Abstract
The initial injury in acute pancreatitis (AP) is characteristically sterile and results in acinar cells necrosis. Intracellular contents released from damaged cells into the extracellular space serve as damage associated molecular patterns (DAMPs) that trigger inflammation. There is increasing evidence that this sterile inflammatory response mediated through DAMPs released from necrotic acinar cells is a key determinant of further pancreatic injury, remote organ injury, and disease resolution in experimental models. A number of DAMPS, including high-mobility group box protein 1 (HMGB1), DNA, ATP, and heat shock protein 70 (hsp70), have been shown to have a role in experimental pancreatitis. Many of these DAMPs are also detectable in the human pancreatitis. Genetic deletion and pharmacologic antagonism demonstrate that specific DAMP receptors, including TOLL-like receptor 4 (TLR4), TOLL-like receptor 9 (TLR9) and P2X7, are also required for inflammation in experimental AP. Down-stream DAMP sensing components include NLRP3, caspase1, interleukin-1β (IL-1), interleukin-18 (IL-18), and IL-1 receptor (IL-1R), and also are required for full experimental pancreatitis. These DAMP-mediated pathways provide novel therapeutic targets using antagonists of TLR’s and other receptors.
Keywords: pancreatitis, TLR9, P2X7, caspase1, DAMP, TLR4
Host Cell Injury and Sterile Inflammation
The adaptive immune system consists primarily of T and B cells and recognizes individual molecules using highly specific receptors.1 This produces an immune response that is focused to a particular pathogen, and recognition of self molecules is limited by purging of potentially self-reactive T and B cells during development. The innate immune system consists of a wider range of cells including macrophages, dendritic cells, neutrophils and mast cells, and uses receptors recognizing molecules with a general pattern shared by a number of pathogens. A good example is lipopolysaccharide (LPS), a molecule that is common to gram-negative bacteria, and requires toll like receptor 4 (TLR4) for its recognition. The TLRs are the best characterized family of such pattern recognition receptors (PRRs), and recognize molecules as diverse as LPS via TLR4, double stranded DNA by TLR9, and single stranded RNA by TLR7.2
Recently it has been demonstrated that TLRs and other PRRs are also be activated by self-molecular patterns which in health are sequestered inside cells and unable to engage these cell surface receptors.3, 4 Such self-molecules are termed damage associated molecular patterns (DAMPs). Mitochondrial DNA is a good example; it is not present in the extracellular space during health, but can activate TLR9.5 After organ injury, intracellular contents including DAMPs are released into the extracellular space, and trigger inflammation through engagement of TLRs and other PRRs.4 Since DAMPs can engage the same receptors as those activated by bacterial and other infectious agents, this process has been designated “sterile inflammation.” A full sterile inflammatory response requires at least two distinct signals to an inflammatory cell such as macrophage. Signal 1 is through engagement of plasma membrane or endosomal receptors of the TOLL-like receptor superfamily (TLRs). This induces gene expression of pro-inflammatory cytokines, including pro-interleukin-1β (pro-IL-1β ) and pro-interleukin-18 (pro-1L-18). Signal 2 is provided by DAMP signaling through plasma membrane P2X7 receptor and cytosolic receptors of the Nod-like receptor (NLR) family activating a cytosolic complex termed the inflammasome, which regulates proteolytic maturation of caspase1.6 Caspase1, previously identified as interleukin converting enzyme (ICE), is a cytosolic cysteine protease which tightly regulates the conversion of the inflammatory cytokines pro-IL-1β and pro-IL-18 into mature forms.7 These two complementary DAMP sensing pathways are the hallmark of sterile inflammation (Figure 1). There is strong experimental evidence that DAMP-mediated sterile inflammation is a key determinant of additional parenchymal damage in acute sterile injury of the liver, kidney, and brain.2
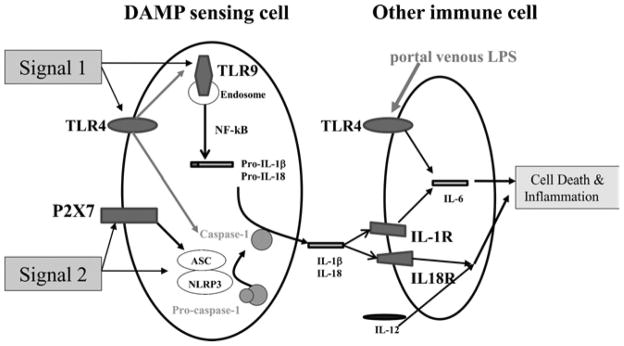
Figure 1.
The sterile inflammatory response in acute pancreatitis. In acute pancreatitis, injured parenchymal cells release DAMPs. These are Signal 1 and Signal 2 for respective receptors in DAMP sensing cells which then produce IL-1β and IL-18. The latter effector cytokines stimulate pro-inflammatory responses in other immune cells promoting further cell death in the pancreas and immune injury in distant organs.
There is now compelling experimental evidence that sterile inflammation has a central role in the pathogenesis of acute pancreatitis. The initial injury in acute pancreatitis (AP) is characteristically sterile, and early pancreatic necrosis is predictive of the disease course including the generation of inflammatory cytokines, local and systemic inflammation, and morbidity.8 Moreover, components of the systemic inflammatory response promote further pancreatic necrosis and intrapancreatic zymogen activation in the setting of experimental AP.9, 10
Signal 1: TLRs and Respective DAMPs in Acute Pancreatitis
Acinar cells and fats cells are the initial cell types to be injured in most forms of acute pancreatitis. Clinical markers of pancreatic injury, amylase and lipase, are released into the circulation by dysregulated basolataral secretion, enhanced ductal permeability as well as by unregulated release of intracellular contents from necrotic acinar cells, but are not thought to induce further injury.11 In contrast, high-mobility group box protein 1 (HMGB1) is also released by necrotic acinar cells in experimental and human disease and is a DAMP, which mediates further tissue injury and inflammation in sterile inflammatory injury through TOLL-like receptor 4 (TLR4).12 HMGB1 is markedly elevated in the serum of patients with AP and also correlates with disease severity.13, 14 Extracellular HMGB1 induces further pancreatic and distant organ injury and inflammation in severe experimental AP in which pancreatic necrosis occurs.15, 16 Exogenous shock protein 70 (hsp70) increases pancreatic injury in rodent models of AP through a TLR4-dependent manner.17 The role of endogenous hsp70 as a potential DAMP is less clear.18
There is substantial evidence that TLR4 is required for maximum injury in acute pancreatitis. Genetic deletion of Tlr4 reduces the severity of pancreatic, lung, and acinar cell injury in edematous and necrotizing experimental AP.19, 20 Of note, LPS and bacteria were not detected in the serum or pancreas in these rodent models of edematous or severe experimental AP, consistent with a role for DAMPs such as HMGB1 and/or hsp70 as endogenous ligands of TLR4.19 TLR4 is expressed on pancreatic ductal and endothelial cells and tissue macrophages, but not acinar cells.21 Endothelial cells and tissue macrophages are also known DAMP sensing cells.3, 22
Extracellular double-stranded DNA of host origin is recognized as a DAMP by TOLL-like receptor 9 (TLR9), and promotes injury through immune activation in sterile injury.12,22 We have recently demonstrated that Tlr9 is required for full pancreatitis response and robust pancreatic IL-1β production in experimental AP.23 Host genomic DNA is markedly elevated in the blood very early in the course of experimental AP, consistent with a role for TLR9 in sensing initial pancreatic injury. Serum DNA is significantly elevated in patients with severe AP.14 Similar to TLR4, TLR9 is expressed by pancreatic ductal and endothelial cells but not acinar cells.23
TLR4 and TLR9 stimulation can induce pancreatic injury in the context of a pro-inflammatory state. Repeated administration of TLR4 and TLR9 ligands induces pancreatic injury and inflammation in mice genetically deficient in interleukin-10 (IL-10), an anti-inflammatory cytokine known to suppress pro-inflammatory responses in the pancreas.24,25, 26
Signal 2: Caspase1, NLRP3 Inflammasome, IL-1β , and IL-18 in Acute Pancreatitis
Caspase1 is a cytosolic protease which is cleaved from a pro-form to an active form. The cytosolic machinery responsible for this cleavage is termed the inflammasome, and is activated by a wide range of by DAMPs, microbial components and particulates such as uric acid, or cholesterol crystals.27 Caspase1 is required for full acinar cell death, and inflammation in experimental AP; its genetic deletion greatly reduces these responses.23, 27 Caspase1-mediated effects in AP have been attributed to its function in mediating release of IL-1β and IL-18. Caspase1 expression is strongly induced in atrophic acinar cells and ductal cells in the setting of pancreatic inflammation.28 Acinar cell expression of caspase1 has not been fully investigated and its role in acute pancreatitis is of great interest.
The NLRP3 inflammasome consist of the DAMP receptor NLRP3, an adaptor molecule ASC and caspase1.29 All three of these components are required for maximum injury in experimental AP and are expressed in tissue macrophages.23 There are several DAMP ligands for NLRP3 inflammasome activation, including uric acid and cholesterol crystals. Additionally, extracellular ATP and NAD released from necrotic parenchymal cells activate the NLRP3 inflammasome via the plasma membrane receptor P2X7.3, 30 P2X7 likely contributes to NLRP3/caspase1 inflammasome activation in AP; genetic deletion of P2X7 decreases pancreatic injury and inflammation in experimental AP.23
IL-1β and IL-18 are key effector cytokines in the innate immune responses to sterile injury. Both are transcriptionally induced by TLR signaling, and activated by caspase-1 cleavage. IL-1β mediated signaling is required for full pancreatic and distant organ injury and inflammation in experimental AP.31, 32 Further supporting a role for IL-1β in mediating pancreatic injury, pancreas-specific over-expression of an interleukin-1β transgene results in chronic pancreatitis.33 Serum IL-1β levels have not been consistently correlated with the severity of AP in humans.34, 35 However, there is evidence suggesting a role for IL-1β in the initiation of a sterile inflammatory response to pancreatic injury in the human disease. Serum IL-1β values peak within 24 hours and are significantly greater in study patients with severe versus mild AP.36 These findings are recapitulated in animal models, where intrapancreatic IL-1β levels exceed serum levels, and peak serum IL-1β values precede peak serum IL-6 values.31, 37 Moreover, IL-1R signaling is required for IL-6 production in experimental AP.31, 38 This suggests an important requirement for IL-1β signaling for induction of IL-6 in AP, and may be of clinical significance as serum IL-6 levels strongly correlate with severity in the human disease.39
IL-18, when given exogenously with IL-12, induces edematous pancreatitis in wild-type mice and pancreatic necrosis in mice with dietary or genetic (ob/ob) obesity.40, 41 In one report, recombinant IL-18 appears to be protective from experimental AP and genetic deficiency of IL-18 results in significantly more pancreatic injury.42 The different effects of IL-18 in AP may reflect context dependence with regard to the presence of other cytokines. In the human disease, IL-18 serum levels consistently correlate with severity and ascitic levels of IL-18 were 20 fold higher than serum levels, suggesting an important role in the local immune response to pancreatic injury.43–45
Priming the Pancreas for Sterile Inflammatory Injury
There has been extensive investigation of the common causes of acute pancreatitis, specifically alcohol and biliary stone mediated disease, with development of clinically relevant experimental models of AP.46–49 It is currently unknown how innate immune components are altered in the pancreas in these etiologies of AP. Both alcohol administration and biliary obstruction are known to induce immunologically significant portal venous endotoxemia.50, 51 Bacterial lipopolysaccharide (LPS) increases the severity of experimental AP, and this is consistent with the clinical finding that endotoxemia is correlated with more severe disease and complications in human AP.52 LPS can up-regulate expression of inflammasome components Nlrp3 and caspase1 as well as Tlr9 in immune cells.53, 54 This LPS priming for innate immune responsiveness is likely of significance to pathophysiology because resident and recruited immune cells are responsible for most of the IL-1β production in experimental AP.55 Additionally, LPS can synergize with TLR9 signaling in pro-inflammatory cytokine production in vitro and in vivo.56, 57
Obesity, a common risk factor for pancreatitis and disease severity, promotes susceptibility to IL-18-mediated pancreatic injury in experimental AP as discussed above. The mechanism of this increased susceptibility to immune mediated injury is not known, and it is of great interest to determine how factors such as smoking alter sterile inflammatory components in AP.
Novel Therapeutic Strategies in AP
Over twenty DAMP receptors have been identified, and the contribution of a number of these in pancreatic injury and inflammation has been explored through pharmacologic antagonism of both signal 1 and signal 2 components. This work has provided insight into novel therapeutics strategies in AP.
Regarding signal 1, pharmacologic inhibition of HMBG1 through the use of blocking antibodies decreases pancreatic injury and lung inflammation in an experimental model of severe AP.15 Similarly, pharmacologic antagonism of both TLR9 and TLR7 decreases acinar cell necrosis and lung injury in an experimental model of severe AP.23 Lysosomal acidification is required for endosomal TLR mediated immune responses, including those of TLR3, TLR7, and TLR9. Chloroquine decreases the severity of pancreatic injury and decreases mortality in an experimental model of severe AP and may be efficacious in part through inhibition of endosomal acidification, a requirement for activation of some TLRs.58, 59
Regarding signal 2, caspase1 inhibitors decrease pancreatic necrosis, pancreatic IL-1β production and inflammation, and mortality in an experimental model of severe AP.60 To a significantly lesser degree, a small molecule inhibitor of P2X7 decreases pancreatic injury and inflammation in experimental models of mild AP.23 There are likely several DAMPs other than P2X7 receptor ligands which induce Nlrp3/caspase1 inflammasome activation in AP and this likely accounts for the lesser degree of protection from antagonism of P2X7 relative to caspase1. Allopurinol is a xanthine oxidase inhibitor which decreases uric acid formation and may thereby decrease uric acid mediated Nlrp3/caspase1 inflammasome activation in the setting of tissue injury. Allopurinol decreases pancreatic injury in experimental AP and has been shown to decrease the incidence of post-ERCP pancreatitis in a randomized controlled trial.61, 62
Finally, regarding the key effector cytokines in sterile inflammatory response, administration of recombinant IL-1 receptor antagonist decreases pancreatic injury and inflammation in an experimental model of severe AP.32 Pharmacologic antagonism of IL-18 has not been similarly explored in experimental AP.
As intriguing as modification of the innate immune response appears to be in mitigating AP, suppression of innate immune signaling may be deleterious in the setting of infection. Tlr4 deficient C3H/HeJ mice have reduced injury to the liver and kidney in the closed loop duodenal obstruction model of AP but increased rates of pancreatic infection.63 Similarly, neutralizing antibodies to high mobility group box 1 (HMGB1) decrease pancreatic and distant organ injury in the closed loop duodenal obstruction model of AP but increase rates of pancreatic infection.15 The relevance to humans of this increased risk of infection with TLR antagonism in animal models of AP is unclear as over a dozen humans with deficiency of most TLRs have been characterized and do not have an increased rate of infections after childhood.
It is also very uncommon to find evidence of infection at the initiation of AP, as infection is documented as the inciting cause in less than 1% of cases.64 However, later in disease local and systemic infections contribute significantly to morbidity and mortality. In the largest available autopsy series of patients with mortality from AP, infectious death occurred mainly in patients with AP of greater than 7 days duration.65 Infection of pancreatic necrosis is a common finding in patients with clinical indications for biopsy of pancreatic tissue in AP, occurring in 60–100% of cases in reported series.66 It should be noted that this patient population is most often a subgroup with extensive radiographic necrosis as well as clinical deterioration suspicious for sepsis. Finally, immunomodulation through the use of probiotics in severe AP within 72 hours of admission resulted in increased mortality, bowel ischemia, and extrapancreatic infectious complications.67 Use of live bacterial probiotics is somewhat different from pharmacologic antagonism of DAMP receptors but how the latter might affect gut-microbiome homeostasis is largely unknown.
Summary
It is clear that acute pancreatitis conforms to the paradigm of a sterile inflammatory injury whereby initial parenchymal cell injury is sensed by resident and circulating immune cells through receptor mediated recognition of DAMPs. Components of the innate immune response, including TLR4, TLR9, P2X7, and the NLRP3/caspase1 inflammasome, are activated and induce pro-inflammatory cytokine production and release that consequently promotes further pancreatic parenchymal cell injury, local inflammation, and remote organ inflammation and injury. Up-regulation of this pathway may occur in specific settings such as the increased levels of serum LPS observed in alcohol abuse. There is great interest in developing therapeutics for acute pancreatitis, as there are currently no specific disease modifying therapies and the clinical course can be severe with significant morbidity and mortality. Innate immune modifying agents are attractive candidates for study with the cautionary note that suppression of innate immunity might predispose patients to an increased risk of infection. Further careful study is warranted in the laboratory and clinical setting to explore these innovative therapeutic avenues and caveats in the setting of this unmet clinical need.
Acknowledgments
This work was supported by NIH R01DK076674-01A2 and VA Merit (WM), NIH T32 DK7356 (RH), DK54021 (FG) and VA Merit (FG).
References
- 1.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125:S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benko S, Philpott DJ, Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine. 2008;43:368–373. doi: 10.1016/j.cyto.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Yazdi AS, Guarda G, D'Ombrain MC, et al. Inflammatory caspases in innate immunity and inflammation. J Innate Immun. 2010;2:228–237. doi: 10.1159/000283688. [DOI] [PubMed] [Google Scholar]
- 8.O'Reilly DA, Kingsnorth AN. A brief history of pancreatitis. J R Soc Med. 2001;94:130–132. doi: 10.1177/014107680109400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoval D, Gukovskaya A, Reavey P, et al. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology. 1996;111:1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- 10.Van Laethem JL, Marchant A, Delvaux A, et al. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917–1922. doi: 10.1016/0016-5085(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 11.Adler G, Rohr G, Kern HF. Alteration of membrane fusion as a cause of acute pancreatitis in the rat. Dig Dis Sci. 1982;27:993–1002. doi: 10.1007/BF01391745. [DOI] [PubMed] [Google Scholar]
- 12.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasuda T, Ueda T, Takeyama Y, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359–363. doi: 10.1097/01.mpa.0000236741.15477.8b. [DOI] [PubMed] [Google Scholar]
- 14.Kocsis AK, Szabolcs A, Hofner P, et al. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology. 2009;9:383–391. doi: 10.1159/000181172. [DOI] [PubMed] [Google Scholar]
- 15.Sawa H, Ueda T, Takeyama Y, et al. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666–7670. doi: 10.3748/wjg.v12.i47.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan H, Jin X, Sun J, et al. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38:143–148. doi: 10.1097/MPA.0b013e31818166b4. [DOI] [PubMed] [Google Scholar]
- 17.Song JM, Liu HX, Li Y, et al. Extracellular heat-shock protein 70 aggravates cerulein-induced pancreatitis through toll-like receptor-4 in mice. Chin Med J (Engl) 2008;121:1420–1425. [PubMed] [Google Scholar]
- 18.McConnell KW, Fox AC, Clark AT, et al. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J Immunol. 186:3718–3725. doi: 10.4049/jimmunol.1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharif R, Dawra R, Wasiluk K, et al. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813–819. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- 20.Ding SQ, Li Y, Zhou ZG, et al. Toll-like receptor 4-mediated apoptosis of pancreatic cells in cerulein-induced acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int. 2010;9:645–650. [PubMed] [Google Scholar]
- 21.Li Y, Zhou ZG, Xia QJ, et al. Toll-like receptor 4 detected in exocrine pancreas and the change of expression in cerulein-induced pancreatitis. Pancreas. 2005;30:375–381. doi: 10.1097/01.mpa.0000160959.21580.41. [DOI] [PubMed] [Google Scholar]
- 22.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 Inflammasome Link Acinar Cell Death With Inflammation in Acute Pancreatitis. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishio A, Asada M, Uchida K, et al. The role of innate immunity in the pathogenesis of experimental autoimmune pancreatitis in mice. Pancreas. 2011;40:95–102. doi: 10.1097/MPA.0b013e3181f3a5d4. [DOI] [PubMed] [Google Scholar]
- 25.Cook JW, Karakozis S, Kim D, et al. Interleukin-10 attenuates proinflammatory cytokine production and improves survival in lethal pancreatitis. Am Surg. 2001;67:237–241. discussion 241–232. [PubMed] [Google Scholar]
- 26.Minter RM, Ferry MA, Murday ME, et al. Adenoviral delivery of human and viral IL-10 in murine sepsis. J Immunol. 2001;167:1053–1059. doi: 10.4049/jimmunol.167.2.1053. [DOI] [PubMed] [Google Scholar]
- 27.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramadani M, Gansauge F, Schlosser S, et al. Overexpression of caspase-1 in pancreatic disorders: implications for a function besides apoptosis. J Gastrointest Surg. 2001;5:352–358. doi: 10.1016/s1091-255x(01)80061-5. [DOI] [PubMed] [Google Scholar]
- 29.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30:628–631. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 30.Scheuplein F, Schwarz N, Adriouch S, et al. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol. 2009;182:2898–2908. doi: 10.4049/jimmunol.0801711. [DOI] [PubMed] [Google Scholar]
- 31.Norman JG, Fink G, Franz M, et al. Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Ann Surg. 1996;223:163–169. doi: 10.1097/00000658-199602000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norman J, Franz M, Messina J, et al. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648–655. doi: 10.1016/s0039-6060(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 33.Marrache F, Tu SP, Bhagat G, et al. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology. 2008;135:1277–1287. doi: 10.1053/j.gastro.2008.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749–755. doi: 10.1097/00003246-199904000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Heresbach D, Letourneur JP, Bahon I, et al. Value of early blood Th-1 cytokine determination in predicting severity of acute pancreatitis. Scand J Gastroenterol. 1998;33:554–560. doi: 10.1080/00365529850172160. [DOI] [PubMed] [Google Scholar]
- 36.Mayer J, Rau B, Gansauge F, et al. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546–552. doi: 10.1136/gut.47.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fink GW, Norman JG. Specific changes in the pancreatic expression of the interleukin 1 family of genes during experimental acute pancreatitis. Cytokine. 1997;9:1023–1027. doi: 10.1006/cyto.1997.0260. [DOI] [PubMed] [Google Scholar]
- 38.Norman JG, Fink GW, Denham W, et al. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783–1788. doi: 10.1023/a:1018886120711. [DOI] [PubMed] [Google Scholar]
- 39.Stimac D, Fisic E, Milic S, et al. Prognostic values of IL-6, IL-8, and IL-10 in acute pancreatitis. J Clin Gastroenterol. 2006;40:209–212. doi: 10.1097/00004836-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Pini M, Sennello JA, Cabay RJ, et al. Effect of diet-induced obesity on acute pancreatitis induced by administration of interleukin-12 plus interleukin-18 in mice. Obesity (Silver Spring) 2010;18:476–481. doi: 10.1038/oby.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sennello JA, Fayad R, Pini M, et al. Interleukin-18, together with interleukin-12, induces severe acute pancreatitis in obese but not in nonobese leptin-deficient mice. Proc Natl Acad Sci U S A. 2008;105:8085–8090. doi: 10.1073/pnas.0804091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueno N, Kashiwamura S, Ueda H, et al. Role of interleukin 18 in nitric oxide production and pancreatic damage during acute pancreatitis. Shock. 2005;24:564–570. doi: 10.1097/01.shk.0000184285.57375.bc. [DOI] [PubMed] [Google Scholar]
- 43.Ueda T, Takeyama Y, Yasuda T, et al. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol. 2006;41:158–165. doi: 10.1007/s00535-005-1735-4. [DOI] [PubMed] [Google Scholar]
- 44.Rau B, Baumgart K, Paszkowski AS, et al. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: high correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit Care Med. 2001;29:1556–1562. doi: 10.1097/00003246-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Wereszczynska-Siemiatkowska U, Mroczko B, Siemiatkowski A. Serum profiles of interleukin-18 in different severity forms of human acute pancreatitis. Scand J Gastroenterol. 2002;37:1097–1102. doi: 10.1080/003655202320378310. [DOI] [PubMed] [Google Scholar]
- 46.Aho HJ, Suonpaa K, Ahola RA, et al. Experimental pancreatitis in the rat. Ductal factors in sodium taurocholate-induced acute pancreatitis. Exp Pathol. 1984;25:73–79. doi: 10.1016/s0232-1513(84)80010-9. [DOI] [PubMed] [Google Scholar]
- 47.Laukkarinen JM, Van Acker GJ, Weiss ER, et al. A mouse model of acute biliary pancreatitis induced by retrograde pancreatic duct infusion of Na-taurocholate. Gut. 2007;56:1590–1598. doi: 10.1136/gut.2007.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandol SJ, Periskic S, Gukovsky I, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117:706–716. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 49.Samuel I, Yuan Z, Meyerholz DK, et al. A novel model of severe gallstone pancreatitis: murine pancreatic duct ligation results in systemic inflammation and substantial mortality. Pancreatology. 2010;10:536–544. doi: 10.1159/000320776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera CA, Bradford BU, Seabra V, et al. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275:G1252–1258. doi: 10.1152/ajpgi.1998.275.6.G1252. [DOI] [PubMed] [Google Scholar]
- 51.Bailey ME. Endotoxin, bile salts and renal function in obstructive jaundice. Br J Surg. 1976;63:774–778. doi: 10.1002/bjs.1800631011. [DOI] [PubMed] [Google Scholar]
- 52.Wig JD, Kochhar R, Ray JD, et al. Endotoxemia predicts outcome in acute pancreatitis. J Clin Gastroenterol. 1998;26:121–124. doi: 10.1097/00004836-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.An H, Xu H, Yu Y, et al. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunol Lett. 2002;81:165–169. doi: 10.1016/s0165-2478(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 55.Fink GW, Norman JG. Intrapancreatic interleukin-1beta gene expression by specific leukocyte populations during acute pancreatitis. J Surg Res. 1996;63:369–373. doi: 10.1006/jsre.1996.0278. [DOI] [PubMed] [Google Scholar]
- 56.De Nardo D, De Nardo CM, Nguyen T, et al. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J Immunol. 2009;183:8110–8118. doi: 10.4049/jimmunol.0901031. [DOI] [PubMed] [Google Scholar]
- 57.Bhan U, Ballinger MN, Zeng X, et al. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS One. 2010;5:9896. doi: 10.1371/journal.pone.0009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guillaumes S, Blanco I, Villanueva A, et al. Chloroquine stabilizes pancreatic lysosomes and improves survival of mice with diet-induced acute pancreatitis. Pancreas. 1997;14:262–266. doi: 10.1097/00006676-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Yasuda H, Leelahavanichkul A, Tsunoda S, et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F1050–1058. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paszkowski AS, Rau B, Mayer JM, et al. Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann Surg. 2002;235:68–76. doi: 10.1097/00000658-200201000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Comert B, Isik AT, Aydin S, et al. Combination of allopurinol and hyperbaric oxygen therapy: a new treatment in experimental acute necrotizing pancreatitis? World J Gastroenterol. 2007;13:6203–6207. doi: 10.3748/wjg.v13.i46.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez-Torres H, Rodriguez-Lomeli X, Davalos-Cobian C, et al. Oral allopurinol to prevent hyperamylasemia and acute pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2009;15:1600–1606. doi: 10.3748/wjg.15.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawa H, Ueda T, Takeyama Y, et al. Role of toll-like receptor 4 in the pathophysiology of severe acute pancreatitis in mice. Surg Today. 2007;37:867–873. doi: 10.1007/s00595-007-3520-x. [DOI] [PubMed] [Google Scholar]
- 64.Parenti DM, Steinberg W, Kang P. Infectious causes of acute pancreatitis. Pancreas. 1996;13:356–371. doi: 10.1097/00006676-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Renner IG, Savage WT, 3rd, Pantoja JL, et al. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- 66.Schneider L, Buchler MW, Werner J. Acute pancreatitis with an emphasis on infection. Infect Dis Clin North Am. 2010;24:921–941. doi: 10.1016/j.idc.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]