Abstract
Objective:
Measures of neuronal damage/dysfunction are likely good surrogates for disease progression in Alzheimer disease (AD). CSF markers of neuronal injury may offer utility in predicting disease progression and guiding prognostic and outcome assessments in therapeutic trials. Visinin-like protein-1 (VILIP-1) has demonstrated potential utility as a marker of neuronal injury. We here investigate the utility of VILIP-1 and VILIP-1/Aβ42 in predicting rates of cognitive decline in early AD.
Methods:
Individuals with a clinical diagnosis of very mild or mild AD (n = 60) and baseline CSF measures of VILIP-1, tau, p-tau181, and Aβ42 were followed longitudinally for an average of 2.6 years. Annual assessments included the Clinical Dementia Rating (CDR), CDR–sum of boxes (CDR-SB), and global composite scores. Mixed linear models assessed the ability of CSF biomarker measures to predict rates of cognitive decline over time.
Results:
Baseline CSF VILIP-1 and VILIP-1/Aβ42 levels predicted rates of future decline in CDR-SB and global composite scores over the follow-up period. Individuals with CSF VILIP-1 ≥560 pg/mL (corresponding to the upper tercile) progressed much more rapidly in CDR-SB (1.61 boxes/year; p = 0.0077) and global scores (−0.53 points/year; p = 0.0002) than individuals with lower values (0.85 boxes/year and −0.15 points/year, respectively) over the follow-up period. CSF tau, p-tau181, tau/Aβ42, and p-tau181/Aβ42 also predicted more rapid cognitive decline in CDR-SB and global scores over time.
Conclusion:
These findings suggest that CSF VILIP-1 and VILIP-1/Aβ42 predict rates of global cognitive decline similarly to tau and tau/Aβ42, and may be useful CSF surrogates for neurodegeneration in early AD.
The aggregation and deposition of amyloid-β and tau, the 2 key proteins involved in Alzheimer disease (AD) pathogenesis, are estimated to begin years prior to the onset of cognitive impairment.1,2 However, it is only after a threshold of neuronal loss is reached in vulnerable brain regions that the first signs of cognitive impairment appear.3
Several lines of evidence suggest that neuronal and synaptic loss is the best surrogate for disease progression and cognitive decline in AD.2,4 While CSF tau and amyloid-beta 1–42 (Aβ42) each predominantly reflect a specific AD pathology, neuronal injury/neurodegeneration likely represents the cumulative outcome of different pathologic substrates. Therefore, CSF markers of neuronal injury, along with CSF tau and Aβ42, may offer utility in predicting disease progression and future cognitive decline in the early stages of disease.
Visinin-like protein-1 (VILIP-1) is a neuronal calcium-sensor protein5 which has demonstrated utility as a marker of neuronal injury.6,7 We have previously demonstrated that CSF VILIP-1 and VILIP-1/Aβ42 offer diagnostic and prognostic utility for AD, and may provide useful biomarker surrogates for neurodegeneration.8 We here investigate the utility of CSF VILIP-1 and VILIP-1/Aβ42 in predicting rates of cognitive decline in a well-characterized cohort of individuals with very mild and mild AD who were followed for 2–3 years.
METHODS
Participants and clinical assessments.
We identified participants (n = 60) enrolled in longitudinal studies of healthy aging and dementia through the Washington University Alzheimer's Disease Research Center (WU-ADRC) who met the following criteria: 1) age 60 years or older, 2) clinical diagnosis of very mild (Clinical Dementia Rating [CDR] 0.5) or mild (CDR 1) AD, 3) baseline CSF measures of VILIP-1, tau, phosphorylated tau-181 (p-tau181), Aβ42, and Aβ40, 4) 2 or more annual cognitive assessments, 5) no other medical or psychiatric illness that could contribute importantly to dementia and no medical contraindication to lumbar puncture (LP). APOE genotypes were obtained as described.9
Cognitive assessments were performed annually and included assignment of the CDR,10,11 CDR–sum of boxes (CDR-SB),12 and a 1.5-hour psychometric test battery.11 Study participants had an average of 3 annual cognitive assessments. A CDR designation of 0 indicating no dementia characterizes individuals who are cognitively normal controls, while a CDR 0.5 and CDR 1 designation denotes very mild and mild dementia, respectively. Clinical diagnoses were made in accordance with standard criteria.13,14 Individuals with CDR 0.5 or greater at baseline (n = 60) in this study all had a clinical diagnosis of AD.
A psychometric test battery assessing a broad spectrum of cognitive functions11 was administered to all participants within 1 to 2 weeks of the annual assessment. Standardized test scores were averaged to form 4 composite scores. The episodic memory composite included the sum of the 3 free recall trials from the Selective Reminding Test,15 associate learning subtest of the Wechsler Memory Scale (WMS),16 immediate recall of the WMS Logical Memory, and Benton Visual Retention test. The semantic memory composite included the Information subtest from the Wechsler Adult Intelligence Scale (WAIS),17 Boston Naming Test,18 and Animal Naming.18 The working memory composite included WMS Mental Control, Digit Span Forward and Digit Span Backward, and Letter Fluency for S and P.19 The visuospatial composite included the WAIS Block Design, Digit Symbol subtests, and Trail-making tests A and B.20 The global psychometric composite score used was prorated based on other tests used to generate the original composite score because of changes in the psychometric test battery across the study period (e-Methods on the Neurology® Web site at www.neurology.org).
The reference (normative) group used to standardize most of the tests prior to forming the composites consisted of 310 participants (mean [SD]; age, 74.5 years [8.6]; education, 14.8 years [3.2]) who were enrolled as CDR 0, had at least one annual follow-up assessment, but never progressed to CDR > 0.21 The means and standard deviations of 3 measures (Selective Reminding Test, Animal Naming, Trail Making B) not included in that report were based on the same robust sample but with slightly smaller sample sizes because these 3 tests were added to the battery after its initiation (e-Methods).
Data from a well-characterized cohort of cognitively normal controls (CDR 0; n = 211) enrolled at the WU-ADRC, who were included in a previous study,8 are reported here to demonstrate differences in baseline CSF biomarker and psychometric characteristics between individuals with AD and controls.
Standard protocol approvals, registration, and patient consents.
Studies were approved by the local ethical review board and the Human Studies Committee at Washington University. Informed consent was obtained from all participants.
CSF collection, processing, and assessment.
CSF samples (20–30 mL) were collected from all participants and analyzed for total tau, p-tau181, Aβ42 (Innotest, Innogenetics, Ghent, Belgium),22 and CSF Aβ4023 by enzyme-linked immunosorbent assays as described. CSF samples were analyzed for VILIP-1 by a microparticle-based immunoassay (Erenna, Singulex, CA).
In vivo amyloid imaging.
In vivo amyloid imaging is described in e-Methods.
Statistical analyses.
The primary aim of the study is to determine whether CSF biomarkers/ratios predict annual change in CDR-SB, global, episodic memory, semantic memory, working memory, or visuospatial composite scores over the follow-up period. For this purpose, we used mixed linear models (PROC MIXED; SAS Institute Inc.) that specified a random subject-specific intercept and a random subject-specific slope. These models allow for heterogeneity among subjects in baseline values and rates of change, and account for correlation among repeated measures on the same subject. Analyses were adjusted for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity (i.e., longitudinal CDR-SB models were adjusted for baseline global scores and global or individual composite models were adjusted for baseline CDR-SB to avoid the issue of circularity).
First, we examined whether CSF biomarkers/ratios, as continuous measures, predicted rates of cognitive decline over the follow-up period. CSF biomarker/ratio measures were standardized to z scores prior to analyses. Estimated effects of CSF biomarkers/ratios on annual change in cognitive measures are reported as β. Analyses were then repeated for CSF biomarkers/ratios as categorical variables (dichotomized at the 33rd or 66th percentile value) to determine whether there were significant differences in rates of cognitive decline between individuals in the upper tercile vs those in the lower 2 terciles for each CSF biomarker/ratio (or the lower tercile vs the upper 2 terciles for Aβ42). Baseline cognitive assessments were the closest assessments prior to the time of the LP. Statistical significance was defined as p < 0.05 (e-Methods).
RESULTS
Baseline characteristics of study participants.
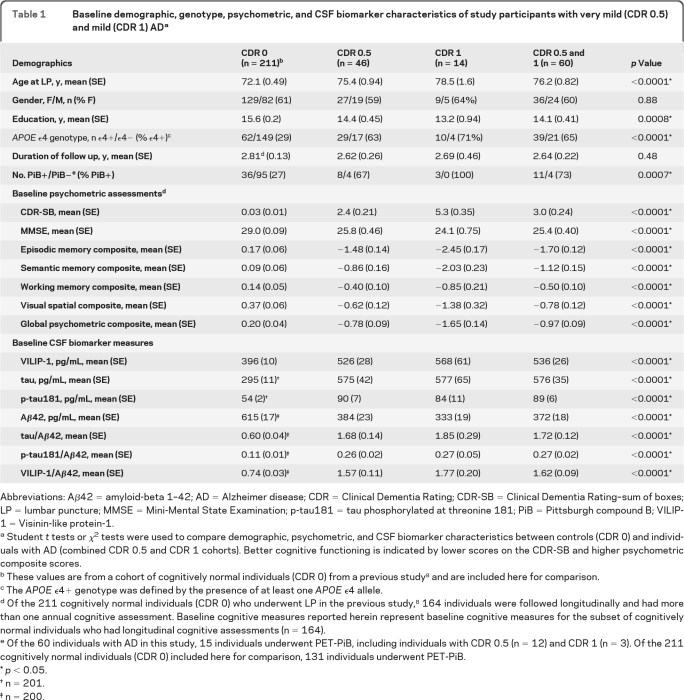
Sixty participants with a clinical diagnosis of AD and a CDR 0.5 (n = 46) or CDR 1 (n = 14) underwent LP and had at least 1 follow-up annual clinical assessment. Mean duration of follow-up was 2.6 years (range 0.9–6.9 years). Table 1 summarizes demographic, psychometric, and CSF biomarker variables at the baseline clinical assessment (median interval, 3.4 months from the LP) for individuals with AD and controls without dementia. Individuals in the CDR 0.5 and CDR 1 cohorts exhibited the typical CSF biomarker phenotype of AD with elevated mean levels of tau and p-tau181 and lower levels of Aβ42. Baseline CSF VILIP-1 and VILIP-1/Aβ42 levels were higher in AD than in controls. Compared to controls, individuals with AD had higher CDR-SB scores, and lower global, episodic memory, semantic memory, working memory, and visuospatial composite scores at the time of the baseline assessment.
Table 1.
Baseline demographic, genotype, psychometric, and CSF biomarker characteristics of study participants with very mild (CDR 0.5) and mild (CDR 1) ADa
Abbreviations: Aβ42 = amyloid-beta 1–42; AD = Alzheimer disease; CDR = Clinical Dementia Rating; CDR-SB = Clinical Dementia Rating–sum of boxes; LP = lumbar puncture; MMSE = Mini-Mental State Examination; p-tau181 = tau phosphorylated at threonine 181; PiB = Pittsburgh compound B; VILIP-1 = Visinin-like protein-1.
Student t tests or χ2 tests were used to compare demographic, psychometric, and CSF biomarker characteristics between controls (CDR 0) and individuals with AD (combined CDR 0.5 and CDR 1 cohorts). Better cognitive functioning is indicated by lower scores on the CDR-SB and higher psychometric composite scores.
These values are from a cohort of cognitively normal individuals (CDR 0) from a previous study8 and are included here for comparison.
The APOEϵ4+ genotype was defined by the presence of at least one APOE ϵ4 allele.
Of the 211 cognitively normal individuals (CDR 0) who underwent LP in the previous study,8 164 individuals were followed longitudinally and had more than one annual cognitive assessment. Baseline cognitive measures reported herein represent baseline cognitive measures for the subset of cognitively normal individuals who had longitudinal cognitive assessments (n = 164).
Of the 60 individuals with AD in this study, 15 individuals underwent PET-PiB, including individuals with CDR 0.5 (n = 12) and CDR 1 (n = 3). Of the 211 cognitively normal individuals (CDR 0) included here for comparison, 131 individuals underwent PET-PiB.
p< 0.05.
n = 201.
n = 200.
No correlations were observed between CSF biomarkers/ratios and cognitive measures at baseline (table e-1). Baseline CSF biomarker measures did not correlate with age or years of education, and did not differ by gender (e-Results).
Correlations between baseline CSF biomarker measures and subsequent change in CDR-SB and global composite scores.
We examined whether baseline CSF biomarker measures (as continuous variables) predicted annual change in CDR-SB and global composite scores over the follow-up period in the AD cohort (n = 60). Analyses were adjusted for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity. The average adjusted rate of cognitive decline (slope ± SE) in the AD cohort was 1.06 ± 0.12 boxes/year in CDR-SB, and −0.28 ± 0.05 points/year in global scores.
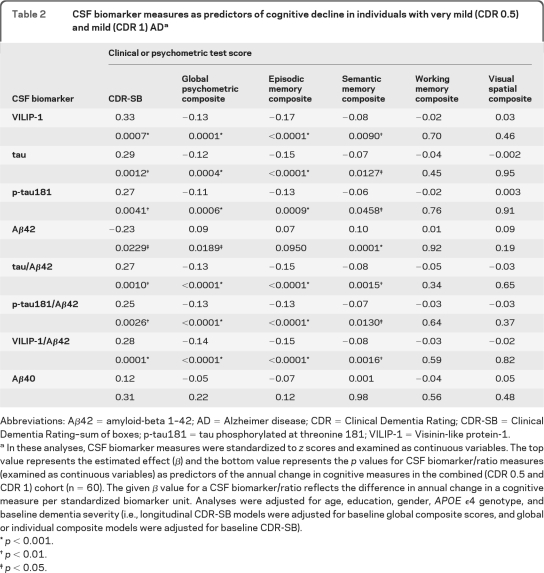
Baseline CSF VILIP-1 and VILIP-1/Aβ42 predicted annual change in CDR-SB and global scores over the follow-up period. With the exception of CSF Aβ40, all other CSF biomarkers/ratios predicted annual change in CDR-SB and global scores over the follow-up period. Table 2 summarizes the estimated effects (β) (top values) and p values (bottom values) for CSF biomarkers/ratios as predictors of annual change in CDR-SB and global scores.
Table 2.
CSF biomarker measures as predictors of cognitive decline in individuals with very mild (CDR 0.5) and mild (CDR 1) ADa
Abbreviations: Aβ42 = amyloid-beta 1–42; AD = Alzheimer disease; CDR = Clinical Dementia Rating; CDR-SB = Clinical Dementia Rating–sum of boxes; p-tau181 = tau phosphorylated at threonine 181; VILIP-1 = Visinin-like protein-1.
In these analyses, CSF biomarker measures were standardized to z scores and examined as continuous variables. The top value represents the estimated effect (β) and the bottom value represents the p values for CSF biomarker/ratio measures (examined as continuous variables) as predictors of the annual change in cognitive measures in the combined (CDR 0.5 and CDR 1) cohort (n = 60). The given β value for a CSF biomarker/ratio reflects the difference in annual change in a cognitive measure per standardized biomarker unit. Analyses were adjusted for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity (i.e., longitudinal CDR-SB models were adjusted for baseline global composite scores, and global or individual composite models were adjusted for baseline CDR-SB).
p < 0.001.
p < 0.01.
p < 0.05.
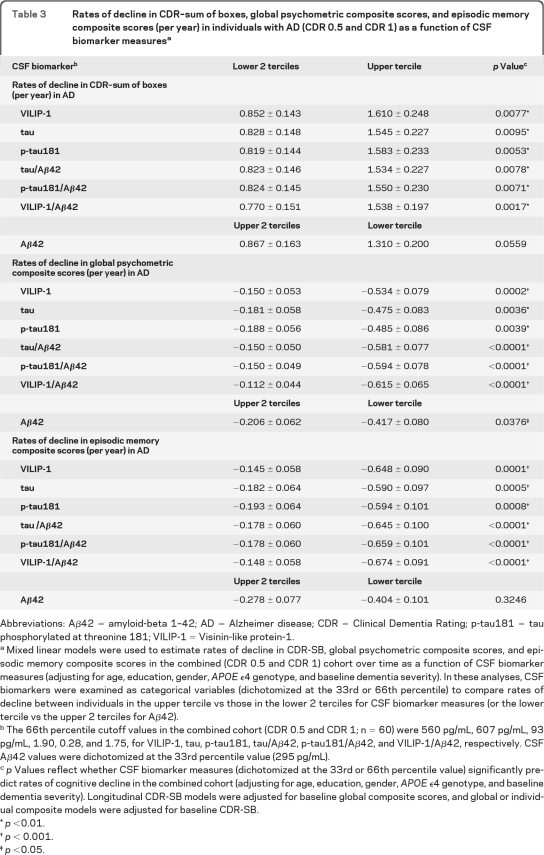
Analyses were then performed for CSF biomarker measures as categorical variables (adjusting for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity). Individuals in the AD cohort (n = 60) were divided into 3 terciles for each CSF biomarker measure (using the 33rd and 66th percentile values as cutoffs). Consistent with our previous results, CSF VILIP-1 and VILIP-1/Aβ42 levels in the upper tercile (corresponding to CSF VILIP-1 ≥560 pg/mL and VILIP-1/Aβ42 ≥1.75) predicted more rapid change in CDR-SB (slope ± SE, 1.61 ± 0.25 and 1.54 ± 0.20 boxes/year, respectively) than those in the lower 2 terciles (0.85 ± 0.14 and 0.77 ± 0.15 boxes/year, respectively). Similarly, CSF VILIP-1 and VILIP-1/Aβ42 levels in the upper tercile predicted more rapid change in global scores than those in the lower 2 terciles. Table 3 and figure e-1, A–D, summarize rates of cognitive decline in CDR-SB and global scores as a function of CSF biomarker terciles in the AD cohort.
Table 3.
Rates of decline in CDR–sum of boxes, global psychometric composite scores, and episodic memory composite scores (per year) in individuals with AD (CDR 0.5 and CDR 1) as a function of CSF biomarker measuresa
Abbreviations: Aβ42 = amyloid-beta 1–42; AD = Alzheimer disease; CDR = Clinical Dementia Rating; p-tau181 = tau phosphorylated at threonine 181; VILIP-1 = Visinin-like protein-1.
Mixed linear models were used to estimate rates of decline in CDR-SB, global psychometric composite scores, and episodic memory composite scores in the combined (CDR 0.5 and CDR 1) cohort over time as a function of CSF biomarker measures (adjusting for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity). In these analyses, CSF biomarkers were examined as categorical variables (dichotomized at the 33rd or 66th percentile) to compare rates of decline between individuals in the upper tercile vs those in the lower 2 terciles for CSF biomarker measures (or the lower tercile vs the upper 2 terciles for Aβ42).
The 66th percentile cutoff values in the combined cohort (CDR 0.5 and CDR 1; n = 60) were 560 pg/mL, 607 pg/mL, 93 pg/mL, 1.90, 0.28, and 1.75, for VILIP-1, tau, p-tau181, tau/Aβ42, p-tau181/Aβ42, and VILIP-1/Aβ42, respectively. CSF Aβ42 values were dichotomized at the 33rd percentile value (295 pg/mL).
p Values reflect whether CSF biomarker measures (dichotomized at the 33rd or 66th percentile value) significantly predict rates of cognitive decline in the combined cohort (adjusting for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity). Longitudinal CDR-SB models were adjusted for baseline global composite scores, and global or individual composite models were adjusted for baseline CDR-SB.
p <0.01.
p < 0.001.
p <0.05.
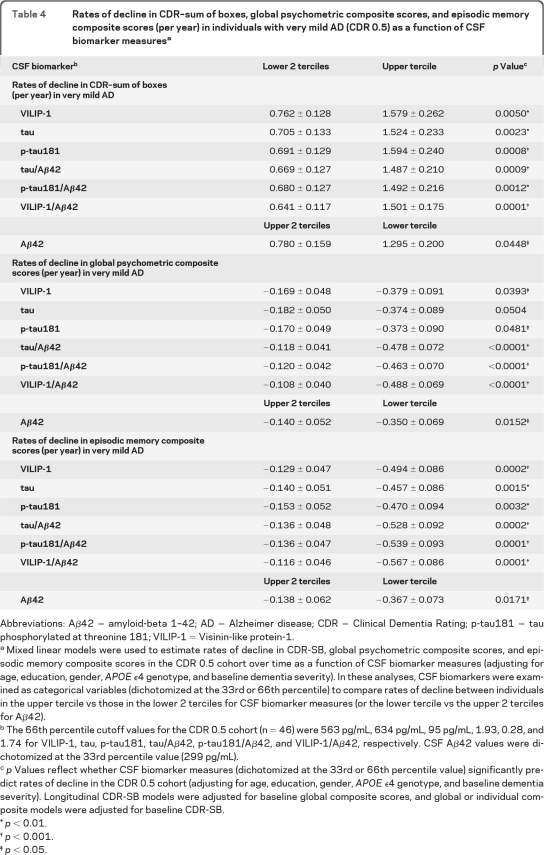
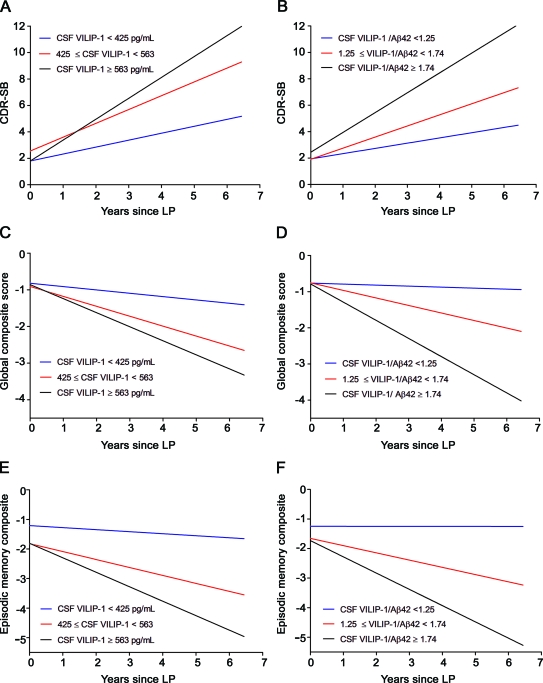
We then examined the utility of CSF biomarkers in predicting cognitive decline in the CDR 0.5 and CDR 1 cohorts separately. Table 4 summarizes rates of cognitive decline in CDR-SB and global scores in the CDR 0.5 cohort as a function of CSF biomarker terciles. In the CDR 0.5 cohort, CSF VILIP-1 or CSF VILIP-1/Aβ42 values in the upper tercile were associated with more rapid change in CDR-SB and global scores than those in the lower 2 terciles, respectively (figure 1). Similar results were seen in the CDR 1 cohort (table e-2 and e-Results).
Table 4.
Rates of decline in CDR–sum of boxes, global psychometric composite scores, and episodic memory composite scores (per year) in individuals with very mild AD (CDR 0.5) as a function of CSF biomarker measuresa
Abbreviations: Aβ42 = amyloid-beta 1–42; AD = Alzheimer disease; CDR = Clinical Dementia Rating; p-tau181 = tau phosphorylated at threonine 181; VILIP-1 = Visinin-like protein-1.
Mixed linear models were used to estimate rates of decline in CDR-SB, global psychometric composite scores, and episodic memory composite scores in the CDR 0.5 cohort over time as a function of CSF biomarker measures (adjusting for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity). In these analyses, CSF biomarkers were examined as categorical variables (dichotomized at the 33rd or 66th percentile) to compare rates of decline between individuals in the upper tercile vs those in the lower 2 terciles for CSF biomarker measures (or the lower tercile vs the upper 2 terciles for Aβ42).
The 66th percentile cutoff values for the CDR 0.5 cohort (n = 46) were 563 pg/mL, 634 pg/mL, 95 pg/mL, 1.93, 0.28, and 1.74 for VILIP-1, tau, p-tau181, tau/Aβ42, p-tau181/Aβ42, and VILIP-1/Aβ42, respectively. CSF Aβ42 values were dichotomized at the 33rd percentile value (299 pg/mL).
p Values reflect whether CSF biomarker measures (dichotomized at the 33rd or 66th percentile value) significantly predict rates of decline in the CDR 0.5 cohort (adjusting for age, education, gender, APOE ϵ4 genotype, and baseline dementia severity). Longitudinal CDR-SB models were adjusted for baseline global composite scores, and global or individual composite models were adjusted for baseline CDR-SB.
p< 0.01.
p< 0.001.
p< 0.05.
Figure 1. Rates of decline in (A and B) Clinical Dementia Rating–sum of boxes (CDR-SB), (C and D) global psychometric composite scores, and (E and F) episodic memory composite scores as a function of CSF VILIP-1 and VILIP-1/Aβ42 terciles in very mild Alzheimer disease (CDR 0.5).
Mixed linear models were used to estimate rates of decline in CDR-SB (A and B), global psychometric composite scores (C and D), and episodic memory scores (E and F) over time in the CDR 0.5 cohort as a function of CSF VILIP-1 and VILIP-1/Aβ42. The slope and intercept for each of the 3 terciles of CSF VILIP-1 and CSF VILIP-1/Aβ42 are plotted. Adjusted rates of cognitive decline in the upper, middle, and lower terciles of VILIP-1 values were 1.58, 1.05, 0.52 boxes/year (respectively) for CDR-SB, −0.38, −0.27, and −0.09 points/year (respectively) for global composite scores, and −0.49, −0.27, and −0.07 points/year (respectively) in episodic memory scores. Adjusted rates of cognitive decline in the upper, middle, and lower terciles of VILIP-1/Aβ42 values were 1.50, 0.84, and 0.40 boxes/year (respectively) in CDR-SB, −0.49, −0.21, and −0.03 points/year (respectively) in global composite scores, and −0.57, −0.25, and −0.001 points/year (respectively) in episodic memory scores. LP = lumbar puncture.
We then examined the utility of CSF biomarker measures in predicting annual change in individual composite scores. CSF VILIP-1, tau, p-tau181, tau/Aβ42, p-tau181/Aβ42, and VILIP-1/Aβ42 predicted change in episodic and semantic memory scores, but not working memory or visuospatial scores in the combined cohort (table 2). Individuals in the upper tercile of VILIP-1 or VILIP-1/Aβ42 values declined more rapidly in episodic memory scores than individuals in the lower 2 terciles. Similar results were seen in the CDR 0.5 and CDR 1 cohorts when examined separately. Table 3 and figure e-1, E–F, summarize rates of decline in episodic memory as a function of CSF biomarker terciles in the AD cohort. Table 4 and figure 1, E–F, summarize rates of decline in episodic memory in the CDR 0.5 cohort, and table e-2 in the CDR 1 cohort.
DISCUSSION
VILIP-1 is a highly expressed neuronal calcium-sensor protein,5 which has demonstrated utility as a neuronal injury marker in brain injury models and gene-array analyses.6 Increased CSF VILIP-1 levels and altered expression patterns of VILIP-1 in AD may reflect the selective vulnerability of VILIP-1-expressing neurons to calcium-mediated neurodegeneration in the presence of AD pathology.24 VILIP-1 is detected in dystrophic neurites and in close association with amyloid plaques and neurofibrillary tangles (NFT), but does not appear to be a component of NFT.8,24 CSF VILIP-1 levels correlate with whole brain and regional atrophy in early symptomatic AD, and with amyloid load in cognitively normal individuals.8 Together, these findings support the potential utility of CSF VILIP-1 as a biomarker surrogate for neurodegeneration in AD.8
We have previously shown that CSF VILIP-1 levels can predict future cognitive impairment in cognitively normal individuals similarly to tau and p-tau181 over a 2- to 3-year follow-up period.8 We here investigate the utility of CSF VILIP-1 in predicting rates of cognitive decline in a well-characterized cohort of cognitively impaired individuals with very mild and mild AD who were followed for 2–3 years. Our results suggest that CSF VILIP-1, alone or in combination with Aβ42 (VILIP-1/Aβ42), predicts rates of cognitive decline over this follow-up period. The clear and ordered separation of the rates of cognitive decline in CDR-SB and global scores among the 3 terciles for CSF VILIP-1 and VILIP-1/Aβ42, and the ability of these markers to predict rates of decline when examined as continuous measures, highlight their predictive ability independently of the cutoff values proposed in this study.
Importantly, CSF VILIP-1 and other CSF markers of AD pathology predicted annual cognitive decline even after adjusting for baseline cognitive performance. Since the rate of cognitive decline in AD is not linear and may differ by disease stage,25 these findings are particularly notable and suggest that CSF biomarkers, including VILIP-1, may complement information provided by clinical assessments in guiding prognostic and therapeutic decisions in clinical practice or in trials of disease-modifying therapies.
Consistent with previous reports,26 CSF tau, p-tau181, tau/Aβ42, and p-tau181/Aβ42 predicted rates of future cognitive decline over this follow-up period. As previously described,27 CSF Aβ42 levels appeared to be a less significant predictor of cognitive decline in our cohort than CSF tau, p-tau181, and VILIP-1; low CSF Aβ42 levels were associated with higher rates of progression in the CDR 0.5 but not in the CDR 1 cohort. While the initial decrease in CSF Aβ42 levels is thought to occur a decade or longer prior to the onset of cognitive impairment,1,2,28,29 once they are low,22 CSF Aβ42 levels remain relatively stable for years in impaired and unimpaired individuals.30,31 Conversely, following the earliest signs of cognitive impairment, progressive increase in NFT pathology and progressive neuronal loss on a background of substantial Aβ accumulation correlates with further cognitive decline and disease progression in AD.28 Therefore, it is likely that this new low set point for Aβ42 can predict rates of decline over the 2- to 3-year follow-up period in the CDR 0.5 cohort, but not in more advanced disease stages, while CSF VILIP-1 (reflective of neuronal/synaptic degeneration) and tau/p-tau181 (reflective of NFT formation) correlate more closely with disease progression in the very mild (CDR 0.5) and mild (CDR 1) stages.
Our findings support the notion that substantial neuronal loss/neurodegeneration is present by the time the earliest signs of cognitive impairment appear, and correlates well with clinical disease progression in AD.28,32 CSF Aβ42 and tau levels each predominantly reflect a specific AD pathology, and do not appear to change considerably with clinical disease progression.30,31 Conversely, neuronal/synaptic loss likely reflects the cumulative outcome of different pathologic substrates. Therefore, CSF markers which capture neuronal loss/neurodegeneration, such as VILIP-1, may offer predictive value for future cognitive decline that is at least comparable to that of CSF markers of tau and amyloid. Theoretically, such markers may also demonstrate response to a treatment that decreases neurodegeneration independently of changes to Aβ42 and tau. In the cohort studied herein, VILIP-1 predicted future cognitive decline over the follow-up period at least as well as tau, p-tau181, and Aβ42. Our results suggest trends for a potentially superior predictive performance for VILIP-1 to tau or Aβ42 in the AD cohort over a 2- to 3-year follow-up period (tables 2 and 3). However, while VILIP-1 is similar to tau in its prognostic ability, we cannot say for sure at this time whether VILIP-1 is better than tau in this regard from our current data. It will be important to study larger cohorts of individuals with longer durations of follow-up, and from different centers, to further evaluate the predictive performance of VILIP-1 in comparison to other markers of AD pathology.
Our study is similar to previous studies examining rates of decline in AD,26,27,32 but differs from other studies in which dichotomous outcomes of conversion/no conversion from very mild (CDR 0.5 or MCI) to mild (CDR 1) AD were examined.33,34 There is a great deal of variability in baseline cognitive scores (CDR-SB or psychometric test scores) among individuals with AD within the same CDR category, and in the degree of further impairment required for the transition between global CDR categories. The use of outcome measures with more gradation to measure cognitive decline (e.g., CDR-SB, psychometric test scores) takes such interindividual variability into consideration, and likely provides better insight into the biological or radiologic correlates of disease progression among different individuals over time.
Consistent with previous reports,21,35,36 our CDR 0.5 cohort showed impairment in baseline episodic memory compared to controls, with less severe impairment in semantic memory, working memory, and visuospatial composite scores. Clinicopathologic and radiologic studies suggest that hippocampal and parahippocampal regions mediate episodic memory functions,35,37 while polar temporal, inferior temporal, and anterior fusiform regions are implicated in semantic memory.38 Our observation that VILIP-1 levels correlate with decline in episodic and semantic memory is consistent with early involvement of medial temporal and fusiform regions (respectively) by AD pathology,39 and with our findings that CSF VILIP-1 levels correlate with atrophy of these regions in early AD.8
Together, these findings highlight the potential utility of CSF VILIP-1 in guiding trial design, outcome assessment, and prognostic decisions in clinical trials of disease-modifying therapies and clinical settings. The incorporation of CSF VILIP-1, along with other CSF biomarkers, into such trials may assist in the accurate selection of homogeneous study participants, by limiting enrollment to individuals who are likely to progress within the study period based on their CSF biomarker values. Our study is limited by the relatively few individuals with mild dementia, and by the short duration of follow-up. Validation of these findings in larger populations of individuals with mild to moderate AD and with longer durations of follow-up will be of interest in the future. The evaluation of a larger number of CSF samples using the VILIP-1 immunoassay utilized in this study and efforts to standardize this assay across different centers will be the focus of future studies, similar to what has been or is currently being done for tau and Aβ42 (e-Comment).
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Kenneth Schechtman, Dr. Catherine Roe, Dr. Chengjie Xiong, Dr. Gina D'Angelo, Dr. Martha Storandt, Dr. Dan Crimmins, Elizabeth Macy, Jay McQuillan, and the Clinical, Psychometrics, Biomarker, Imaging and Biostatistics Cores of the Charles F. and Joanne Knight AD Research Center at Washington University.
GLOSSARY
- Aβ42
amyloid-beta 1–42;
- AD
Alzheimer disease
- CDR
Clinical Dementia Rating
- CDR-SB
Clinical Dementia Rating–sum of boxes
- LP
lumbar puncture
- NFT
neurofibrillary tangle
- VILIP-1
Visinin-like protein-1
- WAIS
Wechsler Adult Intelligence Scale
- WMS
Wechsler Memory Scale
- WU-ADRC
Washington University Alzheimer's Disease Research Center
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Tarawneh: study design, data acquisition, analysis and interpretation of data, statistical analyses, drafting manuscript. Dr. Lee: study design, analysis and interpretation of data, revising manuscript for medical content. Dr. Ladenson: study design, analysis and interpretation of data, obtaining funding, revising manuscript for medical content, contribution of vital reagents/tools. Dr. Morris: study design, obtaining funding, revising manuscript for medical content, contribution of vital reagents/tools. Dr. Holtzman: study design, analysis and interpretation of data, drafting and revising manuscript for medical content, contribution of vital reagents/tools, study coordination, obtaining funding.
DISCLOSURE
Dr. Tarawneh reports no disclosures. Dr. Lee has served as a consultant for Johnson & Johnson and receives research support from AstraZeneca, the NIH/NINDS, the Barnes-Jewish Hospital Foundation, and the McDonnell Foundation. Dr. Ladenson has received research support from Siemens Health Care Diagnostics and is a co-inventor on pending patents filed by Washington University concerning brain biomarkers. Dr. Morris serves on scientific advisory boards for AstraZeneca, Bristol-Myers Squibb, Genentech, Inc., Merck Serono, Novartis, Pfizer Inc, Schering-Plough Corp., Eli Lilly and Company, Wyeth, and Elan Corporation; serves on the editorial advisory board of Alzheimer's Disease and Associated Disorders; receives royalties from publishing Mild Cognitive Impairment and Early Alzheimer's Disease (John Wiley and Sons, 2008), Dementia (Clinical Publishing, 2007), Handbook of Dementing Illnesses, 2nd edition (Taylor & Francis, 2006), and for an editorial in Lancet Neurology (Elsevier, 2008); and receives research support from Elan Corporation, Wyeth, Eli Lilly and Company, Novartis, Pfizer Inc, Avid Radiopharmaceuticals, the NIH, and from the Dana Foundation. Dr. Holtzman serves on scientific advisory boards for Satori Pharmaceuticals, C2N Diagnostics, and EnVivo Pharmaceuticals; serves as an Associate Editor of Annals of Neurology, the Journal of Neuroscience, Neurobiology of Disease, and Experimental Neurology; may accrue revenue on pending patents re: Methods for measuring the metabolism of neurally derived biomolecules in vivo; Use of anti-AB antibody to treat traumatic brain injury; Methods to treat Alzheimer's disease or other amyloid beta accumulation associated disorders; Humanized antibodies that sequester abeta peptide; Diagnostic for early stage Alzheimer's disease; and Predictive diagnostic for Alzheimer's disease; serves as a consultant to Merck Serono, Eli Lilly and Company, Takeda Pharmaceutical Company Limited, Abbott, Comentis, Inc., Eisai Inc., and AstraZeneca; is cofounder of and receives board of directors compensation from C2N Diagnostics LLC; receives research support from AstraZeneca, Pfizer Inc., Eli Lilly and Company, Elan Corporation, Forest Laboratories, Inc., the NIH, Cure Alzheimer's Fund, and Fidelity Foundation; has received compensation from Washington University from license revenue received for licensing of patent applications to C2N Diagnostics LLC; and may receive future royalty payments for Washington University licensing patents to C2N Diagnostics, LLC, and Eli Lilly and Company.
REFERENCES
- 1. Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol 1999;45:358–368 [DOI] [PubMed] [Google Scholar]
- 2. Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Price JL, Ko AI, Wade MJ, et al. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol 2001;58:1395–1402 [DOI] [PubMed] [Google Scholar]
- 4. Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 2005;65:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braunewell K, Riederer P, Spilker C, et al. Abnormal localization of two neuronal calcium sensor proteins, visinin-like proteins (vilips)-1 and -3, in neocortical brain areas of Alzheimer disease patients. Dement Geriatr Cogn Disord 2001;12:110–116 [DOI] [PubMed] [Google Scholar]
- 6. Laterza OF, Modur VR, Crimmins DL, et al. Identification of novel brain biomarkers. Clin Chem 2006;52:1713–1721 [DOI] [PubMed] [Google Scholar]
- 7. Lee JM, Blennow K, Andreasen N, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer's disease patients. Clin Chem 2008;54:1617–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tarawneh R, D'Angelo G, Macy E, et al. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol 2011;70:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talbot C, Lendon C, Craddock N, et al. Protection against Alzheimer's disease with apoE epsilon 2. Lancet 1994;343:1432–1433 [DOI] [PubMed] [Google Scholar]
- 10. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 11. Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology 2006;67:467–473 [DOI] [PubMed] [Google Scholar]
- 12. Berg L, Miller JP, Baty J, et al. Mild senile dementia of the Alzheimer type: 4: evaluation of intervention. Ann Neurol 1992;31:242–249 [DOI] [PubMed] [Google Scholar]
- 13. Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 1998;55:326–335 [DOI] [PubMed] [Google Scholar]
- 14. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216 [DOI] [PubMed] [Google Scholar]
- 15. Grober E, Buschke H, Crystal H, et al. Screening for dementia by memory testing. Neurology 1988;38:900–903 [DOI] [PubMed] [Google Scholar]
- 16. Wechsler D, Stone CP. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973 [Google Scholar]
- 17. Wechsler D. Manual: Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955 [Google Scholar]
- 18. Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders, 2nd ed. Philadelphia: Lea & Febiger; 1983 [Google Scholar]
- 19. Thurstone LL, Thurstone LG. Examiner Manual for the SRA Primary Mental Abilities Test. Chicago: Chicago Science Research Associates; 1949 [Google Scholar]
- 20. Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psychol Monographs 1946;60:1–48 [Google Scholar]
- 21. Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol 2009;66:1254–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59:512–519 [DOI] [PubMed] [Google Scholar]
- 23. Cirrito JR, May PC, O'Dell MA, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci 2003;23:8844–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schnurra I, Bernstein HG, Riederer P, Braunewell KH. The neuronal calcium sensor protein VILIP-1 is associated with amyloid plaques and extracellular tangles in Alzheimer's disease and promotes cell death and tau phosphorylation in vitro: a link between calcium sensors and Alzheimer's disease? Neurobiol Dis 2001;8:900–909 [DOI] [PubMed] [Google Scholar]
- 25. Ito K, Corrigan B, Zhao Q, et al. Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimers Dement 2011;7:151–160 [DOI] [PubMed] [Google Scholar]
- 26. Snider BJ, Fagan AM, Roe C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol 2009;66:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kester MI, van der Vlies AE, Blankenstein MA, et al. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology 2009;73:1353–1358 [DOI] [PubMed] [Google Scholar]
- 28. Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004;62:925–931 [DOI] [PubMed] [Google Scholar]
- 29. Tarawneh R, Holtzman DM. Critical issues for successful immunotherapy in Alzheimer's disease: development of biomarkers and methods for early detection and intervention. CNS Neurol Disord Drug Targets 2009;8:144–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer's disease. Neurosci Lett 2007;419:18–22 [DOI] [PubMed] [Google Scholar]
- 31. Andreasen N, Hesse C, Davidsson P, et al. Cerebrospinal fluid beta-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol 1999;56:673–680 [DOI] [PubMed] [Google Scholar]
- 32. Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology 2009;73:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herukka SK, Hallikainen M, Soininen H, Pirttila T. CSF Abeta42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology 2005;64:1294–1297 [DOI] [PubMed] [Google Scholar]
- 34. Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006;5:228–234 [DOI] [PubMed] [Google Scholar]
- 35. Dudas RB, Clague F, Thompson SA, et al. Episodic and semantic memory in mild cognitive impairment. Neuropsychologia 2005;43:1266–1276 [DOI] [PubMed] [Google Scholar]
- 36. De Jager CA, Hogervorst E, Combrinck M, Budge MM. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer's disease. Psychol Med 2003;33:1039–1050 [DOI] [PubMed] [Google Scholar]
- 37. Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 1999;22:425–444 discussion 444–489 [PubMed] [Google Scholar]
- 38. Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol 2001;11:194–201 [DOI] [PubMed] [Google Scholar]
- 39. Convit A, de Asis J, de Leon MJ, et al. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer's disease. Neurobiol Aging 2000;21:19–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.