Summary
Angioplasty and stenting are emerging alternative treatments to endarterectomy for carotid stenosis. The increasing number of procedures performed carries an increased diagnosis rate of associated asymptomatic intracranial aneurysms, resulting in a clinical and therapeutic dilemma, not fully solved in the literature. When an incidental lesion is found, the first question is whether it is necessary to treat it or not? If treatment is decided, the next question is which should be treated first, the intra or the extracranial lesion?
We review our experience and the literature and discuss our preferred approach of singleprocedure carotid stenting and aneurysm coiling, which we believe is feasible, safe and effective constituting an option when confronted with this difficult therapeutic dilemma.
Key words: angioplasty, stent, carotid, cerebral aneurysm, coiling
Introduction
Endovascular procedures constitute an emerging alternative treatment for extracranial carotid pathology that has resulted in an increased detection rate of asymptomatic intracranial aneurysms.
This retrospective study analyzes the experience in our Centre in patients with concomitant asymptomatic intracranial aneurysms and cervical carotid stenosis. We review the literature and discuss our preferred approach, whenever feasible, of a fully endovascular single procedure.
Methods
We searched our database for patients referred for carotid angioplasty or stenting from 1995 to 2008 who had concomitant significant extracranial carotid stenosis and at least one intracranial aneurysm.
Patient Population
From 1995 to 2008 we performed 277 carotid angioplasties in 269 patients. Patients were referred for diagnostic angiography after extensive neurological investigation and non-invasive carotid doppler sonography. Treatment criteria, according to current approved guidelines, included asymptomatic patients with stenosis greater than 80% or greater than 60% for symptomatic patients. Stenosis grade was assessed according to NASCET criteria.
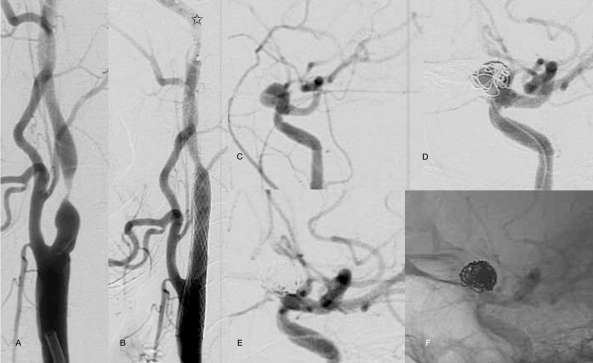
Case 1: A 45-year-old woman who suffered a left middle cerebral artery stroke that was found on angiography to have a preocclusive (98%) left internal carotid artery stenosis as well as an ipsilateral carotid-ophthalmic aneurysm measuring 5 mm. Combined endovascular treatment was planned and achieved successfully (Figure 1).
Figure 1.
Case 1: a 45-year-old female with 98% stenosis in the left internal carotid artery with an ipsilateral incidental carotid-ophthalmic aneurysm measuring 5 mm (A,C). A single procedure combined endovascular treatment was planned. A satisfactory carotid revascularization (B, Protective filter device is present (arrow)) as complete aneurysmal sac exclusion (D intermediate and final (E, F) series) were achieved.
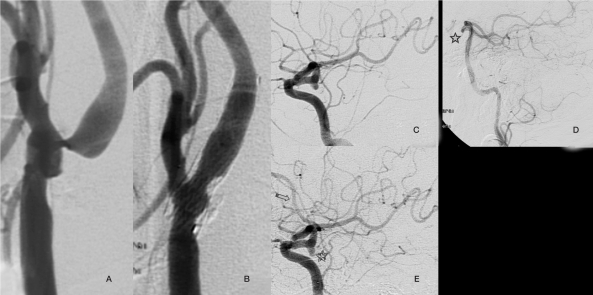
Case 2: A 63-year-old man studied after an episode of amaurosis fugax proved to have on angiography an 80% stenosis in the left carotid bulb as well as a incidental 2 mm ipsilateral posterior communicating artery aneurysm. Carotid angioplasty and stenting were initially performed without associated therapy on the aneurysm. An angiographic study performed six months after the carotid angioplasty showed changes in the intracranial circulation with opacification of the left anterior cerebral artery and posterior cerebral artery whereas previously the flow was inverted in the latter, an appearance in keeping with morphologic changes in the known aneurysm (Figure 2).
Figure 2.
Case 2: a 63-year-old male with 80% stenosis in the left carotid bulb as well as an ipsilateral posterior communicating artery measuring 2 mm. (A,C). Carotid angioplasty and stenting were performed successfully (B). At that time the aneurysm was considered too small to require treatment. A follow-up angiographic performed 6 months after carotid repair showed haemodynamic changes in intracranial circulation with opacification of the anterior cerebral artery (E, arrow) and posterior cerebral artery where as in the previous angiogram there was inversion of flow in the posterior communicating artery (E, star), facts that resulted in changes in the configuration of the aneurysm morphology (E).
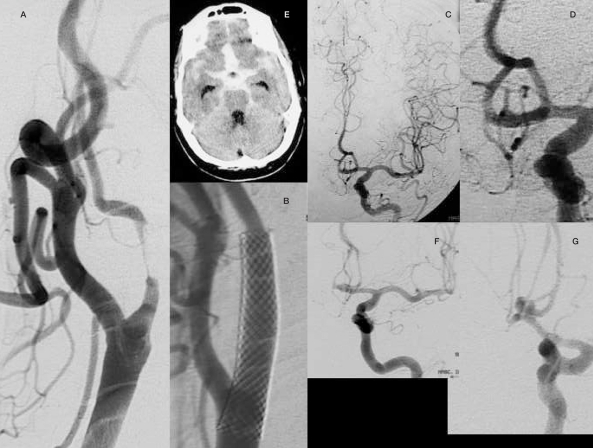
Case 3: A 71-year-old man with a history of right-sided carotid transient ischaemic attacks (TIAs) was found to have on angiography a 95% stenosis in the carotid bulb without associated intracranial lesions. Carotid angioplasty and stenting were performed without complications. One month after the procedure the patient was taken to the emergency department in deep coma and massive subarachnoid bleed was demonstrated on CT. Urgent angiography showed a polylobulated anterior communicating aneurysm measuring approximately 3 mm. the patient suffered a cardiac arrest in the operating room before coiling could be achieved.
Case 4: A 51-year-old man with previous right-sided carotid TIAs with a proven 95% stenosis on angiography. Additionally a 4 mm saccular anterior communicating artery aneurysm was demonstrated. Single procedure combined endovascular treatment was performed without any complications.
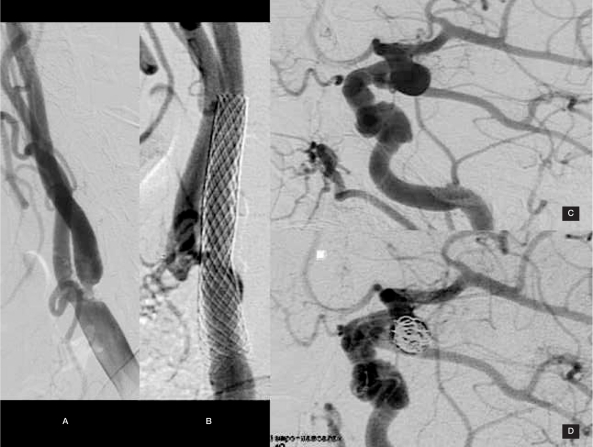
Case 5: A 76-year-old woman with a left basal ganglia infarct was found on angiography to have a 90% stenosis in the left internal carotid artery as well as a 7 mm posterior communicating artery aneurysm coincident with a foetal type left posterior cerebral artery. A combined endovascular procedures was performed successfully (Figure 4).
Figure 3.
Case 3: A 71-year-old female with a high-grade stenosis in the right carotid bulb without associated intracranial lesions (A,C,D).Angioplasty and stenting were performed successfully (B). One month later the patient was admitted to emergency with a subarachnoid haemorrhage (E). An urgent angiogram showed a polylobulated aneurysm in the anterior communicating artery (F,G) that retrospectively could be identified as a minimal irregularity in the previous angiogram (C,D).
Figure 4.
Case 5: A 76-year-old female with 90% left internal carotid artery stenosis (A) and a 7 mm left posterior communicating artery aneurysm with an adjacent foetal-type posterior cerebral artery (C).A combined endovascular treatment was successfully performed with vascular revascularization (B) and aneurysm exclusion (D).
Case 6: A 62-year-old woman with a history of controlled advanced breast carcinoma was being investigated for persistent headaches. A magnetic resonance examination disclosed an incidental aneurysm. Diagnostic angiography showed incidental aneurysms in the right middle cerebral artery bifurcation and right anterior choroidal artery origin respectively as well as a 90% stenosis in the right carotid bulb. Carotid angioplasty and stenting followed by choroidal aneurysm coiling was performed successfully. Unfavourable configuration of the middle cerebral artery with angular branch originating from the sac precluded endovascular treatment. Despite the feasibility of aneurysm clipping, the patient refused to undergo any further treatment.
Case 7: A 56-year-old man suffering from right-sided TIAs was found to have an 80% stenosis in the right carotid bulb as well as a 6 mm saccular aneurysm in the anterior communicating artery. A combined endovascular procedure was performed successfully.
Procedure
Therapeutic procedures were performed by two neuroradiologists from our Unit with patients under conscious sedation and monitored by an anaesthetist. All the procedures were performed after a diagnostic angiography confirmed the carotid stenosis diagnosed by non-invasive techniques (ultrasound doppler or magnetic resonance angiography). This study was used to plan the treatment and choose the required material.
Carotid revascularization was done according to the standardized routine in our Unit which includes the following steps: 1) Distal protective device placement at C1-2 vertebral body level after crossing the stenosis. 2) Depending upon the severity of the stenosis, predilatation with a small diameter balloon is performed prior to stenting. 3) Stent deployment. 4) Balloon angioplasty is done whenever required to achieve full stent expansion.5) Protective distal filter removal.
Using the same sheath, the stent is crossed with a 1.8 F microcatheter and its distal end is placed superselectively into the aneurysm sac which is occluded with GDC coils. Rarely the stent has to be crossed with the sheath due to lack of sufficient support.
Follow-up
Patients were evaluated clinically before, during and immediately after the procedure as well as one and six months afterwards. Besides systemic heparinization during the procedure, all patients had received a double anti-platelet regime with aspirin and clopidogrel that was continued for a month after treatment.
Results
Seven patients were identified retrospectively in our database (three men and four women aged between 45 and 76 years (average 65.7 yrs) with concomitant high-grade extracranial carotid stenosis and cerebral aneurysms. One patient had two lesions, making a total of eight aneurysms which results in an incidence of approximately 2.6% of the carotid stenosis treated (n = 277) during the study period.
Five of the eight aneurysms were located in the same vessel as the stenosis. Three of the eight aneurysms originated in the anterior communicating artery. Aneurysm size ranged from 2 to 10 mm (see Table 1 for details).
Table 1.
Summary of epidemiology data from our cases.
| Patient | Sex | Age | Location of ATP+Stent |
% Carotid stenosis |
Location | Size of aneurysm |
|---|---|---|---|---|---|---|
| 1 | F | 45 | left | 98% | left carotid-ophthalmic artery | 5 mm |
| 2 | M | 63 | left | 80% | left posterior communicating artery | 2 mm |
| 3 | F | 45 | left | 98% | left carotid-ophthalmic artery | 5 mm |
| 4 | M | 51 | right | 95% | anterior communicating artery | 4 mm |
| 5 | F | 76 | left | 90% | left posterior communicating artery | 7 mm |
| 6 | F | 62 | right | 90% | right anterior choroidal artery | 3 mm |
| 6 | F | 62 | right | 90% | right middle cerebral artery bifurcation | 10 mm |
| 7 | M | 56 | right | 80% | anterior communicating artery | 6 mm |
|
(M: male, F: female) (ANT.COM: anterior communicating artery; POST.COM: posterior communicating artery; ANT. Choroidal: anterior choroidal artery; MCA: middle cerebral artery). | ||||||
Carotid stenotic severity ranged from 80 to 98% of the lumen size. Six of the seven patients had a history of neurologic ischaemic events (four transient ischaemic attacks (TIAs) and two brain infarcts). One of the cases was an asymptomatic carotid stenosis (Case 6).
Angiographic follow-up studies performed six months after the treatment did not show any sign of restenosis.
Discussion
Angioplasty and stenting are emerging alternatives to endarterectomy for the treatment of extracranial carotid stenosis. Increased endovascular procedures with full cranial vascular evaluation detect further asymptomatic intracranial lesions. When diagnosed, a dilemma results as to whether to treat this lesion or not and if so,which of the lesions,the carotid stenosis or the intracranial lesion should be treated first.To our knowledge this question is still not definitively clarified in the literature. According to literature concomitant significant carotid stenosis and intracranial lesions are present in 2.8 to 5% of treated patients, an incidence slightly higher than that found in our series 1,2.
The first decision to be made when confronted with a concomitant lesion is whether there is a need to treat an asymptomatic intracranial aneurysm as no definitive consensus is present in the literature in this matter. Natural history studies have shown location and size as risk factors for rupture. However facts are not clear-cut as aneurysms larger than 10 millimetres lesion are associated with an increased risk of rupture but contrary to what one would expect most cases of aneurysmal subarachnoid haemorrhage are due to small aneurysms (3-4 mm). An overall yearly rupture risk of 1-2% is accepted with an estimated mortality risk in the case of rupture of 50% of cases 3,4.
The patient's age is therefore an important element in clinical decision-making.Some studies have shown no statistically significant benefit in repairing an aneurysm related to surgical risks in patients with a life expectancy of less than 15 to 35 years5,6.This becomes even more problematic in the population of our study as most cases of significant carotid stenosis occur in elderly patients as in our group with an average age of 65 years.
However, this risk analysis does not consider the intracranial haemodynamic changes that occur after carotid repair. Increased carotid flow can change the size and morphology of the intracranial aneurysm increasing the risk of rupture. Cases 2 and 3, illustrated in the figures, are example of these changes. In Case 3 we believe hemodynamic changes were responsible for aneurysm rupture and death of the patient.
Some authors favour a conservative approach as concomitant lesions are rare and still carry a low risk of rupture resulting in an overall apparent benign natural history with a low incidence of complications. Isolated carotid repair appears a safe alternative in aneurysms smaller than 10 mm with a low risk of aneurysm rupture in the immediate post-operative period. In this context we have a patient with a successfully revascularized carotid where one aneurysm was left untreated due to patient's refusal to undergo brain surgery that has remained stable on 12 months of follow-up4,8.
Our experience, not necessarily transferable to other centres, is probably influenced by previous fatal events, as in Case 3, and our low rate of treatment morbi-mortality leading us to try aneurysm coiling whenever it is technically feasible.If decided to treat the second question that arises is which lesion should be treated first. Surgical treatment of a cerebral aneurysm in low-flow state secondary to carotid stenosis carries an increased risk of ischaemic events, especially if temporary vascular clips are required during the procedure.
On the other hand, revascularization of a long-standing carotid stenosis that has provoked compensatory intracranial vasodilatory changes to compensate the low-flow status could result in abrupt haemodynamic changes that could negatively influence the aneurysm and result in a catastrophic rupture in the setting of the anticoagulation and anti-platelet regime used for the carotid repair.
Most reports in the literature from the 1980s show a tendency to treat the carotid pathology only as this was the reason for consultation postponing or avoiding treatment of the aneurysms. Recent reports have shown a more aggressive approach undertaking the treatment of both lesions especially in aneurysms larger than 4-5 mm, possibly influenced by the natural history of the disease and increased expertise of the surgical teams 7.
In the surgical references reviewed whenever a combined treatment was performed, carotid endarterectomy preceded aneurysm surgical clipping in most cases. However some authors 7 disagree with this approach and favour clipping the brain aneurysm first as the aneurysmal sac pressure is equivalent to mean systemic blood pressure and there is concern that turbulence within the aneurysm can increase if severe carotid stenosis is repaired and rupture the aneurysm.
Our experience has led us to try to treat both lesions simultaneously. The endovascular route is the best suited for a combined treatment of both lesions, reducing the time the aneurysm is exposed to the haemodynamic changes after carotid revascularization. Vascular instability after endarterectomy occurs in approximately 40% of cases with hypertensive peaks that are difficult to control with medical therapy and induce a significant stress on the intracranial vascular tree. Although haemodynamic instability also occurs after carotid stenting, hypotension is a more common problem and hypertensive peaks are only encountered infrequently. This probably constitutes an additional reason to indicate an endovascular repairing technique.
Conclusions
Combined endovascular treatment of extracranial carotid stenosis and intracranial aneurysms is feasible, safe and effective, and is our preferred option when confronted with this difficult clinical scenario.
References
- 1.Kappelle LG, Eliasziw M, et al. Small, unruptured intracranial aneurysms and management of symptomatic carotid artery stenosis: North American symptomatic carotid endarterectomy trial group. Neurology. 2000;55:307–309. doi: 10.1212/wnl.55.2.307. [DOI] [PubMed] [Google Scholar]
- 2.Pappadà G, Fiori L, et al. Incidence of asymptomatic berry aneurysms among patients undergoing carotid endarterectomy. J Neurosurg Sci. 1997;41:257–262. [PubMed] [Google Scholar]
- 3.The International Study of Unruptured Intracranial Aneurysms Investigators (ISUIA) Engl J Med. 1998;339(24):1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 4.Ladowski JS, Marshall W, et al. Carotid endarterectomy in patients with asymptomatic intracranial aneurysm. Ann Surg. 1984;200(1):70–73. doi: 10.1097/00000658-198407000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiebers DO, Whisnant JP, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 6.Vindlacheruvu RR, Mendelow AD, Mitchell P. Risk-benefit analysis of the treatment of unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2005;76:234–239. doi: 10.1136/jnnp.2003.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappadà G, Fiori L, et al. Management of symptomatic carotid stenoses with coincidental intracranial aneurysms. Acta Neurochir (Wien) 1996;138(12):1386–90. doi: 10.1007/BF01411116. [DOI] [PubMed] [Google Scholar]
- 8.Orecchia PM, Clagett GP, et al. Management of patients with symptomatic extracranial carotid artery disease and incidental intracranial berry aneurysm. J Vasc Surg. 1985;2:158–164. doi: 10.1067/mva.1985.avs0020158. [DOI] [PubMed] [Google Scholar]
- 9.Navaneethan SD, Kannan VS, et al. Concomitant intracranial aneurysm and carotid artery stenosis: a therapeutic dilemma. South Med J. 2006;99(7):757–758. doi: 10.1097/01.smj.0000217190.93989.c9. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Luft AR, et al. Frequency and determinants of postprocedural hemodynamic instability after carotid angioplasty and stenting. Stroke. 1999;30(10):2086–2093. doi: 10.1161/01.str.30.10.2086. [DOI] [PubMed] [Google Scholar]