Abstract
The human steroidogenic cytochromes P450 CYP17A1 (P450c17, 17α-hydroxylase/17,20-lyase) and CYP21A2 (P450c21, 21-hydroxylase) are required for the biosynthesis of androgens, glucocorticoids, and mineralocorticoids. Both enzymes hydroxylate progesterone at adjacent, distal carbon atoms and show limited tolerance for substrate modification. Halogenated substrate analogs have been employed for many years to probe cytochrome P450 catalysis and to block sites of reactivity, particularly for potential drugs. Consequently, we developed efficient synthetic approaches to introducing one or more halogen atom to the 17- and 21-positions of progesterone and pregnenolone. In particular, novel 21,21,21-tribromoprogesterone and 21,21,21-trichloroprogesterone were synthesized using the nucleophilic addition of either bromoform or chloroform anion onto an aldehyde precursor as the key step to introduce the trihalomethyl moieties. When incubated with microsomes from yeast expressing human CYP21A2 or CYP17A1 with P450-oxidoreductase, CYP21A2 metabolized 17-fluoroprogesterone to a single product, whereas incubations with CYP17A1 gave no products. Halogenated steroids provide a robust system for exploring the substrate tolerance and catalytic plasticity of human steroid hydroxylases.
1. Introduction
The human steroidogenic cytochromes P450 CYP17A1 (P450c17, 17α-hydroxylase/17,20-lyase) and CYP21A2 (P450c21, 21-hydroxylase) oxygenate the 17-position and 21-positions of progesterone, respectively. The hydroxylase activity of CYP17A1 leads to the production of the glucocorticoid cortisol, while the activity of CYP21A2 leads to the production of both mineralocorticoids and glucocorticoids. Deficiency of CYP21A2 (21-hydroxylase deficiency, 21OHD, also called congenital adrenal hyperplasia, CAH) is the most common autosomal recessive disease in human beings, with an incidence of 1:16,000 live births in the classical form [1] and at least 1:1,000 in the nonclassical form [2]. Deficiency of CYP17A1 causes hypertension [3], and the CYP17A1 locus has been identified in genome-wide linkage studies as a candidate gene for primary hypertension [4]. In addition to its 17-hydroxylase activity, CYP17A1 also cleaves the 17,20-carbon carbon bond of 17-hydroxysteroids, and because this lyase activity produces 19-carbon androgens, this enzyme is a target to treat prostate cancer [5]. Abiraterone acetate, a potent and specific inhibitor of CYP17A1, received FDA approval for the treatment of castration-resistant prostate cancer unresponsive to docitaxel in early 2011, improving median survival from 10.9 to 14.8 months [6]. Consequently, these enzymes are critically important in human physiology and disease, and a better understanding of their chemistries might lead to better treatments of rare and common diseases.
Interestingly, when progesterone is the substrate, CYP17A1, both 17α- and 16α-hydroxylates in a 3:1 ratio [7]; however when pregnenolone is used as a substrate, 17-hydroxypregnenolone is the only hydroxylation product. CYP21A2 exclusively 21-hydroxylates progesterone and 17-hydroxyprogesterone, but we recently showed that the rationally designed CYP21A2 mutation V359G is primarily a progesterone 16α-hydroxylase [8]. Consequently, these biosynthetic enzymes, known for their high fidelity, also demonstrate intrinsic and latent catalytic plasticity. Because neither the crystal nor the NMR structures are known for both enzymes, insight to structure-function relationships derives largely from computer modeling [9], site-directed mutagenesis [10], human genetics [11], and substrate analog studies. Halogenated substrate analogs have been employed as probes of cytochrome P450 function and as a strategy to prevent drug metabolism for many years. Limited data exist on how steroidogenic P450 enzymes handle halogenated substrates, and few efforts have comprehensively pursued their synthesis and characterization. Consequently, we developed methodologies to incorporate fluorine, chlorine, and bromine atoms on either the 17- or 21-positions of progesterone and pregnenolone, and we tested these compounds as substrates for CYP17A1 and CYP21A2.
2. Experimental Procedures
2.1. Materials and Methods
NMR spectra were obtained using Varian instruments at frequencies for 1H and 13C as specified in the experimental detail. Chemical shifts were referenced to the chloroform peak in the 1H NMR assigned at 7.26 ppm and in the 13C NMR assigned at 77.16 ppm [12]. Reaction progress was determined either by TLC monitoring, or an aliquot was taken and analyzed by NMR. NMR was the only technique used to validate the structures of the new compounds. Pregnenolone was purchased from Waterstone Technologies, and all other reagents and solvents were purchased from Sigma Aldrich or Fisher. NMR spectra are provided in the supporting information.
2.2. Chemical Syntheses
2.2.1. pregn-5-ene-21-N-pyridyl-3β-ol-20-one iodide (21-N-pyridinyl-pregnenolone iodide, compound 19)
Iodine (6.0 g, 23.6 mmol, 1.3 mol eq) was added to a heated stirring solution of pregnenolone (6.0 g, 19.0 mmol) in pyridine, and the reaction was stirred at reflux for 2 hours under a N2 atmosphere. Additional iodine (3.0 g, 11.8 mmol) was added, and the resulting mixture was refluxed overnight. The reaction mixture was cooled to room temperature, diluted with ethyl acetate (100 mL), filtered through cotton, and dried under reduced pressure to afford the crude pyridinium salt 19 as a yellow powder, which was used without purification. 1H NMR (500 MHz, CD3OD) δ 8.78 (d, J = 5.5 Hz, 1H), 8.64 (t, J = 8.0 Hz, 1H), 8.14 (apparent t, J = 7.0 Hz, 2H), 5.76 (d, J = 17.5 Hz, 1H), 5.61 (d, J = 17.5 Hz, 1H), 5.34 (d, J = 5.5 Hz, 1H), 3.39 (ddd, J1 = 15.5, J2 = 10.5, J3 = 4.5 Hz, 1H), 2.83 (apparent t, J = 9 Hz, 1H), 2.25-2.16 (m, 3H), 2.04-1.98 (m, 2H), 1.90-1.85 (m, 2H), 1.79-1.77 (m, 2H), 1.72-1.68 (m, 1H), 1.63-1.43 (m, 4H), 1.31-1.24 (m, 3H), 1.15-1.00 (m, 2H), 1.02 (s, 3H), 0.88-0.83 (m, 1H), 0.74 (s, 3H).
2.2.2. 3β-hydroxy-androst-5-ene-17β-carboxylic acid (etienic acid, compound 20)
To the crude pyridinium salt 19 dissolved in THF (40 mL), NaOH pellets (5.2 g) were added, and the reaction was stirred at reflux. After 10 h, the reaction was cooled to RT, diluted with methanol (4 mL) and CH2Cl2 (30 mL), and washed with saturated aqueous NaHCO3 solution to submerge the carboxylic acid in the aqueous layer (50 mL). The aqueous layer was separated and concentrated via reduced pressure. The resultant solid was filtered through a cotton-plugged funnel with CH2Cl2 (100 mL), and the sodium salt 20a was collected as a white powder (3.8 g, 11.2 mmol, 59% over two steps). The carboxylic acid 20 [13] is obtained by dispersing 20a in diethyl ether, washing with 10% HCl, and concentrating the organic extracts. 1H NMR of carboxylic acid 20 (300 MHz, CDCl3) δ 5.35 (d, J = 5.1 Hz, 1H), 3.53 (ddd, J1 = 16.5, J2 = 10.8, J3 = 5.1 Hz, 1H), 2.40 (apparent t, J = 8.7 Hz, 1H), 2.34-2.23 (m, 2H), 2.11-1.94 (m, 3H), 1.90-1.82 (m, 3H), 1.47-1.80 (m, 7H), 1.34-1.23 (m, 2H), 1.23-1.07 (m, 1H), 1.02 (s, 3H), 1.04-0.94 (m, 1H), 0.75 (s, 3H); 13C NMR (100 MHz, CD3OD) δ 177.8, 142.4, 122.4, 72.5, 57.7, 56.5, 51.8, 44.9, 43.1, 39.5, 38.7, 37.9, 33.5, 33.1, 32.4, 25.7, 24.8, 22.3, 20.1, 13.8.
2.2.3. 21,21,21-trifluoro-3β-hydroxy-pregn-5-en-20-one (21-trifluoropregnenolone, compound 13) and 21,21,21-trifluoro-pregn-4-ene-3,20-dione (21-trifluoroprogesterone, compound 4)
The carboxylic acid 20 (0.2 g, 0.6 mmol, 1.0 mol eq) in dry toluene (20 mL) was cooled to 0 °C. Pyridine (1.0 mL, 8.6 mmol, 14 mol eq) was added via syringe under N2 atmosphere followed by trifluoroacetic anhydride (1.2 mL, 8.6 mmol, 13.7 mol eq). The reaction was stirred at reflux for 16 hours, and a second portion of trifluoroacetic anhydride (1.0 mL) was added via syringe. Once the trifluoroketone was verified by NMR and TLC, water (0.2 mL) was added, and the reaction continued to stir at reflux for 2 h. The reaction was cooled to room temperature, washed with H2O (20 mL), and the aqueous layer was back-extracted with ethyl acetate (2 × 20 mL). The combined organic extracts were concentrated, and the crude material was purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield 13 as a light yellow solid (0.1 g, 0.27 mmol, 43%). 1H NMR (400 MHz, CDCl3) δ 5.32 (d, J = 5.2 Hz, 1H), 3.50 (ddd, J1 = 15.6, J2 = 11.2, J3 = 4.4 Hz, 1H), 2.93 (apparent t, J = 9.2 Hz, 1H), 2.30-2.10 (m, 3H), 2.02-1.93 (m, 3H), 1.89-1.71 (m, 4H), 1.63-1.41 (m, 5H), 1.38-1.18 (m, 2H), 1.10-1.02 (m, 1H), 1.00-0.93 (m, 1H), 0.98 (s, 3H), 0.72 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.9 (q, JCF = 33.9 Hz), 140.9, 121.2, 115.5 (q, JCF = 174.7), 71.6, 57.3, 56.7, 49.8, 47.1, 42.2, 38.04, 38.03, 37.3, 36.5, 32.1, 31.8, 31.6, 24.7, 21.1, 19.4, 13.5.
Compound 4 (21-trifluoroprogesterone) is obtained by Oppenauer oxidation of compound 13 as previously described [14] or the by the procedure in sections 2.2.20 and 2.2.21.
2.2.4. 21,21,21-trichloro-3β-trichloroacetoxy-pregn-5-en-20-one (21-trichloropregnenolone-3-trichloroacetate, compound 23)
To carboxylic acid 20 (1.09 g, 3.5 mmol) in CH2Cl2 (100 mL) was added pyridine (1.5 mL, 18.6 mmol, 5.4 mol eq) and trichloroacetic anhydride (1.0 mL, 8.9 mmol, 2.6 mol eq) under N2 atmosphere at 0 °C. The reaction was warmed to RT and stirred for 18 h. The resulting reaction mixture was concentrated via reduced pressure and purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield trichloroketone 23 (0.65 g, 1.2 mmol, 33%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.42 (m, 1H), 4.80-4.71 (m, 1H), 3.22 (apparent t, J = 8 Hz, 1H), 2.51-2.39 (m, 2H), 2.22-2.11 (m, 1H), 2.07-1.89 (m, 6H), 1.82-1.71 (m, 2H), 1.39-1.31 (m, 1H), 1.27-1.14 (m, 2H), 1.06 (s, 3H), 0.93 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.9, 161.5, 138.9, 123.3, 79.7, 56.9, 54.2, 49.8, 47.3, 38.7, 37.4, 36.9, 36.7, 32.1, 32.0, 31.4, 27.2, 25.4, 21.1, 19.5, 13.6.
2.2.5. methyl 3β-hydroxy-androst-5-ene-17β-carboxylate (etienic acid methyl ester, compound 24)
To pyridinium iodide 19 (6.5 g, 12.5 mmol) dissolved in methanol (10 mL) was added sodium methoxide solution (5.4 M, 10 mL). The reaction was stirred for 48 h and loaded directly on a silica gel column (hexanes to 50% ethyl acetate in hexanes), and methyl ester 24 (3.3 g, 9.9 mmol, 79%) was purified as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.36-5.34 (m, 1H), 3.67 (s, 3H), 3.56-3.49 (m, 1H), 2.37-2.24 (m, 3H), 2.21-2.09 (m, 2H), 2.00-1.94 (m, 2H), 1.89-1.65 (m, 4H), 1.53-1.38 (m, 4H), 1.35-1.23 (m, 3H), 1.15-1.05 (m, 2H), 1.00 (s, 3H), 0.67 (s, 3H).
2.2.6. methyl 3β-(t-butyl)dimethyl-siloxy-androst-5-ene-17β-carboxylate (etienic acid methyl ester-3-TBDMS-ether, compound 25)
Solid t-butyldimethylsilyl chloride (0.61 g, 4.0 mmol, 1.3 mol eq) followed by imidazole (0.28 g, 4.2 mmol, 1.3 mol eq) was added to a stirring solution of methyl ester 24 (1.05 g, 3.2 mmol) in dimethylformamide (50 mL). The reaction was stirred for 1 h, and the resulting mixture was diluted with ethyl acetate (50 mL) and washed with water (at least 2 × 30 mL). The combined organic extracts were concentrated via reduced pressure and purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes), which also removed residual dimethylformamide and yielded TBDMS-ether 25 (0.70 g, 1.6 mmol, 50%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.31(broad s, 1H), 3.66 (s, 3H), 3.50-3.44 (m, 1H), 2.34 (apparent t, J = 12 Hz, 1H), 2.30-2.19 (m, 2H), 2.19-2.09 (m, 2H), 2.05-1.94 (m, 2H), 1.85-1.65 (m, 5H), 1.60-1.37 (m, 4H), 1.32-1.22 (m, 2H), 1.14-1.04 (m, 2H), 0.99 (s, 3H), 1.00-0.89 (m, 2H), 0.88 (s, 12H), 0.66 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 174.7, 141.7, 121.0, 72.7, 61.5, 56.3, 55.3, 51.4, 50.3, 44.1, 42.9, 38.4, 37.5, 36.8, 32.2, 32.0, 26.1, 24.7, 23.8, 21.0, 19.6, 18.4, 13.5, 4.5.
2.2.7. 3β-(t-butyl)dimethyl-siloxy-androst-5-ene-17β-methanol, compound 26
To methyl ester 25 (1.56 g, 3.5 mmol) in CH2Cl2 (100 mL) was added di-isobutyl aluminum hydride (3.0 mL, 16.8 mmol, 4.8 mol eq, neat) at −78 °C under nitrogen atmosphere. The reaction was stirred for 1 h, then ethyl acetate (10 mL) and a saturated aqueous solution of Rochelle’s salt (10 mL) were added. The reaction mixture warmed to RT and stirred until the two layers separated (~1–2 h). The aqueous layer was extracted with ethyl acetate (3 × 40 mL), and the combined organic extracts were dried with MgSO4 and concentrated under reduced pressure. The crude material was purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield alcohol 26 (1.28 g, 3.1 mmol, 84%). 1H NMR (400 MHz, CDCl3) δ 5.31 (broad s, 1H), 3.71 (dd, J1 = 12.0, J2 = 8.0 Hz, 1H), 3.53 (dd, J1 = 12.0, J2 = 8.0 Hz, 1H), 3.53-3.44 (m, 1H), 2.30-2.25 (m, 1H), 2.25-2.15 (m, 1H), 2.05-1.95 (m, 1H), 1.85-1.75 (m, 3H), 1.75-1.40 (m, 10H), 1.00 (s, 3H), 0.89 (s, 9H), 0.66 (s, 3H), 0.06 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 141.7, 121.2, 72.7, 64.8, 56.4, 53.1, 50.6, 43.0, 41.8, 38.8, 37.5, 36.8, 32.2, 32.1, 31.8, 26.1, 25.7, 24.8, 20.9, 19.6, 18.4, 12.6, −4.4.
2.2.8. 3β-(t-butyl)dimethyl-siloxy-androst-5-ene-17β-carboxaldehyde, compound 27
Alcohol 26 (1.03 g, 2.5 mmol) in CH2Cl2 (100 mL) was treated at RT with Dess-Martin periodinane (1.53 g, 3.6 mmol, 1.4 eq), and the reaction was stirred for 2 h. The reaction mixture was washed with saturated aqueous NaHCO3 and extracted with ethyl acetate. The combined organic extracts were dried with MgSO4 and concentrated under reduced pressure. The crude material was purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield aldehyde 27 (0.66 g, 1.6 mmol, 65%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 9.77 (d, J = 4 Hz, 1H), 5.31 (broad s, 1H), 3.51-3.43 (m, 1H), 2.38-1.97 (m, 9H), 1.85-1.71 (m, 6H), 1.35-1.21 (m, 3H), 1.00 (s, 3H), 0.88 (s, 9H), 0.76 (s, 3H), 0.05 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 205.2, 141.7, 120.9, 72.6, 63.0, 56.6, 50.3, 44.9, 42.9, 38.5, 37.5, 36.8, 32.2, 32.0, 31.6, 26.1, 26.0, 25.1, 21.2, 20.7, 19.6, 18.4, 13.9.
2.2.9. 21,21,21-tribromo-pregn-5-ene-3β-(t-butyl)dimethylsiloxy-20-ol, compound 28
To a flask containing aldehyde 27 (0.78 g, 1.9 mmol) was added bromoform (1.4 mL, 16.0 mmol, 8.5 mol eq) followed by DBU (0.32 mL, 2.1 mmol, 1.1 mol eq). The reaction was stirred at RT for 24 h and partially purified via flash column chromatography (hexanes to 30% ethyl acetate in hexanes) to yield a mixture of the 20α- and 20β-carbinols 28 with unreacted 27 (0.56 g total weight), which was used directly for the next step. (See supporting information for NMR spectra).
2.2.10. 21,21,21-tribromo-pregn-5-ene-3β-(t-butyl)dimethylsiloxy-20-one, compound 29
To the mixture of 20α- and 20β-alcohols 28 with unreacted aldehyde 27 (0.56 g, 1.0 mmol) in CH2Cl2 (50 mL) was added Dess-Martin periodinane (0.60 g, 1.4 mmol, 1.3 mol eq). The reaction was stirred for 2 h and purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) directly to yield ketone 29 (0.19 g, 0.28 mmol, 27%, 15% over two steps) as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.31 (broad s, 1H), 3.53-3.44 (m, 1H), 3.37 (apparent t, J = 8 Hz, 1H), 2.32-2.11 (m, 2H), 2.10-1.94 (m, 2H), 1.86-1.75 (m, 2H), 1.65-1.35 (m, 5H), 1.25 (s, 3H), 1.02 (s, 3H), 0.96 (s, 3H), 0.88 (s, 9H), 0.05 (s, 6H); 13C NMR (100 MHz, CD Cl3) δ 192.1, 141.8, 120.9, 72.6, 56.9, 52.9, 50.0, 47.4, 42.9, 39.3, 37.5, 36.8, 33.0, 32.2, 32.1, 29.9, 26.1, 25.6, 21.2, 19.6, 18.4, 13.7, 9.9, −4.4.
2.2.11. 21,21,21-tribromo-pregn-5-en-3β-ol-20-one (21-tribromopregnenolone, compound 15)
To a solution of 29 (0.19 g, 0.28 mmol) in CH2Cl2 (10 mL) and methanol (5 mL) was added camphorsulfonic acid (0.2 mg, 0.85 µmol, 0.003 mol eq). The reaction was stirred for 12 h and directly purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to afford tribromide 15 (90 mg, 0.16 mmol, 58%) as a white solid. Deprotection using p-toluenesulfonic acid was also successful, but longer reaction times led to formation of the dibromide (21,21-dibromopregnenolone) by mono-dehalogenation. 1H NMR (400 MHz, CDCl3) δ 5.36 (m, 1H), 3.58-3.48 (m, 1H), 3.37 (apparent t, J = 8 Hz, 1H), 2.35-2.20 (m, 3H), 2.13-1.93 (m, 3H), 1.90-1.75 (m, 3H), 1.65-1.35 (m, 9H), 1.15-0.95 (m, 1H), 1.03 (s, 3H), 0.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 192.1, 141.0, 121.4, 71.8, 56.9, 52.9, 50.1, 47.4, 42.3, 39.3, 37.4, 36.7, 33.0, 32.2, 32.0, 31.7, 25.6, 21.2, 19.6, 13.7.
2.2.12. 21,21,21-tribromo-pregn-4-ene-3,20-dione (21-tribromoprogesterone, compound 6)
To a solution of 21-tribromopregnenolone 15 (70 mg, 0.13 mmol) in CH2Cl2 (15 mL) was added Dess-Martin periodinane (55 mg, 0.13 mmol, 1.0 mol eq). After stirring for 3 h at RT, the reaction mixture was directly purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield 21-tribromoprogesterone 6 (32 mg, 0.06 mmol, 46%). The Δ5-olefin isomerized to the Δ4-isomer on the silica column. 1H NMR (400 MHz, CDCl3) δ 5.73 (s, 1H), 3.38 (apparent t, J = 8 Hz, 1H), 2.55-2.20 (m, 7 H), 2.10-1.95 (m, 4H), 1.92-1.80 (m, 3H), 1.55-1.35 (m, 2H), 1.30-1.15 (m, 2H), 1.17 (s, 3H), 0.99 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 199.6, 191.9, 170.9, 124.1, 56.0, 53.6, 52.7, 49.8, 47.3, 39.1, 38.7, 35.82, 35.78, 34.1, 32.87, 32.85, 32.1, 25.4, 21.1, 17.5, 13.8.
2.2.13. 21,21,21-trichloro-pregn-5-ene-3β-(t-butyl)dimethylsilyloxy-20-ol, compound 31
To a flask containing aldehyde 27 (0.60 g, 1.4 mmol) was added chloroform (20 mL) followed by DBU (0.50 mL, 3.7 mmol, 2.6 mol eq). The reaction was stirred at RT for 72 h and directly purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to afford epimeric trichlorocarbinols 31 [15] (0.13 g, 17%) as a white solid. 1H NMR (less polar 20-epimer, 400 MHz, CDCl3) δ 5.31 (broad s, 1H), 4.27 (d, J = 4 Hz, 1H), 3.55-3.41 (m, 1H), 2.80 (d, J = 4 Hz, 1H), 2.39 (dd, J1 = 8, J2 = 4 Hz, 1H), 2.30-2.23 (m, 1H), 2.20-1.96 (m, 4H), 1.90-1.80 (m, 2H), 1.76-1.70 (m, 3H), 1.35-1.19 (m, 5H), 1.01 (s, 3H), 0.89 (s, 9H), 0.86 (s, 3H), 0.06 (6H); 1H NMR (more polar 20-epimer, 400 MHz, CDCl3) δ 5.32 (broad s, 1H), 4.02 (dd, J1 = 9, J2 = 6 Hz, 1H), 3.55-3.43 (m, 1H), 2.69 (d, J = 6 Hz, 1H), 2.32-2.14 (m, 5H), 2.05-1.91 (m, 4H), 1.85-1.65 (m, 8H), 1.35-1.15 (m, 3H), 0.89 (s, 9H), 0.86 (s, 3H), 0.06 (s, 6H).
2.2.14. 21,21,21-trichloro-pregn-5-ene-3β-(t-butyl)dimethylsilyloxy-20-one, compound 32
Compound 32 was obtained from 31 in the similar fashion to section 2.2.10 (110 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 5.32 (broad s, 1H), 3.52-3.44 (m, 1H), 3.22 (apparent t, J = 12 Hz, 1H), 2.32-2.10 (m, 4H), 2.10-1.95 (m, 2H), 1.90-1.65 (m, 6H), 1.65-1.40 (m, 6H), 1.01 (s, 3H), 0.92 (s, 3H), 0.88 (s, 9H), 0.05 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 192.0, 141.8, 120.8, 72.6, 57.0, 54.2, 50.0, 47.3, 42.9, 38.8, 37.5, 36.8, 32.18, 32.15, 32.06, 31.4, 26.1, 25.5, 21.2, 19.6, 18.4, 13.6, −4.4.
2.2.15. 21,21,21-trichloro-pregn-5-en-3β-ol-20-one (21-trichloropregnenolone, compound 14)
To a solution of 32 (104 mg, 0.19 mmol) in THF (25 mL) was added p-toluenesulfonic acid monohydrate (66 mg, 0.35 mmol, 1.8 mol eq). The reaction was stirred for 2 h, and the product was purified via flash column chromatography (hexanes to 70% ethyl acetate in hexanes) to yield 14 (71 mg, 0.17 mmol, 87%). 1H NMR (400 MHz, CDCl3) δ 5.36 (broad s, 1H), 3.60-3.45 (m, 1H), 3.22 (apparent t, J = 12 Hz, 1H), 2.35-2.10 (m, 4H), 1.40-1.20 (m, 5H), 1.00 (s, 3H), 0.93 (s, 3H).
2.2.16. 21,21,21-trichloro-pregn-4-ene-3,20-dione (21-trichloroprogesterone, compound 5)
The procedure to oxidize 14 was followed as in section 2.2.12 with isomerization on the silica gel column to yield 5 [16] (7 mg, 58%, over two steps). 1H NMR (400 MHz, CDCl3) δ 5.73 (broad s, 1H), 3.22 (apparent t, J = 12 Hz, 1H), 2.49-2.27 (m, 5H), 2.21-2.11 (m, 1H), 2.08-1.96 (m, 1H), 1.95-1.75 (m, 4H), 1.70-1.56 (m, 4H), 1.55-1.25 (m, 2H), 1.19 (s, 3H), 0.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 199.6, 191.8, 170.8, 124.1, 56.1, 53.6, 53.0, 47.1, 38.6, 35.84, 35.77, 34.0, 32.9, 32.1, 31.3, 25.3, 21.1, 17.5, 13.7.
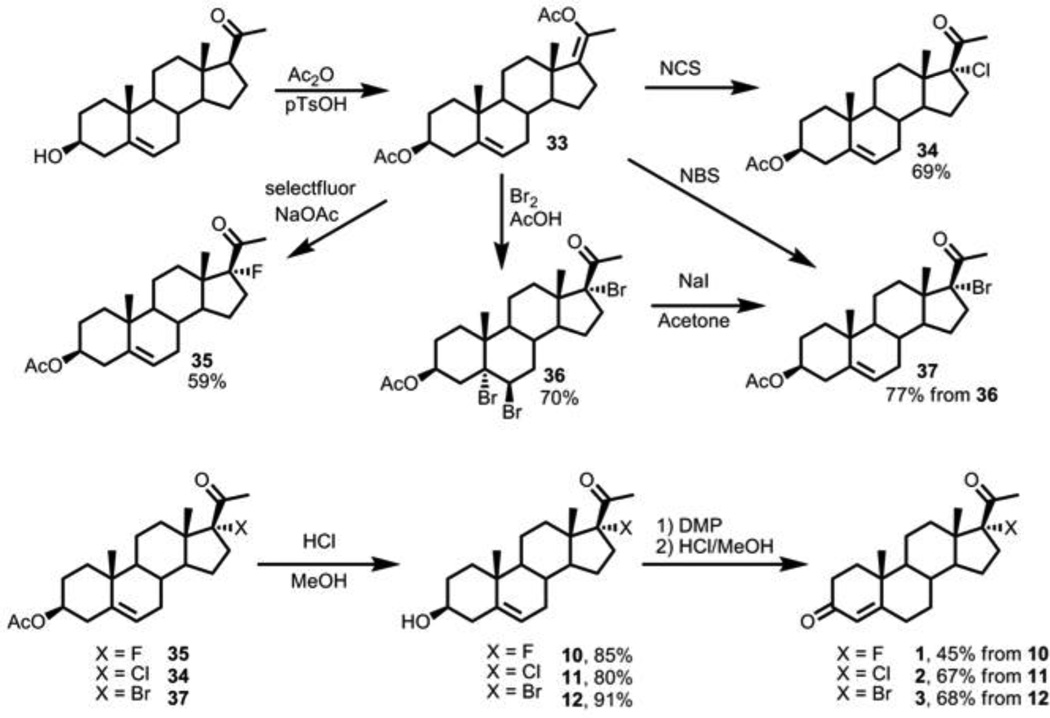
2.2.17. 5,6,17-tribromo-20-oxo-pregn-5-ene-3β-yl acetate, compound 36
Pregnenolone (4.0 g, 12.6 mmol) and p-toluenesulfonic acid (2.4 g, 14.0 mmol, 1.1 mol eq) were refluxed in acetic anhydride (45 mL, 25 mol eq) until 20 mL of solvent was condensed out of the reaction via a Dean-Stark trap and a reflux condenser. The reaction was cooled to room temperature and then maintained at 0 °C for 1 h. The cold reaction solution was diluted with diethyl ether (25 mL) and washed with NaHCO3 (sat. aqueous solution, 3 × 25 mL). The organic layer was concentrated via reduced pressure to yield enol acetate 33 as a brown solid, which was used without purification.
The crude enol acetate 33 (5.0 g, 12.5 mmol) in acetic acid (20 mL) with sodium acetate (2.0 g, 24.3 mmol, 1.9 mol eq) was treated with a mixture of bromine (1.3 mL, 25.4 mmol, 2.0 mol eq) in 5 mL H2O, and the reaction was stirred for 1 h at room temperature. The reaction was quenched with saturated aqueous Na2S2O3 (30 mL), extracted with ethyl acetate (3 × 15 mL), and concentrated under reduce pressure. The crude material was purified via flash column chromatography (10% ethyl acetate in hexanes) to yield the tribromide 36 as a white solid (5.2 g, 8.8 mmol, 70%). 1H NMR (400 MHz, CDCl3) δ 5.41–5.49 (m, 1H), 3.04 (ddd, J1 = 16.0, J2 = 12.0, J3 = 3.6 Hz, 1H), 2.76 (ddd, J1 = 15.6, J2 = 12.4, J3 = 4.0 Hz, 1H), 2.03 (dd, J1 = 14.0, J2 = 10.4 Hz, 1H), 2.35 (s, 3H), 2.23–2.31 (m, 2H), 2.04–2.15 (m, 2H), 2.02 (s, 3H), 1.78–1.99 (m, 5H), 1.69–1.75 (m, 2H), 1.56–1.65 (m, 3H), 1.44 (s, 3H), 1.21–1.32 (m, 2H), 0.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 201.3, 170.4, 87.4, 85.8, 71.9, 55.6, 49.7, 47.3, 46.6, 41.94, 41.91, 37.1, 36.6, 36.1, 35.4, 31.4, 27.5, 26.2, 22.8, 21.5, 21.4, 20.3, 14.4.
2.2.18. 17-bromopregnenolone-3-acetate (17-bromo-20-oxo-pregn-5-ene-3β-yl acetate, compound 37)
Tribromide 36 (2.5 g, 4.2 mmol) and sodium iodide (1.5 g, 10 mmol, 2.4 mol eq) were dissolved in acetone (30 mL), and the reaction was stirred for 1 h. The reaction was quenched with Na2S2O3 (20 mL sat. aqueous), mixed with brine (10 mL), and extracted with diethyl ether (3 × 25 mL). The organic extracts were combined and concentrated via reduced pressure, and the crude material was purified via silica column chromatography (10% ethyl acetate in hexanes) to yield 37 (1.4 g, 3.3 mmol, 77%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.37 (d, J = 5.2 Hz, 1H), 4.55–4.63 (m, 1H), 3.06 (ddd, J1 = 16.0, J2 = 12.0, J3 = 4.0 Hz, 1H), 2.37 (s, 3H), 2.23–2.34 (m, 3H), 2.02, (s, 3H), 1.93–2.00 (m, 2H), 1.82–1.90 (m, 5H), 1.36–1.69 (m, 5H), 1.22–1.36 (m, 1H), 1.12–1.20 (m, 1H), 1.06 (dd, J1 = 12.4, J2 = 4.8 Hz, 1H), 1.01 (s, 3H), 0.76 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 201.6, 170.6, 139,7, 122.3, 86.2, 73.9, 51.1, 49.3, 47.0, 38.1, 37.0, 36.6, 36.1, 35.5, 32.4, 31.2, 27.8, 27.6, 23.1, 21.5, 21.2, 19.4, 14.0.
2.2.19. 17-bromo-3β-hydroxy-pregn-5-en-20-one (17-bromopregnenolone, compound 12)
To a solution of acetate 37 (0.55 g, 1.26 mmol) dissolved in 5 mL CH2Cl2 and 45 mL methanol was added 0.2 mL of 12 M HCl, and the reaction was stirred overnight. The reaction mixture was concentrated via reduced pressure, and the crude product was purified via flash column chromatography (20% ethyl acetate in hexanes) to yield 12 (0.45 g, 1.14 mmol, 91%) as a white solid. 1H NMR (500 MHz, CDCl3) δ 5.36 (d, J = 8.5 Hz, 1H), 3.48–3.59 (m, 1H), 3.08 (ddd, J1 = 19.0, J2 = 7.5, J2 = 5.5 Hz, 1H), 2.38 (s, 3H), 2.23–2.34 (m, 3H), 2.03–2.07 (m, 1H), 1.97–2.02 (m, 2H), 1.82–1.94 (m, 4H), 1.39–1.71 (m, 3H), 1.04–1.36 (m, 5H), 1.01 (s, 3H), 0.78 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 201.6, 140.9, 121.1, 86.3, 71.5, 51.1, 49.3, 46.9, 42.2, 37.3, 36.4, 36.0, 35.5, 32.3, 31.6, 31.5, 27.5, 23.0, 21.1, 19.5, 13.9.
2.2.20. 17α-bromo-pregn-5-ene-3,20-dione (17-bromo-Δ5-progesterone)
Alcohol 12 (0.51 g, 1.28 mmol) and Dess-Martin periodinane (0.52 g, 1.21 mmol, 0.9 mol eq) were dissolved in CH2Cl2 (5 mL) and stirred for 30 min at room temperature, then concentrated via reduced pressure and purified via flash column chromatography (10% ethyl acetate in hexanes) to yield the 3-ketosteroid intermediate (0.36 g, 0.92 mmol, 72%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.30 (apparent t, J = 2.8 Hz, 1H), 3.23 (dd, J1 = 16.4, J2 = 3.6 Hz, 1H), 3.02, (ddd, J1 = 16.4, J2 = 12.0, J3 = 4.0 Hz, 1H), 2.78 (dd, J1 = 16.4, J2 = 2.0, Hz, 1H), 2.44 (ddd, J1 = 13.6, J2 = 13.6, J3 = 6.0 Hz, 1H), 2.34 (s, 3H), 2.20–2.29 (m, 2H), 1.98–2.05 (m, 2H), 1.79–1.96 (m, 4H), 1.60–1.70 (m, 2H), 1.41–1.56 (m, 3H), 1.18–1.29 (m, 1H), 1.14 (s, 3H), 1.06–1.11 (m, 1H), 0.76 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 210.0, 201.4, 138.6, 122.4, 86.0, 51.0, 48.4, 48.3, 47.0, 37.6, 36.9, 36.8, 36.1, 35.4, 32.4, 31.5, 27.5, 23.0, 21.4, 19.3, 14.0.
2.2.21. 17α-bromo-pregn-4-ene-3,20-dione (17-bromo-progesterone, compound 3)
17-Bromo-pregn-5-ene-3,20-dione (0.36 g, 0.92 mmol) was dissolved in 1:1 CH2Cl2:methanol (30 mL), and 0.1 mL of HCl was added. After stirring at RT for 1h, the reaction mixture was concentrated via reduced pressure, and the crude material was purified via flash column chromatography (hexanes to 10% ethyl acetate in hexanes) to yield 3 [17] as a white solid (0.34 g, 0.87 mmol, 94%). 1H NMR (500 MHz, CDCl3) δ 5.75 (s, 1H), 3.07 (ddd, J1 = 17, J2 = 12, J3 = 4 Hz, 1H), 2.38 (s, 3H), 2.29–2.48 (m, 6H), 2.40 (s, 3H), 1.86–2.08 (m, 3H), 1.67–1.79 (m, 2H), 1.54–1.62 (m, 1H), 1.21 (s, 3H), 1.12–1.18 (m, 2H), 1.06 (ddd, J1 = 10.0, J2 = 10.0, J3 = 3.2 Hz, 1H), 0.83 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 201.3, 199.4, 170.6, 124.1, 85.9, 52.9, 50.4, 47.0, 38.6, 36.05, 36.02, 35.7, 35.4, 34.0, 32.8, 31.7, 27.5, 22.9, 21.1, 17.5, 14.1.
2.2.22. 17-fluoro-20-oxo-pregn-5-en-3β-yl acetate (17-fluoropregnenolone-3-acetate, compound 35)
Enol acetate 33 (0.10 g, 0.25 mmol), Selectfluor (0.25 g, 0.71 mmol, 2.8 mol eq) and sodium acetate (0.05 g) were weighed in a screw-cap vial and dissolved in acetonitrile (3 mL). The reaction was stirred at 60 °C for 2 h, then concentrated under reduced pressure and purified via flash column chromatography (10% to 50% ethyl acetate in hexanes) to afford fluoride 35 (56 mg, 0.15 mmol, 59%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.37 (m, 1H), 4.63-4.55 (m, 1H), 2.64-2.49 (m, 1H), 2.37-2.28 (m, 1H), 2.21 (d, JHF = 8 Hz, 3H), 2.01 (s, 3H), 1.98-1.93 (m, 1H), 1.20-1.10 (m, 1H), 1.00 (s, 3H), 0.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 207.7 (d, JCF = 132 Hz), 170.6, 139.7, 122.4 (d, JCF = 60 Hz), 112.2, 110.3, 73.9, 51.6, 49.5, 38.2, 37.1, 36.7, 32.0, 31.3, 30.7, 27.7, 23.9, 21.5, 20.6, 19.4, 14.1 (d, JCF = 20 Hz).
2.2.23. 17-fluoro-3β-hydroxy-pregn-5-en-20-one (17-fluoropregnenolone, compound 10)
Acetate 35 was hydrolyzed as described in section 2.2.19, yielding compound 10 (20 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 5.37-5.34 (m, 1H), 3.58-3.50 (m, 1H), 2.66-2.49 (m, 1H), 2.36-2.23 (m, 1H), 2.23 (d, JHF = 5.2 Hz, 3H), 2.09-1.95 (m, 2H), 1.90-1.72 (m, 3H), 1.72-1.60 (m, 2H), 1.35-1.25 (m, 1H), 1.15-1.05 (m, 1H), 1.01 (s, 3H), 0.67 (s, 3H).
2.2.24. 17-fluoro-pregn-4-ene-3,20-dione (17-fluoroprogesterone, compound 1)
The procedures for oxidation and isomerization of 10 to 1 [18] were followed as described in sections 2.2.20 and 2.2.21 (10 mg, 45%, 2 steps). 1H NMR (400 MHz, CDCl3) δ 5.74 (broad s, 1H), 2.59 (dddd, JHF = 40, J1 = 12, J2 = 4, J3 = 2 Hz, 1H), 2.48-2.26 (m, 1H), 2.23 (d, JHF = 5 Hz, 3H), 2.05-2.01 (m, 1H), 1.92-1.62 (m, 9H), 1.19 (s, 3H), 1.08-0.97 (m, 1H), 0.70 (s, 3H); additional resonances from impurities appeared in the 2.25–2.50 ppm region of the spectrum.
2.2.25. 17-chloro-20-oxo-pregn-5-en-3β-yl acetate (17-chloropregnenolone-3-acetate, compound 34)
To enol acetate 33 (90 mg, 0.22 mmol) in CH2Cl2 (5 mL) in a screw cap vial was added N-chlorosuccinimide (46 mg, 0.34 mmol, 1.5 mol eq). The reaction was stirred at 55 °C for 2 h, concentrated under reduced pressure, and purified via flash column chromatography (10% to 50% ethyl acetate in hexanes) to afford chloride 34 (60 mg, 0.15 mmol, 69%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 5.39-5.38 (m, 1H), 4.67-4.56 (m, 1H), 3.09-2.98 (m, 1H), 2.32 (s, 3H), 2.10-1.95 (m, 3H), 2.04 (s, 3H), 1.95-1.84 (m, 4H), 1.75-1.61 (m, 3H), 1.30-1.05 (m, 4H), 1.02 (s, 3H), 0.74 (s, 3H).
2.2.26. 17-chloro-3β-hydroxy-pregn-5-en-20-one (17-chloropregnenolone, compound 11) and 17α-chloro-pregn-4-ene-3,20-dione (17-chloroprogesterone, compound 2)
Using procedures described in sections 2.2.19–2.2.21, hydrolysis of acetate 34 gave 11 (15 mg, 80%), and oxidation/isomerization of 11 gave 2 [19] (10 mg, 67%, 2 steps). 1H NMR of compound 11 (400 MHz, CDCl3) δ 5.37-5.35 (m, 1H), 3.57-3.50 (m, 1H), 3.01 (ddd, J1 = 15.4, J2 = 11.5, J3 = 2.9 Hz, 1H), 2.32 (s, 3H), 2.32-2.15 (m, 2H), 2.06-1.93 (m, 3H), 1.92-1.80 (m, 4H), 1.79-1.74 (m, 1H), 1.72-1.64 (m, 2H), 1.60-1.37 (m, 3H), 1.15-1.02 (m, 2H), 1.01 (s, 3H), 0.74 (s, 3H).
1H NMR of compound 2 (400 MHz, CDCl3) δ 5.75 (s, 1H), 3.07-2.96 (m, 1H), 2.45-2.26 (m, 3H), 2.32 (s, 3H), 2.09-1.66 (m, 11H), 1.19 (s, 3H), 1.06-0.97 (m, 2H), 0.91-0.79 (m, 3H), 0.77 (s, 3H).
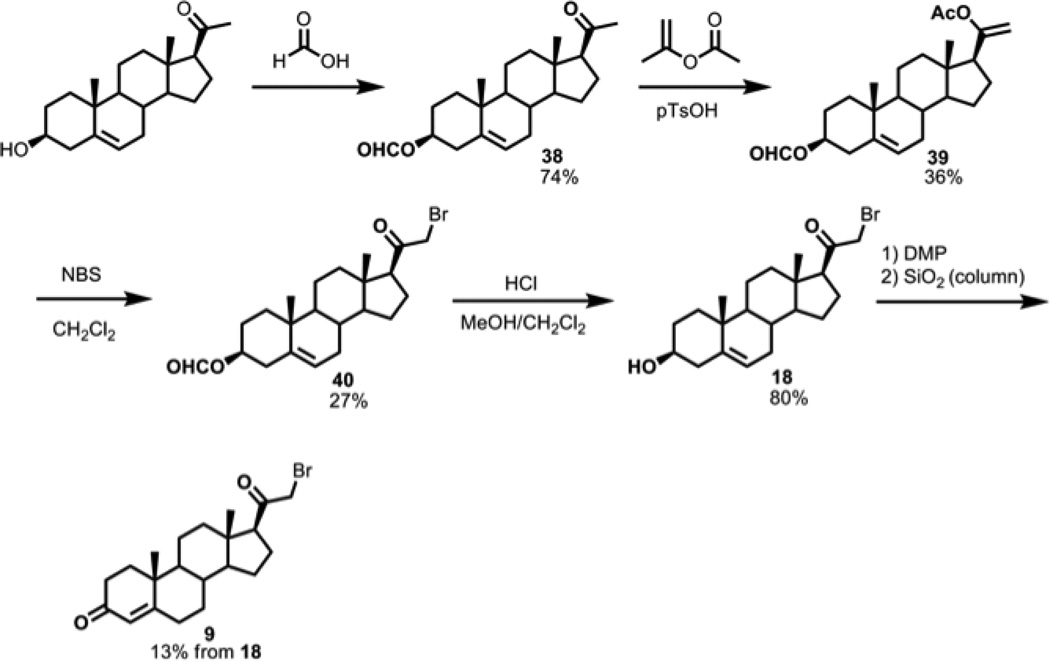
2.2.27. 20-oxo-pregn-5-en-3β-yl formate (pregnenolone-3-formate, compound 38)
Pregnenolone (6.0 g, 19.0 mmol) and formic acid (60 mL) were stirred for 30 min at 70 °C. The reaction was cooled to RT, diluted with diethyl ether (200 mL), and washed sequentially with water (50 mL) and NaHCO3 (2 × 50 mL, saturated aqueous solution). The organic phase was concentrated and crystallized with acetone and diethyl ether to yield formate 38 (5.0 g, 14.0 mmol, 74%). 1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H), 5.38 (m, 1H), 4.77-4.67 (m, 1H), 2.52 (apparent t, J = 9 Hz, 1H), 2.36 (m, 2H), 2.21-2.10 (m, 3H), 2.10 (s, 3H), 2.05-1.95 (m, 2H), 1.90-1.86 (m, 2H), 1.71-1.57 (m, 5H), 1.51-1.39 (m, 4H), 1.01 (s, 3H), 0.61 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 209.57, 160.7, 139.4, 122.8, 73.9, 63.8, 56.9, 50.0, 44.1, 38.9, 38.1, 37.0, 36.7, 31.9, 31.8, 31.6, 27.8, 24.6, 22.9, 21.1, 19.4, 13.3.
2.2.28. pregna-5,20-diene-3β,20-diyl 20-acetate 3-formate, compound 39
To formate 38 (1.0 g, 2.8 mmol) in isopropenyl acetate (50 mL) with p-toluenesulfonic acid monohydrate (0.1 g) was refluxed for 20 h as water was removed with a Dean Stark trap. Longer times (>24 h) produced isomerization to the 17,20-enol acetate. The reaction was diluted with diethyl ether (50 mL) and washed with sodium bicarbonate (3 × 40 mL, saturated aqueous solution). The combined organic extracts were concentrated and purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to afford enol acetate 39 (0.4 g, 1.0 mmol, 36%). 1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H), 5.38 (d, J = 4 Hz, 1H), 4.69 (s, 2H), 4.76-4.65 (m, 1H), 2.40-2.33 (m, 2H), 2.10 (s, 3H), 1.93-1.84 (m, 4H), 1.30-1.02 (m, 5H), 1.01 (s, 3H), 0.66 (s, 3H).
2.2.29. 21-bromo-20-oxo-pregn-5-en-3β-yl formate (21-bromopregnenolone-3-formate, compound 40)
A solution of enol acetate 39 (135 mg, 0.35 mmol) and N-bromosuccinimide (0.062 g, 0.350 mmol, 1 mol eq) in methylene chloride (10 mL) was refluxed for 2 h. The reaction mixture was cooled, concentrated, and purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield 21-bromide 40 (40 mg, 0.10 mmol, 27%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H), 5.40 (m, 1H), 4.77-4.68 (m, 1H), 3.93 (d, J = 12 Hz, 1H), 3.90 (d, J = 12 Hz, 1H), 2.83 (apparent t, J = 8 Hz, 1H), 1.35-1.11 (m, 8H), 1.02 (s, 3H), 0.66 (s, 3H).
2.2.30. 21-bromo-pregn-5-en-3β-ol-20-one (21-bromopregnenolone, compound 18)
Formate 40 (40 mg, 0.1 mmol) was dissolved 5 mL each methanol and CH2Cl2, and 2 drops of concentrated HCl (12 N) was added. The reaction was stirred for 1 h and directly purified via flash column chromatography (10% to 50% ethyl acetate in hexanes) to afford 18 [20] (30 mg, 0.08 mmol, 80%). 1H NMR (400 MHz, CDCl3) δ 5.35 (m, J = 8 Hz, 1H), 3.94 (d, J = 12 Hz, 1H), 3.90 (d, J = 12 Hz, 1H), 3.57-3/47 (m, 1H), 2.83 (apparent t, J = 8 Hz, 1H), 2.31-2.25 (m, 3H), 2.05-1.95 (m, 2H), 1.80-1.70 (m, 4H), 1.35-1.06 (m, 5H), 1.01 (s, 3H), 0.66 (s, 3H).
2.2.31. 21-bromo-pregn-4-ene-3,20-dione (21-bromoprogesterone, compound 9)
To a solution of 18 (15.6 mg, 0.04 mmol) in CH2Cl2 (20 mL) was added Dess-Martin periodinane (17 mg, 0.04 mmol, 1 mol eq). The reaction was stirred for 2 h, and the mixture was directly purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield 9 [21] (2.0 mg, 13%). The Δ5-olefin appeared to isomerize to the Δ4-isomer on the silica column. 1H NMR (400 MHz, CDCl3) δ 5.74 (broad s, 1H), 3.93 (d, J = 12 Hz, 1H), 3.89 (d, J = 12 Hz, 1H), 2.84 (apparent t, J = 8 Hz, 1H), 2.49-2.17 (m, 6H), 1.19 (s, 3H), 1.12-0.85 (m, 5H), 0.70 (s, 3H).
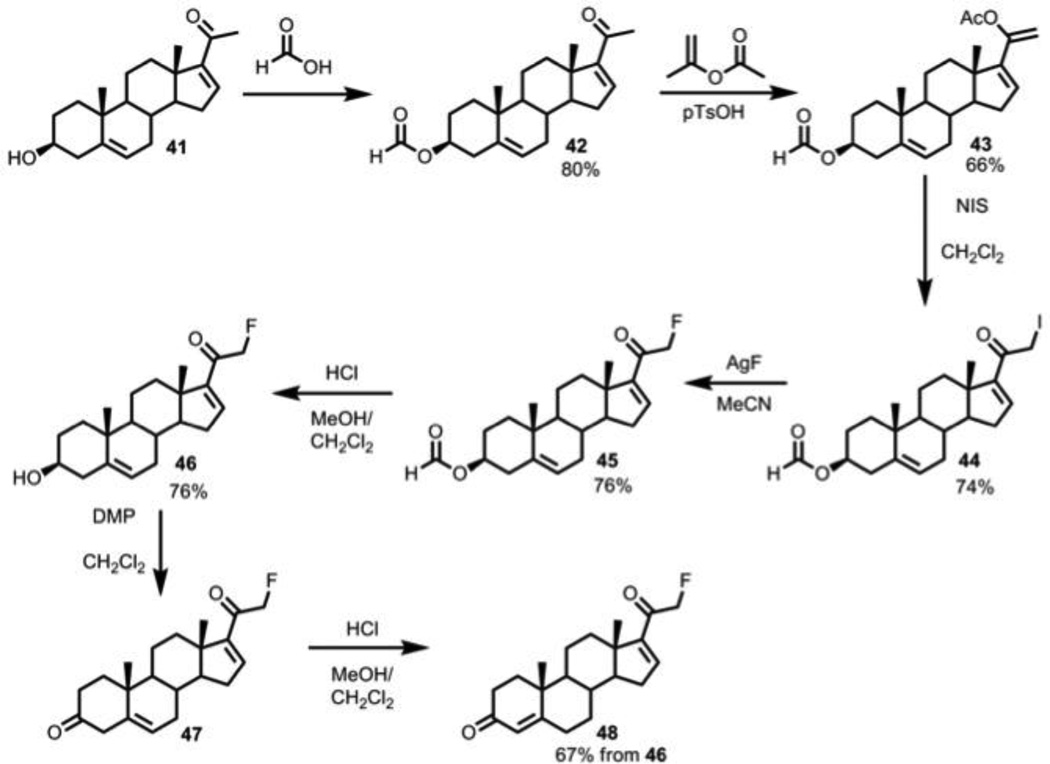
2.2.32. 20-oxo-pregna-5,16-dien-3β-yl formate, compound 42
Formic acid (50 mL) and 16-dehydropregnenolone (5.0 g, 19.2 mmol) were stirred at reflux for 30 min, cooled to RT, washed with H2O, and extracted with diethyl ether (2 × 100 mL). The resulting mixture was crystallized with acetone and diethyl ether to yield formate 42 (5.46 g, 16.0 mmol, 80%). 1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H), 5.37 (m, 1H), 4.77-4.65 (m, 1H), 2.52 (apparent t, J = 9 Hz, 1H), 2.40-2.32 (m, 2H), 2.23-2.10 (m, 3H), 2.10 (s, 3H), 2.05-1.82 (m, 4H), 1.75-1.40 (m, 5H), 1.25-1.10 (m, 3H), 1.01 (s, 3H), 0.61 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 209.6, 160.7, 139.4, 122.8, 63.8, 56.9, 50.0, 44.0, 38.9, 38.1, 37.0, 36.7, 31.9, 31.8, 27.8, 24.6, 22.9, 21.1, 19.4, 13.3.
2.2.33. pregna-5,16,20-triene-3β,20-diyl 20-acetate 3-formate, compound 43
Ketone 42 (1.22 g, 3.56 mmol) and p-toluenesulfonic acid monohydrate (0.15 g, 0.8 mmol, 0.2 mol eq) were refluxed in isopropenyl acetate (50 mL) for 20 h as water was removed with a Dean-Stark apparatus. The reaction was cooled to RT and diluted with diethyl ether (100 mL). The solution was washed with NaHCO3 (saturated aqueous solution, 2 × 50 mL), and the organic layer was washed with brine (2 × 25 mL), dried with MgSO4, and concentrated via reduced pressure. The solid formed during concentration was washed with ice-cold methanol to yield the enol acetate 43 [22] (0.90 g, 2.34 mmol, 66%). 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 5.82 (m, 1H), 5.40 (m, 1H), 5.06 (s, 1H), 4.78 (s, 1H), 4.78-4.65 (m, 1H), 2.39-2.36 (m, 2H), 2.18 (s, 3H), 2.15-2.10 (m, 1H), 2.04-1.99 (m, 1H), 1.95-1.86 (m, 3H), 1.70-1.47 (m, 8H), 1.19-1.13 (m, 1H), 1.07 (s, 3H), 0.98 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 169.2, 160.7, 149.7, 148.2, 139.7, 129.8, 122.7, 104.8, 102.3, 73.9, 57.0, 50.1, 46.1, 38.1, 36.9, 35.3, 31.6, 31.0, 30.2, 27.8, 21.0, 20.9, 19.3, 15.9.
2.2.34. 21-iodo-20-oxo-pregna-5,16-dien-3β-yl formate, compound 44
To enol acetate 43 (0.33 g, 0.86 mmol) in CH2Cl2 (50 mL) was added N-iodosuccinimide (0.29 g, 1.29 mmol, 1.5 eq). The reaction was stirred at RT for 1 h and directly purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to afford iodide 44 (0.30 g, 0.64 mmol, 74%). 1H NMR (500 MHz, CDCl3) δ 8.02 (s, 1H), 6.79 (s, 1H), 5.39 (broad s, 1H), 4.70-4.65 (m, 1H), 4.07 (d, J =12 Hz, 1H), 4.02 (d, J = 12 Hz, 1H), 2.39-2.30 (m, 5H), 2.05-1.98 (m, 1H), 1.92-1.80 (m, 2H), 1.76-1.53 (m, 5H), 1.50-1.43 (m, 1H), 1.37 (ddd, J1 = 12, J2 = 12, J3 = 5 Hz, 1H), 1.14 (apparent t, J = 12 Hz, 1H), 1.06 (s, 3H), 0.96 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 191.2, 160.7, 151.7, 145.7, 140.0, 122.4, 73.9, 56.1, 50.4, 46.5, 38.2, 36.9, 36.8, 34.4, 32.7, 31.6, 30.2, 27.8, 20.7, 19.3, 15.6, 3.8.
2.2.35. 21-fluoro-20-oxo-pregna-5,16-dien-3β-yl formate, compound 45
A solution of iodide 44 (0.70 g, 1.45 mmol) in acetonitrile (50 mL) with silver fluoride (0.54 g, 4.26 mmol, 3.4 mol eq) was stirred at RT for 36 h to complete the reaction. After addition of water, the mixture was extracted with ethyl acetate (2 × 25 mL), and the combined organic extracts were concentrated via reduced pressure. The crude material was purified via flash column chromatography (hexanes to 50% ethyl acetate in hexanes) to yield fluoride 45 [23] (0.39 g, 1.07 mmol, 76%). 1H NMR (500 MHz, CDCl3) δ 8.04 (s, 1H), 6.78 (s, 1H), 5.4 (broad s, 1H), 5.16 (dd, JHF = 24, J1 = 8 Hz, 1H), 5.04 (dd, JHF = 24, J1 = 8 Hz, 1H), 4.80-4.65 (m, 1H), 2.48-2.29 (m, 5H), 2.20-1.95 (m, 4H), 1.95-1.80 (m, 4H), 1.07 (s, 3H), 0.96 (s, 3H).
2.2.36. 21-fluoro-3β-hydroxy-pregna-5,16-dien-20-one (16,17-dehydro-21-fluoropregnenolone, compound 46)
Formate 45 (0.39 g, 1.07 mmol) was hydrolyzed with hydrochloric acid as in section 2.2.19 to give 46 (0.27 g, 0.81 mmol, 76% yield). 1H NMR (500 MHz, CDCl3) δ 6.77 (s, 1H), 5.35 (m, 1H), 5.13 (dd, JHF = 24, J1 = 5 Hz, 1H), 5.04 (dd, JHF = 24, J1 = 5 Hz, 1H), 2.42-2.20 (m, 5H), 2.15-1.95 (m, 2H), 1.88-1.80 (m, 2H), 1.56-1.46 (m, 1H), 1.45-1.32 (m, 2H), 1.12-1.01 (m, 3H), 1.04 (s, 3H), 0.95 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 192.1 (d, JCF = 16 Hz), 151.6, 145.4 (d, JCF = 38 Hz), 141.5, 121.0, 83.4 (d, JCF = 181 Hz), 71.8, 56.0, 50.6, 46.9, 42.4, 37.2, 36.8, 34.5, 33.0, 32.4, 31.7, 30.2, 20.8, 19.4, 16.0.
2.2.37. 21-fluoro-pregna-4,16-diene-3,20-dione (16,17-dehydro-21-fluoroprogesterone, compound 48)
Using the procedure in section 2.2.12, 46 (0.27 g, 0.81 mmol) was oxidized to 47, followed by isomerization as in section 2.2.21 to yield 48 (0.18 g, 0.54 mmol, 67%, 2 steps). 1H NMR (400 MHz, CDCl3) δ 6.78 (s, 1H), 5.74 (broad s, 1H), 5.15 (dd, JHF = 24, J1 = 12 Hz, 1H), 5.05 (dd, JHF = 24, J1 = 12 Hz, 1H), 2.50-2.25 (m, 5H), 2.16-2.10 (m, 1H), 2.06-2.00 (m, 1H), 1.92-1.85 (m, 1H), 1.82-1.50 (m, 6H), 1.46-1.33 (m, 3H), 1.22 (s, 3H), 0.98 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 210.2, 192.1 (d, JCF = 16 Hz), 151.5, 145.3 (d, JCF = 5 Hz), 139.3, 122.3, 104.9, 83.4 (d, JCF = 183 Hz), 55.8, 49.7, 48.5, 47.0, 37.7, 37.2, 34.4, 33.0, 31.6, 30.3, 21.0, 19.2, 16.0.
2.3. Enzymology studies
2.3.1. CYP17A1 and CYP21A2 microsome incubation with halosteroid
Enzyme assays used human CYP21A2 and CYP17A1 with P450-oxidoreductase in microsomes from yeast expressing the recombinant proteins as described [24]. Test steroid (20–50 µM, delivered in <5 µL ethanol) and microsomes (5–10 µL, ~10 pmol P450) were incubated in 1 mL 50 mM potassium phosphate (pH 7.4) with 1 mM NADPH for 2–5 hours at 37 °C. For control incubations with ketoconazole to inhibit P450 activity, microsomes and 100 µM ketoconazole were preincubated at room temperature for 15 min before the addition of steroid followed by NADPH. The incubations were terminated with the addition of CH2Cl2 (1 mL), extraction, and centrifugation. The organic extract was dried under nitrogen flow, dissolved in 50% aqueous methanol (70–90 µL), and analyzed by high performance liquid chromatography (HPLC).
2.3.2. Cholesterol oxidase treatment
For analysis of incubations with Δ5,3β-hydroxy-steroids, substrates and products were converted to their Δ4,3-ketosteroid congeners to generate a UV chromophore. The dried extracts were dissolved in 80 µL of ethanol and diluted in 0.2 mL of 50 mM potassium phosphate (pH 7.4) solution. To this solution, 1 unit of cholesterol oxidase in 40 µL water was added, and the incubation was shaken at 200 rpm and 30 °C for 5–10 h. The reaction was extracted with 1 mL of CH2Cl2, and the dried extracts were dissolved in 50% methanol for HPLC analysis.
2.3.3. HPLC analyses
Steroids were resolved using a Waters Breeze 1525 HPLC equipped with autosampler and dual-wavelength UV-visible detector set at 254 nm. The stationary phase was a Symmetry 3.1 × 150 mm, 3.5 µm C18 (Waters) or either Kinetex 2.1 × 100 mm, 2.6 µm C18 or 3.0 × 50 mm, 2.6 µm, C8 (Phenomenex) reverse-phase column maintained at 40 °C (30 °C for Kinetex 3.0 × 50 mm), and methanol-water gradients at 0.4 mL/min (0.6 mL/min for Kinetex 3.0 × 50 mm) comprised the mobile phase. For the Kinetex 3.0 × 50 mm C8 column in each run, the gradient went from 27% methanol from 0 to 0.5 min, 39% methanol at 0.51 min, 44% methanol to 16 min, 60% methanol to 20 min, 71% methanol to 22 min, 75% methanol to 30 min, and back to 27% methanol to re-equilibrate for 3 min. Authentic standards and starting substrates were chromatographed before and/or after incubation products with each experiment.
3. Results
3.1. Synthesis of Compounds
3.1.1. 21-Trihalo Progesterone Compounds (4, 5, 6, 13, 14, 15)
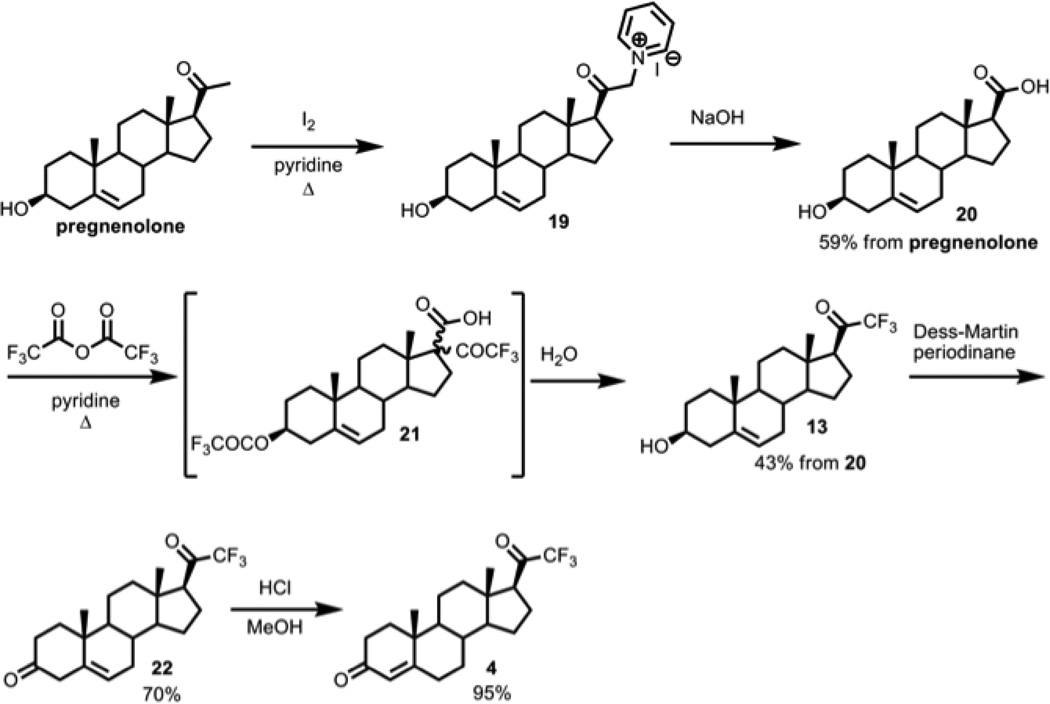
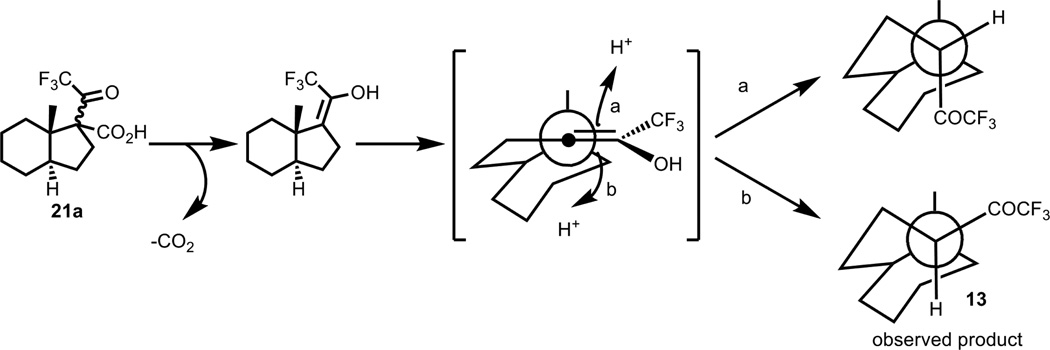
The first approach to 21-trihalo compounds (Scheme 1) used the 20-carbon carboxylic acid 20, derived from the pyridinium salt 19, to attempt Zard’s protocol of transforming carboxylic acids to trifluoromethyl-ketones [25]. Trifluoroacetic anhydride and pyridine converted the carboxylic acid 20 to the trifluoromethyl ketone 13 upon addition of water and heating. The β-ketoacid intermediate 21 is observable by NMR before the addition of water, and the decarboxylation proceeds with the desired stereochemistry at C-17, probably due to torsional strain in the protonation step (Figure 2).
Scheme 1.
Synthesis of 21-F3-progesterone via Zard methodology:
Figure 2.
Explanation of C-17 stereochemistry of 21-F3-pregnenolone – AB ring system omitted for clarity.
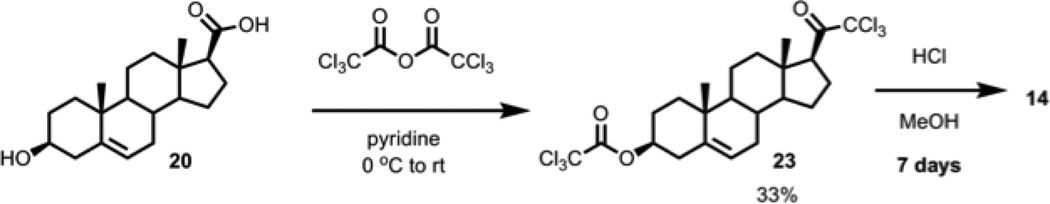
Interestingly, the trichloromethyl ketone 23 was also obtained in a similar manner by using either trichloroacetic anhydride or trichloroacetic acid chloride, except we found that the reaction must be done at 0 °C due to the reactive chloride leaving groups, and the product contained an incidental 3-trichloroacetate group (Scheme 2). Moreover, in the trichloro-case, the β-ketoacid decarboxylation occurred spontaneously, without the addition of water. The base-labile trichloroacetyl group mandated the deprotection of the 3-trichloroacetate group under acidic conditions in methanol, which took a week to complete and prompted investigation of alternative approaches. In a similar manner to the trichloromethyl ketone case, we attempted to use tribromoacetyl chloride to form the tribromomethyl ketone, but too many side reactions were observed.
Scheme 2.
21-Cl3-pregnenolone-3-trichloroacetate (9) synthesis using trichloroacetic anhydride:
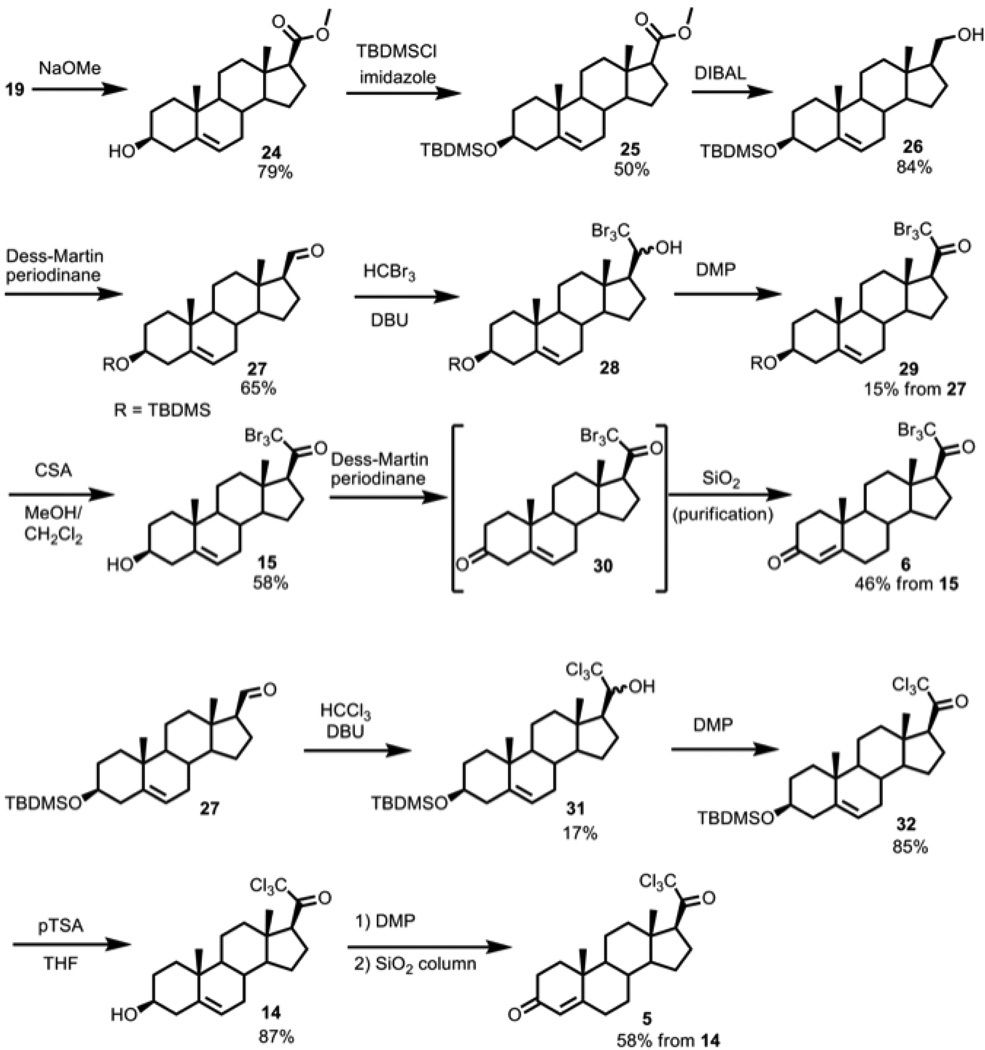
Due to difficulties with the trichloromethyl and tribromomethyl cases, we pursued a second route, which involved the addition of a trihalomethyl anion onto the 20-carbon aldehyde 27 (Scheme 3). One equivalent of DBU and excess bromoform was used to optimize yield and to prevent solidification of the reaction mixture [15]. Aldehyde 27 also served as a versatile intermediate to make alkyl substituents at the 21-position in addition to making the 21-trihalo compounds, including the 21-trichloroketone 14 by using the chloroform anion as the nucleophile. If 3-ketosteroids are sought, the TBDMS ether can be removed before the Dess-Martin periodinane-mediated oxidation step, and the resulting 3,20-diketones are isomerized to the Δ4,3-ketosteroids under mildly acidic conditions, which avoids the harsher Oppenauer oxidation protocol when the compounds contain labile or reactive groups.
Scheme 3.
21-trihaloprogesterone syntheses using haloform:
3.1.2. 17-Monohalo Compounds (1, 2, 3, 10, 11, 12)
The 17-monohalo compounds were afforded from a common intermediate – the 17,20-enol acetate, obtained in literature precedence [17] by refluxing pregnenolone in acetic anhydride and catalytic p-toluenesulfonic acid. The 17,20-enol acetate was a highly versatile intermediate, and we were able to use Selectfluor to fluorinate [26], NCS to chlorinate, and NBS to brominate the 17-position and access compounds 1–3 and 10–12. As an alternative to NBS, we could repeat the reported protocol of bromine in acetic acid [17], but the alkene on the 5-position is dibrominated, adding an additional step in reforming the Δ5-alkene with sodium iodide (Scheme 4). Presence of the 17-halogen did not complicate the facile oxidation and isomerization of pregnenolone halides to the corresponding progesterone series with Dess-Martin periodinane, followed by mild acid or spontaneous isomerization during purification on silica gel chromatography.
Scheme 4.
17-haloprogesterone syntheses:
3.1.3. 21-Monohalo Progesterone Compounds (7, 8, 9)
Analogous to the 17-monohalo steroid series, one can envision that the 21-monohalo compounds can be synthesized by halogenating the less substituted 20,21-enol acetate intermediate (Scheme 5, compound 39). Refluxing pregnenolone-3-formate with isopropenyl acetate and catalytic p-toluenesulfonic acid formed the 20,21-enol acetate as the kinetic product, which isomerized to the thermodynamic product (17,20-enol acetate) if refluxing was prolonged (3 days). We selectively halogenated the 20,21-enol acetate using N-iodo-, N-bromo-, and N-chlorosuccinimide to form the 21-iodo-, 21-bromo, and 21-chloropregnenolone formates, with chlorination requiring heat, providing routes to compounds 7, 8, and 9.
Scheme 5.
21-monobromoprogesterone synthesis:
We also sought to synthesize 21-fluoro-16,17-dehydroprogesterone to use as a potential substrate for CYP17A1. This compound was obtained from the same procedure used to access 21-monohalosteroids, except starting with 16,17-dehydropregnenolone (Scheme 6). Unlike in the 16,17-saturated case, refluxing 16,17-dehydropregnenolone-3-formate in isopropenyl acetate and catalytic p-toluenesulfonic acid cleanly yielded the 20,21-enol acetate, which was iodinated using N-iodosuccinimide. AgF was used to displace the iodide, followed by deformylation, oxidation, and isomerization, affording 21-fluorosteroid 48.
Scheme 6.
16,17-dehydro-21-fluoroprogesterone synthesis:
3.2. Halogenated steroids as substrates for human CYP17A1 and CYP21A2
Fluorinated small molecules have importance in the field of medicine to probe the biological function of compounds in vivo [27]. Consequently, we screened how the incorporation of fluorine atom(s) on either the 17-position or the 21-position of progesterone or pregnenolone altered metabolism by CYP17A1 or CYP21A2.
3.2.1. 17-fluorosteroids as substrates
When yeast microsomes containing CYP17A1 and P450-oxidoreductase were incubated with 17-fluoroprogesterone, under conditions where progesterone is largely metabolized to 17α- and 16α-hydroxylated products, no metabolism of 17-fluoroprogesterone 1 could be detected despite prolonged incubations (not shown). Similarly, CYP17A1-containing microsomes did not metabolize 17-fluoropregnenolone 10 to any demonstrable products, as assessed after conversion via cholesterol oxidase to their cognate Δ4-steroids. In both experiments, which were repeated 3 or more times, only 17-fluoroprogesterone was recovered, without any convincing evidence of product formation (not shown). These results demonstrate that blocking metabolism at the 17-position of both pregnenolone and progesterone by fluorine replacement does not result in metabolic switching to an alternative C-H bond, such as the 16α-position, as might be predicted from the known progesterone 16α-hydroxylase activity of human CYP17A1 [7].
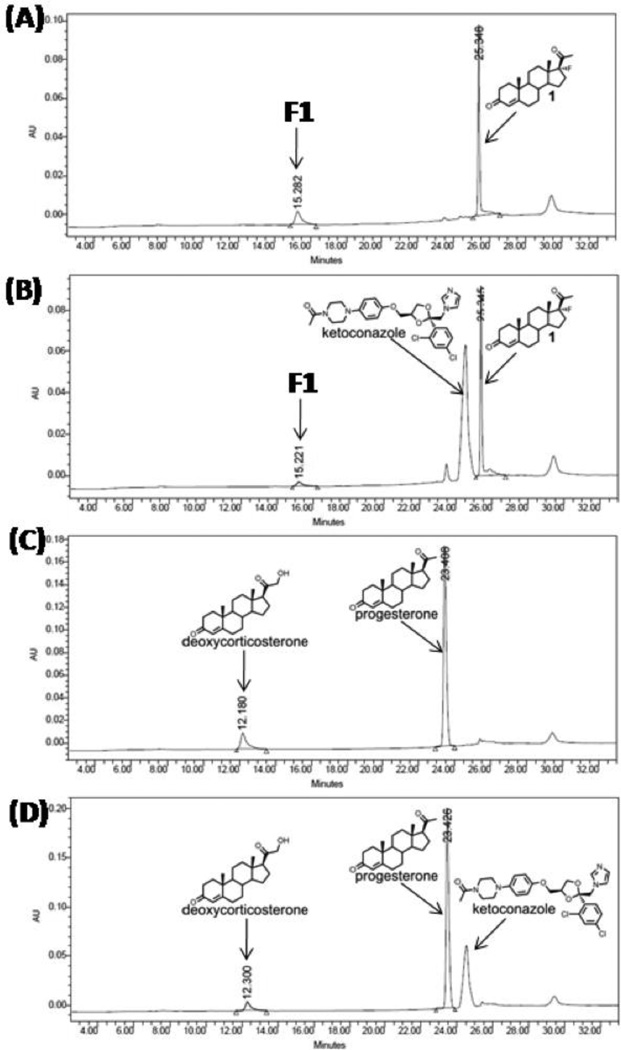
In contrast, when yeast microsomes containing CYP21A2 and P450-oxidoreductase were incubated with 17-fluoroprogesterone, under conditions where progesterone is metabolized to 11-deoxycorticosterone via 21-hydroxylation, comparable amounts of 17-fluoroprogesterone were reproducibly converted to an earlier-eluting peak, consistent with 21-hydroxylation (Figure 3). This metabolism of progesterone and 17-fluoroprogesterone was comparably inhibited by ketoconazole, consistent with P450-mediated oxygenation (Figure 3). This result suggests that 17-fluorination does not significantly impede substrate binding or alter catalysis by CYP21A2.
Figure 3.
In vitro studies of 17-fluoroprogesterone as a substrate for CYP21A2. HPLC traces of incubations: (A) 17-fluoroprogesterone (1) incubation with CYP21A2, (B) 17-fluoroprogesterone (1) incubation with CYP21A2 and ketoconazole, (C) progesterone incubation with CYP21A2, (D) progesterone incubation with CYP21A2 and ketoconazole. The formation and migration of compound F1 is consistent with 17-fluoro-21-hydroxyprogesterone. Conversion of 17-fluoroprogesterone to F1 was inhibited 65% by pre-incubation with ketoconazole, and conversion of progesterone to 11-deoxycorticosterone was inhibited 40% by ketoconazole using the same conditions. The peak at 30 min is due to the change in solvent gradient.
3.2.2. 21-trifluorosteroids as substrates
When 21-trifluoroprogesterone 4 was incubated with yeast microsomes containing CYP17A1 or CYP21A2, two earlier-eluting peaks were variably observed, but their formation was only partially inhibited by ketoconazole. The same pattern of product formation was observed from incubations with CYP17A1-containing microsomes and 21-trifluoropregnenolone 13, followed by cholesterol oxidase treatment (not shown). Consequently, we are unable to conclude if the 21-trifluorosteroids are substrates for CYP17A1 and CYP21A2.
4. Discussion
In summary, by exploring novel and previously reported methodologies, we have synthesized halogenated progesterone analogs to probe the active sites of CYP17A1 and CYP21A2. Zard’s methodology proved useful in accessing 21-trifluoropregnenolone 13 directly from the carboxylic acid intermediate, and we have found that trichloroketones are accessible from carboxylic acid precursors by using trichloroacetyl chloride as the electrophile. Moreover, nucleophilic bromoform addition was required to ultimately access 21-tribromopregnenolone 15. Synthesis of a kinetic 20,21-enol acetate was required to regioselectively halogenate the 21-position of pregnenolone over the Δ5,6-alkene and 17-position.
Njar and colleagues have reported the synthesis of 21-trifluoropregnenolone through the addition of trifluoromethyl silane onto an aldehyde intermediate and have shown that 21-trifluoropregnanes are inhibitors of CYP17A1 [14]. We found some evidence that 21-trifluorinated substrates might also be metabolized by CYP17A1 and CYP21A2, but the inherent difficulties in these experiments precluded unambiguous conclusions. One explanation for these results is that the electron-withdrawing trifluoromethyl group shifts the hydration equilibrium from the C20 ketone of compound 4 to its geminal diol, which is too bulky to bind to either active site. In contrast, we reproducibly demonstrated that CYP21A2 but not CYP17A1 metabolized 17-fluoroprogesterone to a single ketoconazole-inhibited product, possibly 17-fluoro-21-hydroxyprogesterone. These data demonstrate that steroid halogenation might block normal sites of P450 oxygenation but not preclude substrate binding and turnover.
The synthetic methods described here enables the detailed study of 17- and 21-halogenated progesterone and pregnenolone substrate analogs as substrates, inhibitors, and mechanistic probes of steroidogenic cytochrome P450 enzymes. By varying the location and number of halogen atoms, as well as the specific halide employed, variations in steric, electronic, and reactivity properties are introduced in the steroid near the sites of reactivity. Although the classical cytochrome P450 reaction is the hydroxylation of C-H bonds in alkanes, these substituted pregnanes are also reagents to test the capacity of CYP17A1 and CYP21A2 to catalyze halide reductions, as described for other cytochromes P450 [28].
In addition, halogenated steroids have been successfully employed for a variety of purposes in science and medicine. As suggested above, halogenated steroids might be employed as reversible [29] or irreversible [30] enzyme inhibitors, by blocking catalysis and/or increasing affinity for the steroid. Halogenated steroids are also in use as agonists and antagonists of steroid hormone receptors, and halogenation also blocks positions of metabolism by hepatic enzymes such as CYP3A4. This modification increases the half-life of the potential drug, as seen in the potent glucocorticoid dexamethasone (9α-fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione), with a half-life of over 36 hours. Furthermore, fluorinated [18F-] steroids are used as imaging agents for positron-emitted tomography (PET) studies [31,32], by binding to receptors for these steroids. Consequently, improved methods to selective halogenation of steroids might facilitate advances in several disciplines.
Highlights.
We describe the synthesis of 17-halosteroids, X = F, Cl, Br
We describe the synthesis of 21-trihalosteroids, X = F, Cl, Br
We show that 17-fluoroprogesterone is a substrate for CYP21A2
We show that 17-fluoroprogesterone and 17-fluoropregnenolone are not substrates for CYP17A1
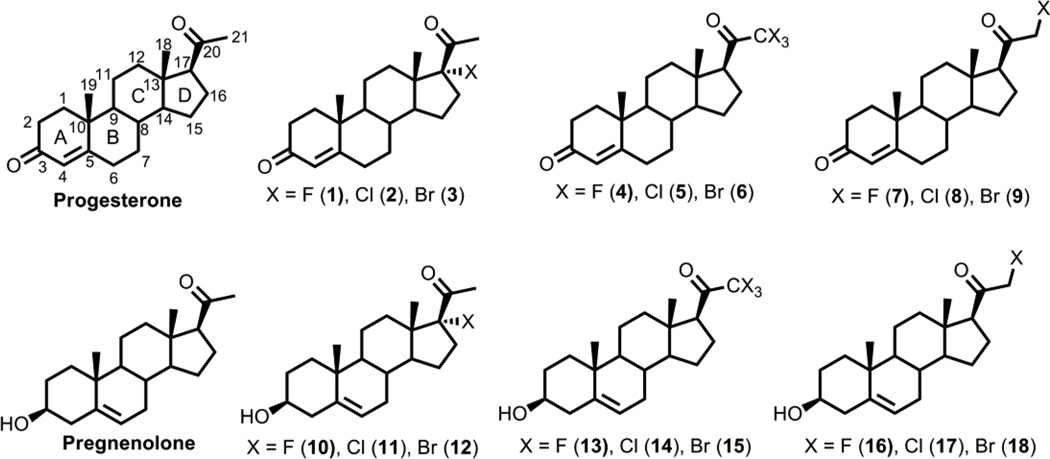
Figure 1.
Halogenated steroid substrates (1–18) synthesized for incubation with steroidogenic cytochromes P450, CYP17A1 and CYP21A2.
Acknowledgments
This research was supported by grants I-1493 from the Robert A. Welch Foundation and 5R01GM0865696-02 from the National Institutes of Health (to R.J.A.) and by a Chemistry and Biology Interface graduate fellowship from the University of Texas (to F.K.Y.). M.C.D. was a UT Southwestern SURF student. This work was also supported in part by 1R13DK092108-01, partially funded by the National Institute of Environmental Health Sciences (NIEHS), National Institute Of Diabetes And Digestive And Kidney Diseases (NIDDK), and Eunice Kennedy Shriver National Institute Of Child Health and Human Development (NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Therrell BLJ, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- 2.Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650–667. [PMC free article] [PubMed] [Google Scholar]
- 3.Costa-Santos M, Kater CE, Auchus RJ. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab. 2004;89:49–60. doi: 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 4.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:463–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swart P, Swart AC, Waterman MR, Estabrook RW, Mason JI. Progesterone 16α-hydroxylase activity is catalyzed by human cytochrome P450 17α-hydroxylase. J Clin Endocrinol Metab. 1993;77:98–102. doi: 10.1210/jcem.77.1.8325965. [DOI] [PubMed] [Google Scholar]
- 8.Mizrachi D, Wang Z, Sharma KK, Gupta MK, Xu K, Dwyer CR, Auchus RJ. Why human cytochrome P450c21 is a progesterone 21-hydroxylase. Biochemistry. 2011;50:3968–3974. doi: 10.1021/bi102078e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auchus RJ, Miller WL. Molecular modeling of human P450c17 (17α-hydroxylase/17,20-lyase): Insights into reaction mechanisms and effects of mutations. Mol Endocrinol. 1999;13:1169–1182. doi: 10.1210/mend.13.7.0326. [DOI] [PubMed] [Google Scholar]
- 10.Lee-Robichaud P, Akhtar ME, Akhtar M. An analysis of the role of active site protic residues of cytochrome P450s: mechanistic and mutational studies on 17α-hydroxylase-17,20-lyase (P45017α also CYP17) Biochem J. 1998;330:967–974. doi: 10.1042/bj3300967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin D, Black SM, Nagahama Y, Miller WL. Steroid 17α-hydroxylase and 17,20 lyase activities of P450c17: Contributions of serine106 and P450 reductase. Endocrinology. 1993;132:2498–2506. doi: 10.1210/endo.132.6.8504753. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb HE, Kotlyar V, Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 13.Kim TK, Chen J, Li W, Zjawiony J, Miller D, Janjetovic Z, Tuckey RC, Slominski A. A new steroidal 5,7-diene derivative, 3β-hydroxyandrosta-5,7-diene-17β-carboxylic acid, shows potent anti-proliferative activity. Steroids. 2010;75:230–239. doi: 10.1016/j.steroids.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Njar VC, Klus GT, Johnson HH, Brodie AM. Synthesis of novel 21-trifluoropregnane steroids: inhibitors of 17α-hydroxylase/17,20-lyase (17α-lyase) Steroids. 1997;62:468–473. doi: 10.1016/s0039-128x(97)00016-0. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal VK, Mereu A. Amidine-promoted addition of chloroform to carbonyl compounds. J Org Chem. 2000;65:7211–7212. doi: 10.1021/jo000584n. [DOI] [PubMed] [Google Scholar]
- 16.Kaspar E, Wiechert R. The action of haloform on the steroid-carbonyl function. Chem Ber. 1958;91:2664–2670. [Google Scholar]
- 17.Engel CR, Jahnke H. Steroids and related products .X. 17α-Bromoprogesterone, a new potent gestogen. Can J Biochem Physiol. 1957;35:1047–1055. [PubMed] [Google Scholar]
- 18.Deghenghi R, Gaudry R. The synthesis of 17α-fluoroprogesterone. Can J Chem. 1961;39:1553–1557. [Google Scholar]
- 19.Marshall DJ, Gaudry R. 17α-halogenated progesterones: Orally-active progestins. Can J Chem. 1960;38:1495–1504. [Google Scholar]
- 20.Banday AH, Shameem SA, Gupta BD, Kumar HM. D-ring substituted 1,2,3-triazolyl 20-keto pregnenanes as potential anticancer agents: Synthesis and biological evaluation. Steroids. 2010;75:801–804. doi: 10.1016/j.steroids.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Numazawa M, Nagaoka M. A facile synthesis of deoxycorticoids. J Org Chem. 1985;50:81–84. [Google Scholar]
- 22.Romo J, Rosenkranz G, Sondheimer F. Steroids. LXXXVIII. A new synthesis of desoxycorticosterone acetate and of 16-dehydro-desoxycorticosterone acetate. J Am Chem Soc. 1957;79:5034–5036. [Google Scholar]
- 23.Eng RR, Spitznagle LA, Trager WF. Preparation of radiolabeled pregnenolone analogs. 21-Fluoro-pregnenolone-21-18F, 21-fluoropregnenolone-3-acetate-21-18F, 21-fluoropregnenolone-7-3H, and 21-fluoropregnenolone-3-acetate-7-3H. J Label Compd Radiopharm. 1983;20:63–72. [Google Scholar]
- 24.Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003;278:48563–48569. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 25.Boivin J, Elkaim L, Zard SZ. A new and efficient synthesis of trifluoromethyl ketones from carboxylic-acids. Tetrahedron. 1995;51:2573–2584. [Google Scholar]
- 26.Fukuzumi T, Shibata N, Sugiura M, Nakamura S, Toru T. Enantioselective fluorination mediated by cinchona alkaloids/selectfluor combinations: A catalytic approach. J Fluorine Chem. 2006;127:548–551. [Google Scholar]
- 27.Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew Chem Int Ed Engl. 2011;50:1922–1925. doi: 10.1002/anie.201006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 29.Marchais-Oberwinkler S, Henn C, Moller G, Klein T, Negri M, Oster A, Spadaro A, Werth R, Wetzel M, Xu K, Frotscher M, Hartmann RW, Adamski J. 17β-Hydroxysteroid dehydrogenases (17β-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development. J Steroid Biochem Mol Biol. 2011;125:66–82. doi: 10.1016/j.jsbmb.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Poirier D. Contribution to the development of inhibitors of 17β-hydroxysteroid dehydrogenase types 1 and 7: key tools for studying and treating estrogen-dependent diseases. J Steroid Biochem Mol Biol. 2011;125:83–94. doi: 10.1016/j.jsbmb.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Teare H, Robins EG, Kirjavainen A, Forsback S, Sandford G, Solin O, Luthra SK, Gouverneur V. Radiosynthesis and evaluation of [18F]Selectfluor bis(triflate) Angew Chem Int Ed Engl. 2010;49:6821–6824. doi: 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]
- 32.Zhou HB, Lee JH, Mayne CG, Carlson KE, Katzenellenbogen JA. Imaging progesterone receptor in breast tumors: synthesis and receptor binding affinity of fluoroalkyl-substituted analogues of tanaproget. J Med Chem. 2010;53:3349–3360. doi: 10.1021/jm100052k. [DOI] [PMC free article] [PubMed] [Google Scholar]