Abstract
Memories for emotionally arousing experiences are typically vivid and persistent. The recurrent, intrusive memories of traumatic events in post-traumatic stress disorder (PTSD) are an extreme example. Stress-responsive neurotransmitters released during emotional arousal are proposed to enhance the consolidation of fear memory. These transmitters may include norepinephrine and epinephrine (NE/E) because stimulating β-adrenergic receptors shortly after training can enhance memory consolidation. However, mice lacking NE/E acquire and consolidate fear memory normally. Here, we show by using pharmacologic and genetic manipulations in mice and rats that NE/E are not essential for classical fear memory consolidation because signaling by the β2-adrenergic receptor is redundant with signaling by dopamine at the D5-dopaminergic receptor. The intracellular signaling that is stimulated by these receptors to promote consolidation uses distinct G proteins to redundantly activate phospholipase C. The results support recent evidence indicating that blocking β-adrenergic receptors alone shortly after trauma may not be sufficient to prevent PTSD.
Introduction
The basolateral nuclei of the amygdala (BLA) is a brain region critical to the consolidation of fear memory (Pape and Pare, 2010). Changes in neuromodulatory signaling in the BLA during emotional arousal are believed to underlie the powerful and persistent consolidation of long-term fear memory. Neuromodulators such as norepinephrine and epinephrine (NE/E) that are released during fearful or traumatic events can promote memory consolidation for such experiences. For example, stimulating β-adrenergic receptors by infusing NE or β-adrenergic selective agonists into the BLA shortly after instrumental fear conditioning enhances long-term memory (McGaugh and Roozendaal, 2002).
In contrast, a requirement for β-adrenergic signaling in fear memory consolidation is less clear. In one study, BLA infusion of β-adrenergic receptor antagonists impaired consolidation of instrumental fear (Gallagher et al., 1977). However, results from other studies suggest that β signaling is not required for consolidation of instrumental fear (Izquierdo and Dias, 1983; Izquierdo et al., 1992). Negative results have also been reported for classical fear. Infusing a β-adrenergic receptor antagonist into the BLA shortly before or immediately after classical fear conditioning does not impair consolidation (Miserendino et al., 1990; Debiec and LeDoux, 2004). Furthermore, instrumental and classical fear memory consolidation are normal in mice completely lacking NE/E (Thomas and Palmiter, 1997; Murchison et al., 2004) or harboring a targeted disruption of either the β1- or β2-adrenergic receptor gene (Schutsky et al., 2011).
A potential resolution to the observation that the adrenergic system can influence, but is not required for, consolidation is that there is redundancy between the adrenergic system and another neurotransmitter system. In redundancy, the loss of a single system will have no effect on consolidation. However, interfering with two relevant but redundant systems simultaneously could have considerable effect. Classically, β-adrenergic receptors couple to Gs proteins that act to stimulate adenylyl cyclase (AC) and elevate intracellular levels of cAMP, a second messenger required in the BLA for fear memory consolidation (Schafe and LeDoux, 2000). It is through Gs and cAMP that β receptors are currently proposed to enhance synaptic plasticity and memory consolidation (Sara, 2009; Tully and Bolshakov, 2010).
For this study, we considered whether DA might serve as a stress-responsive system that acts in a redundant manner with NE/E in promoting consolidation. Like NE/E, extracellular levels of DA are elevated for minutes following fear conditioning (Inglis and Moghaddam, 1999; Anstrom and Woodward, 2005). Like β receptors, the D1 class of DA receptors, consisting of the D1 and D5 receptors (D1,5), can couple to the Gs class of G proteins and elevate cAMP (Sibley and Monsma, 1992), providing an opportunity for redundant signaling between these two classes of catecholamine receptors. Here, we use genetic and pharmacologic approaches in mice and rats to demonstrate that DA and NE/E have redundant roles in the BLA for the consolidation of classical fear memory. Surprisingly, we found that the signaling by these neuromodulators that is critical for consolidation converges on the activation of phospholipase C.
Materials and Methods
Animals.
Wild-type, Dbh+/−, Dbh−/−, Adrb1−/− (β1 KO), Adrb2−/− (β2 KO), and Drd1−/− (D1 KO) mice were on a hybrid 129/Sv × C57BL/6 background, while Drd5−/− (D5 KO) mice were on a C57BL/6 background (Xu et al., 1994; Thomas et al., 1995; Rohrer et al., 1996; Chruscinski et al., 1999; Holmes et al., 2001). Mice were generated by mating either heterozygotes or homozygotes, and genotype was determined by PCR. The prenatal loss of Dbh−/− mice was rescued as previously described (Ouyang et al., 2004). No significant differences were found by gender or parental genotype so data were combined. Female Fischer 344 rats (Harlan) were 3–4 weeks old upon arrival. Animals were maintained on ad libitum food and water and a 12 h light/dark cycle, with lights on beginning at 7:00 A.M. Animals were housed in small, quiet rooms for at least 3 weeks before studies began. Mice were 3–6 months old and rats were 8–11 weeks old when tested. Studies were performed during the light phase, with most experiments taking place between 9:00 A.M. and 5:00 P.M. Studies were in accordance with NIH guidelines and had the approval of the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Classical fear conditioning.
Adjacent to the training room, animals were placed in pairs (mice) or singly (rats) into opaque plastic holding buckets (12 cm diameter) with bedding and lids for 30–60 min before being manipulated further. Animals were given two 3 min preconditioning handling sessions over 2 d in the training room. Saline was injected at the end of handling each day. For conditioning, animals were placed in the training apparatus (ENV-010MC with ENV-414S, Med Associates) for 2 min, after which an 84 dB, 4.5 kHz tone was activated for 30 s. Two seconds before the end of the tone, a 2 s footshock was delivered (1 mA for mice, 1.7 mA for rats). The animal was removed from the apparatus and injected 30 s after shock, and then returned to its home cage. The apparatus was cleaned with Versa-Clean (Thermo Fisher Scientific) between subjects. Experiments examining enhancement of consolidation in mice were conducted identically except that a 0.4 mA footshock was used. Pseudoconditioning was similar to conditioning except that animals received the 2 s, 1 mA footshock immediately after being placed in the conditioning apparatus, while activation of the 30 s tone occurred at the normal time 2 min later. Contextual fear was tested for 5 min in the conditioning apparatus in the absence of the tone. Cued fear was tested in a Plexiglas cylinder (21 cm diameter, 24 cm tall) with green wire grid floor and vertical green and white wall stripes 240° around, and was cleaned with lemon-scented Ajax between subjects. After 2 min, the training tone was turned on for 3 min. Percentage freezing was estimated by scoring the presence or absence of nonrespiratory movement every 5 s. Tests were conducted 1 d after training.
Instrumental fear conditioning.
Animals were handled as described above. Training consisted of placing an animal in the lighted chamber of the apparatus used for classical conditioning and timing its latency to fully enter (except for the tail) the dark chamber. Once the animal entered the dark chamber, the retractable partition separating the two chambers was lowered and a footshock was delivered for 2 s (0.35 mA). The animal was removed from the apparatus and injected 15 s after shock, and then returned to its home cage. Animals that did not enter the dark chamber after 100 s during conditioning were excluded (<4% of mice, independent of genotype). Testing was identical to training except that no shock was delivered and the partition remained up. Latencies to enter the dark chamber were recorded. If an animal did not enter the dark chamber within 10 min, it was returned to its cage and assigned a latency of 10 min. Tests were conducted 1 d after training.
Drugs.
SCH 23390 HCl, ecopipam (SCH 39166 HBr), SKF 38393 HBr, SKF 83959 HBr, SKF 83822 HBr, (−)-propranolol HCl, CGP 20172A HCl, ICI 118,551 HCl, procaterol HCl, pertussis toxin, edelfosine (all Tocris Bioscience) and m-3M3FBS (Sigma) were administered intraperitoneally or infused into the BLA immediately after training. The phospholipase C (PLC) inhibitor U73122 could not be used in these studies because its delivery requires high percentage organic vehicles that disrupt memory consolidation on their own. Xamoterol hemifumarate (Tocris Bioscience) was administered intraperitoneally 60 min before testing contextual fear in Dbh−/− mice to rescue memory retrieval (Murchison et al., 2004). Drugs were dissolved in 0.9% saline (SKF compounds and procaterol also contained 0.1 mg/ml ascorbic acid, pH 7.4, Sigma). Vehicle was saline with or without 0.1 mg/ml ascorbic acid. Systemic injection volumes were 10 μl/g body weight.
CNS infusion.
Two guide cannulae mounted on a base plate (C315GS system, Plastics One) were implanted under pentobarbital anesthesia (72.5 mg/kg) using a stereotax (SAS75/EM40M, Cartesian Research). The guides were placed 1.25 mm posterior to bregma and 3.5 mm bilateral for BLA infusions. The guide and dummy cannulae projected 3 mm below the base plate. Habituation of the animals to the investigator and the infusion procedure began a couple of days later with a 3 min handling session followed by 3 min of immobilization (gently holding the nape of the neck and body) that mimicked infusion. Five handling sessions were given, with two of them being on the 2 d immediately preceding training and the final one being 1 h before training. Immediately after training, mice were infused bilaterally using injection cannulae that extended 2.8 mm below the tip of the guide cannulae. Infusion was 0.2 μl/side at 0.08 μl/min, with the injection cannulae being left in place for 30 s before the mouse was returned to its home cage. Because studies indicate that the effects of PTx are best evaluated 3 d after infusion, PTx was infused into the BLA 3 d before training (Goh and Pennefather, 1989; Stratton et al., 1989). For sites adjacent to the BLA, infusions were displaced 0.75 mm from the BLA coordinates in the direction indicated. As a result, dorsal was in the posterior striatum, ventral was near the ventral piriform cortex, medial was at the central/medial amygdala border, lateral was in the dorsal piriform cortex, rostral was in the extreme anterior amygdala, and caudal was in the extreme posterior amygdala.
IP3 levels.
Mice were anesthetized with CO2, killed by cervical dislocation and brains were rapidly removed, frozen in isopentane on dry ice and stored at −80°C. Two frozen coronal sections (400 μm) that contained the BLA were cut by cryostat (HM505E, Microm) from each mouse, and a 0.5 mm diameter punch of BLA tissue was collected bilaterally from each slice. The four punches per mouse were pooled and homogenized on ice with three 2 s pulses (5 s interval) in 125 μl of 4% perchloric acid using a Sonic Dismembrator 100 set on level 3 (Thermo Fisher Scientific). After 15 min on ice, samples were stored overnight at −80°C. The next day samples were centrifuged at 4°C and 2000 × g for 15 min, and the pellet was stored at −80°C for subsequent Bradford assay to determine total protein. Supernatants were neutralized on ice with 10 m KOH (to precipitate the perchloric acid) and centrifuged at 4°C and 2000 × g for 15 min. Supernatant (100 μl) was then used in the [3H]-IP3 radioreceptor assay (PerkinElmer) according to instructions. Pilot experiments indicated that IP3 levels were elevated 30 min after systemic agonist injection, but not at 15, 22, or 35 min.
Statistics.
Data were analyzed with Statistica 9.1 (StatSoft) using one- or two-way ANOVA with α = 0.05. The Bartlett Chi-square test was used to analyze homogeneity of variances. Post hoc comparisons were made using Duncan's range test. In Figures 1–9, data are presented as mean ±SE. Comparisons marked as significant are to the reference group except where indicated.
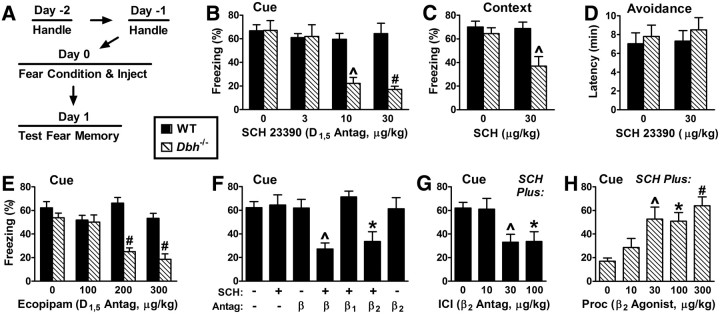
Figure 1.
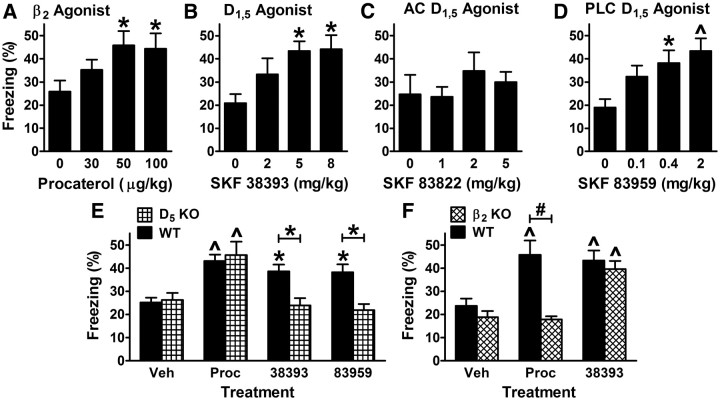
D1/5-dopaminergic signaling is redundant with β2-adrenergic signaling for fear memory consolidation. A, General time line for fear memory experiments, indicating that drugs were administered immediately after conditioning and testing was performed the next day. More extensive pretraining handling was performed for CNS infusion experiments. For this figure, conditioning was with intense shock (1 mA). B—E, A D1/5 receptor antagonist (SCH 23390 or ecopipam) was injected intraperitoneally. B, Cued fear test. p = 0.0001 for the main effect of dose; p < 0.0001 for the main effect of genotype; and p = 0.0004 for the interaction of dose and genotype (6/group). C, Contextual fear test. The β1-adrenergic receptor agonist xamoterol (3 mg/kg) was administered 60 min before testing contextual fear in Dbh−/− mice to rescue their contextual memory retrieval deficit (Murchison et al., 2004). p = 0.027 for the main effect of treatment; p = 0.005 for the main effect of genotype; and p = 0.039 for the interaction of treatment and genotype (6/group). D, Cued fear test. p = 0.0006 for the main effect of dose; p < 0.0001 for the main effect of genotype; and p = 0.0003 for the interaction of dose and genotype (5–8/group). E, Instrumental fear test. Main effects and their interaction were not significant (7–8/group). F, G, Cued fear test. The D1/5 antagonist SCH 23390 (30 μg/kg) was administered to wild-type mice either in saline (− or 0) or in combination with a β-adrenergic receptor antagonist. F, The β antagonists used were either the nonselective β blocker (-)-propranolol (β), the β1-selective blocker CGP 20712A (β1), or the β2-selective blocker ICI 118,551 (β2), each at 1 mg/kg. p = 0.0005 for the main effect of treatment (6–9/group). G, ICI tested at lower doses in combination with SCH (30 μg/kg). p = 0.003 for the main effect of dose (6–12/group). H, SCH (30 μg/kg) was administered with the β2 agonist procaterol. p = 0.0008 for the main effect of dose (6/group). *p < 0.05, ^p < 0.01, #p < 0.001.
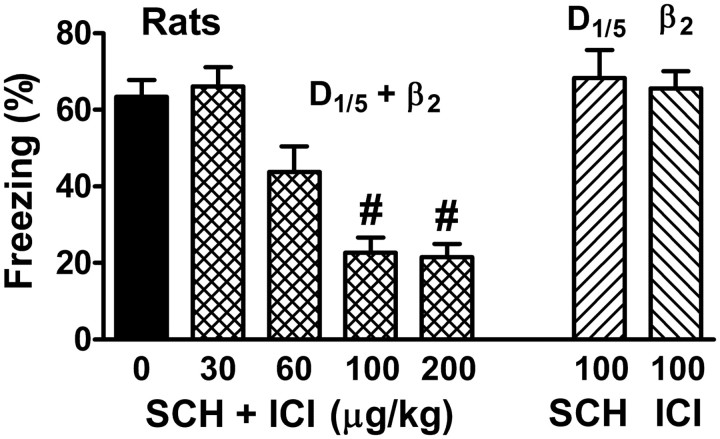
Figure 2.
Redundancy for fear memory consolidation also occurs in rats. Experimental design was as depicted in Figure 1A using intense shock (1.7 mA for rats). Rats were treated with either saline (0), SCH, ICI or the combination. p < 0.0001 for the main effect of treatment (4–8/group). #p < 0.001.
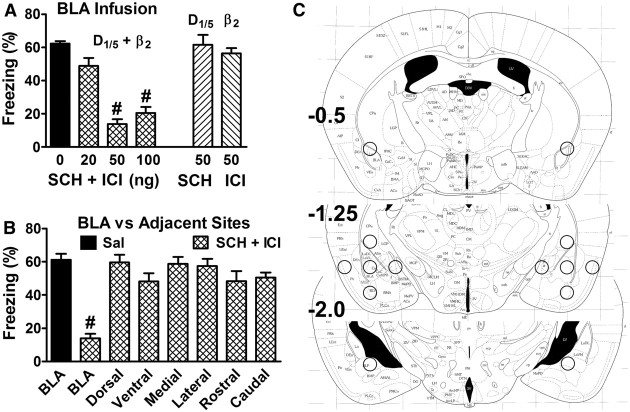
Figure 3.
BLA is the locus of redundant signaling in fear memory consolidation. Experimental design was as depicted in Figure 1A using intense shock (1 mA for mice). A, Drugs were infused bilaterally into the BLA of wild-type mice. p < 0.0001 for the main effect of treatment (5–8/group). B, The combination of SCH plus ICI (each 50 ng) was infused bilaterally into the BLA or into adjacent brain regions displaced 0.75 mm in the direction indicated. p < 0.0001 for the main effect of treatment (5–6/group). C, Injection cannula tips were located within spheres marked by the circles on the atlas drawings (Frankland and Paxinos, 1997). #p < 0.001.
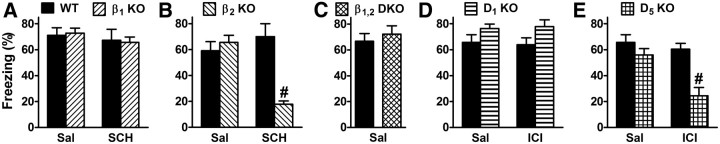
Figure 4.
The role of DA in fear memory consolidation is mediated by D5 receptors. Experimental design was as depicted in Figure 1A using intense shock (1 mA). A, B, Either saline (Sal) or a D1/5 antagonist (SCH, 30 μg/kg) was administered to wild-type (WT) and β receptor knock-out (KO) mice. For β2 mice, p = 0.016 for the main effect of treatment; p = 0.0044 for the main effect of genotype; and p = 0.0006 for the interaction of treatment and genotype (5/group). C, Sal was administered to WT and β1,2 double KO mice. No significant difference was observed (5/group). D, E, Either Sal or a β2 antagonist (ICI, 30 μg/kg) was administered to WT and DA receptor KO mice. For D5 mice, p = 0.004 for the main effect of treatment; p = 0.0006 for the main effect of genotype; and p = 0.029 for the interaction of treatment and genotype (5–8/group). #p < 0.001.
Figure 5.
Agonists of β2 or D5 receptors enhance fear memory consolidation. Experimental design was as depicted in Figure 1A using moderate shock (0.4 mA). A, Various doses of the β2 agonist procaterol were injected immediately after conditioning. p = 0.043 for the main effect of dose (6–11/group). B–D, Various doses of either a nonselective D1/5 agonist (SKF 38393), a D1/5 agonist that selectively activates AC (SKF 83822), or a D1/5 agonist that selectively activates PLC (SKF 83959) were tested. p = 0.017 and p = 0.0038 for the main effect of dose for SKF 38393 and SKF 83959, respectively (8–13/group). E, F, Procaterol (50 μg/kg) or D1/5 agonist (5 mg/kg for SKF 38393 or 2 mg/kg for SKF 83959) was administered to β2 KO and D5 KO mice and their wild-type littermate controls. E, p = 0.0001 for the main effect of treatment; p = 0.013 for the main effect of genotype; and p = 0.015 for the interaction of treatment and genotype (8–31/group). F, p < 0.0001 for the main effect of treatment; p = 0.0009 for the main effect of genotype; and p = 0.009 for the interaction of treatment and genotype (5–13/group). *p < 0.05, ^p < 0.01, #p < 0.001.
Figure 6.
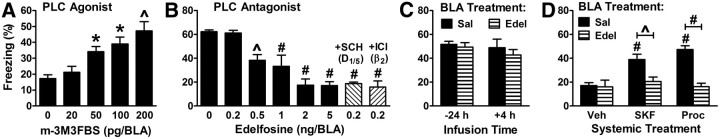
PLC is a critical regulator of consolidation. Experimental design was similar to that depicted in Figure 1A using moderate or intense shock (0.4 or 1 mA). A, The PLC agonist m-3M3FBS was infused into the BLA of wild-type mice after training with 0.4 mA. p = 0.001 for the main effect of dose (5/group). B, The PLC inhibitor edelfosine alone or in combination with either ICI (50 ng) or SCH (50 ng) was infused into the BLA after training with 1 mA. p < 0.0001 for the main effect of treatment (5–7/group). C, Infusion time is relative to conditioning. Cued fear testing was performed 1 d after training. The main effects of treatment and of time, as well as their interaction, were not significant (5/group). D, Mice were fear conditioned using 0.4 mA. Saline (Sal) or edelfosine (2 ng) was then infused into the BLA, and immediately afterward either vehicle, SKF 83959 (2 mg/kg) or procaterol (50 ng/kg) was injected intraperitoneally. p = 0.001 for the main effect of agonist; p < 0.0001 for the main effect of antagonist; and p = 0.0027 for the interaction of agonist and antagonist (5/group). *p < 0.05, ^p < 0.01, #p < 0.001.
Figure 7.
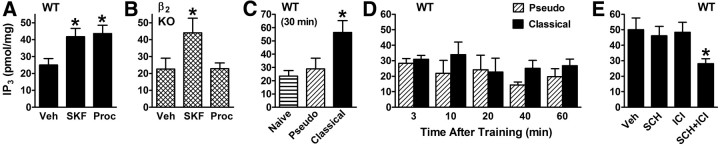
Redundancy between β2 and D5 signaling occurs via activation of PLC. Experimental design was similar to that depicted in Figure 1A using moderate or intense shock (0.4 or 1 mA). A, Wild-type mice were injected intraperitoneally with either vehicle, SKF 83959 (2 mg/kg) or procaterol (50 ng/kg) and killed 30 min later. Punches from the BLA were assayed for IP3 (pmol/mg protein). p = 0.045 for the main effect of agonist (10/group). B, β2 KO mice were treated as described in A. p = 0.044 for the main effect of agonist (9–10/group). C, Mice were treated as indicated and killed 30 min later. Shock intensity was 1 mA for the pseudo- and classical-conditioned groups. The classical-conditioned group exhibited significantly higher IP3 levels in the BLA relative to the pseudo-conditioned (p = 0.033) and naive (p = 0.016) groups. p = 0.021 for the main effect of conditioning (6–10/group). D, Mice were handled as in (C) and killed at the times indicated. Main effects and their interaction were not significant (5–7/group). E, Mice were fear conditioned using 1 mA and then injected intraperitoneally with either Sal, SCH (30 μg/kg), ICI (30 μg/kg), or the combination of SCH and ICI, and killed 30 min later. Only combined treatment caused a significant reduction in conditioning-induced IP3 levels in the BLA. p = 0.039 for the main effect of treatment (21/group). *p < 0.05.
Figure 8.
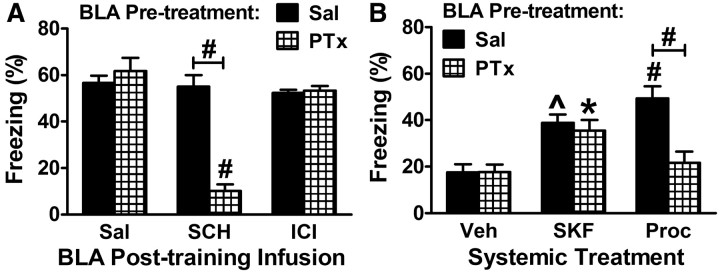
β2 activation of PLC in the BLA is mediated by Gi/o. Experimental design was similar to that depicted in Figure 1A using moderate or intense shock (0.4 or 1 mA). A, Pertussis toxin (PTx, 1 ng) or Sal was infused into the BLA 3 d before training. Immediately after training with 1 mA, either Sal, SCH (50 ng), or ICI (50 ng) was infused into the BLA. p = 0.0002 for the main effect of pretreatment; p < 0.0001 for the main effect of treatment; and p < 0.0001 for the interaction of pretreatment and treatment (5/group). B, Sal or PTx (1 ng) was infused into the BLA 3 d before training. Immediately after training with 1 mA, either Veh, SKF 83959 (2 mg/kg), or procaterol (50 μg/kg) was injected intraperitoneally. p = 0.018 for the main effect of pretreatment; p = 0.0009 for the main effect of treatment; and p = 0.014 for the interaction of pretreatment and treatment (5–10/group). *p < 0.05; ^p < 0.01, #p < 0.001.
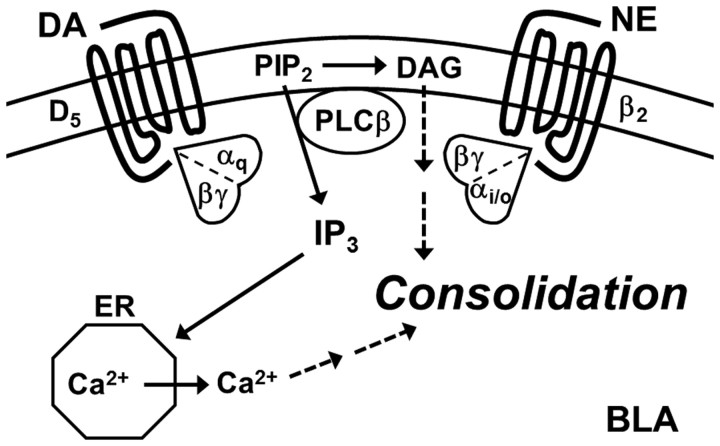
Figure 9.
Schematic diagram of functional redundancy. DA and NE are functionally redundant for fear memory consolidation in the BLA due to activation of PLC by D5-Gqα and β2-Gi/oβγ receptor signaling. Whether downstream signaling activated by the second messengers IP3/Ca2+, diacylglycerol (DAG), or both is required is currently unclear, but both are likely required for activating a conventional isozyme of protein kinase C that is implicated in fear memory consolidation (Weeber et al., 2000). The cell type(s) in which this signaling occurs has yet to be defined. Phosphatidylinositol 4,5-bisphosphate (PIP2) is a substrate of PLC. The endoplasmic reticulum (ER) stores Ca2+ for cytosolic release that is induced by IP3.
Results
Redundancy between D1/5-dopaminergic and β2-adrenergic signaling
To determine whether signaling by DA might be redundant with that for NE/E, we first examined the effect of the D1,5 antagonist SCH 23390 (SCH) on memory consolidation when administered immediately after classical fear conditioning in wild-type mice and mice completely lacking NE/E (Iorio et al., 1983). Mice that lacked NE/E were homozygous for targeted disruption of the dopamine β-hydroxylase gene (Dbh−/−) (Thomas et al., 1995). The same general treatment protocol was used for this and subsequent experiments (Fig. 1A). One day after fear conditioning, Dbh−/− mice treated immediately after training with the D1,5 antagonist SCH at 10–30 μg/kg body weight exhibited low freezing in response to the training cue (a tone that immediately preceded shock) compared with Dbh−/− mice treated with vehicle or with wild-type mice treated with either SCH or vehicle (Fig. 1B).
In a separate group of mice tested for their contextual fear of the training apparatus (no tone), SCH also impaired consolidation selectively in Dbh−/− mice relative to wild-type mice (Fig. 1C). Mice lacking NE/E exhibit impaired retrieval of contextual fear 1 d after conditioning due to lack of β1 signaling (Murchison et al., 2004). Thus, to examine potential effects of NE/E deficiency on consolidation, retrieval was rescued in the contextual fear experiment by administering the β1-selective agonist xamoterol shortly before testing (Hicks et al., 1987; Murchison et al., 2004). For simplicity, subsequent experiments using classical fear conditioning focused on cued fear, for which retrieval is independent of NE/E.
In addition to classical fear, we examined whether consolidation of instrumental fear requires NE/E and/or D1,5 signaling. Wild-type and Dbh−/− mice were treated with either vehicle or SCH immediately after conditioning. Neither the absence of NE/E by itself nor treatment of wild-type mice with SCH impaired consolidation of instrumental fear (Fig. 1D), confirming previous observations with Dbh−/− mice (Thomas and Palmiter, 1997). Furthermore, and in contrast to the results for classical fear, treatment of Dbh−/− mice with SCH also had no effect on the consolidation of instrumental fear. Differences in signaling mechanisms between instrumental and classical fear conditioning may parallel differences in their reliance on the amygdala (Wilensky et al., 2000).
A potential confound of the above results for classical fear conditioning is that SCH is also an agonist at serotonin 5-HT2C receptors (Ramos et al., 2005). To further examine a role for D1,5 receptors, we used a less potent but more selective D1,5 antagonist, ecopipam, that lacks serotonin receptor activity (Chipkin et al., 1988). Similar to SCH, ecopipam impaired cued fear memory consolidation selectively in Dbh−/− mice (Fig. 1E).
The results to this point are consistent with two distinct potential roles for DA in classical fear memory consolidation. First, DA might not normally play a role in consolidation, but instead might compensate for the chronic lack of NE/E in Dbh−/− mice. Alternatively, NE/E and DA might both contribute to consolidation, but their roles would be redundant. In the latter case, stimulation of either system might facilitate consolidation, but only impairments in signaling by both systems would cause deficits in consolidation. To evaluate these possibilities, wild-type mice were treated with SCH, the β-adrenergic receptor antagonist propranolol, or the combination. Mice treated with SCH or propranolol alone exhibited normal cued fear memory, while mice treated with the combination of SCH plus propranolol exhibited impaired memory (Fig. 1F). Furthermore, a selective β2 antagonist (ICI 118,551 = ICI) but not a selective β1 antagonist (CGP 20712A) impaired consolidation when combined with SCH, while ICI alone had no effect (O'Donnell and Wanstall, 1980; Dooley et al., 1986). Dose-response data indicated that ICI was fully effective at 30 μg/kg when it was combined with SCH (Fig. 1G). The results provide strong initial support for the idea that DA and NE/E act in a redundant manner to consolidate classical fear memory.
Results from the antagonist studies suggest that β2 signaling is required for the role of NE/E in consolidation. To determine whether β2 signaling is sufficient for the role of NE/E, Dbh−/− mice were treated with both SCH (to impair consolidation) and various doses of the selective β2 agonist procaterol (Waelbroeck et al., 1983). Procaterol provided a dose-dependent rescue of cued fear memory consolidation, suggesting that β2 signaling is sufficient for mediating the role of NE/E in consolidation in the absence of D1,5 signaling (Fig. 1H).
Redundancy also occurs in rats, is localized to the BLA, and is mediated by D5 receptors
Many prior studies examining the roles of catecholamines in fear memory have used rats rather than mice. To determine whether redundancy between catecholamines generalizes across species, rats were fear conditioned and treated with SCH and/or ICI. Consistent with the results obtained from mice, only concurrent administration of SCH and ICI impaired cued fear memory consolidation in rats (Fig. 2).
Because the BLA is critical for fear memory consolidation, we asked whether redundant catecholamine signaling occurs in this brain region. One week before conditioning, mice were cannulated to permit infusions into the BLA. When SCH and/or ICI were infused bilaterally immediately after training, only the combination of drugs impaired memory consolidation (Fig. 3A). To determine whether drug infusions into the BLA impair consolidation by affecting adjacent brain regions instead of the BLA, the SCH/ICI combination was infused into each of six locations surrounding the BLA (Fig. 3C). These infusions did not impair consolidation, indicating that the BLA is the site of drug action (Fig. 3B).
To genetically test a role for β receptors, mice with a targeted disruption of the gene for either the β1- or β2-adrenergic receptor were treated with SCH or vehicle. While SCH had no effect on consolidation in β1 knock-out (KO) mice (Fig. 4A), SCH impaired consolidation in β2 KO mice (Fig. 4B). As a genetic complement to nonselective β receptor blockade, cued fear memory was also examined in β1,2 double KO mice, and no deficit was observed (Fig. 4C). Importantly, the pharmacologic studies to this point were unable to distinguish between the potential roles of D1 and D5 receptors in consolidation due to a lack of receptor subtype selectivity of the drugs. For this purpose, gene-targeted mice were used. While the β2 antagonist ICI had no effect on consolidation in D1 KO mice (Fig. 4D), ICI impaired consolidation in D5 KO mice (Fig. 4E), indicating that the role of DA in consolidation is mediated by the D5 receptor.
Agonists of β2 or D5 receptors enhance fear memory consolidation
Given the roles for β2 and D5 receptors defined above, we asked whether activating these specific receptors shortly after conditioning would enhance fear memory consolidation in mice when trained with a lower shock intensity. For NE/E, enhancement of consolidation by β receptor stimulation has not been demonstrated for classical fear, although this has been demonstrated for instrumental fear (McGaugh and Roozendaal, 2002). For DA, results from BLA infusion of a D1,5 receptor agonist before classical fear conditioning suggest that stimulation of these receptors enhances either acquisition or consolidation (Guarraci et al., 1999).
When wild-type mice were systemically injected with the β2 agonist procaterol immediately after conditioning, cued fear memory was significantly enhanced (Fig. 5A). When mice were injected with the D1,5 agonist SKF 38393 immediately after conditioning, cued fear memory was also significantly enhanced (Fig. 5B). Of note, D1,5 receptors can activate various downstream effectors, including AC and PLC, and certain D1,5 agonists can induce the selective activation of either AC or PLC (Undie et al., 1994; Jin et al., 2003). To gain insight into the initial mechanism by which D1,5 receptors might enhance consolidation, effector-selective D1,5 agonists were used. While an AC-selective D1,5 agonist did not significantly alter consolidation, a PLC-selective D1,5 agonist enhanced consolidation to an extent similar to that for the nonselective D1,5 agonist used initially (Fig. 5B–D). Because D1,5 agonists do not distinguish between these two receptors, receptor KO and wild-type littermate control mice were used. The ability of the nonselective and PLC-selective D1,5 agonists to enhance consolidation was absent in D5 KO mice, although the β2 agonist procaterol remained effective (Fig. 5E). Similarly, the ability of procaterol to enhance consolidation was absent in β2 KO mice, although a D1,5 agonist remained effective (Fig. 5F).
β2 and D5 signaling in consolidation converge on the activation of PLC
Given that D5 may activate PLC to enhance fear memory consolidation, we asked whether directly stimulating PLC activity with the agonist m-3M3FBS enhances consolidation (Bae et al., 2003). In support of a role for PLC, infusion into the BLA of this PLC agonist also enhanced consolidation (Fig. 6A). We next asked whether inhibiting PLC activity would impair consolidation by infusing the PLC inhibitor edelfosine into the BLA immediately after training (Powis et al., 1992). Edelfosine dose-dependently impaired consolidation (Fig. 6B), suggesting that activation of PLC may be required for fear memory consolidation, and that this could be a site of convergence for D5 and β2 signaling. Infusion of edelfosine either 1 d before or 4 h after conditioning had no effect on cued fear memory, indicating that edelfosine does not lesion the BLA or impair expression (Fig. 6C). Although prior studies support the possibility that D5 may signal via Gq and PLC in the amygdala (Friedman et al., 1997; Leonard et al., 2003; Sahu et al., 2009), the only data indicating that β2 could signal via PLC come from heterologous expression of β2 receptors in HEK-293 cells in vitro (Keiper et al., 2004).
To determine whether D5, β2, or both receptors might signal via PLC to promote consolidation, we used edelfosine in two complementary experiments. The first experiment was based on the idea that PLC activity could be reduced to a point where it becomes rate-limiting for consolidation. To achieve this, edelfosine was infused into the BLA at the highest dose (0.2 ng) that did not impair consolidation. We then examined the combinatorial effects of PLC and receptor blockade. Edelfosine at 0.2 ng was administered with a dose (50 ng) of either SCH or ICI that impairs consolidation when combined with each other but not when given alone (Fig. 3A). The combination of edelfosine plus SCH and the combination of edelfosine plus ICI each impaired consolidation (Fig. 6B).
The second experiment examined the ability of agonists to enhance consolidation when PLC was inhibited. Edelfosine, at the smallest dose (2 ng) that impaired consolidation of high-shock intensity training, was infused into the BLA while administering a D1,5 or β2 agonist systemically immediately after training with lower shock intensity. Edelfosine blocked the enhancements of consolidation induced by the D1,5 agonist and by the β2 agonist (Fig. 6D).
Results from the above two experiments suggested that D5 and β2 receptors may both signal via PLC to promote consolidation. To further test this possibility, inositol-1,4,5-trisphosphate (IP3), a second messenger molecule generated by PLC activity, was measured in the BLA following systemic administration of a D1,5 or β2 agonist. Significant increases in IP3 levels in the BLA were observed ex vivo with each agonist in wild-type mice (Fig. 7A). Because this is a novel finding for β2 stimulation, β2 KO mice were also examined. In these mice, a D1,5 agonist but not a β2 agonist caused BLA IP3 levels to increase (Fig. 7B). For D5, others have shown that the ability of D1,5 agonists to augment IP3 levels in various brain regions is absent in D5 KO mice (Sahu et al., 2009).
Finally, we asked whether learning-specific activation of PLC occurs in the BLA by measuring IP3 levels ex vivo following fear conditioning. IP3 levels were elevated in the BLA 30 min after fear conditioning when compared with pseudoconditioning or no conditioning (Fig. 7C). The elevation in IP3 was selective for this time point, as several earlier and later time points from 3–60 min did not show elevation (Fig. 7D). Systemic administration of SCH or ICI immediately after conditioning had no effect on IP3 levels 30 min after conditioning (Fig. 7E). In contrast, administration of SCH and ICI combined significantly reduced IP3 levels relative to vehicle administration, demonstrating that redundancy in receptor function extends to the learning-induced production of IP3 in the BLA.
β2 activation of PLC in the BLA is mediated by Gi/o
While there is considerable evidence indicating that D5 couples to Gq to activate PLC, evidence for the coupling of β2 to Gq is lacking. A study in HEK-293 cells suggested that by coupling to Gs, β2 receptors can sequentially activate AC, Epac, Rap2, and PLCε. However, recent results from our laboratory suggested an alternate potential mechanism for coupling β2 to PLC in the brain. Retrieval of contextual fear memory requires NE, β1, Gs, and AC signaling in the hippocampus (Ouyang et al., 2008). β2 signaling impairs retrieval by stimulating the inhibitory Gi class of G proteins, causing cAMP levels to decrease in the hippocampus (Schutsky et al., 2011). Of potential relevance, Gi signaling can also result in the activation of PLC by releasing βγ subunits that are capable of stimulating PLCβ isoforms. Therefore, we asked whether such a mechanism might apply to β2 signaling in the BLA for consolidation.
Pertussis toxin (PTx) inactivates Gi/o proteins through ADP ribosylation, uncoupling them from their receptors. Because it takes several days to observe optimal efficacy when PTx is administered in vivo, PTx was infused into the BLA 3 d before conditioning (Goh and Pennefather, 1989; Stratton et al., 1989). BLA pretreatment with PTx had no effect on consolidation when saline was infused immediately after training, suggesting that Gi/o signaling is not essential for consolidation (Fig. 8A). That outcome was expected if only one of the redundant pathways uses Gi/o. Interestingly, pretreatment with PTx impaired consolidation when SCH was infused immediately after training. This outcome suggested that PTx pretreatment might be mimicking β2 blockade. In support of this idea, pretreatment with PTx did not affect consolidation when ICI was infused immediately after training. Together, these results suggest that β2 but not D5 signaling in the BLA is mediated by Gi/o. To further test this possibility, PTx was infused into the BLA before treating with receptor agonists to enhance consolidation. PTx pretreatment blocked the enhancement of consolidation normally observed following systemic treatment with a β2 agonist, but had no effect on the ability of a D1,5 agonist to enhance consolidation (Fig. 8B).
Discussion
In summary, our experiments identify an important but redundant role in classical fear memory consolidation for adrenergic signaling by β2 receptors and dopaminergic signaling by D5 receptors (Fig. 9). It is well recognized that there are multiple stress-response mediators with distinct but overlapping temporal and mechanistic attributes (Joëls and Baram, 2009). Redundancy in systems responsible for potentially life-preserving processes such as long-term fear memory consolidation could be advantageous for survival. While we have found that NE and DA act in a redundant manner to consolidate classical fear memory, it is also possible that each has additional unique roles during (but not essential to) consolidation that may be identified in future studies.
While it has been widely hypothesized that endogenous NE/E and β-adrenergic signaling play critical roles in amygdala-dependent emotional memory consolidation, there is a paucity of evidence indicating that such signaling is uniquely required for this. In one study suggesting a unique role, various β receptor antagonists (including ICI) were infused into the BLA of rats immediately after cued fear conditioning, and impairment of cued fear was reported 1 d later (Qu et al., 2008). However, the doses of antagonist used were 100-fold higher than those found to be effective for ICI (when combined with SCH) in the current study, and are considerably higher than what should be necessary for the size difference between rats and mice. Another study suggested that β receptors contribute to the acquisition but not consolidation of cued fear memory (Bush et al., 2010). However, it is difficult to reconcile the above observations with results from mouse genetic models, which do not support a unique role for β receptors in the acquisition, consolidation, or retrieval of cued fear memory: Dbh−/−, β1 KO, β2 KO, or β1/β2 double KO mice do not exhibit cued fear deficits. Furthermore, our pharmacologic data from the current study and a previous study using mice and rats support the genetic findings (Murchison et al., 2004).
With respect to the role for DA, results from some studies suggest that D1,5 signaling might be required for fear memory acquisition or consolidation (Guarraci et al., 1999; Greba and Kokkinidis, 2000). However, doses of SCH used in those studies (systemic or intra-BLA) were 10- to 100-fold higher than those found to be effective here, potentially lacking specificity for D1,5 signaling. Relevant to this, we did not observe a fear conditioning deficit in D1 or D5 KO mice, confirming previous results (El-Ghundi et al., 1999; Holmes et al., 2001). These and our results contrast with a recent study reporting a deficit in either the acquisition or consolidation of fear-potentiated startle in D1 KO mice (Fadok et al., 2009). In that study, conditioning used 30 trials reinforced by mild footshocks (0.2 mA for 0.5 s), while the current study examined the consolidation of more intense fear resulting from a single, strongly aversive footshock (1 mA for 2 s). It is possible that the mechanisms underlying the consolidation of multiple weakly reinforced training trials are different from those for a single, strongly reinforced event.
Given that β2 and D5 receptors can couple to Gs, and that cAMP signaling is required for classical fear memory consolidation (Schafe and LeDoux, 2000), it is interesting that neither β2 nor D5 signaling in the amygdala may increase cAMP levels. DA and D1,5 agonists fail to elevate cAMP in the BLA (Leonard et al., 2003), and stimulation of β2 receptors in hippocampal slices causes a decrease in cAMP (Schutsky et al., 2011). However, our results are consistent with observations indicating that D1,5 agonists activate PLC rather than AC in the BLA, and that activation of PLC by DA is greatly diminished in D5 but not D1 KO mice (Friedman et al., 1997; Leonard et al., 2003; Sahu et al., 2009). Our results are also consistent with observations indicating that signaling by β2 receptors in the heart and hippocampus depends predominantly on Gi/o rather than Gs (Rockman et al., 2002; Schutsky et al., 2011).
Results from the current study indicate that β2 and D5 receptor signaling converge and become redundant by activating PLC. Remarkably, there is little evidence that canonical neurotransmitter signaling pathways that activate Gq/PLC are required for fear memory. Gene-targeted mice lacking expression of either metabotropic glutamate receptor mGluR1 or mGluR5, muscarinic acetylcholine receptor M1 or M3, serotonergic receptor 5-HT2a or 5-HT2c, adrenergic receptor α1d, or histaminergic receptor H1 all exhibit intact cued fear memory (Aiba et al., 1994; Lu et al., 1997; Tecott et al., 1998; Anagnostaras et al., 2003; Sadalge et al., 2003; Weisstaub et al., 2006; Dai et al., 2007; Poulin et al., 2010). Pharmacologically, there is evidence for and against mGluR5 signaling being required for cued fear memory (Nielsen et al., 1997; Rodrigues et al., 2002; Gravius et al., 2006), although stimulating mGluR1/5 receptors can enhance fear memory (Rudy and Matus-Amat, 2009). For NE/E, antagonist treatment suggests that α1-adrenergic signaling is not required for fear memory (Lazzaro et al., 2010). On the other hand, mice with a targeted disruption of the gene for PLC-β1 exhibit greatly reduced contextual fear, although this could be due to a deficit in hippocampus-dependent memory rather than BLA-dependent fear memory per se (McOmish et al., 2008a,b).
Stimulation of PLC is likely to activate Ca2+- and diacylglycerol-dependent signaling such as that mediated by protein kinase C (PKC) and calmodulin-dependent kinases (CaMKs). Genetic and pharmacologic data support a role for these kinases in fear memory. Genetic disruption of the PKCβ gene or the CaMKIV gene results in impaired cued and contextual fear (Weeber et al., 2000; Wei et al., 2002), and inhibitors of PKC infused into the BLA shortly after conditioning impairs consolidation of instrumental fear (Bonini et al., 2005). In addition to the generation of IP3, it will be valuable in future studies to identify the signaling events that are altered when β2 and D5 receptors are antagonized. It will also be valuable to determine in what cell type(s) these receptors act to promote consolidation, given that the potential expression and physiological effects of these receptors in the BLA are broad and diverse (Ciliax et al., 2000; Qu et al., 2008; Farb et al., 2010).
In humans, some studies indicate that blocking β receptors eliminates enhanced memory for emotionally arousing items, although other studies have not corroborated these findings (Cahill et al., 1994; O'Carroll et al., 1999). If β blockers have this effect, it would suggest a lack of redundancy under these conditions. This could be due to differences in arousal systems engaged by viewing aversive material versus experiencing a potentially life-threatening event, such as may occur with fear conditioning or events that can lead to the development of PTSD. Results from recent clinical trials suggest that β blockers are of limited efficacy in the prevention of PTSD (Stein et al., 2007; McGhee et al., 2009; Nugent et al., 2010). Our results suggest that combined D5/β2 blockade might be more efficacious.
Footnotes
This work was supported by Department of Defense GrantPT075099 and NIH Grant5R01MH063352 to S.A.T. We thank Dainippon Sumitomo Pharma (Osaka, Japan) for their generous gift of l-threo-3,4-dihyroxyphenylserine used to rescue Dbh−/− mice prenatally, and B. Kobilka (Stanford University), M. Xu (University of Chicago), and D. Sibley (National Institutes of Health) for providing stock for the β receptor and DA receptor KO mouse lines.
The authors declare no conflict of interest.
References
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a compound that directly stimulates phospholipase C activity. Mol Pharmacol. 2003;63:1043–1050. doi: 10.1124/mol.63.5.1043. [DOI] [PubMed] [Google Scholar]
- Bonini JS, Cammarota M, Kerr DS, Bevilaqua LR, Izquierdo I. Inhibition of PKC in basolateral amygdala and posterior parietal cortex impairs consolidation of inhibitory avoidance memory. Pharmacol Biochem Behav. 2005;80:63–67. doi: 10.1016/j.pbb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Bush DE, Caparosa EM, Gekker A, LeDoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Chipkin RE, Iorio LC, Coffin VL, McQuade RD, Berger JG, Barnett A. Pharmacological profile of SCH39166: a dopamine D1 selective benzonaphthazepine with potential antipsychotic activity. J Pharmacol Exp Ther. 1988;247:1093–1102. [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse. 2000;37:125–145. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A, Sakurai E, Kato M, Okamura N, Kuramasu A, Yanai K. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res. 2007;57:306–313. doi: 10.1016/j.neures.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Bittiger H, Reymann NC. CGP 20712 A: a useful tool for quantitating beta 1- and beta 2- adrenoceptors. Eur J Pharmacol. 1986;130:137–139. doi: 10.1016/0014-2999(86)90193-7. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O'Dowd BF, George SR. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb CR, Chang W, LeDoux JE. Ultrastructural characterization of noradrenergic axons and Beta-adrenergic receptors in the lateral nucleus of the amygdala. Front Behav Neurosci. 2010;4:162. doi: 10.3389/fnbeh.2010.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic; 1997. [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: evidence for a specific neurochemical system in the amygdala. Science. 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Goh JW, Pennefather PS. A pertussis toxin-sensitive G protein in hippocampal long-term potentiation. Science. 1989;244:980–983. doi: 10.1126/science.2543072. [DOI] [PubMed] [Google Scholar]
- Gravius A, Barberi C, Schäfer D, Schmidt WJ, Danysz W. The role of group I metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats - a comparison. Neuropharmacology. 2006;51:1146–1155. doi: 10.1016/j.neuropharm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Greba Q, Kokkinidis L. Peripheral and intraamygdalar administration of the dopamine D1 receptor antagonist SCH 23390 blocks fear-potentiated startle but not shock reactivity or the shock sensitization of acoustic startle. Behav Neurosci. 2000;114:262–272. doi: 10.1037//0735-7044.114.2.262. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- Hicks PE, Cavero I, Manoury P, Lefevre-Borg F, Langer SZ. Comparative analysis of beta-1 adrenoceptor agonist and antagonist potency and selectivity of cicloprolol, xamoterol and pindolol. J Pharmacol Exp Ther. 1987;242:1025–1034. [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- Izquierdo I, Dias RD. The influence of adrenergic receptor antagonists on the amnestic and antiamnestic actions of adrenaline and tyramine. Psychopharmacology. 1983;80:181–183. doi: 10.1007/BF00427966. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiper M, Stope MB, Szatkowski D, Böhm A, Tysack K, Vom Dorp F, Saur O, Oude Weernink PA, Evellin S, Jakobs KH, Schmidt M. Epac- and Ca2+ -controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J Biol Chem. 2004;279:46497–46508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- Lazzaro SC, Hou M, Cunha C, LeDoux JE, Cain CK. Antagonism of lateral amygdala alpha1-adrenergic receptors facilitates fear conditioning and long-term potentiation. Learn Mem. 2010;17:489–493. doi: 10.1101/lm.1918210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SK, Anderson CM, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. Amygdaloid D1 receptors are not linked to stimulation of adenylate cyclase. Synapse. 2003;50:320–333. doi: 10.1002/syn.10272. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGhee LL, Maani CV, Garza TH, Desocio PA, Gaylord KM, Black IH. The effect of propranolol on posttraumatic stress disorder in burned service members. J Burn Care Res. 2009;30:92–97. doi: 10.1097/BCR.0b013e3181921f51. [DOI] [PubMed] [Google Scholar]
- McOmish CE, Burrows EL, Howard M, Hannan AJ. PLC-beta1 knockout mice as a model of disrupted cortical development and plasticity: behavioral endophenotypes and dysregulation of RGS4 gene expression. Hippocampus. 2008a;18:824–834. doi: 10.1002/hipo.20443. [DOI] [PubMed] [Google Scholar]
- McOmish CE, Burrows E, Howard M, Scarr E, Kim D, Shin HS, Dean B, van den Buuse M, Hannan AJ. Phospholipase C-beta1 knockout mice exhibit endophenotypes modeling schizophrenia which are rescued by environmental enrichment and clozapine administration. Mol Psychiatry. 2008b;13:661–672. doi: 10.1038/sj.mp.4002046. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear- potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nielsen KS, Macphail EM, Riedel G. Class I mGlu receptor antagonist 1-aminoindan-1,5-dicarboxylic acid blocks contextual but not cue conditioning in rats. Eur J Pharmacol. 1997;326:105–108. doi: 10.1016/s0014-2999(97)85402-7. [DOI] [PubMed] [Google Scholar]
- Nugent NR, Christopher NC, Crow JP, Browne L, Ostrowski S, Delahanty DL. The efficacy of early propranolol administration at reducing PTSD symptoms in pediatric injury patients: a pilot study. J Trauma Stress. 2010;23:282–287. doi: 10.1002/jts.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Memory for emotional material: a comparison of central versus peripheral beta blockade. J Psychopharmacol. 1999;13:32–39. doi: 10.1177/026988119901300104. [DOI] [PubMed] [Google Scholar]
- O'Donnell SR, Wanstall JC. Evidence that ICI 118, 551 is a potent, highly Beta 2-selective adrenoceptor antagonist and can be used to characterize Beta-adrenoceptor populations in tissues. Life Sci. 1980;27:671–677. doi: 10.1016/0024-3205(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci. 2008;105:11993–11997. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin B, Butcher A, McWilliams P, Bourgognon JM, Pawlak R, Kong KC, Bottrill A, Mistry S, Wess J, Rosethorne EM, Charlton SJ, Tobin AB. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc Natl Acad Sci. 2010;107:9440–9445. doi: 10.1073/pnas.0914801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G, Seewald MJ, Gratas C, Melder D, Riebow J, Modest EJ. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res. 1992;52:2835–2840. [PubMed] [Google Scholar]
- Qu LL, Guo NN, Li BM. Beta1- and beta2-adrenoceptors in basolateral nucleus of amygdala and their roles in consolidation of fear memory in rats. Hippocampus. 2008;18:1131–1139. doi: 10.1002/hipo.20478. [DOI] [PubMed] [Google Scholar]
- Ramos M, Goñi-Allo B, Aguirre N. Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5-HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology. 2005;30:2180–2191. doi: 10.1038/sj.npp.1300735. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. DHPG activation of group 1 mGluRs in BLA enhances fear conditioning. Learn Mem. 2009;16:421–425. doi: 10.1101/lm.1444909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadalge A, Coughlin L, Fu H, Wang B, Valladares O, Valentino R, Blendy JA. alpha 1d Adrenoceptor signaling is required for stimulus induced locomotor activity. Mol Psychiatry. 2003;8:664–672. doi: 10.1038/sj.mp.4001351. [DOI] [PubMed] [Google Scholar]
- Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75:447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. Stress and glucocorticoids impair memory retrieval via {beta}2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci. 2011;31:14172–14181. doi: 10.1523/JNEUROSCI.2122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20:923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Cole AJ, Pritchett J, Eccles CU, Worley PF, Baraban JM. Intrahippocampal injection of pertussis toxin blocks adenosine suppression of synaptic responses. Brain Res. 1989;494:359–364. doi: 10.1016/0006-8993(89)90604-5. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Logue SF, Wehner JM, Kauer JA. Perturbed dentate gyrus function in serotonin 5-HT2C receptor mutant mice. Proc Natl Acad Sci. 1998;95:15026–15031. doi: 10.1073/pnas.95.25.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Disruption of the dopamine beta-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav Neurosci. 1997;111:579–589. doi: 10.1037//0735-7044.111.3.579. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem. 1994;62:2045–2048. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M, Taton G, Delhaye M, Chatelain P, Camus JC, Pochet R, Leclerc JL, De Smet JM, Robberecht P, Christophe J. The human heart beta-adrenergic receptors. II. Coupling of beta 2-adrenergic receptors with the adenylate cyclase system. Mol Pharmacol. 1983;24:174–182. [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002;5:573–579. doi: 10.1038/nn0602-855. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]