Abstract
Objectives. Innate immune responses in the rheumatoid synovium contribute to inflammation and joint destruction in RA. Two IκB kinase (IKK)-related kinases, TNF receptor associated factor (TRAF) family member-associated nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) activator (TANK)-binding kinase 1 (TBK1) and IKKε, potentially regulate synovitis by activating IFN response genes. These kinases induce the expression of inflammatory mediators such as C-X-C motif ligand 10 (CXCL10)/IFN-γ-induced protein 10 kDa (IP-10) in fibroblast-like synoviocytes (FLS). Since IP-10 is a promising therapeutic target in RA, we evaluated whether blocking TBK1 might be an effective way to modulate IP-10 expression.
Methods. Wild-type (WT) and IKKε−/− FLS were transfected with TBK1 or control small interfering RNA (siRNA) and stimulated with polyinosinic acid : polycytidylic acid [poly(I:C)]. Gene expression was assayed using quantitative PCR. Cytokine production in culture supernatants was measured by Luminex multiplex analysis. IFN-regulatory factor (IRF3) dimerization was determined by native PAGE. IFN-β and IP-10 promoter activity was measured using luciferase reporter constructs.
Results. Initial studies showed that siRNA markedly decreased TBK1 expression in cultured FLS. Poly(I:C)-induced IRF7 gene expression was inhibited in the absence of TBK1, but not IKKε. IRF3 gene expression was similar to WT cells in TBK1 or IKKε-deficient FLS. IRF3 dimerization required both TBK1 and IKKε. Surprisingly, IRF3-mediated gene and protein expression of IFN-β and IP-10 was dependent on TBK1, not IKKε. Promoter constructs showed that TBK1 decreased IP-10 gene transcription and IP-10 mRNA stability was unaffected by TBK1 deficiency.
Conclusion. Based on the selective regulation of IP-10 in FLS, TBK1 appears to be the optimal IKK-related kinase to target in RA.
Keywords: rheumatoid arthritis, innate immunity, C-X-C motif ligand 10, TANK-binding kinase 1, IκB-kinase ε, fibroblast-like synoviocytes, Toll-like receptor 3 ligand
Introduction
RA is a systemic immune-mediated disease characterized by chronic synovial inflammation and joint destruction [1–3]. Although adaptive immune responses contribute to many aspects of the disease, innate immunity and Toll-like receptors (TLRs) have been implicated in its initiation and perpetuation [4, 5]. Regulation of the IFN signature by TLR3 is particularly interesting because expression of the receptor is elevated in RA synovial lining, primarily in fibroblast-like synoviocytes (FLS) [6, 7]. Nuclear components from damaged synovial tissue activate the TLR3 pathway in FLS [8], inducing the production of metalloproteinases, cytokines and chemokines that contribute to synovitis and matrix destruction [9–11].
The binding of dsRNA or its mimetic, polyinosinic acid : polycytidylic acid [poly(I:C)], to endosomal TLR3 activates the IFN-response pathway in FLS [12]. TLR3 dimerization leads to the phosphorylation of two IκB kinase (IKK)-related kinases, IKKε and TNF receptor associated factor (TRAF) family member-associated nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) activator (TANK)-binding kinase 1 (TBK1) [13–15]. These kinases, in turn, phosphorylate IFN regulatory factors (IRFs) 3 and 7, transcription factors that induce the expression of IFN response genes. IKKε and TBK1 contribute to chronic inflammation by regulating production of pro-inflammatory mediators such as C-X-C motif ligand 10 (CXCL10)/IFN-γ-induced protein 10 kDa (IP-10) (16, 17).
A recent clinical trial has demonstrated the efficacy of anti-IP-10 neutralizing antibody in RA and ulcerative colitis [18, 19]. A potential alternative to a biologic that blocks IP-10 is a small molecule inhibitor that targets components of the IKK-related kinase pathway. Both IKKε and TBK1 are possible targets that regulate this chemokine, but their functional hierarchy is not known in FLS, which are the primary producers of IP-10 in the synovium. In this study, we evaluated the effects of TBK1 and IKKε deficiency in poly(I:C)-stimulated FLS. The data suggest that TBK1 would be the optimal target for modulating IP-10 production.
Materials and methods
Reagents
Polyinosinic acid : polycytidylic acid [poly(I:C)] and anti-β-actin antibody were obtained from Sigma (St Louis, MO, USA). Anti-mouse IRF3 antibody was purchased from Invitrogen (Carlsbad, CA, USA). Anti-TBK1 antibody was purchased from Epitomics (San Diego, CA, USA). Actinomycin D was purchased from USB Corporation (Cleveland, OH, USA).
Mice
IKKε−/− (C57/b6) mice were derived from breeding pairs purchased from Dr Tom Maniatis (Harvard University, Cambridge, MA, USA). Wild-type (WT) mice (C57/b6) were purchased from Charles River Laboratories (Wilmington, MA, USA). All experimental protocols involving animals were reviewed and approved by the University of California San Diego Institutional Animal Care and Use Committee (IACUC; La Jolla, CA, USA).
FLS and culture conditions
Synovia from IKKε−/− and WT mice were micro-dissected from ankles, minced and digested with 1 mg/ml collagenase in serum-free Roswell Park Memorial Institute medium for 2 h at 37°C. The cell suspension was washed extensively and cultured in complete Dulbecco's modified Eagle medium (DMEM) [supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, gentamicin and l-glutamine] in a humidified 5% CO2 atmosphere. After overnight culture, non-adherent cells were removed and adherent cells were cultivated in 10% FBS/DMEM. At confluence, cells were trypsinized and split at a 1 : 3 ratio. FLS were used from passages 3–4 at 90% confluence. Cells were synchronized in 0.1% FBS/DMEM for 18 h before addition of poly(I:C) [20, 21].
Small interfering RNA transfection
WT and IKKε−/− FLS (5 × 105 cells) were transfected with 2 µg of TBK1 or scramble (sc) control Smartpool small interfering RNA (siRNA) (Dharmacon, Lafayette, CO, USA). The mouse embryonic fibroblast-2 (MEF-2) nucleofection kit was used according to the manufacturer’s instruction (Amaxa, Gaithersburg, MD, USA). Knockdown efficiency at 72-h post-transfection was ∼60%.
Cytokine analysis
Cytokine gene expression was measured by quantitative real-time PCR using the GeneAmp 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA) as previously described [22]. The Ct values were normalized to Hypoxanthine-guanine phosphoribosyltransferase1 (HPRT1) expression. Cytokines in cell supernatants were quantified using murine multiplex assays (Bio-Rad, Hercules, CA, USA).
Nuclear extract preparation and native PAGE
Nuclear extracts were obtained using the nuclear extraction kit (Panomics, Fremont, CA, USA), according to the manufacturer’s instruction. The protein concentration was measured using DC protein assay (Bio-Rad, Hercules, CA, USA). For detecting IRF3 dimers, native PAGE was performed [23]. The nuclear extracts (10 µg) were separated using a 7.5% non-denaturing gel (Bio-Rad, Hercules, CA, USA). The samples in the gel were electrophoresed at 25 mA for 60 min after pre-running (at 40 mA for 30 min). The gel was soaked in SDS electrophoresis buffer (25 mM Tris pH 8.3, 250 mM glycine, 0.1% SDS) for 60 min at room temperature. The gel was transferred to PVDF membrane and analysed by standard Western blot protocol. Densitometry was performed with the Versadoc software (Bio-Rad, Hercules, CA, USA).
Reporter gene assays
pGL3-mouse IFN-β promoter construct was a generous gift from Dr Taniguchi (University of Tokyo, Japan) and pGL3-mouse IP-10 promoter construct was a kind gift from Dr Bhat (Medical University of South Carolina, USA). Seventy-two hours after siRNA transfection, 4 × 105 FLS were transfected with 2 µg of reporter plasmid DNA and 0.2 µg of Renilla reniformis luciferase construct as internal control (a gift from Dr David, University of California San Diego, USA). Eighteen hours after transfection, the cells were stimulated with 20 µg/ml poly(I:C). Luciferase activity was measured after 24 h using a dual luciferase assay kit (Promega, Madison, WI, USA).
Measurement of mRNA stability
WT FLS were transfected with TBK1 or sc control siRNA for 48 h, after which the cells were serum starved with 0.1% FCS/DMEM for 24 h. FLS were stimulated with 20 µg/ml poly(I:C) for 6 h and then incubated with 10 µg/ml actinomycin D for 0 (t0), 2, 4 and 18 h. Total RNA was isolated and IP-10 mRNA was quantified by quantitative real-time PCR and normalized to β-actin. The data are expressed as a percentage of mRNA at t0. Control untransfected WT FLS (WT NT) were used as control.
Statistical analysis
Data are expressed as mean (s.e.m.). Comparisons between two groups were performed using Student’s t-test, unless otherwise stated. A comparison was considered statistically significant if P < 0.05.
Results
TBK1- and IKKε-deficient cells
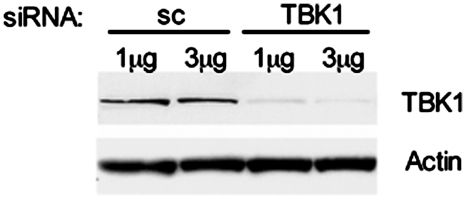
To evaluate the functions of TBK1 and IKKε, especially as it relates to IP-10 expression and activation of the IRF pathway, we initially used siRNA to knock down the respective genes. As shown in Fig. 1, TBK1 expression decreased 72 h after transfection with TBK1 siRNA, but not with sc negative control siRNA. IKKε expression could not be decreased sufficiently by IKKε-specific siRNA in the activated cells (data not shown), most likely due to markedly increased expression as previously described [24]. Since TBK1 deficiency in mice is embryonic lethal [25], we used siRNA to decrease TBK1 expression and IKKε−/− FLS to evaluate the role of IKKε. TBK1 siRNA decreased TBK1 expression in IKKε−/− FLS (data not shown) and was used to evaluate combined IKKε/TBK1 deficiency.
Fig. 1.
siRNA-mediated TBK1 knockdown. WT FLS were transfected with either 1 or 3 µg of Smartpool TBK1 siRNA or sc negative control for 72 h. Western blot analysis showed that TBK1 siRNA efficiently reduced protein expression compared with sc control. Two micrograms of siRNA was used in all further experiments.
Poly(I:C)-induced IRF gene expression in TBK1- and IKKε-deficient FLS
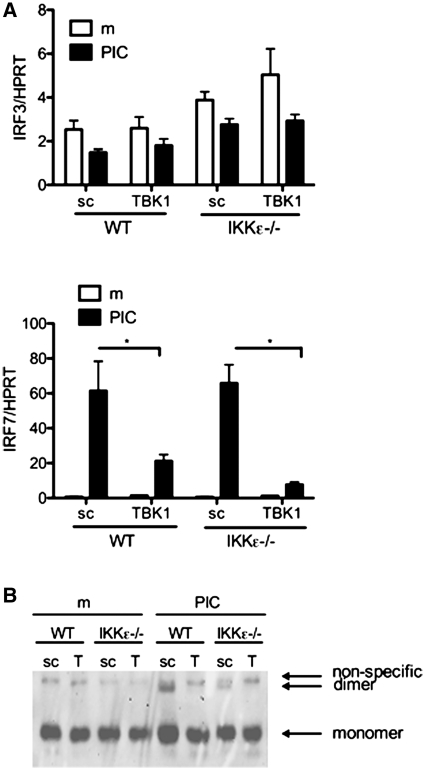
IRF3 and IRF7 are critical transcription factors that regulate of TLR3-induced IFN response genes and signalling downstream of the IKK-related kinases [26, 27]. While IRF3 function is generally regulated by post-translational phosphorylation, IRF7 is an inducible gene. Initial studies were performed to determine whether TBK1 and IKKε play a role in the expression of these IRFs (Fig. 2A). WT and IKKε−/− cells transfected with TBK1 siRNA or sc control were assayed for IRF3 and IRF7 gene expression by quantitative PCR (qPCR). TBK1- or IKKε-deficient cells or combined deficiency had no significant effect on IRF3 gene expression in resting or poly(I:C)-stimulated cells. IRF7 expression, however, was significantly increased by poly(I:C) in WT and IKKε−/− FLS compared with medium (P = 0.02, n = 3/group). TBK1 deficiency, but not IKKε deficiency, prevented IRF7 induction (P = 0.003). Therefore, poly(I:C)-mediated induction of IRF7, but not IRF3, requires TBK1 in FLS.
Fig. 2.
Effect of TBK1 and IKKε deficiency on IRF expression and activation. (A) WT and IKKε−/− FLS transfected with TBK1 siRNA were stimulated with 20 µg/ml poly(I:C) for 24 h and total RNA was isolated. IRF3 and IRF7 gene expression was determined by qPCR and normalized to HPRT. IRF7 expression was significantly decreased in TBK1-deficient FLS, regardless of IKKε deficiency (*P < 0.04, n = 3 lines/group). IRF3 expression was not altered by the lack of TBK1 or IKKε. (B) TBK1-deficient WT and IKKε−/− FLS were treated for 2 h with poly(I:C) (20 µg/ml) and nuclear extracts were prepared. Native PAGE and western blot analysis were performed using anti-IRF3 antibody. IRF3 monomeric and dimeric forms (←). IRF3 dimerization was inhibited in TBK1-deficient WT and IKKε−/− cells, indicating that both kinases regulate IRF3 activation in FLS. The figure is representative of three separate experiments.
Regulation of IRF3 activation in FLS
Poly(I:C) stimulation leads to phosphorylation of IRF3, which dimerizes and functions as a transcription factor for IFN response genes such as IFN-β. Our previous studies suggested that IKKε deficiency can decrease IRF3 phosphorylation in human FLS [24]. The next step in IFN response gene expression is IRF3 dimerization. The contribution of TBK1 and IKKε to poly(I:C)-induced IRF3 dimerization and nuclear translocation was analysed in nuclear extracts of TBK1-deficient WT and IKKε−/− FLS by native gel electrophoresis. Figure 2B shows that IRF3 dimer formation occurred within 2 h of poly(I:C) stimulation. TBK1 deficiency significantly decreased the level of nuclear IRF3 dimers in both WT and IKKε−/− FLS (45 and 41%, respectively). IRF3 dimerization was also decreased by 32% in IKKε−/− FLS.
TBK1-dependent gene expression in synoviocytes
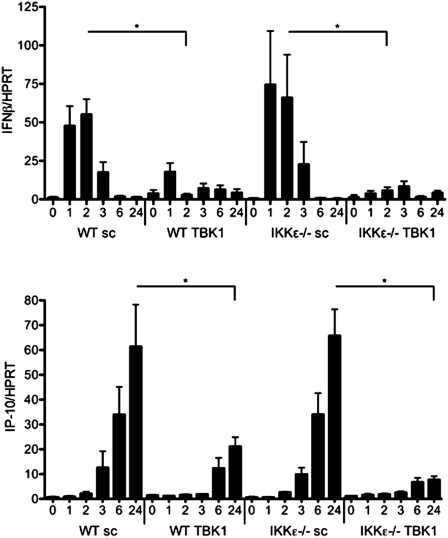
IRF3 and IRF7 function as transcription factors along with nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) and activator protein-1 to initiate transcription of inflammatory mediators. Since TBK1 regulates IRF7 expression and IRF3 dimerization, we determined whether it alters expression of prototypical IFN response genes in synoviocytes. TBK1-deficient WT and IKKε−/− FLS were stimulated with poly(I:C) and cytokine gene expression was assayed by qPCR (Fig. 3). IFN-β gene expression peaked within 2 h of poly(I:C) stimulation in WT FLS and decreased to baseline levels by 6 h. TBK1 deficiency in both WT and IKKε−/− FLS significantly decreased IFN-β gene expression [WT: 95 (12)% vs IKKε−/−: 91 (3)% inhibition at peak, n = 3/group, P < 0.05]. IKKε−/− cells transfected with sc control siRNA had normal IFN-β levels, despite reduced IRF3 dimerization. Unlike IFN-β, IP-10 expression peaked 24 h after stimulation in WT FLS. IP-10 expression was dependent on TBK1 after poly(I:C) stimulation [WT: 76 (10)% vs IKKε−/−: 97 (1)% decrease at peak, n = 3/group, P = 0.008]. In contrast, IP-10 expression was normal in sc control siRNA-transfected IKKε−/− FLS.
Fig. 3.
Time course of TBK1-dependent cytokine gene expression in FLS. TBK1-deficient WT and IKKε−/− FLS were stimulated with 20 µg/ml poly(I:C) for various times and IFN-β and IP-10 gene expression was assayed by qPCR. IFN-β was induced within 2 h of poly(I:C) stimulation, while IP-10 peaked at 24 h. TBK1 deficiency significantly decreased IFN-β (*P = 0.01) and IP-10 expression (*P = 0.01), while lack of IKKε did not alter their expression.
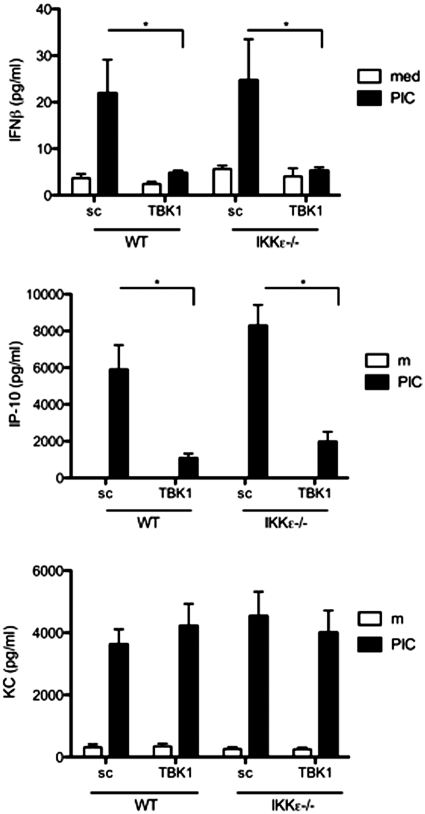
TBK1-dependent cytokine profile in FLS
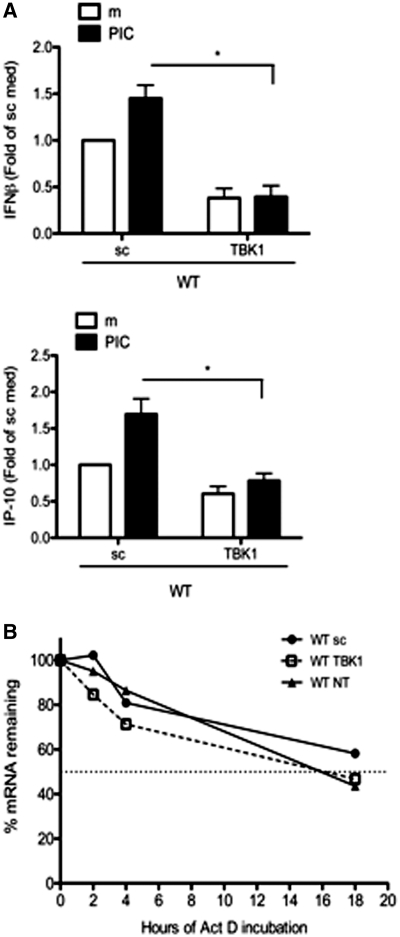
As noted above, TBK1 is the primary IKK-related kinase required for IFN-β and IP-10 gene expression in FLS stimulated with a TLR3 ligand. Previous studies show that IP-10 protein expression can be induced by over-expression of IKKε in human embryonic kidney cells [28], while others observed an inhibition of IP-10 production in TBK1-deficient MEFs stimulated with poly(I:C) [29]. To evaluate the contribution of TBK1 to IP-10 production in FLS, we evaluated the cytokine profiles in TBK1-deficient WT and IKKε−/− FLS using multiplex analysis of the 24 h culture supernatants (Fig. 4). IP-10 levels were significantly reduced in TBK1-knockdown FLS, regardless of IKKε status [WT: 88 (5)% inhibition and IKKε−/− 85 (4)% inhibition, n = 3, P < 0.01, t-test]. As with IP-10, IFN-β levels were reduced significantly in the absence of TBK1 [WT: 68 (16)% and IKKε−/− 70 (11)% inhibition, n = 3, P < 0.05]. In contrast, expression of the NF-κB-target gene keratinocyte-derived chemokine (KC) (mouse IL-8 homologue) was not inhibited in TBK1- or IKKε-deficient FLS.
Fig. 4.
TBK1-dependent cytokine profile in FLS. WT and IKKε−/− FLS were transfected with TBK1 siRNA and stimulated for 24 h with poly(I:C). Cytokines in the culture supernatants were measured using multiplex analysis. IP-10 and IFN-β protein expression was significantly reduced in TBK1-knockdown FLS (*P < 0.05, n = 3 lines/group).
TBK1-mediated regulation of IFN-β and IP-10 promoter activity
To understand how TBK1 regulates the expression of IFN response genes in FLS, we evaluated the effects of TBK1 deficiency on gene transcription using IFN-β and IP-10 promoter constructs (Fig. 5A). TBK1 deficiency significantly reduced IFN-β in WT FLS compared with stimulated sc control [74 (6)% inhibition, P = 0.003, n = 3 different lines]. However, IKKε deficiency did not alter IFN-β promoter activity (data not shown). Like IFN-β, IP-10 promoter activity was also significantly reduced in TBK1-knockdown WT FLS compared with stimulated sc control [53 (4)% inhibition, n = 3, P = 0.008].
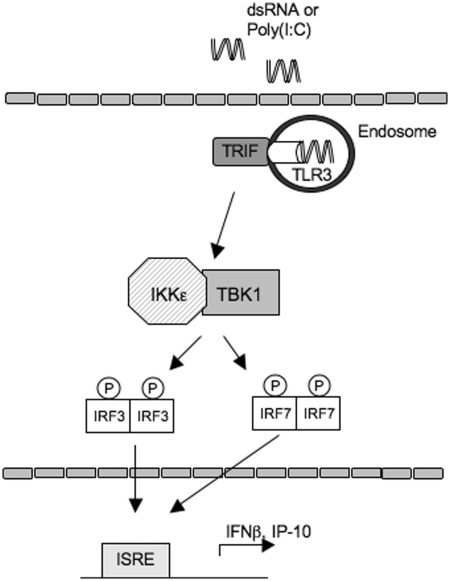
Fig. 6.
Activation of the TLR3 pathway. Endosomal poly(I:C)-bound TLR3 activates Toll/IL1R-domain containing adapter-inducing interferon beta, a TLR adaptor molecule as well as members of the TRAF family, leading to the activation of the TBK1–IKKε complex. This complex phosphorylates IRF3 and IRF7, transcription factors that regulate IFN response genes such as IFN-β and IP-10. ISRE: interferon-stimulated response elements.
Fig. 5.
Effect of TBK1 deficiency on IP-10 promoter activity and mRNA stability. (A) WT FLS were transfected with either sc or TBK1 siRNA. After 72 h, the cells were co-transfected with mouse IFN-β or IP-10 promoter constructs. The cells were stimulated with poly(I:C) for 24 h and assayed for luciferase activity normalized to R. reniformis luciferase. The data are expressed as fold of sc medium. TBK1 deficiency significantly reduced the promoter activity of IFN-β and IP-10 compared with stimulated sc control (*P < 0.01). (B) IP-10 expression was determined by qPCR and normalized to β-actin mRNA. Data are expressed as a percentage of IP-10 in cells not treated with actinomycin D. IP-10 mRNA decay is linear in untransfected WT FLS and the half-life is ∼16 h. TBK1 deficiency did not significantly alter IP-10 mRNA half-life in FLS.
Role of TBK1 in IP-10 mRNA stability
Our data show that TBK1 regulates IP-10 transcription in synoviocytes. Recent studies, however, indicate that IP-10 expression can also be regulated post-transcriptionally via the degradation of the AU-rich 3′′-untranslated region (UTR) [30, 31]. Therefore, we evaluated whether TBK1 deficiency also alters IP-10 mRNA stability in poly(I:C)-stimulated FLS. IP-10 mRNA half-life in untransfected WT cells was ∼16 h (Fig. 5B). Although there was a trend towards slightly faster decay in TBK1-deficient cells, it did not reach statistical significance.
Discussion
Innate immunity participates in the pathogenesis of numerous inflammatory diseases. For instance, IFN signatures have been demonstrated in the blood and tissues of patients with diverse diseases such as SLE, scleroderma and RA [5]. Innate immune response contributes to adaptive immune responses and potentially to a breakdown in tolerance [32]. While the stimuli that activate innate immunity in RA are uncertain, co-expression and activation of TLRs by endogenous ligands in the synovium is one possible mechanism. Bacterial DNA, peptidoglycan and endogenous ligands such as necrotic debris and stress-induced proteins (high mobility group protein B1) [33] are present in the inflamed joint and can activate local TLR2, TLR4 and TLR3. Cultured synoviocytes also express these receptors and can be activated by the same ligands in vitro to produce pro-inflammatory mediators that contribute to joint destruction.
The effects of poly(I:C) on synoviocyte function are rapid and dramatic and trigger a cascade of signalling events leading to the activation of IKKs and IKK-related kinases (IKKε and TBK1, Fig. 6). These, in turn, phosphorylate transcription factors such as the IRFs that increase expression of key genes, such as IP-10, that are involved in chronic inflammation. IP-10 plays a critical role in recruiting activated T cells and macrophages into RA synovium and contributes to bone erosion by enhancing osteoclastogenesis [34]. Inhibition of IP-10 function significantly attenuated bone loss in CIA [35]. Clinical studies demonstrating the efficacy of IP-10 neutralization in RA suggest that TBK1 or IKKε might also be potential therapeutic targets.
TBK1 and IKKε are homologous proteins with overlapping, non-redundant functions since IKKε−/− mice are viable and healthy [36], while TBK1 deficiency is embryonic lethal [25]. Mouse and human TBK1 proteins share >99% homology, indicating that this protein is highly conserved in mammals. In humans, TBK1 is constitutively and ubiquitously expressed in lymphoid organs such as peripheral blood lymphocytes and spleen as well as in non-lymphoid organs such as brain, kidney and skeletal muscle [37]. In contrast, IKKε is expressed at low basal levels in immune cells as well as synoviocytes, but is rapidly induced in response to TLR activation. The goals of this study were to evaluate the function of the two IKK-related kinases in regulating FLS biology and determine whether either kinase is particularly attractive as a target for modulating a promising chemokine such as IP-10.
The function of TBK1 and IKKε in the TLR3 signalling pathway was evaluated using IKKε−/− and TBK1 knockdown FLS stimulated with poly(I:C). We first examined the effects of TBK1 or IKKε deficiency on IRF3 and IRF7 gene expression. While IRF3 is constitutively expressed in synoviocytes, IRF7 expression is low at basal conditions, but is induced within 24 h. In contrast, plasmacytoid dendritic cells constitutively express IRF7, demonstrating the cell specificity of the function of IKK-related kinases [27]. TBK1 is the primary regulator of IRF7 expression in FLS while neither IKK-related kinase affected IRF3 gene expression.
Phosphorylation of IRF3 causes its dimerization and translocation into the nucleus. In FLS, optimal IRF3 dimerization requires TBK1 as well as IKKε. This observation is consistent with previous reports demonstrating impaired poly(I:C)-mediated IRF3 activation in TBK1−/− murine embryonic fibroblasts (MEFs) [29]. In contrast, IRF3 activation is completely abolished in TBK1−/− IKKε−/− double knockout MEFs [38]. IFN-β and IP-10 gene and protein expression required TBK1, which is similar to TBK1−/− MEFs stimulated with either poly(I:C), lipopolysaccharide (LPS) or single-stranded RNA viruses [29]. Interestingly, although IRF3 dimerization was inhibited in IKKε−/− FLS, IFN-β and IP-10 gene or protein expression remained unaltered in these cells.
Activation of TBK1 and IKKε in FLS results in the rapid transcription of primary response genes such as IFN-β, which binds to the IFN-α receptor (IFNαR) in an autocrine manner. The IFNαR triggers activation of the Janus kinase-signal transducers and activators of transcription signalling cascade leading to the production of multiple IFN-β-dependent secondary response genes such as IP-10 and IFN-α. IP-10 can activate FLS in an autocrine manner by binding CXCR3, which is constitutively expressed on the cell surface [11]. The secondary response cytokine production varies with cell lineage since plasmacytoid dendritic cells, MEFs and macrophages produce IFN-α, but FLS do not (data not published) [27, 39].
We then defined the contribution of TBK1 in the poly(I:C)-induced cytokine response in FLS. TBK1 was required for IFN-β and IP-10 gene as well as protein expression, which is similar to TBK1−/− MEFs stimulated with either poly(I:C), LPS or single-stranded RNA viruses [29]. Expression of the NF-κB target gene, KC (mouse IL-8 homologue), was not inhibited in TBK1-deficient FLS, suggesting a preservation of NF-κB activity in the absence of TBK1. This contrasts with studies showing inhibition of p65/RelA phosphorylation in TBK1−/− and IKKε−/− MEFs [15]. Unlike TBK1, IKKε does not regulate IP-10 production in FLS, which differs from other cell lineages that over-express IKKε [28]. We next determined whether IP-10 expression is regulated by post-transcriptional mechanisms in TBK1-deficient FLS. Our data show that TBK1 deficiency does not alter IP-10 mRNA stability in FLS. Therefore, TBK1 deficiency decreases IP-10 expression primarily by inhibiting transcription in FLS. Furthermore, we found no cytoplasmic vesicles with pre-formed IP-10, which would have suggested post-translational regulation of this chemokine in FLS.
Conclusions
Recent research on TLR biology and IFN response genes has identified new targets for inflammatory and autoimmune diseases. The contribution of IKK-related kinase signalling and IFN signatures to RA pathogenesis suggests that these kinases might be useful therapeutic targets. Based on the profiles of the two kinases, especially as it pertains to IP-10, TBK1 might be more relevant than IKKε as a key contributor to the pathogenesis of the disease.
Acknowledgements
We are grateful to Josh Hillman and Katharyn Topolewski for their technical assistance.
Funding: This work was supported by the US National Institute of Arthritis and Musculoskeletal, and Skin Diseases (NIAMS) (Grant number RO1 AI067752 to G.S.F.).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–55. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11(Suppl. 3):S39–44. doi: 10.1097/01.rhu.0000166673.34461.33. [DOI] [PubMed] [Google Scholar]
- 4.Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–64. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drexler SK, Foxwell BM. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2010;42:506–18. doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Ospelt C, Brentano F, Rengel Y, et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58:3684–92. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 7.Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–22. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 8.Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52:2656–65. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- 9.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 10.Kim KW, Cho ML, Oh HJ, et al. TLR-3 enhances osteoclastogenesis through upregulation of RANKL expression from fibroblast-like synoviocytes in patients with rheumatoid arthritis. Immunol Lett. 2009;124:9–17. doi: 10.1016/j.imlet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50:3866–77. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–12. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald KA, McWhirter SM, Faia KL, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–6. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–51. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 15.Clement JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18:889–99. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 16.Kravchenko VV, Mathison JC, Schwamborn K, Mercurio F, Ulevitch RJ. IKKi/IKKepsilon plays a key role in integrating signals induced by pro-inflammatory stimuli. J Biol Chem. 2003;278:26612–9. doi: 10.1074/jbc.M303001200. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg AM, Drexler SK, Monaco C, et al. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007;110:3245–52. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- 18.Kuhne M, Preston B, Wallace S, et al. MDX-1100, a fully human anti-CXCL10 (IP-10) antibody, is a high affinity, neutralizing antibody that has entered Phase I clinical trials for the treatment of Ulcerative Colitis (UC) J Immunol. 2007;178:131–120. [Google Scholar]
- 19.Yellin M, Paliienko I, Balanescu A, et al. A phase II, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate (MTX) in patients with rheumatoid arthritis (RA) [abstract] Arthritis Rheum. 2009;60(Suppl 10):414. doi: 10.1002/art.34330. [DOI] [PubMed] [Google Scholar]
- 20.Kullmann F, Judex M, Neudecker I, et al. Analysis of the p53 tumor suppressor gene in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 1999;42:1594–600. doi: 10.1002/1529-0131(199908)42:8<1594::AID-ANR5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren S, Boyle DL, Firestein GS. Acquisition, culture, and phenotyping of synovial fibroblasts. Methods Mol Med. 2007;135:365–75. doi: 10.1007/978-1-59745-401-8_24. [DOI] [PubMed] [Google Scholar]
- 22.Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5:R352–60. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwamura T, Yoneyama M, Yamaguchi K, et al. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells. 2001;6:375–88. doi: 10.1046/j.1365-2443.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney SE, Mo L, Firestein GS. Antiviral gene expression in rheumatoid arthritis: role of IKKepsilon and interferon regulatory factor 3. Arthritis Rheum. 2007;56:743–52. doi: 10.1002/art.22421. [DOI] [PubMed] [Google Scholar]
- 25.Bonnard M, Mirtsos C, Suzuki S, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–85. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle S, Vaidya S, O’Connell R, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–63. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 27.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 28.Sankar S, Chan H, Romanow WJ, Li J, Bates RJ. IKK-i signals through IRF3 and NFkappaB to mediate the production of inflammatory cytokines. Cell Signal. 2006;18:982–93. doi: 10.1016/j.cellsig.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci USA. 2004;101:233–8. doi: 10.1073/pnas.2237236100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanmugam N, Ransohoff RM, Natarajan R. Interferon-gamma-inducible protein (IP)-10 mRNA stabilized by RNA-binding proteins in monocytes treated with S100b. J Biol Chem. 2006;281:31212–21. doi: 10.1074/jbc.M602445200. [DOI] [PubMed] [Google Scholar]
- 31.Galbis-Martinez M, Saenz L, Ramirez P, Parrilla P, Yelamos J. Poly(ADP-ribose) polymerase-1 modulates interferon-gamma-inducible protein (IP)-10 expression in murine embryonic fibroblasts by stabilizing IP-10 mRNA. Mol Immunol. 2010;47:1492–9. doi: 10.1016/j.molimm.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Lang KS, Recher M, Junt T, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–45. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 33.Park JS, Svetkauskaite D, He Q, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 34.Kwak HB, Ha H, Kim HN, et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 2008;58:1332–42. doi: 10.1002/art.23372. [DOI] [PubMed] [Google Scholar]
- 35.Byrne FR, Winters A, Brankow D, et al. An antibody to IP-10 is a potent antagonist of cell migration in vitro and in vivo and does not affect disease in several animal models of inflammation. Autoimmunity. 2009;42:171–82. doi: 10.1080/08916930802629547. [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Takeuchi O, Sato S, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–50. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tojima Y, Fujimoto A, Delhase M, et al. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–82. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi O, Hemmi H, Akira S. Interferon response induced by Toll-like receptor signaling. J Endotoxin Res. 2004;10:252–6. doi: 10.1179/096805104225005896. [DOI] [PubMed] [Google Scholar]
- 39.Solis M, Romieu-Mourez R, Goubau D, et al. Involvement of TBK1 and IKKepsilon in lipopolysaccharide-induced activation of the interferon response in primary human macrophages. Eur J Immunol. 2007;37:528–39. doi: 10.1002/eji.200636090. [DOI] [PubMed] [Google Scholar]