Abstract
Mercury is an environmental toxicant that can disrupt brain development. However, while progress has been made in defining its neurotoxic effects, we know far less about available therapies that can effectively protect brain in exposed individuals. We previously developed an animal model in which we defined the sequence of events underlying neurotoxicity: Methylmercury (MeHg) injection in postnatal rat acutely induced inhibition of mitosis and stimulated apoptosis in the hippocampus, that later resulted in intermediate term deficits in structure size and cell number. NAC is the N-acetyl derivative of L-cysteine used clinically for treatment of drug intoxication. Here, based on its known efficacy in promoting MeHg urinary excretion, we evaluated NAC for protective effects in the developing brain. In immature neurons and precursors MeHg (3µM) induced a >50% decrease in DNA synthesis at 24hr, an effect that was completely blocked by NAC co-incubation. In vivo, injection of MeHg (5µg/gbw) into 7 day-old rats induced a 22% decrease in DNA synthesis in whole hippocampus and a 4-fold increase in activated caspase-3 immunoreactive cells at 24hr, and reduced total cell numbers by 13% at 3 weeks. Treatment of MeHg exposed rats with repeated injections of NAC abolished MeHg toxicity. NAC prevented the reduction in DNA synthesis and the marked increase in caspase-3 immunoreactivity. Moreover, the intermediate term decrease in hippocampal cell number provoked by MeHg was fully blocked by NAC. Altogether, these results suggest that MeHg toxicity in the perinatal brain can be ameliorated by using NAC, opening potential avenues for therapeutic intervention.
Keywords: mercury, hippocampus, N-acetyl cysteine, neurogenesis, programmed cell death
INTRODUCTION
Methylmercury (MeHg) is an environmental toxicant that poses serious health risks in humans and especially children, whose brains are still developing and are therefore particularly vulnerable to exogenous toxicants (Adams et al. 2000; Stein et al., 2002; Spurgeon 2006). MeHg exposure results primarily from the consumption of contaminated food. MeHg is easily absorbed from the diet into the bloodstream and distributes to all tissues including the brain. Indeed, MeHg exhibits high mobility in the body, due to its ability to form thiol complexes with small molecules such as the amino acid cysteine (Clarkson et al. 2007). The kinetics of MeHg accumulation in the brain differs from that of peripheral organs, such as liver or kidney (Burbacher et al., 2005), raising the possibility that therapeutic interventions may be organ-specific. In the central nervous system, MeHg interferes with developmental processes, such as neurogenesis and cell survival, as demonstrated in both humans and animal models (Chang et al., 1977; Lapham et al. 1995; Newland et al. 2004; Burke et al. 2006; Falluel-Morel et al. 2007). Indeed, previous studies demonstrated that an acute MeHg exposure by subcutaneous injection in 7 day old (P7) rats induces cell cycle arrest and cell death of neuronal precursors in the dentate gyrus of the hippocampus (Burke et al. 2006; Falluel-Morel et al. 2007; Sokolowski et al., 2011). In humans, it is difficult to estimate the level of exposure in the fetus and in children in affected areas, and effective treatments for brain toxicity have yet to be defined. The cellular mechanisms underlying mercury neurotoxicity are not fully understood, although several studies now indicate that ROS production plays a central role (Falluel-Morel et al., 2007; Haase et al., 2011).
N-acetyl cysteine (NAC) is a compound used clinically for the treatment of drug intoxication, such as acetaminophen, and recent animal studies suggest it is a useful antidote for peripheral organ metal toxicity (Ballatori et al., 1998). In the adult, NAC reduced body MeHg levels by promoting rapid urinary excretion. In parallel, NAC can increase the reserves of the antioxidant glutathione in the body. Like its homologue glutathione, NAC contains a thiol group that confers antioxidant properties, potentially enhancing cellular resistance against reactive oxygen species (ROS). However, the utility of NAC as an antidote in the preweanling animal (<P21), whose brain is still immature and vulnerable, was unclear because during this period, NAC induced urinary excretion of only a small fraction of MeHg (Aremu et al., 2008). In turn, the effects of NAC on preweanling brain MeHg concentrations and neurotoxicity remain virtually unexplored.
In the present study, we investigated the effects of NAC in P7 rats exposed to a single MeHg injection, a model used successfully in previous studies (Burke et al. 2006; Falluel-Morel et al. 2007; Sokolowski et al., 2011). We employed this MeHg exposure paradigm (5µg/gbw) because it allows sequential observations of molecular mechanisms that acutely injure the brain and produce later deleterious effects on developing brain cell composition, structure and function. This approach has defined a cascade of detectable changes that now serve as biomarkers of neurotoxicity and therefore possible protection. It was hypothesized that if NAC successfully prevented MeHg induced neurotoxicity, it should counteract its acute cellular effects on DNA synthesis and caspase activation as well as prevent MeHg intermediate term effects on hippocampal development assessed on P21.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley rats with 4-day-old pups (P4) or pregnant rats (E14.5) were purchased from Hilltop Lab Animals (Philadelphia, PA). They were housed in a temperature- and light-controlled animal care facility and given food and water ad libitum. All animal procedures were approved by the Robert Wood Johnson Medical School institutional animal care and utilization committee and conformed to NIH Guidelines for animal use.
Exposure Models
Methylmercury chloride (CH3HgCl) was purchased from Sigma (St Louis, MO). A 1.5 mg/mL stock solution in 0.1M phosphate buffered saline (PBS) was prepared immediately before use and dissolved by agitation. Mercury administration and disposal procedures were approved by the Environmental and Occupational Health Sciences Institute (EOHSI) institutional committee. N-acetyl cysteine (NAC) was obtained from Sigma (St. Louis, MO). A 2 mg/ml stock solution was prepared in PBS. In vitro, stock solutions were dissolved in the culture medium to reach the final desired concentrations of MeHg (0.1 – 6 µM) and NAC (3 – 1,000 µM). In vivo, P7 rats were injected subcutaneously (sc) with vehicle or MeHg (5 µg/gbw) in a 100µL bolus. Animals were also injected intraperitoneally (IP) with vehicle or NAC (10 µg/gbw) every two hours during an 8 hour period. According to experimental needs, animals tissues were dissected and processed immediately, or frozen at −80 °C until assay.
Cortical Neuron and Precursor Culture
To obtain a relatively homogeneous neuronal population, the dorsolateral cerebral cortex from E14.5 rat embryos was separated from the basal ganglia and overlying meninges. At this stage, immature neurons as well as mitotic precursors exist in the embryo, and after plating, additional precursors undergo cell cycle exit to begin neuronal differentiation, as shown previously (Carey et al., 2002). Cells were dissociated mechanically, plated on 0.1 mg/mL poly-D-lysine coated culture dishes, and incubated at 37°C with 5% CO2 in defined media (Lu and DiCicco-Bloom 1997) composed of DMEM and F12 (50:50 v/v; Invitrogen, Grand Island, NY) and containing penicillin (50U/mL), streptomycin (50 µg/mL), transferrin (100µg/mL) (Calbiochem, La Jolla, CA), putrescine (100 µM), progesterone (20 nM), selenium (30 nM), glutamine (2 mM), glucose (6 mg/mL), and bovine serum albumin (10 mg/mL). Unless otherwise noted, components were obtained from Sigma (St. Louis, MO). Cells (3 × 105) were added to 24-well plates and were treated with MeHg/drugs 1hr after plating so that initial adhesion was not disturbed by the treatments.
[3H]-Thymidine Incorporation
Tritiated thymidine (5 µCi/gbw; Amersham Bioscience, UK) was injected sc into animals 2 hrs prior to analysis. DNA synthesis was evaluated using a ‘percent incorporation’ assay, as described (Burke et al. 2006; Cheng et al. 2002; Wagner et al. 1999). Frozen tissues were manually homogenized in distilled water using a 22 gauge needle and syringe. An aliquot was removed for determination of total isotope uptake into the tissue. In an equal aliquot, DNA was precipitated with 10% trichloroacetic acid, sedimented by centrifugation, and washed by resuspension and resedimentation. The final pellet was dissolved and counted along with the original aliquot in a scintillation spectrophotometer. Since radiolabel incorporation into DNA depends on the amount of label taken up by the tissue, incorporation was calculated as the fraction of total tissue uptake. This method assures that experimental effects do not reflect possible differences in tissue region dissection or individual animal injection, absorption or blood flow, but rather changes in specific regional DNA synthesis.
DNA synthesis in vitro
Plated cells were incubated with tritiated thymidine (5 µCi/mL) during the last 2 hrs of total incubation, detached with a trypsin-EDTA solution, and collected onto filter paper with a semi-automatic cell harvester (Skatron) (Lu and DiCicco-Bloom 1997). After addition of the luminating solution Eco-Lite (MP Biomedicals), radioactivity was measured by scintillation spectrophotometry.
Immunohistochemistry
Animals were perfused with 0.9% NaCl followed by 4 % paraformaldehyde (PFA) in 0.1 M PBS. Brains were postfixed in 4% PFA in PBS for 12 hrs at 4°C, cryoprotected in 30% sucrose in PBS and cut, after embedding in Tissue-Tek (Sakura, Tokyo, Japan), into 12-µm-thick coronal sections on a cryostat (Leica, Heidelberg, Germany). Sections were incubated in boiling 0.01 M citrate buffer (3 × 30 min), hydrogen peroxide (0.3 % in methanol, 10 min), 5 % normal serum for 1 hr, followed by cleaved-caspase-3 antibody (1:200; Cell Signaling, Beverly, MA) overnight at 4°C in 0.5 % Triton X-100 and 1 % normal serum in PBS. Biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) was applied as secondary antibody and avidin–biotin–peroxidase complex (ABC kit, Vector Laboratories) served as amplification reagent. Diaminobenzidine served as chromogen to localize the peroxidase, followed by counterstain with Toluidine Blue (Sigma). Quantification of positive cells was performed on 9 sections/animal, 3 animals/group, from 3 independent litters.
DNA quantification
P21 hippocampii were manually homogenized in distilled water. DNA was pelleted with 10% trichloroacetic acid (TCA) and centrifugation. After the pellet was hydrolyzed with 1 N KOH and neutralized, DNA was resedimented with 5% TCA and denatured at 90°C. Samples were quantified with a diphenylamine reagent that reacts proportionally with DNA to generate a colored reaction product that can be assessed by spectroscopy for each experiment. Sample values were converted to total DNA based on a standard curve run in parallel for each assay, as previously described (Wagner et al. 1999; Cheng et al. 2002).
Inductively coupled plasma mass spectrometry (ICP-MS)
Animals were perfused with 0.9% NaCl to flush blood from tissues. Whole hippocampi were collected for analysis. Hippocampal tissues (<100mg wet weight) were placed into conical tubes. To each tube 0.25 mL of concentrated nitric acid (EMD OmniTrace Ultra High Purity, VWR Scientific) was added and the samples were allowed to react with sonication. They were subsequently digested using a MARSX microwave sample digester (CEM Corp., Mathews NC). Final concentrations were diluted to 5% acid using DI water (MilliQ Ultrapure De-ionized, Millipore Corp., Billerica, MA) for a total volume of 7 mL. Internal standards and controls included: acid blank, acid spike, matrix blank and matrix spike. The acid blank had no tissue and no Hg added, while the acid spike had a known amount of Hg added. The matrix spike was an untreated piece of tissue with a known amount of Hg added. Samples were analyzed for Hg using an X5 (Thermo Electron, MA) inductively coupled plasma mass spectrometer (ICP/MS). The m/z of 202 was used for quantitiation, while m/z of 199, 200 and 201 were also observed as a QC measure.
Statistical evaluation
A one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison test was used to determine degrees of significance. Data were analyzed using GraphPad Instat Version 3.05 (GraphPad Software, San Diego, CA).
RESULTS
Effects of NAC on MeHg induced neurotoxicity in culture
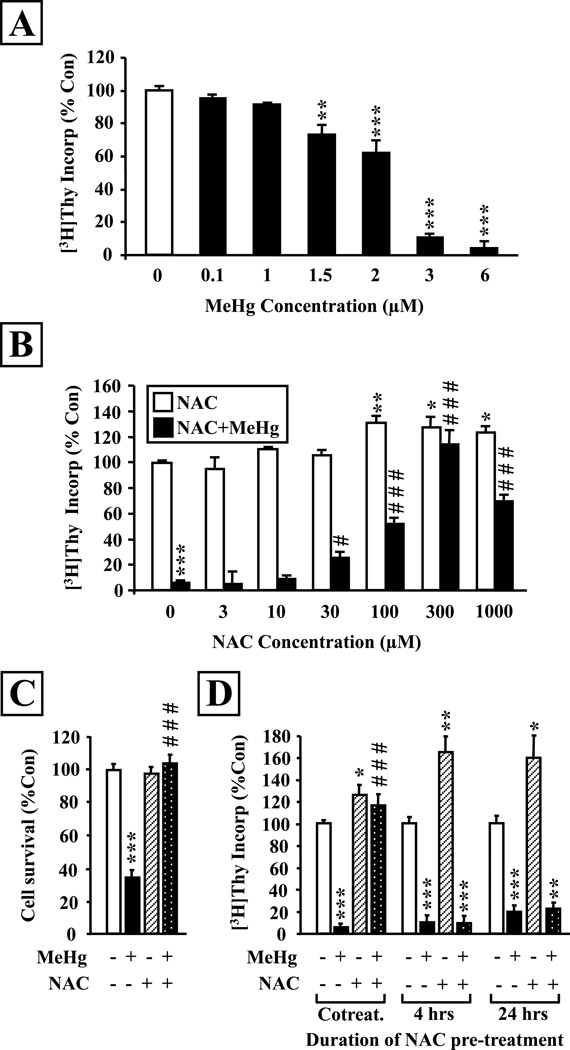
To investigate the potential protective effects of NAC on brain cells, we first used an in vitro model of embryonic cortical neurons and precursors which was previously shown to be suitable for the study of mercury neurotoxicity (Burke et al. 2006; Falluel-Morel et al. 2007; Sokolowski et al., 2011). We treated cortical cultures with various concentrations of MeHg ranging from 0.1 to 6 µM and measured [3H]-Thymidine ([3H]-Thy) incorporation at 24 hours. In this model, MeHg induced a concentration-dependent decrease in DNA synthesis significant at 1.5 µM and higher (Fig. 1A). The 3 µM MeHg concentration that induced a 90% decrease in [3H]-Thy incorporation was used to investigate the potential effects of NAC. Several concentrations of NAC were assessed, ranging from 3 to 1,000 µM (Fig. 1B). NAC induced a concentration-dependent protective effect, significant at 30 µM and above, with a complete protection at 300 µM. Interestingly, when administered alone, 100–300 µM NAC induced a ~30% increase in DNA synthesis. Moreover, the induction of cell death at 24 hrs following MeHg exposure was completely abolished by NAC co-incubation (Fig. 1 C).
Figure 1. NAC prevents MeHg toxic effects on DNA synthesis and cell survival in embryonic cortical cultures.
A: Concentration-dependent effects of MeHg on [3H]-Thy incorporation in cortical cultures. Cells were exposed for 24 hrs to vehicle or 0.1, 1, 1.5, 2, 3 or 6µM MeHg at zero time. [3H]-Thy was added at 20 hrs and cells were collected at 24 hrs for analysis. MeHg exposure induced a concentration-dependent reduction in DNA synthesis. B: Concentration-dependent effect of NAC on MeHg-induced inhibition of DNA synthesis. Cells were exposed or not to MeHg (3µM) and/or NAC (3 – 1,000µM) for 24 hrs. NAC treatment increased thymidine incorporation at high concentrations (>100µM) and induced a dose-dependent protection against the negative effects of MeHg. C: Protective effect of NAC against MeHg-induced cell death. Cells were exposed or not to MeHg (3µM) and/or NAC (300µM) for 24 hrs and total numbers were assessed under phase microscopy. NAC cotreatment completely abolished MeHg-elicited neuronal death. D: Influence of treatment paradigm on NAC protective effects in vitro. NAC (300µM) exerted protective effects only when it was administered at zero time concurrently with MeHg (3µM). However, when NAC was added to the culture media either 4 or 24 hours before MeHg and removed prior to mercury exposure, no protection was measured. Data are expressed as the mean ± sem of 3 independent experiments for all groups, performed in quadruplicates for every condition. *P<0.05; **P<0.01; ***P<0.001 vs control; #P<0.05; ###P<0.001 vs MeHg.
As a metabolic precursor, some studies have used culture preincubation with NAC to allow extended time for cellular uptake and glutathione biosynthesis (Shimizu et al., 2002). Thus we examined the impact of pretreatment with NAC on its protective effects against MeHg toxicity (Fig. 1D). Although NAC was highly effective in counteracting MeHg toxicity when co-administered, it proved entirely ineffective when added to the culture media for a period of either 4 or 24 hours and then removed prior to mercury exposure.
Effects of NAC on MeHg induced neurotoxicity in vivo
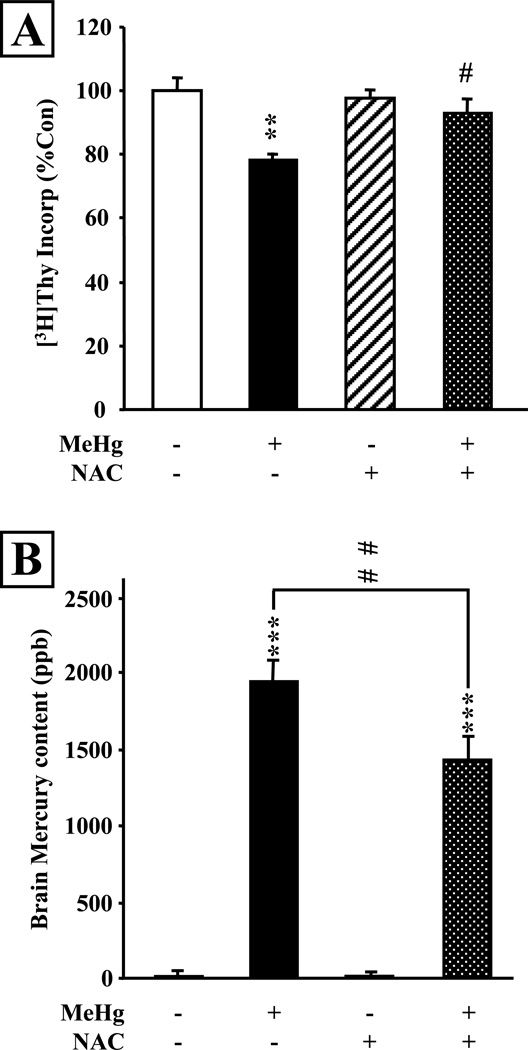
Because NAC appeared to be a potent and efficacious inhibitor of MeHg-induced neurotoxicity in cultured cortical neurons and precursors, we investigated its effects further in a developmental model in vivo. We performed these studies using a paradigm of acute exposure of perinatal rats to MeHg and assessment of the hippocampus. In this model, MeHg induces cell cycle arrest and programmed cell death of dentate gyrus neuronal precursors, leading to intermediate term modification of hippocampal structure and function (Falluel-Morel et al., 2007). In the current experiments, P7 rats were given 5 injections of NAC (10 µg/gbw) with an interval of 2 hours between injections, spanning a total of 8 hours. Concurrent with the second NAC administration, animals received a single injection of saline or 5 µg/gbw MeHg, and [3H]-Thy incorporation was measured 24 hours later (Fig. 2A). This injection paradigm was chosen amongst several administration protocols (see Discussion below) to ensure sufficient NAC blood levels without producing side effects. MeHg induced a 20% decrease in DNA synthesis in the total hippocampus, reproducing the inhibitory effects defined in previous studies (Burke et al., 2005; Falluel-Morel et al., 2007). However, in the presence of NAC, the negative effects of MeHg were almost completely blocked. NAC alone had no significant effect. Thus, similar to our in vitro data, NAC administration in vivo prevented the inhibitory effects of MeHg on hippocampal DNA synthesis.
Figure 2. NAC administration reduces mercury uptake into the hippocampus and prevents inhibition of DNA synthesis in developing P7 rats in vivo.
P7 rats were injected with saline or MeHg (5µg/gbw) and received 5 repeated injections of NAC (10µg/gbw per injection) over 8 hours with 2 hours intervals between each injection. NAC exposure was initiated 2 hours before MeHg exposure. A: [3H]-Thy incorporation into the whole hippocampus was measured 24 hours after MeHg exposure. The inhibitory effect of MeHg on DNA synthesis was almost completely abolished by NAC. B: ICP-MS measurement of hippocampal mercury content 24 hours after treatment with NAC and/or MeHg. Mercury was almost undetectable in the control and NAC treated animals. MeHg sc injection led to a massive Hg uptake into the hippocampus, which was significantly reduced by NAC. Values are expressed as the means ± sem of 4 independent experiments for all groups, with 3 animals per group in each experiment (N=12 per group). **, P < 0.01 vs control; ***, P < 0.001 vs control; #, P < 0.05 vs MeHg; ##, P < 0.01 vs MeHg.
Effects of NAC on mercury levels in the hippocampus
The ability to block MeHg neurotoxic effects in the perinatal rat raised the possibility that NAC may have altered the metal’s access to the brain, especially given its ability to promote MeHg renal uptake and excretion (Koh et al., 2002). To examine this issue, we measured hippocampal mercury levels 24 hours following exposure (Fig. 2B). The single 5 µg/gbw injection led to the accumulation of ~2,000 ppb Hg in the hippocampus, whereas mercury levels were almost undetectable in vehicle injected animals. Significantly, in the presence of NAC, hippocampal Hg levels were decreased by ~25%, indicating that NAC co-administration with MeHg reduced mercury accumulation in the hippocampus (P<0.001 vs MeHg).
Effects of NAC on MeHg induced hippocampal cell death
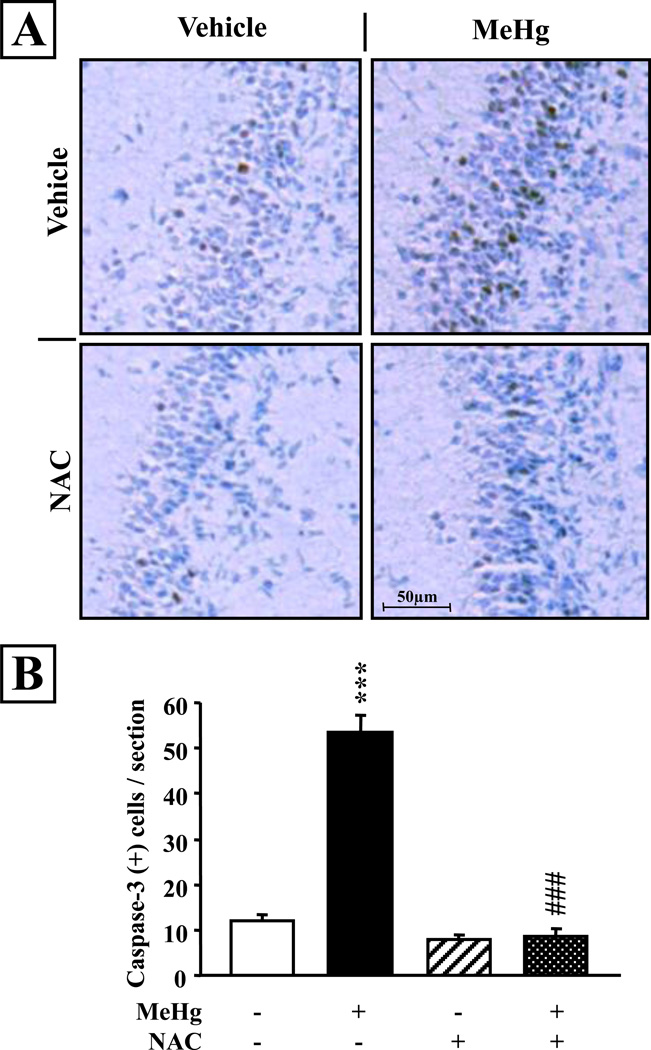
In our previous studies of MeHg neurotoxicity, reduction of DNA synthesis was a process downstream to activation of caspases during apoptotic cell death. To determine whether NAC treatment affected cell death, we performed immunohistochemical analysis for activated caspase-3, a marker of apoptosis, on hippocampal sections obtained from P8 animals injected on the previous day (P7) with NAC and/or MeHg. We observed few caspase-3-immunopositive cells in the dentate gyrus of the hippocampus of the control and NAC injected animals (Fig. 3A). However, following MeHg exposure, there was a greater than 4-fold increase in the number of caspase-3 positive cells (P<0.001; Fig. 3B), primarily in the hilus and the granule cell layer of the dentate gyrus. In marked contrast, there was no increase present in animals that were injected with both MeHg and NAC. Quantification revealed that MeHg exposed animals exhibited a highly significant increase in active caspase-3 positive cell number at 24 hrs (P<0.001; Fig. 3B). NAC administration fully blocked MeHg induced cell death (P<0.001 vs MeHg).
Figure 3. NAC injection prevents acute induction of apoptotic cell death elicited by MeHg exposure in the P7 hippocampal dentate gyrus.
A: Detection of activated caspase-3 immunoreactivity in the dentate gyrus following MeHg and/or NAC exposure. P7 rats were injected with vehicle, 5.0 µg/gbw MeHg and/or 5×10µg/gbw NAC, sacrificed at 24 hrs and processed for immunostaining. Scale bar = 100 µm. B: Quantification revealed that the MeHg induced increase in caspase-3 positive cell number was completely blocked by NAC. Values are expressed as the means ± sem per section of 9 sections per animal, 3 animals per group. ***, P < 0.001 vs control; ###, P < 0.001 vs MeHg.
Effects of NAC on intermediate term structural deficits elicited by MeHg
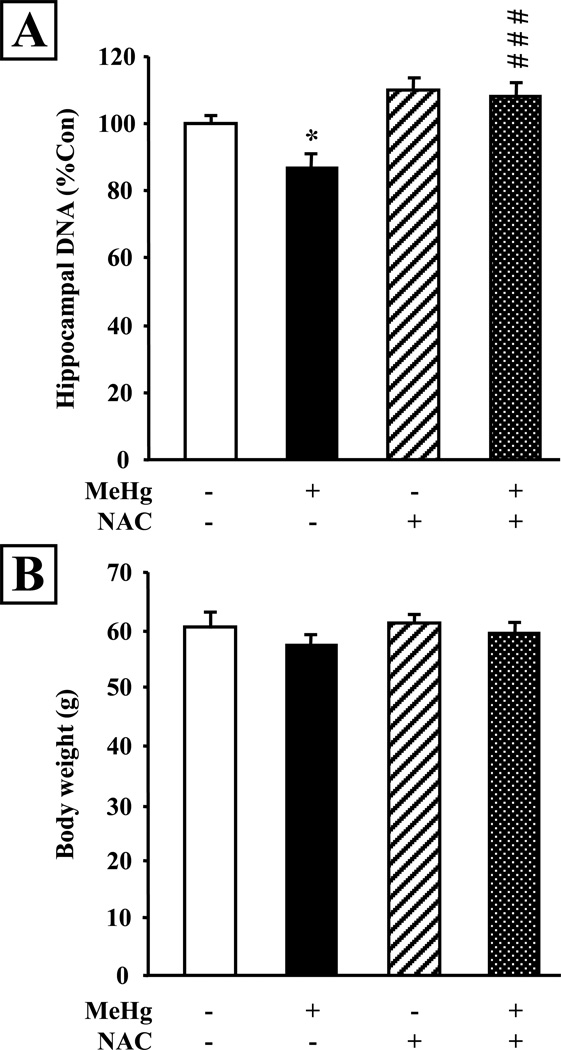
We investigated the consequences of postnatal MeHg exposure and NAC administration on the cell number of the hippocampus at P21. We quantified total DNA content, which is proportional to total cell number, in the hippocampus. MeHg exposure on P7 produced a 13% reduction in total DNA content in the P21 hippocampus (P<0.05; Fig. 4A), reproducing developmental consequences reported previously (Burke et al. 2006; Falluel-Morel et al. 2007). In contrast, in the presence of NAC, the inhibitory effects of MeHg were entirely prevented (P<0.001 vs MeHg). In contrast to effects on hippocampus, MeHg did not elicit any changes in overall body weight (Fig. 4B).
Figure 4. NAC administration prevents MeHg-induced reduction of hippocampal cell content at P21.
A: Measurement of hippocampal DNA content at 2 weeks (P21) after MeHg exposure on P7. Total DNA was significantly reduced in MeHg treated animals, and NAC injection prevented this effect. B: Body weights of the animals were measured prior to sacrifice and no significant differences were observed between animals. Values are expressed as the means ± sem of 3 independent experiments, with 3 animals per group in each experiment. *, P < 0.05 vs control ; ###, P < 0.001 vs MeHg.
DISCUSSION
Our observations indicate that NAC administration prevents the deleterious effects of an acute MeHg exposure on developing neurons in vitro and in vivo. MeHg toxicity is characterized in these neurons and precursors by a reduction in DNA synthesis and an increase in cell death, as evaluated by measuring thymidine incorporation and caspase-3 activation. Using the same markers, we were able to observe a virtually complete protection from MeHg effects by NAC treatment, whereas the brain MeHg level was decreased by only 25%. Acute MeHg exposure led to lasting reductions in hippocampal cell number at P21, an effect that was also prevented by NAC administration. Altogether, these results suggest that NAC or its derivative may constitute a promising lead for the prevention of teratogenic effects of heavy metals such as mercury in the developing brain.
In culture studies using embryonic cortical neurons and precursors, we found that co-administration of NAC reduced MeHg toxicity, preventing reductions in DNA synthesis and cell number. However, it was unclear whether NAC binds directly to mercury or alternatively, is converted into the antioxidant tripeptide glutathione to exert its protective effects. To begin addressing this question, we compared various protocols where NAC was added to the culture media for either 4 or 24 hours before MeHg, potentially allowing cells to synthesize additional glutathione as shown by others (Dringen and Hamprecht 1999; Shimizu et al., 2002; Kaur et al. 2007), and then removed NAC prior to mercury exposure. Under these conditions, NAC was not able to counteract the MeHg-induced decrease in thymidine incorporation, suggesting that NAC may interact directly with mercury to prevent its deleterious effects. However, in vivo, the mechanism(s) by which NAC exerts its neuroprotective effects remains to be elucidated.
Our observations in vivo however are consistent with the concept that a fraction of methylmercury binds to cysteine in the blood, forming a compound which can be actively transported by hepatic and kidney cells into bile and urine (Clarkson et al., 2007). This excretion is described as a two-step process, with the uptake of MeHg-NAC from blood into epithelial cells by organic anion transporters, such as Oat1 (Koh et al., 2002), followed by active excretion of the complex into bile or urine by the apical Multidrug resistance-associated protein-2 (Mrp2/Abcc2) (Madejczyk et al., 2007). Significantly, however, Aremu and coworkers have shown that this mechanism, which allows efficient, NAC-enhanced renal excretion of MeHg in adult rat, was not effective in young animals (<P21) in which developmental expression of the transporters has not yet attained mature levels (Buist et al., 2002; Aremu et al., 2008). Thus the value of NAC treatment for perinatal toxicity (Aremu et al., 2008), especially in brain where effects were not observed (Madejczyk et al., 2007), was drawn into question. Apparently consistent with limited NAC-induced excretion in preweanling rats, we found that while NAC reduced the uptake of mercury into the hippocampus, 75% of the toxicant still remained in this organ after 24 hrs. Nonetheless, the rescue was almost complete (as measured by thymidine incorporation, caspase-3 activity and total DNA content) suggesting that a mechanism other than altered transport was involved. It is possible that following NAC treatment, mercury remaining in the brain was sequestered as a complex (MeHg-NAC) that may have attenuated neurotoxicity. Or, perhaps MeHg-NAC was taken up by different brains cells, such as neurons versus glial cells, a question we could address using available culture models and the radiolabeled tracer (Koh et al., 2002). While the nature of brain mercury is yet undefined, a fraction of the cysteine-coupled metal may be converted into intracellular MeHg-glutathione (Clarkson et al. 2007). MeHg-glutathione may then be released into the circulation and excreted into the intestinal lumen. However, on the other hand, recent study suggests this excretion mechanism may be counterproductive, as the thiol-bound mercury can exhibit an increased rate of re-uptake into kidney and brain (Zalups and Ahmad 2005; Rooney 2007), challenging its value as a therapy. In at least one rat model, administration of GSH itself did not reduce mercury levels in the brain when instituted following the exposure period (Aposhian et al. 2003). With regard to a possible indirect action of NAC through GSH production, our in vitro data are consistent with recent literature, and indicate that NAC fails to protect cells when it is not present in the culture medium with MeHg (Zieminska et al., 2010). Indeed, a preincubation period allowing time for cells to metabolize NAC into GSH had no beneficial effect on neuronal survival, thereby excluding the hypothesis for a stimulation of GSH pathway in this culture model.
Several administration protocols are commonly used for NAC treatment in humans, depending on the age of the patients and nature of the insult. The recommended regimen for NAC therapy in human intoxications, such as for acetaminophen, is an intravenous bolus of 150 mg/kgbw, followed by 50 mg/kgbw every four hours. We investigated the effects of a similar range of doses in our P7 rat model. First we performed a single injection of NAC in a bolus and measured thymidine incorporation 24 hours later. The two doses we used appeared to be toxic for rat pups: a 200 mg/kgbw injection of NAC alone provoked a 40% decrease in hippocampal DNA synthesis, while 100 mg/gbw still induced a 10% reduction (data not shown). To reduce NAC toxicity by decreasing peak drug exposure, we finally decided on 5 repeated lower dose NAC injections (10 mg/kgbw) with 2 hour inter-injection intervals. Under these conditions, NAC exhibited no apparent toxicity alone and this allowed us to test and characterize its efficacy against MeHg-induced brain toxicity. The possibility that higher doses of NAC may produce deleterious effects has been described in mice (Palmer et al. 2007). Indeed, high doses of NAC cause heart and lung damage through the production of the metabolite S-nitroso-N-acetylcysteine (SNOAC). Similar effects have been described in humans (Hildebrandt et al. 2002). These various data suggest that NAC dosage regimens may need to be tailored based on both animal species as well as developmental age.
In addition to insights into preventing MeHg neurotoxicity, the current studies provide information on the possible mechanism by which the toxicant induces hippocampal teratogenicity. It is possible that MeHg exposure elicits acute effects on proliferation of hippocampal neural precursors through blockade of G1/S phase transition and induction of caspase-dependent apoptosis (Falluel-Morel et al., 2007; Sokolowski et al., 2011), whereas later effects on hippocampal cell number would be mediated by other pathways. The current studies however, showing that NAC prevents acute mitotic inhibition as well as later hippocampal cell deficits, suggest MeHg’s main teratogenic mechanism is its acute inhibition of neural precursor proliferation/survival. While NAC prevented MeHg induced reduction in hippocampal cell number, future studies will determine whether NAC can also prevent the spatial learning deficits occurring in this model at puberty (Falluel-Morel et al. 2007).
In conclusion, our studies indicate that injection of NAC is an efficient method to prevent MeHg-induced toxicity in the perinatal brain in vivo These studies support the mounting evidence that NAC may be preferable to the currently available thiol-containing MeHg chelators, meso-2,3- dimercaptosuccinic acid (DMSA) and 2,3-dimercapto-1-propanesulfonate (DMPS) that unfortunately mobilize and deplete other minerals (especially divalent cations) that are essential for normal physiologic function (discussed in Aremu et al., 2007). Our studies in developing hippocampus contrast with other work that suggests NAC exposure may enhance mercury induced damage by serving as a molecular transporter (Zalups et al., 2005; Rooney et al., 2007). However, to produce protective effects, low dose NAC administration was initiated 2 hours prior to MeHg exposure. In preliminary studies, we did not detect protection when NAC administration was begun 2 hours after MeHg exposure, suggesting that MeHg was distributed rapidly to sites of injury and/or that proper NAC dosing for post-MeHg treatment remains to be determined, a possibility under active investigation. Nonetheless, the current evidence, contrary to previous studies, suggests that NAC may provide some degree of benefit from brain injury during the perinatal period, when the sources of mercury exposure are predictable or sustained. Since fish remains a good source of nutrients and oils beneficial for neurodevelopment, potential deleterious effects of mercury-containing fish consumption may be ameliorated through the use of NAC or a related compound. Therefore, it is of interest to develop compounds able to bind heavy metals with high affinity without producing toxic effects by themselves to prevent developmental neurotoxicity in populations exposed to organomercurials.
Acknowledgments
Grant information: National Institutes of Health (ES11256 to E.D-B., ES05022 to E.D-B., ES07148 to K.S.. NS062591 to K.S., NIH-NIEHS 1R21ES019762 to E.D-B.); UMDNJ Center for Environmental Exposures and Disease (P30ES005022); Fondation pour la Recherche Médicale (SPE20051105 to A.F-M.); US Environmental Protection Agency (R82939101 to E.D-B.).
REFERENCES
- Adams J, Barone S, LaMantia A, Philen R, Rice DC, Spear L, Susser E. Workshop to identify critical windows of exposure for children's health: neurobehavioral work group summary. Environ Health Perspect. 2000;108:535–544. doi: 10.1289/ehp.00108s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aremu DA, Madejczyk MS, Ballatori N. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect. 2008;116:26–31. doi: 10.1289/ehp.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aposhian HV, Morgan DL, Queen HL, Maiorino RM, Aposhian MM. Vitamin C, glutathione, or lipoic acid did not decrease brain or kidney mercury in rats exposed to mercury vapor. J Toxicol Clin Toxicol. 2003;41:339–347. doi: 10.1081/clt-120022000. [DOI] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther. 2002;301:145–151. doi: 10.1124/jpet.301.1.145. [DOI] [PubMed] [Google Scholar]
- Burke K, Cheng Y, Li B, Petrov A, Joshi P, Berman RF, Reuhl KR, DiCicco-Bloom E. Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin E. Neurotoxicology. 2006;27:970–981. doi: 10.1016/j.neuro.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environm Health Pespect. 2005;113:1015–1021. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RG, Li B, DiCicco-Bloom E. Pituitary adenylate cyclase activating polypeptide anti-mitogenic signaling in cerebral cortical progenitors is regulated by p57Kip2-dependant CDK2 activity. J Neuroscience. 2002;22:1583–1591. doi: 10.1523/JNEUROSCI.22-05-01583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LW, Reuhl KR, Lee GW. Degenerative changes in the developing nervous system as a result of in utero exposure to methulmercury. Environm Res. 1977;14:414–423. doi: 10.1016/0013-9351(77)90049-4. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Black IB, DiCicco-Bloom E. Hippocampal granule neuron production and population size are regulated by levels of bFGF. Eur J Neurosci. 2002;15:3–12. doi: 10.1046/j.0953-816x.2001.01832.x. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50:757–764. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hamprecht B. N-acetylcysteine, but not methionine or 2-oxothiazolidine-4-carboxylate, serves as cysteine donor for the synthesis of glutathione in cultured neurons derived from embryonal rat brain. Neurosci Lett. 1999;259:79–82. doi: 10.1016/s0304-3940(98)00894-5. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem. 2007;103:1968–1981. doi: 10.1111/j.1471-4159.2007.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H, Engelhardt G, Hebel S, Rink L. Mercuric ions inhibit mitogen-activated protein kinase dephosphorylation by inducing reactive oxygen species. Toxicol Aplli Pharmacol. 2011;250:78–86. doi: 10.1016/j.taap.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Hildebrandt W, Alexander S, Bartsch P, Droge W. Effect of N-acetyl-cysteine on the hypoxic ventilatory response and erythropoietin production: linkage between plasma thiol redox state and O(2) chemosensitivity. Blood. 2002;99:1552–1555. doi: 10.1182/blood.v99.5.1552. [DOI] [PubMed] [Google Scholar]
- Kaur P, Aschner M, Syversen T. Role of glutathione in determining the differential sensitivity between the cortical and cerebellar regions towards mercury-induced oxidative stress. Toxicology. 2007;230:164–177. doi: 10.1016/j.tox.2006.11.058. [DOI] [PubMed] [Google Scholar]
- Koh AS, Simmons-Willis TA, Pritchard JB, Grassl SM, Ballatori N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: transport by the renal organic anion transporter-1. Mol Pharmacol. 2002;62:921–926. doi: 10.1124/mol.62.4.921. [DOI] [PubMed] [Google Scholar]
- Lapham LW, Cernichiari E, Cox C, Myers GJ, Baggs RB, Brewer R, Shamlaye CF, Davidson PW, Clarkson TW. An analysis of autopsy brain tissue from infants prenatally exposed to methymercury. Neurotoxicology. 1995;16:689–704. [PubMed] [Google Scholar]
- Lu N, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proc Natl Acad Sci U S A. 1997;94:3357–3362. doi: 10.1073/pnas.94.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madejczyk MS, Aremu DA, Simmons-Willis TA, Clarkson TW, Ballatori N. Accelerated urinary excretion of methylmercury following administration of its antidote N-acetylcysteine requires Mrp2/Abcc2, the apical multidrug resistance-associated protein. J Pharmacol and Exp Ther. 2007;322:378–384. doi: 10.1124/jpet.107.122812. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol. 2004;26:179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JP. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology. 2007;234:145–156. doi: 10.1016/j.tox.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Komatsu N, Iyo M. Roles of endogenous glutathione levels on 6-hydroxydopamine-induced apoptotic neuronal cell death in human neuroblastoma SK-N-SH cells. Neuropharmacology. 2002;43:434–443. doi: 10.1016/s0028-3908(02)00108-9. [DOI] [PubMed] [Google Scholar]
- Sokolowski K, Falluel-Morel A, Zhou X, DiCicco-Bloom E. Methylmercury MeHg) elicits mitochondrial-dependent apoptosis in developing hippocampus and acts at low exposures. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.06.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon A. Prenatal methylmercury exposure and developmental outcomes: review of the evidence and discussion of future directions. Environ Health Perspect. 2006;114:307–312. doi: 10.1289/ehp.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Schettler T, Wallinga D, Valenti M. In harm's way: toxic threats to child development. J Dev Behav Pediatr. 2002;23:S13–S22. doi: 10.1097/00004703-200202001-00004. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Transport of N-acetylcysteine s-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J Pharmacol Exp Ther. 2005;314:1158–1168. doi: 10.1124/jpet.105.086645. [DOI] [PubMed] [Google Scholar]
- Zieminska E, Toczylowska B, Stafiej A, Lazarewick JW. Low molecular weight thiols reduce thimerosal neurotoxicity in vitro: modulation by proteins. Toxicology. 2010;276:154–163. doi: 10.1016/j.tox.2010.07.023. [DOI] [PubMed] [Google Scholar]