Abstract
Objectives To develop a measure of decision-making involvement in children and adolescents with cystic fibrosis, diabetes, and asthma. Methods Parent–child dyads completed the Decision-Making Involvement Scale (DMIS) and measures of locus of control and family communication. DMIS items were subjected to exploratory and confirmatory factor analysis (CFA). Temporal stability and construct validity were assessed. Results The parent form was reduced to 20 items representing five factors. CFA showed that the five factors were an acceptable fit to the parent- and child-report data. Internal consistency values ranged from 0.71 to 0.91. Temporal stability was supported by moderate–substantial intraclass correlation coefficients. DMIS subscales were associated with child age, child locus of control, and family communication. Conclusions The DMIS can be used to inform our understanding of the transition to greater independence for illness management. Additional research is needed to examine outcomes of decision-making involvement, including treatment adherence and responsibility.
Keywords: adolescents, asthma, children, chronic illness, cystic fibrosis, decision making; diabetes, involvement, participation
Introduction
The treatment regimen for a pediatric chronic illness can require attention to nutrition, physical activity, medications and treatments, and symptom management. Parents usually have responsibility for managing these issues when the child is young, but this can become more difficult when the child begins to desire more decision making autonomy. The family's approach to this transition is likely to influence the development of effective self-management skills, including decision making related to illness tasks.
Children's decision-making involvement (DMI) is a potentially important component of the transition to greater independence (Liprie, 1993; White, 1996; Wills, Blechman, & McNamara, 1996). We define DMI as the ways in which children can contribute to the decision-making process, independent of who makes the final decision. For example, children can be provided with information, express an opinion, suggest ideas, share information, choose among options provided by parents, or negotiate with parents (Baylis, Downie, & Kenny, 1999; Joffe, 2003; McCabe, 1996; Miller, 2009; Weithorn, 1983). DMI is hypothesized to teach the factors to consider when making decisions, consequences of different decisions, and communication skills that are necessary to influence decisions (White, 1996; Wills et al., 1996). In addition, DMI may enhance self-efficacy (Liprie, 1993; White, 1996; Wills et al., 1996), promote the ability to cope with illness (McCabe, 1996; Walker & Doyon, 2001; Schmidt, Petersen, & Bullinger, 2003), and impact the development of effective self-management, by allowing the child to practice decision making without bearing the full weight of the decision (Wills et al., 1996).
Children's DMI for chronic illness management has received little empirical attention, and a review of the literature found no instruments to assess it. Prior research and instruments have focused on treatment responsibility, which has to do with who performs treatment tasks (e.g., Anderson, Auslander, Jung, Miller, & Santiago, 1990); decision-making autonomy, which has to do with who makes decisions about normative or illness-related issues (e.g., Devine, Wasserman, Gershenson, Holmbeck, & Essner, 2011); or parent involvement/support, which has to do with parental assistance with illness-related tasks and emotions (e.g., Hanna, DiMeglio, & Fortenberry, 2005; Nansel et al., 2009). In contrast, DMI focuses on the multiple ways in which children can be involved in decision making, which may be more important for the development of autonomy and effective self-management than who ultimately makes the decision. Unlike constructs assessed by existing instruments, children's DMI focuses on the process of decision-making interactions so is not bound to illness-specific issues.
The aim of this study was to develop a measure of DMI, called the Decision-Making Involvement Scale (DMIS), in children and adolescents with type 1 diabetes, cystic fibrosis, or asthma. These conditions have differences in terms of prognosis and treatment, but all are life-threatening, can involve a burdensome and complex regimen, and may pose challenges to adherence and the transition to independence. Empirical data related to DMI are important for each group, and the various ways for children to be involved in decision making are the same regardless of the illness type. In addition, our intent was to develop a generic measure that was applicable across conditions, so it was important to test the instrument in more than one group.
Our approach to measure development proceeded in several phases and included (a) item development based on qualitative data and a literature review; (b) administration of the experimental item pool to children and adolescents and their parents; (c) factor analysis to identify underlying dimensions of children's DMI, which we expected to include parent behaviors that engage the child in the decision, such as expressing an opinion or advice, providing options to the child, sharing information, and asking for the child's opinion or ideas; child behaviors that indicate involvement in decision making, such as expressing an opinion, sharing information, or asking for the parent's advice or opinion; and joint behaviors, such as brainstorming and negotiating; (d) item, subscale, and composite score analysis to explore the properties of the new instrument and document shared variance; (e) tests of measurement invariance between parent- and child-report versions of the DMIS; and (f) examination of the temporal stability of DMIS scores. We expected that parent and child perceptions of children's DMI in the context of a specific parent–child discussion would be stable across a 1 to 2 week time period.
We also assessed the convergent and discriminant validity of the new instrument, by testing associations of the DMIS with child age, child health locus of control, and family communication. First, we hypothesized that DMI would change with the child's age. We expected that children's tendency to express an opinion would increase with age, while parents' tendency to express an opinion would decrease with age, consistent with qualitative research on health decision making (Geller, Tambor, Bernhardt, Fraser, & Wissow, 2003; Miller, Reynolds, & Nelson, 2008). We expected joint decision-making behaviors to increase with age (Smetana, Campione-Barr, & Daddis, 2004). We did not expect parents’ and children's tendency to seek information or opinions from each other to change with age, due to the importance of information-sharing (Miller et al., 2008) and parental involvement (Nansel et al., 2009) across development. Although not specifically hypothesized, we tested for a curvilinear relationship between all DMIS subscales and child age, given prior research regarding developmental patterns in parent–child interactions. For example, Larson and colleagues (Larson et al., 1996) found that adolescents’ positive affect in family interactions decreased in early adolescence and increased in late adolescence, and Smetana 1995 reported that parent–adolescent conflict and communication worsen during early adolescence and improve thereafter. Thus, we wanted to test for the possibility that parent–child decision-making interactions would also show nonlinear relationships with age.
Second, we hypothesized that DMI would be associated with the child's health locus of control. Specifically, we expected children with an internal orientation to be more likely to express their opinions because they believe they can influence the decision that is made. In contrast, we expected children with an orientation toward powerful others to be less likely to express an opinion and more likely to seek information and advice from the parent, because of the belief that those with authority control what happens (Crittendon, 1990; Gordon, 1996).
Finally, we hypothesized that DMI would be associated with family communication. Specifically, we expected that better family communication would be associated with children's tendency to seek information and advice from parents (Brown & Mann, 1990; Pianta & Harbers, 1996) and to express an opinion, because the family environment is one that promotes exchange of opinions and feelings (Brown & Mann, 1990). We also expected that better family communication would be associated with more joint decision making (Geller et al., 2003).
Methods
Recruitment
Participants were recruited from June 2008 through May 2010 at an urban, tertiary care pediatric hospital in the northeastern USA. Eligible participants included children and adolescents between the ages of 8 and 19 years, with a diagnosis of either asthma, cystic fibrosis (CF), or type 1 diabetes for at least 6 months, and their parents. Eligibility criteria also included that the child lived with the parent, the parent and child had a discussion about illness management in the last 2 weeks, the child did not have a major developmental delay, and the child's treatment regimen involved daily management. The latter criterion was straightforward for the diabetes and CF groups; for the asthma group, we defined daily management as being prescribed daily controller medication according to the parent. One parent and child per family were eligible to participate. We identified potential participants from clinic schedules and inpatient census data, contacted them by phone or in person, assessed eligibility, explained the study, and solicited their willingness to participate.
A total of 301 parent–child dyads were contacted and assessed for eligibility. Of these, 28 were ineligible due to age, illness type or duration of illness (n = 6); treatment regimen did not involve daily management (n = 6); developmental delay (n = 4); child/adolescent not living at home (n = 3); no parent–child discussion about illness management in the last two weeks (n = 3); and miscellaneous other reasons (n = 6). Of the 273 parent–child dyads who were eligible for the study, 267 (97.8%) agreed to participate. Of these, 28 (10.5%) could not be scheduled or could not be reached again and 239 (89.5%) participated in the study. Of those who participated, eight (3.3%) were withdrawn from the database due to data validity issues (e.g., child's understanding of items was questionable). An additional five (2.1%) dyads started the questionnaire packet but did not complete it. The final sample for the analysis consisted of 226 participants. A comparison of the final sample to those who were eligible but not included in the sample (n = 47) showed that they did not differ in terms of child sex, race, illness group, or age.
Procedures
The study was approved by the institutional review board. Study personnel first explained the elements of informed consent to the parent and provided a developmentally appropriate explanation to the child. After the parent provided consent and permission and the child assented (or consented in the case of 18- to 19-year olds), study personnel described the questionnaires and reviewed the instructions. Following completion of the questionnaires, participants received $20 each in appreciation for their time and effort.
Temporal Stability
We asked a subset of participants to complete the DMIS a second time and return it in a self-addressed, stamped envelope. We instructed them to complete it during a 1-week window, starting 1 week after the initial administration. On the first page of the retest DMIS, study personnel documented the specific discussion parents and children should refer to when completing the items, which was the same discussion that was identified for the original administration (see ‘Measures’ section). Initially, we asked every third dyad to complete the retest materials, but a low response rate (see ‘Results’ section) necessitated that we ask every participant to complete them.
Measures
DMIS
The experimental item pool for the DMIS was generated from a literature review and qualitative data (see Miller, 2009). Drawing on the construct definition, literature review, and qualitative data, we maintained a list of potential items. Items were reviewed by the team1 for content validity, readability, grammar, and developmental appropriateness (i.e., making sure items could be understood by younger children). The final experimental item pool consisted of 23 items (see Appendix Table A1).
To administer the DMIS, the interviewer first assisted the dyad in identifying a discussion they had about chronic illness management in the last 2 weeks. We did not require the dyad to identify a specific decision, because much of what happens around illness management, including decisions, is embedded in the context of daily life and may not be perceived as discrete events by families (Angst & Deatrick, 1996; Miller & Drotar, 2003). However, we assumed that by restricting discussions to those having to do with illness management, most would deal with a problem/barrier for which a course of action would need to be identified or decided. Parents and children then independently completed the items. The response options were Not at all = 1, A little bit = 2, A moderate amount = 3, and A lot = 4. An additional seven items were included as filler to reduce socially desirable responding (see Appendix Table A1). Participants also responded “yes” or “no” to the following three items: “My child made a decision,” “I made a decision,” and “We made a decision together.” These three items and the seven filler items were not included in the factor analysis.
The DMIS was piloted with 12 parent–child dyads. Based on their feedback, minor changes were made to the sequence and wording of items. These 12 dyads were not part of the final sample of 226 dyads used in the analyses.
Demographics
Parents completed a demographic form that assessed characteristics of the child, parent, and family. It also included a single item to assess perceived severity of the child's illness on a 1 to 5 scale (1 = Insignificant, 3 = Moderate, and 5 = Severe).
Multidimensional Health Locus of Control Scales
Children completed the Multidimensional Health Locus of Control Scales (MHLOC) (Thompson, Webber, & Berenson, 1987), which assesses the extent to which children believe they control their health versus the influence of chance or powerful others. It contains 18 items and yields three subscales that were derived from factor analysis (Thompson et al., 1987). The MHLOC has been used in prior research in children ages 7 through 17 years (Malcarne, 2005). We planned to use both the Internal and Powerful Others subscales to test our hypotheses, but the Cronbach's α for the Internal scale was unacceptably low in our sample (.49); this finding is similar to prior research (e.g., Malcarne, 2005; Stanton, Raja, & Langley, 1995). As such, we used only the Powerful Others subscale, which had a minimally acceptable (DeVellis, 1991) α of .67. Scores on the Powerful Others subscale were calculated as the average of the six items on the subscale and ranged from 1 to 4; higher scores indicate a greater orientation towards powerful others.
Family Adaptability and Cohesion Evaluation Scales
Parents and children ages 12 years and older completed the Family Adaptability and Cohesion Evaluation Scales (FACES-IV), a 42-item measure that assesses several domains of family functioning (Gorall, Tiesel, & Olson, 2004). The validity of the FACES-IV was supported by associations with general family functioning and family satisfaction (Gorall et al., 2004). It has been used in prior research with children as young as 11 years (Franklin, Streeter, & Springer, 2001). We used the Communication subscale, which consists of 10 items and had a Cronbach's α of .86 for parent report and .84 for child report in the present sample. Possible scores range from 10 to 50, with higher scores indicating better communication.
Analytic Plan
Coding of Discussion Categories
We categorized the discussions participants identified into content areas, so that we could better understand the context participants were referring to when completing items. The second author read through the descriptions of the discussions for all dyads and developed preliminary categories. The first author reviewed and edited these, ending up with six categories. The second author then categorized all of the discussions. The first author reviewed all of the data; we resolved discrepancies via discussion and made corrections to the database if necessary. Since the discussion categories were for descriptive purposes and consensus between the two coders was achieved, the calculation of inter-rater reliability was not necessary.
Exploratory and Confirmatory Factor Analysis
For parent report on the DMIS, repeated exploratory factor analysis (EFA), with principal axis factoring and promax rotation, was used to identify the most appropriate number of underlying factors and describe those factors using the fewest and most informative items. We chose an oblique solution because of the potential for correlated factors. Criteria for the number of factors to retain included consideration of eigenvalues >1.0, scree plots, and interpretability (Ford, MacCallum, & Tait, 1986). We removed or retained items with each iteration based on several criteria, including strength of factor loadings (≥.40) (Ford et al., 1986) and minimal co-loadings across factors (Costello & Osborne, 2005). We defined co-loading as an item having a factor loading of .30 or higher on a second factor and separation of <.10 between the two factor loadings. However, co-loading did not necessarily lead to item removal, because it is not unexpected for there to be multiple high loadings in a factor solution (Ford et al., 1986). Overall, the goal was to find the best-fitting solution that was also conceptually interpretable.
After obtaining an acceptable factor solution using EFA, we applied confirmatory factor analysis (CFA) to determine if the model was a good fit to the data. Error terms were allowed to correlate if they improved fit (Byrne, Shavelson, & Muthen, 1989). We assessed goodness-of-fit by examining the root mean squared error of approximation (RMSEA) and the comparative fit index (CFI) (Bentler & Chou, 1987; Browne & Cudeck, 1993). For RMSEA, values of <.05 are good, values between .05 and .08 are acceptable, and values between .08 and .10 are marginal (Bentler & Chou, 1987; Browne & Cudeck, 1993). For CFI, values >.95 are good and values >0.90 are adequate (Bentler & Chou, 1987; Browne & Cudeck, 1993). We did not examine chi-square as an index of goodness-of-fit, because it is sensitive to sample size and likely to yield a significant difference even when actual differences are small (Bentler & Bonett, 1980).
We expected the underlying factors of the DMIS to be similar for parent and child report, and we wanted to avoid interpretive difficulties as a result of different factor structures. As such, we tested whether the obtained factor structure for the parent-report data was a good fit to the child-report data using CFA.
When we were satisfied with the results of EFA and CFA, we calculated DMIS subscale and total scores as the average of the participant's responses on the relevant items and compared these scores by illness group and child sex, using ANOVAs and t-tests.
Reliability Estimates and Item, Subscale, and Composite Correlations
We calculated reliability estimates using Cronbach's α, for the combined sample and for each illness group. We categorized values of .65–.70 as minimally acceptable and values of .70 and above as acceptable (DeVellis, 1991; Nunnally & Bernstein, 1994). We computed corrected item–total, subscale–total, corrected item–subscale, and subscale–subscale correlations to document shared variance among items and subscales. We expected item–subscale correlations to be >.40 and higher than item–total correlations, as evidence of the item's discriminant validity (Ware & Gandek, 1998). We expected subscale–subscale correlations to be less than the subscale reliability coefficients, as evidence that each subscale measures unique and reliable variance (Ware & Gandek, 1998).
Measurement Invariance Between Parent and Child Report
We used multiple-group CFA to test for measurement invariance between parent- and child report on the DMIS. This approach involves testing increasingly constrained models, beginning with configural invariance (equality of form), followed by weak/metric (equality of factor loadings), strong/scalar (equality of item intercepts), and strict (equality of unique error terms) invariance (Gregorich, 2006; Meredith & Teresi, 2006). Chi-square difference tests were used to determine if a model was significantly different from the prior, less constrained model. Chi-square difference tests with nonsignificant p-values (p > .05), decrements in CFI of ≥.01, and increases in RMSEA of ≥.015 were interpreted as supporting measurement invariance (Cheung & Rensvold, 2002; Vandenberg & Lance, 2000).
Temporal Stability
We computed intraclass correlation coefficients, utilizing two way random effects models, to assess the temporal stability of DMIS scores (Shrout & Fleiss, 1979). We report the single-measure ICC, because the unit of analysis we are interested in is scores on a single administration of the DMIS, not mean DMIS scores across administrations (see Shrout & Fleiss, 1979, for more detail on different forms of the ICC). We expected coefficients to be >.40, which would be considered at least moderate agreement across observations (Landis & Koch, 1977).
Validity Analyses
We utilized multivariate regression analyses in the general linear model framework to test the hypotheses related to child age, child health locus of control, and family communication. We included a single set of covariates in all of the analyses. These included demographic variables that were significantly different between the three illness groups (child age, parent age, child race, income, and illness duration; Table I) and three additional variables that have been related to shared management of chronic illness in prior research: child sex, perceived illness severity, and parent education level. We also tested for curvilinear relationships between DMI and child age using quadratic regression. If the quadratic term for age was not significant, we removed it from the final model. We report the unstandardized beta and the semipartial eta-square (η2), a measure of the proportion of variance in the dependent variable uniquely accounted for by the independent variable. Data were analyzed using SPSS v16 and SAS v9.1.
Table I.
Demographic and Illness Characteristics
| Variable, n (%) or M (SD) | Combined sample (n = 226) | Cystic fibrosis (n = 68) | Type 1 diabetes (n = 90) | Asthma (n = 68) | Test statistica | p-value |
|---|---|---|---|---|---|---|
| Child age | 12.97 (3.14) | 13.01 (2.94) | 13.61 (3.20) | 12.09 (3.08) | F = 4.71 | .01 |
| Parent age | 42.44 (7.23) | 42.68 (6.51) | 43.64 (7.70) | 40.60 (7.02) | F = 3.54 | .03 |
| Child sex (female) | 112 (49) | 32 (47) | 52 (58) | 28 (41) | χ2 = 4.51 | .12 |
| Parent sex (female) | 199 (88) | 56 (82) | 79 (88) | 64 (94) | χ2 = 4.48 | .11 |
| Child race | χ2 = 33.14 | <.0001 | ||||
| White | 163 (72) | 61 (90) | 70 (78) | 32 (47) | ||
| African-American/Black | 48 (21) | 5 (7) | 16 (18) | 27 (40) | ||
| Asian | 4 (2) | 0 | 2 (2) | 2 (3) | ||
| Amer. Indian/Alaskan Native | 1 (<1) | 1 (1) | 0 | 0 | ||
| Other | 10 (4) | 1 (1) | 2 (2) | 7 (10) | ||
| Parent education | χ2 = 12.21 | .27 | ||||
| Some high school | 5 (2) | 0 (0) | 1 (1) | 4 (6) | ||
| Completed high school | 40 (18) | 14 (21) | 12 (13) | 14 (21) | ||
| Some college or technical school | 78 (35) | 24 (35) | 32 (36) | 22 (32) | ||
| College graduate | 59 (26) | 14 (21) | 30 (33) | 15 (22) | ||
| Some post-graduate education | 17 (7) | 7 (10) | 5 (6) | 5 (7) | ||
| Masters, PhD, MD, law degree | 27 (12) | 9 (13) | 10 (11) | 8 (12) | ||
| Incomeb | χ2 = 25.90 | .004 | ||||
| Less than $19 999 | 19 (9) | 3 (4) | 5 (6) | 11 (16) | ||
| $20 000–$39 999 | 32 (15) | 8 (12) | 6 (7) | 18 (26) | ||
| $40 000–$59 999 | 36 (16) | 15 (22) | 13 (15) | 8 (12) | ||
| $60 000–$79 999 | 34 (15) | 9 (13) | 16 (18) | 9 (13) | ||
| $80 000–$99 999 | 35 (16) | 10 (15) | 17 (19) | 8 (12) | ||
| More than $100 000 | 65 (29) | 21 (31) | 31 (35) | 13 (19) | ||
| Illness duration (years) | 8.53 (4.85) | 11.63 (3.93) | 5.62 (3.95) | 9.61 (4.46) | F = 42.21 | <.0001 |
| Illness severity (1–5; parent report) | 3.52 (0.97) | 3.09 (0.84) | 3.86 (0.94) | 3.51 (0.98) | F = 13.38 | <.0001 |
aFor comparison of illness groups.
bData related to income were missing for five participants.
Results
Participants
Table I presents demographics for the combined sample and by illness group.
Description of DMIS Discussions
The most frequent type of discussion identified by participants from all three illness groups had to do with the child's routine treatment regimen and staying healthy, such as how to fit in airway clearance, how much insulin to inject, or avoiding asthma triggers (n = 143; 63.2%). The next most frequent categories had to do with activities and their impact on treatment (n = 28; 12.4%) and dealing with symptoms (n = 28; 12.4%). Additional categories were interactions with the healthcare system (n = 16; 7.1%), changing the treatment regimen (n = 9; 4%), and psychosocial issues (n = 2; 0.9%).
Ninety-four percent (n = 213) of parents answered “yes” to at least one of the following items: “My child made a decision,” “I made a decision,” and “We made a decision together,” suggesting that, as expected, there was a decision involved in the majority of discussions.
Descriptive Information for the DMIS Experimental Item Pool
Missing data for the DMIS items were minimal. Four out of 226 parents (1.8%) were missing one item, and 11 out of 226 children (4.9%) were missing one or two items. Given this low percentage (Cohen & Cohen, 1983), we used sample mean replacement for these missing data. For the 23 DMIS items, each of the four response options was selected by at least three parents and seven children. For parents, item means ranged from 1.9 to 3.5 (SDs ranged from 0.8 to 1.3), while for children they ranged from 2.1 to 3.4 (SDs 0.8 to 1.2).
Exploratory and Confirmatory Factor Analysis
For parent report, five factors based on 20 items emerged from the exploratory factor analysis (Table II). The three items that were omitted were 4, 14, and 28 (see Appendix Table A1). Based on the conceptual meaning of the factors, we named the factors as follows: child express (e.g., express an opinion or give information to parent), child seek (e.g., ask for advice or information from parent), parent express (e.g., express an opinion, give advice, or information to child), parent seek (e.g., ask for child's opinion or listen to child), and joint/options (e.g., negotiate, provide options to child). Item 29 (“My child gave me information”) had a primary loading of .41 on child express and a secondary loading of .35 on child seek. Item 15 (“I asked my child for his/her opinion”) had a primary loading of .42 on parent seek and a secondary loading of .35 on child express. The total variance accounted for by the five-factor model was 66%. The CFA applied to the parent-report data attained an acceptable goodness-of-fit, with an RMSEA of .0722 (90% confidence interval: 0.0617–0.0826) and a CFI of .9065. All items served as significant indicators of their respective factors.
Table II.
Factor Loadings of the Final DMIS Items Based on Exploratory Factor Analysis: Parent Report
| Item | Mean (SD) | F1 Child seek | F2 Parent express | F3 Joint/ options | F4 Child express | F5 Parent seek |
|---|---|---|---|---|---|---|
| 24. My child asked me for information | 1.9 (1.0) | 0.95 | 0.04 | 0.06 | −0.02 | −0.12 |
| 27. My child asked questions | 2.2 (1.0) | 0.81 | 0.00 | 0.13 | 0.15 | −0.15 |
| 26. My child asked for my advice or opinion | 2.0 (1.0) | 0.75 | 0.06 | −0.01 | 0.07 | 0.08 |
| 12. I gave my child information | 3.0 (1.0) | 0.20 | 0.80 | −0.24 | −0.13 | 0.08 |
| 11. I expressed my opinion | 3.3 (0.8) | −0.15 | 0.69 | −0.03 | 0.20 | −0.06 |
| 20. I gave my child feedback about how he/she has been taking care of his/her illness | 3.0 (1.0) | 0.05 | 0.58 | −0.08 | 0.12 | −0.02 |
| 10. I suggested ideas or gave advice | 2.9 (1.0) | −0.07 | 0.54 | 0.29 | 0.01 | 0.08 |
| 8. I tried to teach my child something related to the illness | 2.4 (1.1) | 0.19 | 0.45 | 0.27 | −0.18 | −0.03 |
| 5. I explained different options about what to do | 2.5 (1.1) | −0.14 | 0.32 | 0.63 | −0.01 | −0.16 |
| 9. I gave my child options to choose from | 2.2 (1.1) | −0.09 | 0.13 | 0.63 | 0.08 | 0.02 |
| 1. We negotiated | 2.2 (1.0) | 0.04 | −0.11 | 0.57 | 0.16 | −0.21 |
| 3. We brainstormed about what to do | 1.9 (1.0) | 0.24 | −0.16 | 0.54 | −0.02 | −0.02 |
| 6. I asked my child if he/she had any questions | 2.2 (1.2) | 0.21 | −0.07 | 0.53 | −0.28 | 0.26 |
| 23. My child expressed an opinion | 2.8 (1.0) | 0.01 | 0.19 | 0.03 | 0.73 | −0.13 |
| 21. My child suggested ideas | 2.3 (1.0) | 0.18 | −0.14 | 0.09 | 0.72 | 0.03 |
| 29. My child gave me information | 2.5 (1.0) | 0.35 | −0.03 | −0.21 | 0.41 | 0.21 |
| 17. I listened to what my child had to say | 3.5 (0.8) | −0.17 | −0.06 | −0.14 | −0.06 | 0.96 |
| 19. I asked my child for information | 2.7 (1.0) | 0.15 | 0.15 | −0.14 | −0.02 | 0.52 |
| 16. I told my child that his/her opinion was important | 2.7 (1.3) | 0.08 | −0.03 | 0.29 | 0.11 | 0.47 |
| 15. I asked my child for his/her opinion | 2.6 (1.1) | −0.08 | 0.09 | 0.28 | 0.35 | 0.42 |
Note. Values in bold typeface indicate the factor onto which each item loaded.
We then tested whether the factor structure for the parent-report data was a good fit to the child-report data using CFA. The results showed that the model was an acceptable fit to the data; the RMSEA was .0668 (90% confidence interval: 0.0560–0.0775) and the CFI was .9043. All items served as significant indicators of their respective factors. All factor loadings were >.40, with the exception of item 11 (“My mom/dad expressed his/her opinion”), which had a factor loading of .32 on parent express (Table IV).
Table IV.
Factor Loadings of the Final DMIS Items Based on Confirmatory Factor Analysis: Child Report
| Item | Mean (SD) | F1 Child seek | F2 Parent express | F3 Joint/ options | F4 Child express | F5 Parent seek |
|---|---|---|---|---|---|---|
| 24. I asked my mom/dad for information | 2.2 (1.1) | .78 | ||||
| 27. I asked questions | 2.3 (1.1) | .71 | ||||
| 26. I asked for my mom/dad's advice or opinion | 2.3 (1.1) | .80 | ||||
| 12. My mom/dad gave me information | 2.7 (1.1) | .64 | ||||
| 11. My mom/dad expressed his/her opinion | 3.1 (0.9) | .32 | ||||
| 20. My mom/dad gave me feedback about how I have been taking care of my illness | 2.7 (1.0) | .55 | ||||
| 10. My mom/dad suggested ideas or gave advice | 2.7 (1.0) | .67 | ||||
| 8. My mom/dad tried to teach me something related to my illness | 2.1 (1.1) | .63 | ||||
| 5. My mom/dad explained different choices about what to do | 2.4 (1.0) | .65 | ||||
| 9. My mom/dad gave me choices about what to do | 2.4 (1.1) | .69 | ||||
| 1. We negotiated | 2.1 (1.1) | .43 | ||||
| 3. We brainstormed together about what to do | 2.1 (1.0) | .55 | ||||
| 6. My mom/dad asked me if I had any questions | 2.1 (1.1) | .65 | ||||
| 23. I expressed an opinion | 2.7 (1.0) | .66 | ||||
| 21. I suggested ideas | 2.3 (1.0) | .75 | ||||
| 29. I gave my mom/dad information | 2.3 (1.1) | .66 | ||||
| 17. My mom/dad listened to what I had to say | 3.3 (1.0) | .57 | ||||
| 19. My mom/dad asked me for information | 2.2 (1.0) | .57 | ||||
| 16. My mom/dad told me that my opinion was important | 2.5 (1.2) | .77 | ||||
| 15. My mom/dad asked me for my opinion | 2.6 (1.1) | .79 |
Mean subscale and total scores, for the combined sample and by illness group, are presented in Table III. There were no differences between the three illness groups on any of the DMIS subscales or the total score, for parent or child report. Parents of male children reported higher levels of parent express compared to parents of female children [t(214) = 2.23, p < .03]. Male children reported lower levels of child express compared to female children [t(224) = −2.84, p = .005].
Table III.
Internal Consistency, Means, SDs, and Range for DMIS Subscales
| DMIS Subscale | Combined sample (n = 226) | Cystic fibrosis (n = 68) | Type 1 diabetes (n = 90) | Asthma (n = 68) |
|---|---|---|---|---|
| Parent report | ||||
| Child seek | ||||
| α | .91 | .89 | .91 | .92 |
| M (SD) | 2.02 (.94) | 1.90 (.86) | 2 (.94) | 2.15 (1.02) |
| Range | 1–4 | 1–4 | 1–4 | 1–4 |
| Child express | ||||
| α | .76 | .80 | .76 | .74 |
| M (SD) | 2.52 (.84) | 2.37 (.83) | 2.54 (.83) | 2.64 (.85) |
| Range | 1–4 | 1–4 | 1–4 | 1–4 |
| Parent seek | ||||
| α | .77 | .73 | .80 | .75 |
| M (SD) | 2.86 (.80) | 2.70 (.77) | 2.91 (.82) | 2.97 (.80) |
| Range | 1.25–4 | 1.25–4 | 1.50–4 | 1.25–4 |
| Parent express | ||||
| α | .78 | .76 | .83 | .72 |
| M (SD) | 2.91 (.71) | 2.93 (.66) | 2.91 (.77) | 2.88 (.69)1 |
| Range | 1–4 | 1.40–4 | 1–4 | –4 |
| Joint/options | ||||
| α | .72 | .71 | .76 | .68 |
| M (SD) | 2.21 (.72) | 2.25 (.68) | 2.26 (.75) | 2.10 (.73) |
| Range | 1–4 | 1–4 | 1–4 | 1–3.40 |
| Total | ||||
| α | .89 | .89 | .91 | .87 |
| M (SD) | 2.53 (.59) | 2.48 (.56) | 2.56 (.62) | 2.56 (.58) |
| Range | 1.35–4 | 1.40–3.95 | 1.35–4 | 1.45–3.85 |
| Child report | ||||
| Child seek | ||||
| α | .81 | .85 | .83 | .73 |
| M (SD) | 2.27 (.93) | 2.19 (.96) | 2.20 (.90) | 2.44 (.92) |
| Range | 1–4 | 1–4 | 1–4 | 1–4 |
| Child express | ||||
| α | .73 | .77 | .77 | .62 |
| M (SD) | 2.44 (.84) | 2.28 (.84) | 2.47 (.87) | 2.54 (.81) |
| Range | 1–4 | 1–4 | 1–4 | 1–4 |
| Parent seek | ||||
| α | .76 | .74 | .72 | .81 |
| M (SD) | 2.64 (.82) | 2.59 (.79) | 2.57 (.77) | 2.78 (.90) |
| Range | 1–4 | 1–4 | 1–4 | 1–4 |
| Parent express | ||||
| α | .71 | .74 | .63 | .76 |
| M (SD) | 2.68 (.69) | 2.65 (.70) | 2.71 (.61) | 2.66 (.79) |
| Range | 1–4 | 1–4 | 1.20–4 | 1–4 |
| Joint/options | ||||
| α | .74 | .71 | .76 | .75 |
| M (SD) | 2.22 (.75) | 2.19 (.71) | 2.22 (.75) | 2.24 (.80) |
| Range | 1–4 | 1–4 | 1–4 | 1–4 |
| Total | ||||
| α | .91 | .91 | .90 | .92 |
| M (SD) | 2.46 (.64) | 2.40 (.63) | 2.45 (.60) | 2.53 (.69) |
| Range | 1.15–4 | 1.15–4 | 1.15–3.80 | 1.20–3.85 |
Reliability Estimates and Item, Subscale, and Composite Correlations
Cronbach's α's are presented in Table III, for the combined sample and by illness group. For parent report, α's ranged from 0.72 to 0.91. The α's were similar across the illness groups and >.70, with the exception of joint/options, which had an α of .68 in the asthma group. Item–total correlations for the 20-item set ranged from a low of .34 for item 1 to a high of .73 for item 15 (M = 0.51, SD = 0.11). As expected, item–subscale correlations were higher than these and were >.40, with the exception of item 1 (“We negotiated”): child express = .55–.68, child seek = .80–.82, parent express = .48–.62, parent seek = .45–.67, and joint/options = .39–.57. Subscale–total correlations ranged from .65 to .82 (M = 0.74, SD = 0.06). As expected, subscale–subscale correlations (M = 0.44, SD = 0.14, range: 0.18 to 0.58) were less than the subscale α's.
For child report, α's ranged from .71 to .91. The α's were similar across the illness groups and >.70, with the exception of parent express, which had an α of .63 in the diabetes group, and child express, which had an α of .62 in the asthma group. Item–total correlations for the 20-item set ranged from a low of .31 for item 11 to a high of .69 for item 26 (M = 0.54, SD = 0.09). As expected, item–subscale correlations were higher than these and were >.40, with the exception of item 11 (“My mom/dad expressed her/his opinion”): child express = .52–.60, child seek = .61–.71, parent express = .32–.54, parent seek = .45–.67, and joint/options = .41–.59. Subscale–total correlations ranged from .72 to .84 (M = 0.80, SD = 0.05). As expected, subscale–subscale correlations (M = 0.56, SD = 0.08, range: 0.39–0.62) were less than the subscale α's.
Measurement Invariance Between Parent and Child Report
Finally, we tested for measurement invariance between parent- and child-report on the DMIS (Table V). Model 1 was unconstrained and tested whether the number and pattern of factor loadings was the same for parent and child report. This model had an acceptable goodness-of-fit, with a CFI of .9055 and an RMSEA of .0695. Model 2 was compared to Model 1 and added the constraint that the factor loadings were the same for child and parent report. This model also had an acceptable goodness-of-fit, with a CFI of .9031 and an RMSEA of .0688. The change in chi-square was not significant (p = .07), and changes in CFI and RMSEA were minimal. Thus, weak/metric invariance between the parent and child report forms was supported. Model 3 was compared to Model 2 and had the additional constraint that the item intercepts were the same for child and parent report. This model did not have an acceptable goodness-of-fit, with a CFI of .8788 and an RMSEA of .0747. The change in chi-square was significant (p < .0001), and changes in CFI and RMSEA were substantial. Thus, strong/scalar invariance between the parent and child report forms was not supported.
Table V.
Multiple-Group Confirmatory Factor Analysis Tests of Measurement Invariance across Parent and Child Report
| Model fit |
Model difference |
||||||
|---|---|---|---|---|---|---|---|
| Models | df | χ2 | CFI | RMSEA [90% CI] | Δ χ2 | Δ CFI | Δ RMSEA |
| M1: Configural invariance | 314 | 655.59 | .9055 | .0695 (0.0620–.0770) | – | – | – |
| M2: Weak/metric invariance | 329 | 679.44 | .9031 | .0688 (0.0615–0.0761) | 23.85 | −.0024 | −.0007 |
| M3: Strong/scalar invariance | 349 | 787.09 | .8788 | .0747 (0.0678–0.0816) | 107.65* | −.0243 | .0057 |
*p < .0001.
Temporal Stability
We gave the retest materials to 137 dyads and 69 (50%) returned them. Forty-nine (71%) of the dyads completed the materials within the correct time frame. Of these, one parent left an entire page blank. Thus, the retest sample consisted of 48 parents and 49 children. The retest sample did not differ from the rest of the sample in terms of illness group, child age, or child duration of illness. However, children in the retest sample were more likely to be Caucasian [X2(1) = 5.36, p < .03] and male [X2(1) = 4.88, p < .03]. The average number of days between the initial completion of the DMIS and the retest was 10.98 (SD = 2.26, range 7–14).
ICCs were as follows: parent report of child express = .45; parent report of child seek = .66; parent report of parent express = .70; parent report of parent seek = .58; parent report of joint/options = .69; parent total score = .65; child report of child express = .68; child report of child seek = .68; child report of parent express = .50; child report of parent seek = .69; child report of joint/options = .64; child total score = .77. All of these values indicated moderate to substantial agreement across observations (March & Sullivan, 1999).
Construct Validity
Associations of DMI with Age
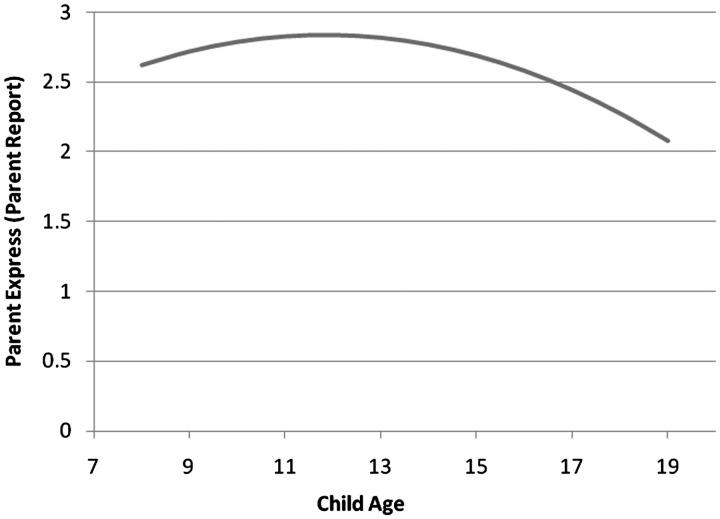
As expected, parent report of child express (B = 0.08, SE = 0.02, p < .001, semipartial η2 = .06) and child report of child express (B = 0.08, SE = 0.02, p < .001, semipartial η2 = .05) increased with child age. The quadratic term testing for a curvilinear relationship between parent report of parent express and child age was significant (B = −0.01, SE = 0.01, p < .02, semipartial η2 = .03). The estimation function showed that parent report of parent express increased with child age until the age of 12 years and decreased thereafter (Figure 1). As expected, there was no relationship between child age and parent or child report of child seek or parent seek. Contrary to expectation, there was no relationship between child age and child report of parent express or parent or child report of joint/options.
Figure 1.
Estimation Function for Relationship between Child Age and Parent Express (Parent Report).
Associations of DMI with Child Health Locus of Control
As expected, scores on the powerful others subscale were positively associated with parent report of child seek (B = .27, SE = 0.13, p < .04, semipartial η2 = .02) and child report of child seek (B = 0.43, SE = .13, p = .001, semipartial η2 = .05). In other words, children with a locus of control oriented toward powerful others were more likely to seek information and advice from parents, according to both child and parent report. Scores on the powerful others subscale were associated with child report of child express but in the opposite direction of what was predicted (B = 0.28, SE = 0.11, p < .02, semipartial η2 = .03). In other words, children with a locus of control oriented toward powerful others were more likely to express an opinion and information to parents. Contrary to expectation, scores on the powerful others' subscales were not associated with parent report of child express.
Associations of DMI With Family Communication
As expected, parent report of family communication was positively associated with parent report of child express (B = 0.02, SE = 0.01, p < .03, semipartial η2 = .02) and parent report of child seek (B = 0.03, SE = 0.01, p < .01, semipartial η2 = .03). Contrary to the hypotheses, parent report of family communication was not associated with parent report of joint/options or child report of child seek, child express, or joint/options.
As expected, child report of family communication (n = 134; ages 12 and above only) was positively associated with parent report of child express (B = 0.03, SE = 0.01, p < .05, semipartial η2 = .03), child report of child express (B = 0.03, SE = 0.01, p < .02, semipartial η2 = .05), parent report of child seek (B = 0.04, SE = 0.01, p < .02, semipartial η2 = .05), and child report of child seek (B = 0.06, SE = 0.01, p < .0001, semipartial η2 = .14). As expected, child report of family communication was positively associated with child report of joint/options (B = 0.02, SE = 0.01, p < .05, semipartial η2 = .03), but it was not associated with parent report of joint/options.
Discussion
The DMIS is a promising new instrument that addresses a gap in the literature by measuring children's involvement in decisions about chronic illness management. The DMIS adds to the body of instruments assessing related constructs by focusing on different ways for children and adolescents to be involved in decisions, which may be the primary means by which they learn to make decisions on their own. Existing measures focus on decision-making autonomy, treatment responsibility, or parental involvement; these measures cannot shed light on how parents and children interact about decisions and whether different types of child involvement are more or less helpful at different ages. Given that it is the child who must eventually assume responsibility for illness management, the child's behaviors related to this transition are critical. The DMIS subscales, derived from exploratory and confirmatory factor analysis, are internally consistent and have moderate to substantial temporal stability. The subscales reflect conceptual domains related to expressing and seeking information, advice, or opinions, joint decision making (i.e., negotiating and brainstorming), and parental provision of options. The preliminary validity of the DMIS is supported by associations with child age, child health locus of control, and family communication. Strengths of the DMIS include that it is a generic measure that can be used with various chronic illness groups, assesses both child and parent behaviors indicative of child involvement, and reflects a variety of ways for children to be involved in decision making, independent of who makes the decision.
The DMIS also has some weaknesses. The tests of measurement invariance between the parent- and child-report versions of the instrument supported weak/metric invariance, but not strong/scalar invariance. In other words, the factor loadings were equal, which suggests that the construct can be conceptualized the same way across groups (Steenkamp & Baumgartner, 1998; Gregorich, 2006). However, the mean item intercepts were not equal across groups; influences unrelated to the latent variables may have caused higher or lower item responses in one group compared to the other (Gregorich, 2006). As such, group differences in observed DMIS means may not correspond to differences in the underlying factor means (Meredith & Teresi, 2006). There are conflicting recommendations in the literature regarding whether strong invariance is needed to make valid group comparisons, especially in the context of basic research or when test bias is not a concern (Cheung & Rensvold, 1999; Meredith & Teresi, 2006; Steenkamp & Baumgartner, 1998). We suggest a conservative approach and argue that additional work on the DMIS is needed if differences between parent and child report are of interest; in the meantime, such differences should be interpreted with caution.
Not all of the hypothesized relationships were supported by the data or consistent across reporters. While we hypothesized that child express would be negatively associated with powerful others, there was actually a positive relationship, indicating that children with an orientation toward powerful others were more likely to express an opinion and give information to parents. One possible explanation for this is that behaviors related to sharing an opinion and information reflect generalized engagement with parents around decision making, which may be more likely in children who are oriented to powerful others. Children with a lower tendency toward powerful others may not engage with parents in this way if they feel they can handle the decision on their own. As expected, a greater orientation toward powerful others was associated with a greater tendency to seek information and advice from parents, according to both parent and child report. Unfortunately, we could not test hypotheses related to internal locus of control, due to the unacceptably low α of the subscale in our sample.
We expected aspects of children's DMI to change with the child's age. Both parent and child report of child express increased with child age. It is not surprising that child express increased, due to changes in cognitive development and/or desire for autonomy. Parent report of parent express increased slightly with child age until 12 years, when one might expect these behaviors to be decreasing (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Hanna, Juarez, Lenss, & Guthrie, 2003). One potential explanation is that parents may be responding to children's greater engagement in discussions or amplifying their advice giving in reaction to children's tendency to spend more time away from the family during adolescence (Larson & Richards, 1991; Larson et al., 1996). As expected, parent seek and child seek did not change with the child's age, perhaps reflecting the importance of being “on the same page” with respect to the child's illness management (Miller, 2009). Contrary to expectation, joint decision making (e.g., negotiating and brainstorming together) did not increase with child age. This finding could be due to the fact that the subscale assessing joint decision making also included items assessing parental provision of options and solicitation of questions, behaviors that may be more likely with younger children.
This study has several limitations. First, the format of the DMIS allows for variability in the types of discussions and decisions identified by parent–child dyads and not all dyads identified a specific decision. Different discussions (e.g., routine management issues vs. single event decisions) may have different implications for the child and family (Angst & Deatrick, 1996). Second, the sample may not be representative of those with low levels of DMI. The DMIS requires that the dyad, at a minimum, had a discussion related to the illness in the prior two weeks and were willing to answer questions about such a discussion. Families with high conflict or infrequent engagement related to illness management may not be included in our sample. A related point is that, because the instrument was designed to measure children's involvement (versus lack of involvement), there is a potential floor effect for families scoring at the low end of child involvement. Additional items would need to be developed to gain a better understanding of these families and their decision-making interactions. Third, the participants were primarily Caucasian, and we cannot determine the extent to which the DMIS is reliable or valid for more ethnically diverse samples. Similarly, the sample consisted of primarily mothers, so the findings are not necessarily generalizable to father–child dyads. Fourth, a single parent-report item was used to measure illness severity. Although the relationship between illness severity and DMI was not a focus of this study, a more comprehensive assessment may have been informative and is currently being addressed in additional research.
Future directions of this research include testing associations of the DMIS with outcomes related to effective self-management of childhood chronic illness, such as adherence and health status. One benefit of the DMIS is that it allows for the determination of whether different types of child involvement are more or less beneficial at different ages. For example, parent express may be more helpful at younger ages, when children lack some of the knowledge and skills required to make effective decisions. Second, although we have argued that the DMIS provides useful information above and beyond what can be assessed with existing measures of related constructs (e.g., treatment responsibility and parental involvement), future research should test this assumption by examining the discriminant validity of the DMIS and whether it predicts health-related outcomes above and beyond these other measures. Third, additional research is needed to explore the role of emotional tone on parent–child decision-making interactions. For example, parental advice giving is likely to have a different effect if it is delivered in a nonjudgmental or positive manner, versus an evaluative or negative manner. Fourth, future work using the DMIS should seek to document the nature of discussions and decisions more carefully, so that we can begin to understand differences in child involvement depending on contextual factors. Finally, future research should consider using the DMIS to assess health-related decisions for which there is debate about children's most appropriate role, such as decisions about research enrollment (Miller & Nelson, 2006). Longitudinal research examining some of these questions is currently underway.
Future research using the DMIS will have important clinical implications, by leading to the development of interventions to enhance parent–child interactions and promote children's self-management skills, particularly as they relate to decision making. Prior research has demonstrated the success of a parent–child teamwork intervention (Anderson, Brackett, Ho, & Laffel, 1999; Laffel et al., 2003), but future interventions may be enhanced by targeting specific child and parent behaviors that are more or less beneficial at different points in development. In addition, it is important to identify early patterns of interacting that either facilitate or impede adherence and responsibility later on, so that clinicians can intervene with families before problems develop. Given the decreases in treatment adherence that are typically seen during adolescence, the development of preventive efforts is a critical area of investigation.
Funding
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (grant K23HD055304 to V.A.M.).
Conflicts of interest: None declared.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the children and parents who participated in this study. We thank Robert Nelson and Anne Kazak for their input regarding the instrument; Xuemei Zhang for conducting statistical analyses; and the Diabetes Center for Children, Cystic Fibrosis Center, and Division of Pulmonary Medicine at The Children's Hospital of Philadelphia for their support of this research. We also thank the anonymous manuscript reviewers and Associate Editor, whose comments contributed to an improved paper and instrument.
Appendix
Table A1.
Experimental Item Pool and Filler Items of the DMIS (Parent Report)a
| 1 | We negotiated. |
| 2 | We argued.b |
| 3 | We brainstormed about what to do. |
| 4 | We agreed about what to do.c |
| 5 | I explained different options about what to do. |
| 6 | I asked my child if he/she had any questions. |
| 7 | I was quiet during the discussion.b |
| 8 | I tried to teach my child about something related to the illness. |
| 9 | I gave my child options to choose from. |
| 10 | I suggested ideas or gave advice. |
| 11 | I expressed my opinion. |
| 12 | I gave my child information. |
| 13 | I kept my opinion to myself.b |
| 14 | I asked my child if he/she had any ideas about what to do.c |
| 15 | I asked my child for his/her opinion. |
| 16 | I told my child that his/her opinion was important. |
| 17 | I listened to what my child had to say. |
| 18 | I was distracted during the discussion.b |
| 19 | I asked my child for information. |
| 20 | I gave my child feedback about how he/she has been taking care of his/her illness. |
| 21 | My child suggested ideas. |
| 22 | My child was quiet during the discussion.b |
| 23 | My child expressed an opinion. |
| 24 | My child asked me for information. |
| 25 | My child was distracted during the discussion.b |
| 26 | My child asked for my advice or opinion. |
| 27 | My child asked questions. |
| 28 | My child listened to what I had to say.c |
| 29 | My child gave me information. |
| 30 | My child kept his/her opinion to him/herself.b |
aChild report form had analogous items (e.g., Item 5 on the Child Report form: “My mom/dad explained different choices about what to do.”)
bFiller item to reduce socially desirable responding; not included in factor analysis
cItem dropped after factor analysis.
Footnotes
1 The team reviewing the items consisted of the two authors (a pediatric psychologist and postdoctoral fellow with a doctorate in health studies and a Master of Bioethics), a physician with expertise in pediatric ethics and decision-making, a senior pediatric psychologist, and a developmental psychologist.
References
- Anderson B J, Auslander W, Jung K, Miller J P, Santiago J. Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology. 1990;15(4):477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- Anderson B J, Brackett J, Ho J, Laffel L M. An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Diabetes Care. 1999;22(5):713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Anderson B J, Ho J, Brackett J, Finkelstein D, Laffel L M. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1997;130(2):257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Angst D B, Deatrick J A. Involvement in health care decisions: Parents and children with chronic illness. Journal of Family Nursing. 1996;2(2):174–194. [Google Scholar]
- Baylis F, Downie J, Kenny N. Children and decisionmaking in health research. IRB: A Review of Human Subjects Research. 1999;21(4):5–10. [PubMed] [Google Scholar]
- Bentler P M, Bonett D G. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–606. [Google Scholar]
- Bentler P M, Chou C. Practical issues in structural modeling. Sociological Methods and Research. 1987;16:78–117. [Google Scholar]
- Brown J, Mann L. The relationship between family structure and process variables and adolescent decision making. Journal of Adolescence. 1990;13:25–37. doi: 10.1016/0140-1971(90)90039-a. [DOI] [PubMed] [Google Scholar]
- Browne M W, Cudeck R. Alternative ways of assessing model fit. In: Bollen K A, Long J S, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Byrne B M, Shavelson R J, Muthen B. Testing for the equivalence of factor covariance and mean structures: The issue of partial measurement invariance. Psychological Bulletin. 1989;105(3):456–466. [Google Scholar]
- Cheung G W, Rensvold R B. Testing factorial invariance across groups: A reconceptualization and proposed new method. Journal of Management. 1999;25(1):1–27. [Google Scholar]
- Cheung G W, Rensvold R B. Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling. 2002;9(2):233–255. [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- Costello A B, Osborne J W. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical Assessment, Research & Evaluation. 2005;10(7):1–9. [Google Scholar]
- Crittendon P M. Toward a concept of autonomy in adolescents with a disability. Children's Health Care. 1990;19:162–168. [Google Scholar]
- DeVellis R F. Scale development: Theory and applications. Vol. 26. Newbury Park: Sage Publications; 1991. [Google Scholar]
- Devine K A, Wasserman R M, Gershenson L S, Holmbeck G N, Essner B S. Mother-adolescent agreement regarding decision-making autonomy: A longitudinal comparison of families of adolescents with and without spina bifida. Journal of Pediatric Psychology. 2011;36(3):277–288. doi: 10.1093/jpepsy/jsq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J K, MacCallum R C, Tait M. The application of exploratory factor analysis in applied psychology: A critical review and analysis. Personnel Psychology. 1986;39:291–314. [Google Scholar]
- Franklin C, Streeter C L, Springer D W. Validity of the FACES IV family assessment measure. Research on Social Work Practice. 2001;11(5):576–596. [Google Scholar]
- Geller G, Tambor E S, Bernhardt B A, Fraser G, Wissow L. Informed consent for enrolling minors in genetic susceptibility research: A qualitative study of at-risk children's and parents' views about children's role in decision-making. Journal of Adolescent Health. 2003;32:260–271. doi: 10.1016/s1054-139x(02)00459-7. [DOI] [PubMed] [Google Scholar]
- Gorall D M, Tiesel J, Olson D H. FACES IV: Development and validation. Minneapolis, MN: Life Innovations, Inc; 2004. [Google Scholar]
- Gordon C P. Adolescent decision making: A broadly based theory and its application to the prevention of early pregnancy. Adolescence. 1996;31(123):561–584. [PubMed] [Google Scholar]
- Gregorich S E. Do self-report instruments allow meaningful comparisons across diverse population groups? Medical Care. 2006;44(11 S3):S78–S94. doi: 10.1097/01.mlr.0000245454.12228.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna K M, DiMeglio L A, Fortenberry J D. Parent and adolescent versions of the diabetes-specific parental support for adolescents' autonomy scale: Development and initial testing. Journal of Pediatric Psychology. 2005;30(3):257–271. doi: 10.1093/jpepsy/jsi036. [DOI] [PubMed] [Google Scholar]
- Hanna K M, Juarez B, Lenss S S, Guthrie D. Parent-adolescent communication and support for diabetes management as reported by adolescents with Type I diabetes. Issues in Comprehensive Pediatric Nursing. 2003;26:145–158. doi: 10.1080/01460860390223871. [DOI] [PubMed] [Google Scholar]
- Joffe S. Rethink “affirmative agreement,” but abandon “assent”. American Journal of Bioethics. 2003;3(4):9–11. doi: 10.1162/152651603322614409. [DOI] [PubMed] [Google Scholar]
- Laffel L M, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson B J. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with Type I diabetes. Journal of Pediatrics. 2003;142:409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- Landis R, Koch G G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- Larson R W, Richards M H. Daily companionship in late childhood and early adolescence. Child Development. 1991;62:284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Larson R W, Richards M H, Moneta G, Holmbeck G, Duckett E. Changes in adolescents' daily interactions with their families from ages 10 to 18: Disengagement and transformation. Developmental Psychology. 1996;32(4):744–754. [Google Scholar]
- Liprie M L. Adolescents' contributions to family decision making. Marriage and Family Review. 1993;18:241–253. [Google Scholar]
- Malcarne V L. Children's health-related locus of control beliefs: Ethnicity, gender, and family income. Children's Health Care. 2005;34(1):47–59. [Google Scholar]
- March J S, Sullivan K. Test-retest reliability of the Multidimensional Anxiety Scale for Children. Journal of Anxiety Disorders. 1999;13(4):349–358. doi: 10.1016/s0887-6185(99)00009-2. [DOI] [PubMed] [Google Scholar]
- McCabe M A. Involving children and adolescents in medical decision making: Developmental and clinical considerations. Journal of Pediatric Psychology. 1996;21(4):505–516. doi: 10.1093/jpepsy/21.4.505. [DOI] [PubMed] [Google Scholar]
- Meredith W, Teresi J A. An essay on measurement and factorial invariance. Medical Care. 2006;44(11 Suppl 3):S69–S77. doi: 10.1097/01.mlr.0000245438.73837.89. [DOI] [PubMed] [Google Scholar]
- Miller V A. Parent-child collaborative decision making for the management of chronic illness: A qualitative study. Families, Systems, & Health. 2009;27(3):249–266. doi: 10.1037/a0017308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V A, Drotar D. Discrepancies between mother and adolescent perceptions of diabetes-related decision-making autonomy and their relationship to diabetes-related conflict and adherence to treatment. Journal of Pediatric Psychology. 2003;28(4):265–274. doi: 10.1093/jpepsy/jsg014. [DOI] [PubMed] [Google Scholar]
- Miller V A, Nelson R M. A developmental approach to child assent for non-therapeutic research. Journal of Pediatrics. 2006;149(1 Supp):S25–S30. doi: 10.1016/j.jpeds.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Miller V A, Reynolds W W, Nelson R M. Parent–child roles in decision-making about medical research. Ethics & Behavior. 2008;18(2–3):161–181. [Google Scholar]
- Nansel T R, Rovner A J, Haynie D, Iannotti R J, Simons-Morton B, Wysocki T, Anderson B, Weissberg-Benchell J, Laffel L. Development and validation of the Collaborative Parent Involvement Scale for youths with type 1 diabetes. Journal of Pediatric Psychology. 2009;34(1):30–40. doi: 10.1093/jpepsy/jsn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally J C, Bernstein I H. Psychometric theory. New York: McGraw-Hill; 1994. [Google Scholar]
- Pianta R C, Harbers K L. Observing mother and child behavior in a problem-solving situation at school entry: Relations with academic achievement. Journal of School Psychology. 1996;34(3):307–322. [Google Scholar]
- Schmidt S, Petersen C, Bullinger M. Coping with chronic disease from the perspective of children and adolescents–a conceptual framework and its implications for participation. Child: Care, Health & Development. 2003;29(1):63–75. doi: 10.1046/j.1365-2214.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- Shrout P E, Fleiss J L. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Smetana J G. Conflict and coordination in adolescent-parent relationships. In: Shulman S, editor. Close relationships and socioemotional development. Vol. 7. Norwood, NJ: Ablex Publishing Corporation; 1995. pp. 155–184. [Google Scholar]
- Smetana J G, Campione-Barr N, Daddis C. Longitudinal development of family decision making: Defining healthy behavioral autonomy for middle-class African American adolescents. Child Development. 2004;75(5):1418–1434. doi: 10.1111/j.1467-8624.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- Stanton W R, Raja S N, Langley J. Stability in the structure of health locus of control among adolescents. British Journal of Clinical Psychology. 1995;34:279–287. doi: 10.1111/j.2044-8260.1995.tb01462.x. [DOI] [PubMed] [Google Scholar]
- Steenkamp J B E M, Baumgartner H. Assessing measurement invariance in cross-national consumer research. Journal of Consumer Research. 1998;25(1):78–107. [Google Scholar]
- Thompson B, Webber L, Berenson G. Factor structure of a children's health locus of control measure: A confirmatory maximum-likelihood analysis. Educational and Psychological Measurement. 1987;47(4):1071–1080. [Google Scholar]
- Vandenberg R J, Lance C E. A review and synthesis of the measurement invariance literature: Suggestions, practices, and recommendations for organizational research. Organizational Research Methods. 2000;3(1):4–69. [Google Scholar]
- Walker N E, Doyon T. ‘Fairness and reasonableness of the child's decision:' A proposed legal standard for children's participation in decision making. Behavioral Sciences and the Law. 2001;19:611–636. doi: 10.1002/bsl.461. [DOI] [PubMed] [Google Scholar]
- Ware J E, Gandek B. Methods for testing data quality, scaling assumptions, and reliability: The IQOLA Project approach. Journal of Clinical Epidemiology. 1998;51(11):945–952. doi: 10.1016/s0895-4356(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Weithorn L A. Involving children in decisions affecting their own welfare. In: Melton G B, Koocher G P, Saks M J, editors. Children's competence to consent. New York: Plenum Press; 1983. pp. 235–260. [Google Scholar]
- White F. Parent-adolescent communication and adolescent decision-making. Journal of Family Studies. 1996;2(1):41–56. [Google Scholar]
- Wills T, Blechman E, McNamara G. Family support, coping, and competence. In: Hetherington E M, Blechman E, editors. Stress, coping, and resiliency in children and families. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 107–133. [Google Scholar]