecule tyrosine kinase inhibitor) treatment (Ali et al., 2010) (Figure 1). In addition, as reported by our group and other investigators, SDF-1 is one of the potent chemo attractant for bone marrow derived endothelial progenitor cells due to the presence of CXCR4 receptors in these cells (Jin et al., 2006;Arbab et al., 2008) and may be involved in enhanced angiogenesis and invasiveness of the tumor following treatment with VEGFRs inhibitors. Moreover, we have also reported the role of inflammatory cytokine RANTES, which also act as a chemo attractant for these cells (Silverman et al., 2007;Janic et al., 2010). Any therapy may cause enhanced inflammation in the tumor sites and invite more endothelial progenitor cells and possible angiogenesis.
Figure 1.
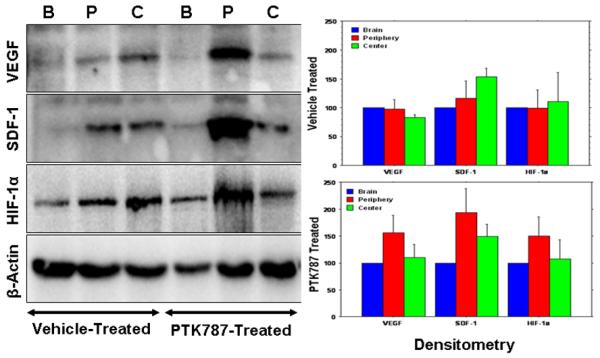
Expression of different angiogenic factors (left panel) in the vehicle- and PTK787-treated tumors from representative cases at the peripheral (P), central part of the tumors (C) and contralateral brains (B). Note the increased expression of VEGF, SDF-1α and HIF-1α at the peripheral part of PTK787 treated tumors. Right panel shows the densitometry analysis of the blot (%β-actin and normalized to contralateral brain). The analysis also confirmed the finding of the blot. Note the patterns of VEGF, SDF-1α and HIF-1α in PTK787 treated tumors.
Therefore, it is important to gain insight into the possible mechanisms that are activated during anti-angiogenic treatment to understand and potentially prevent its failure. It is of extreme importance to document these changes in vivo and develop a novel therapeutic approach for overcoming these paradoxic effects.
Current challenge in preventing vasculogenesis
Current evidences from recent publications indicate the involvement of both angiogenesis and vasculogenesis processes for glioma growth (tumor growth) (Dome et al., 2007;Folkins et al., 2009;Yu et al., 2010). As discussed above, most of the agents that target neovascularization are in fact against angiogenesis. With emerging new insights into vasculogenesis, investigators are looking into possible mechanisms for how bone marrow derived progenitor cells or EPCs migrate and incorporate into tumor neovascularization (Patenaude et al., 2010). One of the mechanisms that has been pointed out is the involvement of SDF-1-CXCR4 axis (Jin et al., 2006;Petit et al., 2007;Shichinohe et al., 2007). SDF-1 is a chemokine that is expressed in tumor cells and released in the circulation following hypoxia in the tumor (with the up regulation of HIF-1α) (Moore et al., 2001;Ceradini et al., 2004;Arbab et al., 2008). In an experiment, Heissig et al (Heissig et al., 2002) determined the mechanisms of releasing hematopoietic stem cells (HSC) and EPCs from bone marrow. Under steady-state conditions, quiescent c-Kit+ HSCs or EPCs reside in a niche in close contact with stromal cells. Membrane-bound cytokines, such as mKitL not only convey survival signals, but also support the adhesion of stem cells to the stroma. Increased chemokine/cytokine such as SDF-1 and VEGF induce up-regulation of MMP-9 resulting in the release of sKitL (soluble Kit ligand). sKitL confers signals that transfer c-Kit+ HSCs or EPCs from quiescent to proliferative state and enhances mobility of VEGFR2+ EPCs and Lin-Sca-1+c-Kit+ repopulating cells, translocating them into a vascular-enriched niche favoring differentiation and mobilization to the peripheral circulation. SDF-1 is a strong chemo-attractant for CXCR4 positive cells. Preventing interaction of SDF-1-CXCR4 is thought to be a mechanism to block vasculogenesis. AMD3100, a receptor (CXCR4) antagonist was initially developed as anti HIV drug and later used to mobilize CD34+ HSCs cells to the peripheral circulation (Petit et al., 2007). Although AMD3100 increased the number of peripheral CD34+ or progenitor cells, the recent investigations pointed out that continuous treatment with AMD3100 or similar CXCR4 receptor antagonists inhibit vasculogenesis in tumors causing inhibition of tumor growth (Petit et al., 2007;Kioi et al., 2010). Similarly anti SDF-1α antibody or siRNA techniques can be used to block the release of SDF-1α and interaction for the mobilization of bone marrow progenitor cells responsible for vasculogenesis. In vivo imaging that shows the effect of chemokine receptor antagonist on the tumor growth and vasculogenesis will be very helpful for future clinical trials.
Role of in vivo imaging in the detection of angiogenesis/vasculogenesis
DCE MRI or perfusion CT has been used to determine the vascular permeability and tumor blood volume, which are the indirect measurements of neovascularization and total vascular densities (Taylor et al., 1999;Hoang et al., 2004;Preda et al., 2006;Provenzale et al., 2006;Raatschen et al., 2009). However, DCE MRI or perfusion CT cannot predict the involvement of bone marrow progenitor cells (BMPC) in the neovascularization processes in tumors before or after anti-angiogenic treatments. There has been no in vivo imaging modality capable of detecting migration and incorporation of host BMPC to the sites of angiogenesis. Investigators have shown the migration and accumulation of host BMPC or EPC to the implanted tumor or in different cancers or lesions by in vitro studies (Asahara et al., 1997;Asahara et al., 1999;Yu et al., 2010). Involvement of stem cells in the formation of tumor vasculatures has been determined by in vivo imaging modalities, however, the stem cells in questions were manipulated ex vivo then administered in animal models (Anderson et al., 2005;Arbab et al., 2006). Different imaging modalities are used to track the migration and incorporation of administered cells to the sites of tumors (Brenner et al., 2004;Arbab and Frank, 2008;Mani et al., 2008). We have pioneered the technique to track administered EPCs to the site of tumor angiogenesis/vasculogenesis by in vivo cellular MRI (Arbab et al., 2006;Arbab et al., 2008). We have also used nuclear medicine technique to track the migration and accumulation of administered stem cells to the sites of tumors (Figure 2).
Figure 2. Migration and accumulation of administered EPCs in PTK treated tumor.
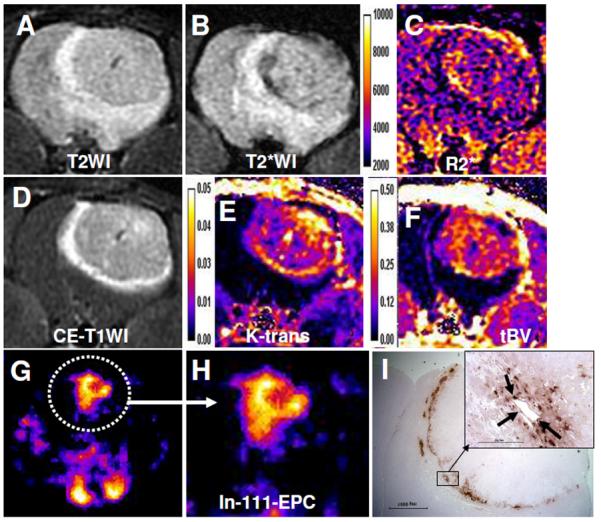
Five million In-111 labeled EPCs followed by 5 million magnetically labeled EPCs were administered in the same rat. SPECT images were obtained on day 0, 1 3 and 7. MRI was obtained by a clinical 3T system on day 7 following last SPECT. (A) T2WI with a TE of 35ms, (B) T2*WI with a TE of 20 and corresponding R2* map (C). (D) Contrast enhanced T1WI and corresponding Ktrans (E) and tumor blood volume (F) maps. Note the low signal intensity areas on T2*WI (B, black arrows) and corresponding R2* map (C, yellow arrows) indicating accumulation of iron positive cells, which is proved by DAB enhanced Prussian blue staining (I). SPECT images of the tumor (G & H) obtained at 24 hours showed increased activity at the site of tumor indicating accumulation of In-111 labeled EPCs. DAB enhanced Prussian blue staining showed accumulation of iron positive cells around the tumor (I). Note a few of the iron positive cells also make the lining of blood vessels (inset, black arrows).
In vivo determination of the involvement of host BMPC in the formation of neovascularization in tumors is challenging. To be detected by in vivo imaging modalities, the host bone marrow cells should carry a reporter, such as fluorescent protein or genes that can be targeted later (such as luciferase or sodium iodide symporter or specific promoter mediated activation). However, this reporter should only be present in BMPC but not in other cell types. Making of transgenic animal model having such conditional gene expression would be difficult. The alternate way would be to make chimeric animal models, where bone marrow cells of recipient animals should be replaced with bone marrow cells from animals expressing different reporters, such as GFP+ bone marrow cells (Sengupta et al., 2003;Sheikh et al., 2007;Yu et al., 2010). Recently a chimeric animal model has been developed in our laboratory to determine the involvement of BMPC in the tumor neovascularization (Figure 3). Sub-lethally irradiated athymic mice received bone marrow cells from green fluorescent protein positive (GFP+) transgenic mice (Schaefer et al., 2001). GFP+ bone marrow cells were transplanted in athymic mice 24 hours following sub-lethal irradiation and the tumors were implanted after 28 days when flowcytometric analysis showed more than 70% engraftment of GFP+ cells. Migration and accumulation of transplanted bone marrow cells in the implanted breast cancer were determined by optical imaging (Kodak, Carestream multi-spectral system, Carestream, USA) with proper excitation and emission profiles. Optical imaging showed gradual increase in GFP intensity in the tumors and multiple GFP+ cells lining the blood vessels and other infrastructures of the tumors were observed under fluorescent microscope. Our ongoing studies using different antiangiogenic agents will determine the involvement of bone marrow derived progenitor cells in the development of resistance to anti-angiogenic treatments, and development of recurrence and distal metastases of tumors
Figure 3. (Upper panel).
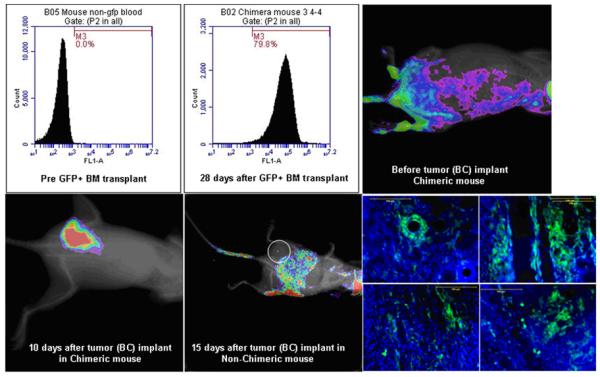
Engraftment of transplanted GFP+ cells in athymic mouse. Analysis of peripheral blood show about 80% GFP+ cells in chimeirc mouse (middle), whereas non-chimeric athymic mouse show no GFP+ cells (left). Optical imaging show fluorescent intensity in the area of pelvic bones (right).
(Lower panel): Optical imaging of implanted breast cancer (10 days old) in chimeric mouse (left) and in non-chimeric mouse (middle, white circle, 15 days old). Optical imaging show increased fluorescent intensity at the site of tumor in chimeric mouse, which is confirmed to be GFP+ cells (right) detected by fluorescent microscope. Distribution of GFP+ cells are all over the tumor. Lining of vascular structures is seen to be from GFP+ cells. The fluorescent intensity at the center of non-chimeric mouse is due to activity in the intestine, which could be due to food intake.
Conclusions
In this short review possible mechanisms for the development of resistance to anti-angiogenic treatments in GBM are discussed. Activation of alternate angiogenic pathways and involvement of bone marrow derived progenitor cells could be the mechanisms for the resistance and in vivo imaging should be utilized or developed to determine the involvement of bone marrow cells in tumor vasculogenesis.
Acknowledgement
I like to thank Drs. Nadimpalli RS Varma and Asm Iskander for their help in immunohistochemistry and western blot. This work was funded by NIH grant R01CA122031
References
- Ahluwalia MS, Gladson CL. Progress on antiangiogenic therapy for patients with malignant glioma. J. Oncol. 2010;2010:689018. doi: 10.1155/2010/689018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MM, Janic B, Babajani-Feremi A, Varma NR, Iskander AS, Anagli J, Arbab AS. Changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in rat glioma. PLoS ONE. 2010;5:e8727. doi: 10.1371/journal.pone.0008727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Glod J, Arbab AS, Noel M, Ashari P, Fine HA, Frank JA. Noninvasive mr imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105:420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- Arbab AS, Frank JA. Cellular mri and its role in stem cell therapy. Regen. Med. 2008;3:199–215. doi: 10.2217/17460751.3.2.199. [DOI] [PubMed] [Google Scholar]
- Arbab AS, Pandit SD, Anderson SA, Yocum GT, Bur M, Frenkel V, Khuu HM, Read EJ, Frank JA. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells. 2006;24:671–678. doi: 10.1634/stemcells.2005-0017. [DOI] [PubMed] [Google Scholar]
- Arbab AS, Janic B, Knight RA, Anderson SA, Pawelczyk E, Rad AM, Read EJ, Pandit SD, Frank JA. Detection of migration of locally implanted ac133+ stem cells by cellular magnetic resonance imaging with histological findings. FASEB J. 2008;22:3234–3246. doi: 10.1096/fj.07-105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. Azd2171, a pan-vegf receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann. Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature Review Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner W, Aicher A, Eckey T, Massoudi S, Zuhayra M, Koehl U, Heeschen C, Kampen WU, Zeiher AM, Dimmeler S, Henze E. 111in-labeled cd34+ hematopoietic progenitor cells in a rat myocardial infarction model. J. Nucl. Med. 2004;45:512–518. [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through hif-1 induction of sdf-1. Nat. Med. 2004;10:858–864. doi: 10.1038/nm1075. Epub 2004 Jul 2004. [DOI] [PubMed] [Google Scholar]
- Chang SM, Butowski NA, Sneed PK, Garner IV. Standard treatment and experimental targeted drug therapy for recurrent glioblastoma multiforme. Neurosurg. Focus. 2006;20:E4. [PubMed] [Google Scholar]
- de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: Radiographic and pathologic correlation in humans and mice. Neuro-Oncology. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhermain F, Ducreux D, Bidault F, Bruna A, Parker F, Roujeau T, Beaudre A, Armand JP, Haie-Meder C. use of the functional imaging modalities in radiation therapy treatment planning in patients with glioblastoma. Bull Cancer. 2005;92:333–342. [PubMed] [Google Scholar]
- Dietrich J, Norden AD, Wen PY. Emerging antiangiogenic treatments for gliomas - efficacy and safety issues. Curr. Opin. Neurol. 2008;21:736–744. doi: 10.1097/WCO.0b013e3283131370. [DOI] [PubMed] [Google Scholar]
- Dome B, Hendrix MJC, Paku S, Tovari J, Timar J. Alternative vascularization mechanisms in cancer: Pathology and therapeutic implications. Am. J. Pathol. 2007;170:1–15. doi: 10.2353/ajpath.2007.060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas J-L, Eichmann A, Delattre J-Y, Maniotis AJ, Sanson M. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain. 2010a;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL, Eichmann A, Delattre JY, Maniotis AJ, Sanson M. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain. 2010b;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, Reinmuth N. Overview of angiogenesis: Biologic implications for antiangiogenic therapy. Semin. Oncol. 2001;28:94–104. doi: 10.1016/s0093-7754(01)90287-8. [DOI] [PubMed] [Google Scholar]
- Folberg R, Maniotis AJ. Vasculogenic mimicry. A.P.M.I.S. 2004;112:508–525. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. [DOI] [PubMed] [Google Scholar]
- Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am. J. Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Clinical applications of research on angiogenesis. N. Engl. J. Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. Journal of Biological Chemistry. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Gerstner ER, Frosch MP, Batchelor TT. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J. Clin. Oncol. 2010;28:e91–93. doi: 10.1200/JCO.2009.25.0233. [DOI] [PubMed] [Google Scholar]
- Goldbrunner RH, Bendszus M, Wood J, Kiderlen M, Sasaki M, Tonn JC. Ptk787/zk222584, an inhibitor of vascular endothelial growth factor receptor tyrosine kinases, decreases glioma growth and vascularization. Neurosurgery. 2004;55:426–432. doi: 10.1227/01.neu.0000129551.64651.74. discussion 432. [DOI] [PubMed] [Google Scholar]
- Guzman G, Cotler SJ, Lin AY, Maniotis AJ, Folberg R. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch. Pathol. Lab. Med. 2007;131:1776–1781. doi: 10.5858/2007-131-1776-apsovm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen F, Griffioen A. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer and Metastasis Reviews. 2007;26:489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang BH, Dyke JP, Koutcher JA, Huvos AG, Mizobuchi H, Mazza BA, Gorlick R, Healey JH. Vegf expression in osteosarcoma correlates with vascular permeability by dynamic mri. Clinical Orthopaedics & Related Research. 2004:32–38. doi: 10.1097/01.blo.0000141492.52166.20. [DOI] [PubMed] [Google Scholar]
- Hormigo A, Ding BS, Rafii S. A target for antiangiogenic therapy: Vascular endothelium derived from glioblastoma. Proc. Natl. Acad. Sci. U S A. 2011;108:4271–4272. doi: 10.1073/pnas.1019656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotfilder M, Nowak-Gottl U, Wolff JE. Tumorangiogenesis: A network of cytokines. Klin Padiatr. 1997;209:265–270. doi: 10.1055/s-2008-1043960. [DOI] [PubMed] [Google Scholar]
- Janic B, Guo AM, Iskander AS, Varma NR, Scicli AG, Arbab AS. Human cord blood-derived ac133+ progenitor cells preserve endothelial progenitor characteristics after long term in vitro expansion. PLoS ONE. 2010;5:e9173. doi: 10.1371/journal.pone.0009173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. Epub 2002 Jun 2020. [DOI] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of sdf-1 induces revascularization through recruitment of cxcr4(+) hemangiocytes. Nat. Med. 2006;12:557–567. doi: 10.1038/nm1400. Epub 2006 Apr 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liekens S, De Clercq E, Neyts J. Angiogenesis: Regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Los M, Roodhart JM, Voest EE. Target practice: Lessons from phase iii trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist. 2007;12:443–450. doi: 10.1634/theoncologist.12-4-443. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Medicine. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Calleri A, Cassi C, Gobbi A, Capillo M, Pruneri G, Martinelli G, Bertolini F. Circulating endothelial cells as a novel marker of angiogenesis. Adv. Exp. Med. Biol. 2003;522:83–97. doi: 10.1007/978-1-4615-0169-5_9. [DOI] [PubMed] [Google Scholar]
- Mani V, Adler E, Briley-Saebo KC, Bystrup A, Fuster V, Keller G, Fayad ZA. Serial in vivo positive contrast mri of iron oxide-labeled embryonic stem cell-derived cardiac precursor cells in a mouse model of myocardial infarction. Magn. Reson. Med. 2008;60:73–81. doi: 10.1002/mrm.21642. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of sdf-1, vegf, and angiopoietin-1. Annals of the New York Academy of Sciences. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45-37. [DOI] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY. Antiangiogenic therapy in malignant gliomas. Curr. Opin. Oncol. 2008a;20:652–661. doi: 10.1097/CCO.0b013e32831186ba. [DOI] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008b;7:1152–1160. doi: 10.1016/S1474-4422(08)70260-6. [DOI] [PubMed] [Google Scholar]
- Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology. 2008c;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude A, Parker J, Karsan A. Involvement of endothelial progenitor cells in tumor vascularization. Microvascular Research. 2010;79:217–223. doi: 10.1016/j.mvr.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Petit I, Jin D, Rafii S. The sdf-1-cxcr4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends in Immunology. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preda A, van Vliet M, Krestin GP, Brasch RC, van Dijke CF. Magnetic resonance macromolecular agents for monitoring tumor microvessels and angiogenesis inhibition. Invest. Radiol. 2006;41:325–331. doi: 10.1097/01.rli.0000186565.21375.88. [DOI] [PubMed] [Google Scholar]
- Provenzale JM, York G, Moya MG, Parks L, Choma M, Kealey S, Cole P, Serajuddin H. Correlation of relative permeability and relative cerebral blood volume in high-grade cerebral neoplasms. AJR Am. J. Roentgenol. 2006;187:1036–1042. doi: 10.2214/AJR.04.0676. [DOI] [PubMed] [Google Scholar]
- Raatschen HJ, Fu Y, Brasch RC, Pietsch H, Shames DM, Yeh BM. In vivo monitoring of angiogenesis inhibitory treatment effects by dynamic contrast-enhanced computed tomography in a xenograft tumor model. Invest. Radiol. 2009;44:265–270. doi: 10.1097/RLI.0b013e31819f1b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy? Nature Reviews. Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- Rahman R, Smith S, Rahman C, Grundy R. Antiangiogenic therapy and mechanisms of tumor resistance in malignant glioma. J. Oncol. 2010;2010:251231. doi: 10.1155/2010/251231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, Dropcho EJ, Wittemer SM, Nippgen J, Picard M, Nabors LB. Randomized phase ii study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J. Clin. Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- Remer S, Murphy ME. The challenges of long-term treatment outcomes in adults with malignant gliomas. Clin. J. Oncol. Nurs. 2004;8:368–376. doi: 10.1188/04.CJON.368-376. [DOI] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow.[see comment] Journal of Clinical Investigation. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annual Review of Cell and Developmental Biology. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Saidi A, Javerzat S, Bellahcène A, De Vos J, Bello L, Castronovo V, Deprez M, Loiseau H, Bikfalvi A, Hagedorn M. Experimental anti-angiogenesis causes upregulation of genes associated with poor survival in glioblastoma. International Journal of Cancer. 2008;122:2187–2198. doi: 10.1002/ijc.23313. [DOI] [PubMed] [Google Scholar]
- Samejima N, Yamazaki K. A study on the vascular proliferation in tissues around the tumor in breast cancer. Jpn. J. Surg. 1988;18:235–242. doi: 10.1007/BF02471439. [DOI] [PubMed] [Google Scholar]
- Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent cd8+ t-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Investigative Ophthalmology & Visual Science. 2003;44:4908–4913. doi: 10.1167/iovs.03-0342. [DOI] [PubMed] [Google Scholar]
- Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of sdf-1/cxcr4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138–147. doi: 10.1016/j.brainres.2007.08.091. [DOI] [PubMed] [Google Scholar]
- Sierra JR, Cepero V, Giordano S. Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol. Cancer. 2010;9:75. doi: 10.1186/1476-4598-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MD, Haas CS, Rad AM, Arbab AS, Koch AE. The role of vascular cell adhesion molecule 1/ very late activation antigen 4 in endothelial progenitor cell recruitment to rheumatoid arthritis synovium. Arthritis Rheum. 2007;56:1817–1826. doi: 10.1002/art.22706. [DOI] [PubMed] [Google Scholar]
- Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari S, Verma IM. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl. Acad. Sci. U S A. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JS, Tofts PS, Port R, Evelhoch JL, Knopp M, Reddick WE, Runge VM, Mayr N. Mr imaging of tumor microcirculation: Promise for the new millennium. J. Magn. Reson. Imaging. 1999;10:903–907. doi: 10.1002/(sici)1522-2586(199912)10:6<903::aid-jmri1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Schatteman GC. Angiogenesis: New insights and therapeutic potential. Anat. Rec. 2000;261:126–135. doi: 10.1002/1097-0185(20000615)261:3<126::AID-AR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ, Leenders WP, van Furth WR. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ET, Brem S. Taming glioblastoma by targeting angiogenesis: 3 years later. J. Clin. Oncol. 2011;29:124–126. doi: 10.1200/JCO.2010.32.5282. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Saya H. Molecularly targeted therapies for glioma. Ann. Neurol. 2009;66:717–729. doi: 10.1002/ana.21793. [DOI] [PubMed] [Google Scholar]
- Yu L, Su B, Hollomon M, Deng Y, Facchinetti V, Kleinerman ES. Vasculogenesis driven by bone marrow-derived cells is essential for growth of ewing’s sarcomas. Cancer Res. 2010;70:1334–1343. doi: 10.1158/0008-5472.CAN-09-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circulation Research. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]


