Abstract
Purpose of Review
Failure to control viral infections such as HIV, results in TCR and inhibitory receptor driven exhaustion of antigen-specific T cells. Persistent signaling by these receptors during chronic viral infection sculpts the transcriptional regulatory programs of virus-specific T cells. The resulting gene expression profile is tailored to temper the potentially damaging effector functions of cytotoxic T cells and adapt them to an antigen and inflammation rich environment. Here we review recent studies investigating mechanisms of transcriptional regulation of effector, functional memory, and exhausted T cell functions during acute versus chronic infections.
Recent Findings
Patterns of gene expression in virus-specific CD8 T cells are a result of a combination of pro and inhibitory signals from antigen presentation (TCR-mediated) and co-inhibitory receptor ligation (PD-1, 2B4). Further, memory-specific transcriptional regulation of 2B4 expression and signaling impose a self-limiting secondary effector response to a prolonged viral infection. Additionally, differentiation of functional memory CD8 T cells is coupled to acquisition of a repressive epigenetic program for PD-1 expression. However, chronic infection provides a signal which blocks the acquisition of these epigenetic modifications reinforcing the suppression of CTL functions in exhausted cells.
Summary
Current findings suggest that the mechanism(s) that delineate functional memory versus exhaustion are coupled to acquisition of transcriptional programs at the effector stage of differentiation, reinforced by cessation or persistence of TCR signaling.
Keywords: Chronic Infection, CD8 T cell, Exhaustion, Transcription Factor, Epigenetic
CD8 T Cell Memory Differentiation
Immunological memory plays a vital role in curtailing secondary infections. A better understanding of the basic mechanisms involved in T cell memory differentiation should facilitate the development or improvement of prophylactic and/or restorative therapeutic strategies targeting human pathogens. Recent efforts have focused on defining the mechanisms involved in differentiation of memory CD8 T cells in response to acute vs. chronic viral infections. Upon initial exposure to antigen (Figure 1a step 1), virus-specific CD8 T cells undergo clonal expansion differentiating into effector T cells with acquired functions including heightened expression of IFNg, TNFa, and IL-2, antigen-specific cytotoxicity via granzyme b and perforin expression, and an ability to localize to sites of infection (Figure 1a step 2). Under conditions where viral infection is efficiently resolved, ~ 5 to 10 % of effector cells survive through the contraction phase of the CD8 T cell response. These cells further differentiate into self-renewing highly functional memory CD8 T cells, persisting in lymphoid as well as nonlymphoid tissues for the life of the host as a result of the acquired ability to homeostatically proliferate (Figure 1a step 3). Relative to the naïve precursors, memory CD8 T cells have an enhanced ability to recall an effector response due in part to their ability to rapidly re-express cytokines and an overall increase in quantity of antigen-specific cells (Figure 1a step 4). Thus, memory CD8 T cells are poised to rapidly resolve previously encountered pathogens due to their increase in quantity, anatomical redistribution, homeostasis, and reprogramming of effector molecule expression providing enhanced CTL quality 1–11.
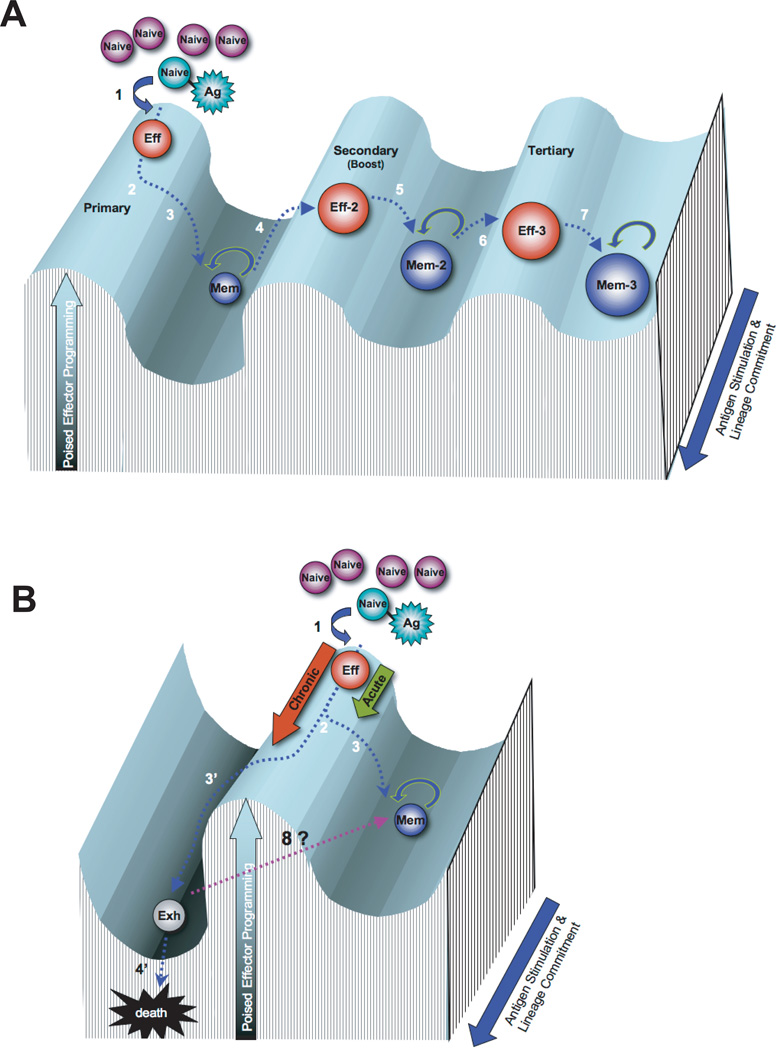
Figure 1.
Rolling hill diagram of antigen-mediated transcriptional reprogramming of CD8 T cells during memory differentiation. A) Differentiation of antigen-specific CD8 T cells during primary, secondary, and tertiary acute viral infection. B) Differentiation of antigen-specific CD8 T cells during chronic viral infection versus acute viral infection. The peaks and troughs of the transcriptional landscape of the Y-axis represent the potential to achieve an effector response through the modification of transcriptional programs. The Z-axis (blue arrow) represents the duration of antigen exposure and the lineage commitment of the antigen-specific CD8 T cell. Step 1: Antigen-specific naïve CD8 T cells encounter an antigen-presenting cell and differentiate into cytolytic effector cells. Step 2: During the ~ 105 fold expansion of effector cells a subset of cells commit to a memory fate. Step 3: Following antigen clearance the effector population contracts, with ~5–10% of the population surviving to differentiate into resting memory CD8 T cells, poised to rapidly recall the effector functions. Step 4: Memory CD8 T cells efficiently recall an effector transcriptional profile upon secondary exposure to the antigen or a vaccine boost regimen. Step 5: Secondary effector CD8 T cells undergo less contraction resulting in a greater quantity of self-renewing memory CD8 T cells with a gene expression profile that retains some effector-like transcriptional status. Steps 6 & 7: The resulting memory population generated from a tertiary infection are more effector-like and increased in quantity relative to the primary and secondary memory cells. Step 3’: If the infection persists the antigen-specific CD8 T cells further differentiate with progressive restriction in the ability to recall effector functions and ultimately loss of the virus-specific cells. Step 8: Future efforts will determine if exhausted CD8 T cells can be reprogrammed to obtain the transcriptional regulation of a resting memory CD8 T cell.
Unfortunately, many human infections are not efficiently resolved. Chronic viral infections result in a divergence from the functional memory CD8 T cell differentiation program described above. As the viral infection persists (Figure 1b step 2 to 3’), antigen-specific CD8 T cells progressively lose the ability to recall IL-2, TNFa, and IFNg expression in a hierarchal manner. Coincident with loss of cytokine expression, the cells also lose cytolytic potential and proliferative capacity 12 (Figure 1b step 3’). Further, the development of this exhaustion is coupled to the duration of the infection and becomes more pronounced in the absence of CD4 help 1, of which both conditions contribute to the exhausted CTL response during HIV infection 13. It is now known that the retained increased expression of cell surface inhibitory receptors such as PD-1, 2B4, LAG3, CTLA-4, Tim-3 and others play a key role in T cell exhaustion by negatively regulating T cell function and skewing differentiation. Blocking signals from inhibitory receptors such as PD-1, can resuscitate exhausted T cells, improving function and immunity in animal models and humans during chronic infections and cancer 14-17.
The cell intrinsic mechanisms that dictate the functional, or lack thereof, capabilities of virus-specific CD8 T cells are initiated at the effector stage of differentiation, dependent upon strength and duration of TCR signaling 18, 19. The “instructions” for development of a functional memory versus exhausted CD8 T cell that come from the multiple surface receptor signals are manifest through differential transcriptional regulation. These acquired transcriptional mechanisms provide programs for upregulation, poised, or repression of gene expression specific to the state of cellular differentiation and include the retained transcriptional upregulation of inhibitory receptors listed above in exhausted T cells (Figure 1b steps 1–3 & 3’). Recent efforts have focused on identifying the molecular signaling pathways and the downstream gene regulatory targets that contribute to the antigen-specific response during different stages of acute and chronic viral infections. In this review we discuss recent reports defining the contribution of TCR and inhibitory receptor signaling events in the acquisition of exhaustion-specific transcriptional regulation in virus-specific CD8 T cells.
CD8 T Cell Gene Expression Patterns are Modified in Response to Antigen Persistence
Initial gene expression profile studies of virus-specific CD8 T cells generated in response to acute viral infection demonstrated that differentiation of naïve CD8 T cells into effector then memory cells is coupled to modifications of the gene regulatory programs. Furthermore, this study demonstrated that the transcriptional profile of memory CD8 T cells continues to change following antigen clearance, and the acquired modifications in gene regulation were propagated from parental to daughter cell.20. The analyzed transcripts cells were grouped based on 6 distinct expression patterns, including the expression patterns of “off-on-off” and “on-off-on” in naïve, effector, and memory cells respectively. Importantly, it was later shown that several of the genes that were grouped into the on-off-on expression pattern, including IL7Ra, CCR7, and L-selectin were found to contribute to the development of functional memory cells. 18, 19, 21. Comparatively, these genes remained repressed during chronic infection, raising the possibility that the acquired transcriptional programming of antigen-specific CD8 T cells generated in response to acute versus chronic infections gradually deviate due to the protracted TCR signaling that occurs after the effector stage of differentiation during chronic infection. Indeed, transcriptional profiles of exhausted memory CD8 T cells relative to functional memory CD8 T cells were found to have hundreds of genes that were differentially upregulated and downregulated 22 (Figure 1b steps 3 vs. 3’). These principal gene signature studies now serve as an additional metric to gauge the quality of the CD8 T cell response elicited by various vaccine strategies.
Acquired Transcriptional Programming Adapts to Repetitive Stimulation
Past efforts aimed at improving the quantity and quality of memory CD8 T cells utilizing a heterologous prime-boost vaccination strategy illustrated that repetitive immunization results in an increase in quantity of antigen-specific memory CD8 T cells that develop an effector-memory phenotype 23, 24. The merit of such a strategy was recently demonstrated by Louis Picker’s laboratory, which showed that prime-boost vaccination using a combination of CMV and Adenovirus based vectors harboring SIV genes elicits an immune response in rhesus macaques that is capable of protecting against an SIV challenge that would normally result in chronic infection 25.
Indeed, one of the challenges in the design of a vaccine that establishes protective T cell immunity is generating a high quantity of functional memory cells. If the quantity of the initial memory population is not sufficient to control the virus, how is the recall effector response impacted at a cellular and molecular level? Using the lymphocytic choriomeningitis virus (LCMV) mouse model system of viral infection, West et al. addressed this question by performing a series of co-transfer experiments comparing a low vs. high quantity of congenically labeled naïve and memory antigen-specific CD8 T cells into mice that were challenged with acute vs. chronic LCMV infection 26. Interestingly, under conditions where viral infection was not resolved with the low quantity transfer of naïve and memory CD8 T cells, the proliferative and functional capacity of the secondary effectors declined relative to the primary effectors as a result of predisposed tighter regulation of the recall response in the memory cells. Ultimately the secondary effectors reached a state of terminal differentiation much faster than the primary effector cells. Comparison of the transcriptional profiles of the primary and secondary effectors from the chronic infection revealed a striking difference in many genes including cell cycle regulators and inhibitory receptors. In particular, the elevated expression of the inhibitory receptor CD244 (2B4) in the secondary effectors relative to the primary effectors was only observed during chronic infection. Further, deletion of the 2B4 inhibitory receptor resulted in a partial recovery of the secondary effector expansion during chronic infection. These data demonstrate that memory cells acquire additional modes of transcriptional control. Thus it stands to reason that the transcriptional regulatory mechanisms of memory cells are still pliable and susceptible to boosting.
In order to better understand the quality of memory CD8 T cells generated from boosting regimens, Wirth et al. analyzed the gene expression profiles of primary, secondary, tertiary, and quaternary memory CD8 T cells derived from repetitive acute infection 27. They reported that all four memory populations contained many uniquely up and downregulated transcripts, while conserving an underlying memory gene signature. Further, using Gene Set Enrichment Analysis (GSEA), the authors compared their quaternary memory CD8 T cell expression profile with historical exhaustion profiles. They reported that only 1/4 of the upregulated quaternary-specific genes were enriched in the exhausted profile, and only 1/3 of the downregulated genes were enriched. They concluded that memory CD8 T cells exposed to repetitive stimulation that includes periods of rest between antigen exposure retain an ability to adapt the transcriptional program, without developing an exhaustion transcriptional profile. (Figure 1a steps 4, 5, 6, & 7).
Collectively, these studies reveal that the execution of the acquired recall response program in memory CD8 T cells is dependent upon the persistence of the antigen, as well as demonstrating that memory CD8 T cells possess an acquired self-limiting instruction that is implemented when the infection is not resolved. Therefore, even though memory CD8 T cells have a superior ability to respond to viral re-challenge, if the initial quantity of memory CD8 T cells is insufficient to control the virus at an effector stage of the immune response, then the memory CD8 T cell population will decline without resolution of the infection. Thus prime-boost vaccine regimens may provide a strategy to increase the quantity of resting memory CD8 T cells while retaining a fundamental memory transcriptional program that maintains the quality of the recall response.
Gene Expression Profiles of HIV-specific CD8 T Cells from Controllers vs. Progressors
The discovery that some individuals, known as elite-controllers, are able to control HIV infection and stave off the development of AIDS provides hope that design of a protective immunization strategy against HIV infection is achievable 13, 28. Specific efforts to better understand the mechanisms for acquisition of the unique transcriptional profiles in CD8 T cells differentiating in response to acute vs. chronic HIV infection has lead researchers to more precisely define the contribution of TCR signaling vs. the inhibitory signals from receptors such as PD-1 to the modification of the transcriptional program. PD-1 signaling directly represses the functional and proliferative capacity of antigen-specific CD8 T cells at the later stages of chronic infection, but the precise mechanism is undefined. Using a combination of in vitro and in vivo analysis of mouse and human virus-specific CD8 T cells, Quigley et al. report that a subset of up and downregulated transcripts in the gene expression profile of exhausted HIV-specific CD8 T cells can be attributed to PD-1 signaling 29. Included in this enriched profile was the upregulation of the transcription factor BATF. The authors went on to demonstrate that overexpression of BATF in CD8 T cells impaired T cell function, while knocking down BATF expression in HIV-specific T cells impaired cytokine production and proliferation. This study highlights the involvement of inhibitory receptor signaling in the development of a CD8 T cell exhaustion gene signature in HIV progressors vs. elite controllers. Further, it establishes that TCR and inhibitory signaling work in a combinatorial manner, culminating in the observed gene expression profile of the virus-specific cell during an in vivo response to viral infection.
It is clear that the cell intrinsic mechanism that mediates infection-dependent functional properties of memory cells is coupled to the acquired transcriptional regulation. It remains to be determined how these transcriptional programs are propagated during homeostatic cell division. Recent efforts to better understand the underlying mechanism involved in propagating transcriptional regulation in dividing cells have focused on epigenetic modifications to chromatin.
Cell-transmissible Transcriptional Regulation in Functional and Exhausted Memory CD8 T Cells
Several mechanisms have been proposed to explain the transmission of transcriptional programs during CD8 T cell homeostatic proliferation, including expression of master regulatory transcription factors and epigenetic modifications to the chromatin 30–32. Epigenetic modifications provide a mechanism for propagation of gene regulation during cell division via replication of chromatin modifications to the newly synthesized strand of DNA without modifying the sequence 33, 34. Initial investigations on the dichotomy of helper CD4 T cell fate and transcriptional “memory” reported that transcriptional repression of IFNg or IL-4 loci in Th1 vs. Th2 was coupled to the presence of DNA methylation, and these repressive DNA methylation marks were maintained during cell division 35–37. Deletion of the maintenance DNA methyltransferase Dnmt1 or methylation binding protein Mbd2 resulted in a dysregulation of effector molecule and lineage associated transcription factor gene expression 36, 38. These studies established that modification of the epigenetic program in T cells is coupled to TCR-mediated changes to the transcriptional program. Following these initial studies, it was demonstrated that antigen-specific CD8 T cells that lack Mbd2 mount an efficient effector response but are impaired in memory differentiation 39. Thus, proper programming at the DNA methylation level is critical for memory differentiation. Recently, there have been several reports on changes in epigenetic modifications at the loci of the effector molecules IFNg, IL-2, perforin, and Granzyme B during memory differentiation 40–45. Consistent among each of these studies was the reported correlation of transcriptional upregulation and the loss of repressive epigenetic marks (DNA and histone modifications) at the proximal promoter region. Moreover, these studies established that the epigenetic program at each of these loci is pliable during CD8 T cell differentiation in response to acute viral infection. In some cases, the repressive epigenetic marks were re-instated in the resting memory CD8 T cells. Given the gross differences in transcriptional regulation between functional memory and exhausted CD8 T cells, it is likely that a general mechanism for adaptive transcriptional regulation involves an altered specificity for epigenetic modification during chronic antigen exposure.
Commitment To an Exhausted Fate is Reinforced by Epigenetic Modifications
We have recently reported dynamic changes in transcriptional regulation at the epigenetic level of the locus of the PD-1 inhibitory receptor during differentiation of functional memory and exhausted CD8 T cells (Youngblood et al. In press 46). Using the model system of LCMV infection in mice, we reported that upregulated transcriptional expression of PD-1 is coupled to transient DNA demethylation of the PD-1 locus during the differentiation of naïve into effector CD8 T cells. Upon control of the infection, the downregulation of PD-1 in the antigen-specific effector CD8 T cells is coupled with the remethylation of the PD-1 locus (Figure 2). Further, terminal effector and memory precursors at an effector stage of differentiation had similar levels of DNA methylation at the PD-1 locus, suggesting that reacquisition of the fully methylated sites in memory CD8 T cells did not occur due to selective survival of memory precursors. In addition, we observed that the human PD-1 locus in virus-specific CD8 T cells generated in response to the yellow fever vaccine was also transiently demethylated at an effector stage of differentiation, then remethylated in memory cells. It is possible that the reacquired epigenetic modifications arise through a de novo mechanism since every antigen-specific memory CD8 T cell transitions through an effector stage 47, 48. Furthermore, if the reacquisition of repressive epigenetic marks, which follows antigen clearance, occurs through de novo programming, then it is likely that the PD-1 locus remains unmethylated during chronic infection.
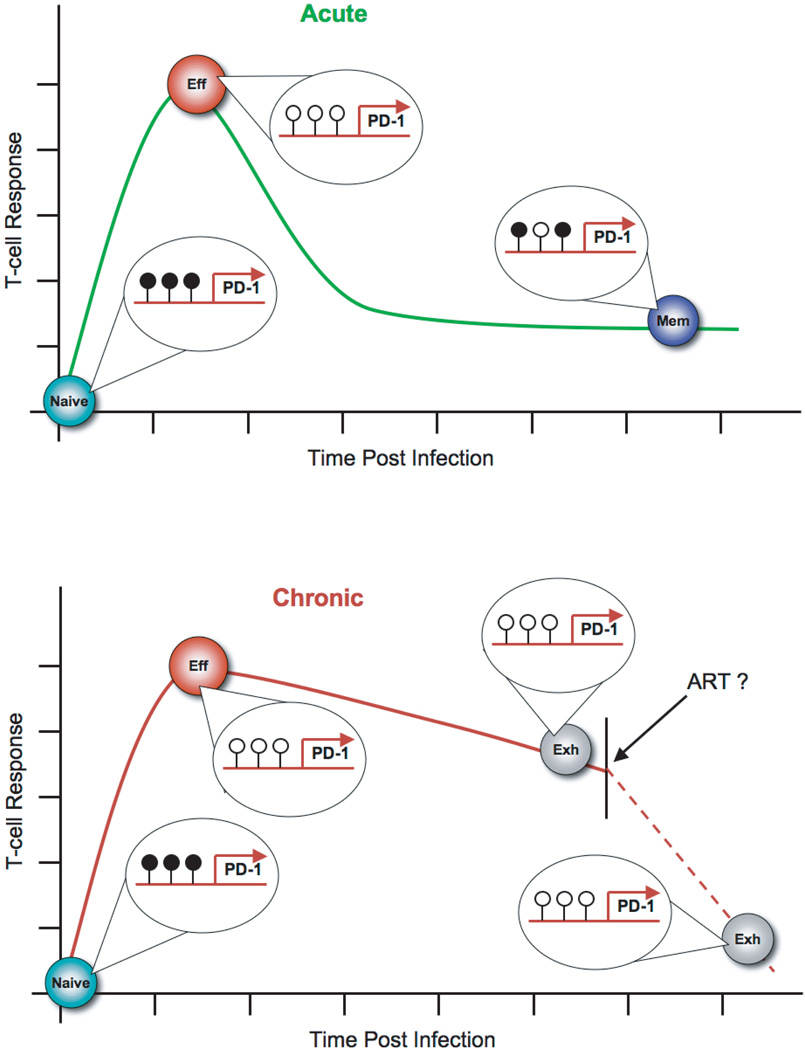
Figure 2.
Epigenetic modifications at the PD-1 locus are coupled to persistence of antigen. During an acute viral infection (green line) the PD-1 locus in early effector antigen-specific CD8 T cells becomes unmethylated (open lollipops) and the chromatin more accessible relative to naïve and memory CD8 T cells. Functional memory CD8 T cells have reacquire a unique DNA methylation pattern (filled lollipops) relative to their naïve precursors. Exhausted CD8 T cells retain an unmethylated and accessible PD-1 locus during chronic viral infection (red line). Anti-retroviral therapies can reduce viral load, but it remains to be determined if the PD-1 locus becomes remethylated in exhausted virus-specific CD8 T cells.
To investigate the connection between antigen persistence and epigenetic programming, we performed a longitudinal analysis of the methylation status in mouse antigen-specific CD8 T cells that were persistently exposed to antigen. Indeed, we observed that the PD-1 locus was unmethylated at an effector stage of differentiation during a chronic viral infection and remained unmethylated at later stages of the infection (Figure 2). Furthermore, virus-specific CD8 T cells differentiated in response to two different human chronic infections each contained an unmethylated PD-1 locus. Following prolonged and elevated viremia, a decrease in viremia still results in reduced PD-1 expression on antigen-specific CD8 T cells. Interestingly, the reduction in PD-1 expression was not coupled to remethylation of the PD-1 locus (Figure 2). To better define the role of DNA methylation in regulating PD-1 expression we measured the re-expression kinetics of PD-1 in memory cells with an unmethylated PD-1 locus. The virus-specific CD8 T cells with an unmethylated locus reached maximal PD-1 expression much faster than fully functional memory CD8 T cells with a methylated PD-1 locus. Take together these data suggest that retention of an unmethylated PD-1 locus leaves the cells poised to re-express the inhibitory receptor and prematurely inhibit the CTL response. Thus, epigenetic programming at the PD-1 locus reinforce the signals that cause T cell exhaustion.
Concluding Remarks
Characterization of the gene expression and epigenetic fingerprint of memory CD8 T cells generated from vaccine regimens will provide further insight into the protective quality of the poised effector recall response. In addition to providing a finer assessment of immunological memory generated from vaccination, a better understanding of the mechanism(s) for acquired and maintained transcriptional programs may facilitate the treatment of established chronic infections. The successes observed with autologous cell transfer therapy to treat tumors raise the possibility that re-programming of autologous exhausted CD8 T cells (Figure 1b step 8) in combination with antiviral therapies, may facilitate control of chronic infections 49, 50. It is important to keep in mind that the immune system likely evolved the transcriptional regulatory mechanisms of exhausted CD8 T cells to restrain immune pathology, and efforts to engineer these mechanisms to recover function in virus-specific cells should be carried out with considerable caution. Many significant challenges remain regarding the transcriptional re-programming of exhausted CD8 T cells. In order to recover poised functions, will an exhausted cell have to be de-differentiated to an effector stage to then transition to a memory stage of differentiation (Figure 1b step 3’)? If de novo epigenetic modifications are acquired in functional memory CD8 T cells what role do they play in regulating transcription in exhausted cells, and can the marks be erased and the specificity of the enzymes modified to directly reprogram an exhausted cell into a functional memory cell? More work is needed to better understanding the cellular signaling pathways that promote memory-specific epigenetic transcriptional programming. Insights gained from these investigations may bring within reach our ability to erase epigenetic memory and provide a means to reprogram exhausted CD8 T cells.
Key Points.
Gene expression analyses of functional and exhausted virus-specific memory CD8 T cells have reported the differential expression of hundreds of genes between the two populations of cells, and now serve as a benchmark to gauge the quality of a CD8 T cell response elicited by various vaccine strategies.
Gene expression profile studies of prime-boost vaccine regimens reveal that repetitive stimulation of virus-specific CD8 T cells skews the transcriptional programming towards an effector-memory stage of differentiation.
TCR and inhibitory receptor signaling work in a combinatorial manner to modify transcriptional regulatory programs to adapt virus-specific T cell functions to acute or persistent antigenic environments.
Antigen-specific CD8 T cells acquire epigenetic transcriptional regulatory programs at genes that control cellular function such as PD-1, thereby reinforcing the functional status of the cell.
Acknowledgments
We thank J. Scott Hale for critical reading of our manuscript. R. Ahmed is supported by the National Institutes of Health (P01 AI080192-01, R01 AI030048-20), E.J. Wherry is supported by the National Institutes of Health (AI071309, AI083022, AI095608, AI078897, AI082630, and HHSN266200500030C) , B. Youngblood is supported by the American Cancer Society (PF-09-134-01-MPC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J.Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science (New York, N.Y. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 3.Bevan MJ, Goldrath AW. T-cell memory: You must remember this. Curr Biol. 2000;10:R338–R340. doi: 10.1016/s0960-9822(00)00461-9. [DOI] [PubMed] [Google Scholar]
- 4.Doherty PC, Topham DJ, Tripp RA. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunological reviews. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 5.Lefrancois L, Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Current opinion in immunology. 2002;14:503–508. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 6.Parish IA, Kaech SM. Diversity in CD8(+) T cell differentiation. Current opinion in immunology. 2009;21:291–297. doi: 10.1016/j.coi.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature immunology. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 8.McKinstry KK, Strutt TM, Swain SL. The effector to memory transition of CD4 T cells. Immunologic research. 2008;40:114–127. doi: 10.1007/s12026-007-8004-y. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. European journal of immunology. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 10.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 11.Blattman JN, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. The Journal of experimental medicine. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature immunology. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 13.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 15.Golden-Mason L, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. Journal of virology. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamoto N, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS pathogens. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 18.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature immunology. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of experimental medicine. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 21.Kalia V, et al. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 24. Vezys V, et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486.. *This study demonstrates that prime-boost vaccination regimens can dramatically increase the quantity of a particular antigen-specific population without compromising the quantity of existing an memory T cell population specific to a different pathogen.
- 25. Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003.. *This report provides a proof-of-principle that priming alone or a prime-boost vaccine regimen with a vector that elicits a robust CD8 T cell response can provide protection against SIV infection.
- 26.West EE, et al. Tight Regulation of Memory CD8(+) T Cells Limits Their Effectiveness during Sustained High Viral Load. Immunity. 2011;35:285–298. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wirth TC, et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014.. *This report demonstrates that repetitive boosting progressively modifies the transcriptional profile in the secondary, tertiary, and quaternary memory CD8 T cells relative to primary memory cells without developing an exhaustion transcriptional profile.
- 28.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. The New England journal of medicine. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 29. Quigley M, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature medicine. 2011;16:1147–1151. doi: 10.1038/nm.2232.. *This study demonstrates that inhibitory receptor signaling modifies gene expression profiles by directly upregulating the expression of transcription factor(s), and this has a negative impact on HIV-specific CTL responses.
- 30.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 31.Youngblood B, Davis CW, Ahmed R. Making memories that last a lifetime: heritable functions of self-renewing memory CD8 T cells. International immunology. 2010 doi: 10.1093/intimm/dxq437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zediak VP, Wherry EJ, Berger SL. The contribution of epigenetic memory to immunologic memory. Current opinion in genetics & development. 21:154–159. doi: 10.1016/j.gde.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat.Rev.Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 34.Ting AH, McGarvey KM, Baylin SB. The cancer epigenome--components and functional correlates. Genes & development. 2006;20:3215–3231. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- 35.Young HA, et al. Differentiation of the T helper phenotypes by analysis of the methylation state of the IFN-gamma gene. J Immunol. 1994;153:3603–3610. [PubMed] [Google Scholar]
- 36.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 38.Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol. 2009;182:6648–6652. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 39.Kersh EN. Impaired memory CD8 T cell development in the absence of methyl-CpG-binding domain protein 2. J Immunol. 2006;177:3821–3826. doi: 10.4049/jimmunol.177.6.3821. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. European journal of immunology. 1999;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J.Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 42.Kersh EN, et al. Rapid demethylation of the IFN-gamma gene occurs in memory but not naive CD8 T cells. J.Immunol. 2006;176:4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 43.Araki Y, Fann M, Wersto R, Weng NP. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B) J Immunol. 2008;180:8102–8108. doi: 10.4049/jimmunol.180.12.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juelich T, et al. Interplay between chromatin remodeling and epigenetic changes during lineage-specific commitment to granzyme B expression. J Immunol. 2009;183:7063–7072. doi: 10.4049/jimmunol.0901522. [DOI] [PubMed] [Google Scholar]
- 45.Zediak VP, Johnnidis JB, Wherry EJ, Berger SL. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol. 2011;186:2705–2709. doi: 10.4049/jimmunol.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youngblood B, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity. 2011;35:13. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science (New York, N.Y. 2009;323:505–509. doi: 10.1126/science.1166831.. **This report definitively demonstrates that memory CD8 T cells transition through an effector stage of differentiation.
- 48.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 49.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Current opinion in immunology. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clinical & developmental immunology. 2010;2010 doi: 10.1155/2010/689171. [DOI] [PMC free article] [PubMed] [Google Scholar]