Abstract
Rationale
Recent clinical and preclinical studies have demonstrated that systemic lupus erythematosus (SLE) is associated with an increased risk for cardiovascular disease (CVD). However, unlike in the general population, little is known regarding the efficacy of atheroprotective interventions in patients with SLE. The current study aims to determine the benefit of lymphocyte inhibition on reducing the atherosclerotic burden in SLE-susceptible LDLr-deficient mice.
Methods
Female LDLr−/− mice were lethally irradiated and reconstituted with bone marrow from C57Bl/6 mice (LDLr.B6) or the SLE-susceptible B6.Sle1.2.3 mice (LDLr. Sle). At 16 weeks post transplant, mice were treated with atorvastatin (10 mg/kg), mycophenolate mofetil (MMF; 40 mg/kg), or both (MMF-A) for 8 weeks, after which the extent of atherosclerosis and the presence of SLE were assessed.
Results
Following 8 weeks of treatment, we observed that atorvastatin-mediated reduction in cholesterol levels attenuated atherogenesis in LDLr.B6 mice but failed to significantly reduce atherosclerotic lesion size in LDLr. Sle mice, in spite of a significant reduction in serum cholesterol levels. Treatment with MMF and MMF-A attenuated atherogenesis in LDLr.B6 and LDLr.Sle mice. In addition, MMF-containing regimens inhibited recruitment of CD4+ T cells to atherosclerotic lesions in LDLr.Sle mice. In these mice, MMF also reduced the proportion of activated splenic T cells, as well as interleukin 10 secretion by T cells. With regard to lupus activity, MMF had no overt effect on anti-double-stranded DNA (dsDNA) antibody titres or kidney function and pathology.
Conclusions
The current study demonstrates that reduction of cholesterol levels alone is not atheroprotective in lupus-mediated atherogenesis. This is the first study to demonstrate that MMF reduces the atherosclerotic burden in a model of lupus-accelerated atherosclerosis. Our results suggest that MMF treatment may prove beneficial in preventing CVD in patients with SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease characterised by the dysfunction of T cells, B cells and dendritic cells as well as the production of anti-nuclear autoantibodies.1, 2 SLE is associated with a myriad of comorbidities including renal failure, haemolytic anaemia and neurological disorders. A potentially life-threatening complication of SLE is the increased risk of cardiovascular disease (CVD). The propensity towards increased CV risk cannot be attributed to traditional CV risk factors. Hence the increased CVD risk in SLE is thought to be caused by interactions between classical risk factors, immune cell activation and inflammation.3–6
One of the most successful clinical therapies for atherosclerosis is statin-mediated lowering of the atherogenic low-density lipoprotein cholesterol (LDL). A recent placebo-controlled trial in 200 patients with SLE, however, indicated atorvastatin did not reduce progression of carotid intima–media thickness, a surrogate marker for atherosclerosis.7 Paradoxically, a post hoc analysis indicated significantly more patients with SLE benefitted from atorvastatin than from placebo. These conflicting findings suggest more research is needed regarding the efficacy of statin treatment in patients with SLE and that studies of other immunomodulation treatments for CVD in SLE are warranted.
Mycophenolate mofetil (MMF) is an inhibitor of the enzyme inosine monophosphate dehydroxygenase (IMPDH) and has a strong cytostatic effect on lymphocytes by interfering with DNA synthesis.8 MMF has emerged as an efficacious agent in patients with lupus nephritis but has also been shown to exert atheroprotective effects in patients without lupus recovering from cardiac transplantation or undergoing carotid endarterectomy.9, 10 However, it is not known whether MMF has beneficial effects on lupus-mediated atherogenesis. To examine the effects of MMF on SLE-associated atherosclerosis we examined treatment in the LDLr.Sle bone marrow chimaeric mice. These mice lack the LDL receptor and develop appreciable lesions in the aortic root by 8 weeks feeding high fat diet. LDLr−/− mice reconstituted with B6.Sle1.2.3 bone marrow, a model of spontaneous SLE, have increased atherosclerotic lesion burden at this timepoint without increased SLE-associated mortality.11 The aim of the current preclinical study was to establish the antiatherosclerotic potential of MMF in SLE-prone LDLr−/− mice.
Here we show that MMF, but not atorvastatin, reduces the atherosclerotic burden in lupus-susceptible LDLr.Sle mice. The reduction in atherosclerosis in MMF-treated mice was accompanied by a reduction in CD4+ T cell infiltration to the atherosclerotic lesion, a decrease in activated CD4+ T cells and decreased interleukin 10 (IL-10) secretion by T cells. Our results provide strong evidence to suggest that MMF-based therapies may decrease CVD in patients with SLE.
MATERIALS AND METHODS
Mice
C57BL/6J and B6.129S7-Ldlrtm1her/J mice (hereafter referred to as B6 and LDLr−/−, respectively) were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA) and maintained in the Vanderbilt University animal care facility. Lupus-susceptible B6.Sle1.2.3 congenic mice were originally obtained from Edward K Wakeland (UTSW, Dallas, Texas, USA) and have been described previously.12 All mice were given food and water ad libitum and all procedures received Vanderbilt University Medical Center’s Institutional Animal Care and Use Committee approval.
Atherosclerosis studies
Lethally irradiated female LDLr−/− mice were transplanted with bone marrow from either lupus-susceptible B6.Sle1.2.3 (LDLr. Sle) or C57BL/6 (LDLr.B6) mice as described previously.11 At 16 weeks after transplantation, 4 groups of 15 LDLr.B6 mice and 4 groups of 15 LDLr.Sle mice were placed on a Western diet (20% milk fat, 0.15% cholesterol) containing either no treatment (control), MMF (40 mg/kg/day), atorvastatin (10 mg/kg/day) or MMF and atorvastatin (MMF-A; 40 and 10 mg/kg/day) for 8 weeks. At the end of these 8 weeks, animals were killed and analysed for the extent of atherosclerosis and the presence of SLE.
Serum lipoprotein analysis
Following a 4 h fast, circulating levels of total cholesterol and triglycerides were measured using a colourimetric assay as described previously.11 Lipoprotein distribution was determined by using fast protein liquid chromatography (FPLC).
Histopathology and immunohistochemistry
Atherosclerotic lesion burden was quantified in the proximal aorta by Oil-red-O staining. Staining for macrophages (monocyte and macrophage antibody 2 (MOMA-2)) and CD4+ T cells was performed as described previously.11 Cells were visualised and staining quantified using Image-Pro Plus software (Media Cybernetics, Bethesda, Maryland, USA). For immunoglobulin deposition, frozen kidney sections (7 μm) were stained by incubating with a biotinylated anti-mouse heavy and light chain antibody (Southern Biotech, Birmingham, Alabama, USA) at 37°C for 30 min, followed by incubation with Streptavidin-Texas Red (Vector Laboratories, Burlingame, California, USA) for another 30 min. Gross kidney pathology was assessed by Jones staining.
Autoantibodies
Serum titres of double-stranded DNA (dsDNA) were measured according to the method of Shivakumar et al. and anti-oxidised LDL (oxLDL) antibodies were measured as described previously.11, 13
Kidney function
Functional analysis of kidneys was conducted by measuring serum creatinine as described previously.11
In vitro T cell stimulation
Splenocytes were stimulated with phorbol myristate acetate (PMA) (20 ng/ml) and ionomycin (2 μg/ml) and cultured in the presence of Golgi plug/stop for 5 h and stained for surface markers, followed by membrane permeabilisation and staining for intracellular cytokine. For cytokine analysis, splenocytes were cultured in CD3/CD28-coated plates and supernatant was collected after 24 h stimulation. Cytokine secretion was measured using a Milliplex MAP Mouse Cytokine custom kit from Millipore (Billerica, Massachusetts, USA).
Flow cytometry
Spleens were processed and stained with appropriate antibodies for 20 min at 4° C. Cells were then washed, fixed and analysed using a MACS Quant flow cytometer (Militenyi Biotec, Auburn, California, USA). Flow data was analysed using FCS Express (De Novo Software, Los Angeles, California, USA). Cells were excluded based on the forward and side scatter profile. The following antibodies were used: anti-mouse TCRβ-V450, CD4-PE/APC, CD8-APC.Cy7/PE.Cy5.5, CD44-FITC, CD69-FITC, interferon γ (IFNγ) and IL-10-PE and were obtained from BD Biosciences (San Jose, California, USA).
Statistical analysis
Statistical analyses were conducted using PRISM V.5.0 software (GraphPad Software, La Jolla, California, USA). For comparisons between two groups, an unpaired Student t test was used if the variance was normally distributed and a Mann–Whitney U test was used for comparisons with a variance that was not normally distributed. For atherosclerosis studies, values 2 SD above the mean were excluded from the analysis. For Milliplex analysis, values less than 0.5 the mean were considered non-responders. Comparisons made between three or more groups were performed using one-way analysis of variance (ANOVA). Values are expressed as mean±SEM unless otherwise specified. A p value of <0.05 was considered significant.
RESULTS
Effect of atorvastatin and MMF on atherosclerotic lesion size in lupus-susceptible mice
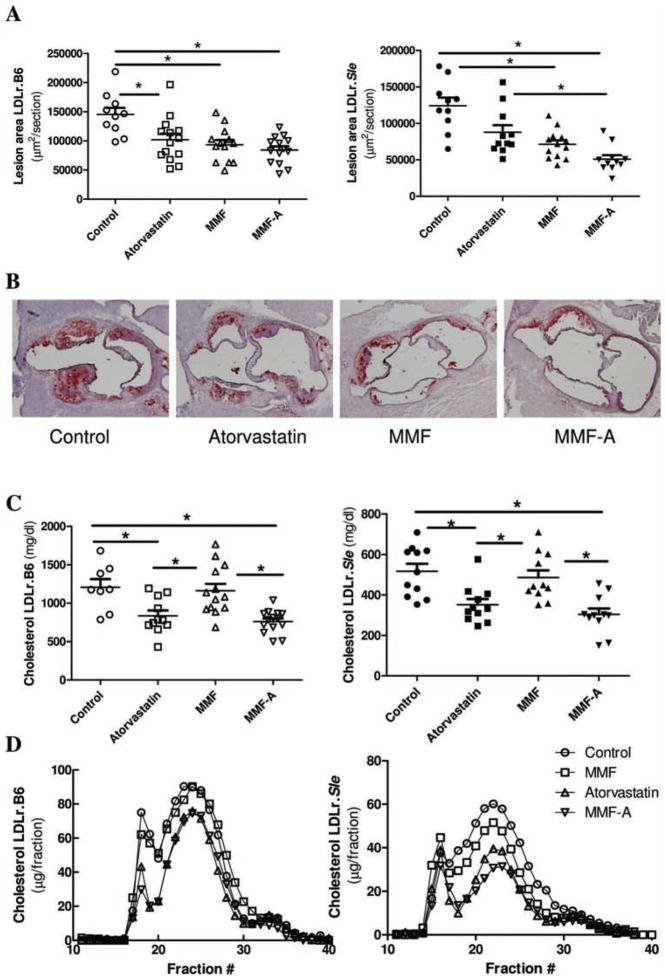
To determine the effects of lymphocyte inhibition and statin treatment on atherosclerosis in lupus, female LDLr.Sle and LDLr. B6 mice were treated with MMF, atorvastatin or a combination of both and placed on a Western-type diet for 8 weeks. Evaluation of the effect of statin and immunosupressive treatment on lesion area by means of Oil-red-O staining in LDLr.B6 mice demonstrated that treatment with atorvastatin, MMF and MMF-A diminished atherosclerotic lesion area by 29.8%, 35.7% and 41.9%, respectively (figure 1A, left panel and B). There was no statistical difference between the atorvastatin, MMF or MMF-A treatment groups, indicating that combination treatment did not provide additional benefit against atherogenesis in LDLr.B6 mice. In contrast, atorvastatin monotherapy in LDLr.Sle mice, although showing a trend toward lesion reduction, did not significantly diminish lesion area compared to control mice (figure 1A, right panel and B). MMF and MMF-A, however, significantly attenuated atherosclerotic lesion area by 42.5% and 59.0%, respectively, when compared to untreated mice. Moreover, combination treatment (MMF-A) reduced the atherosclerotic burden in LDLr.Sle mice even when compared to atorvastatin-treated mice.
Figure 1.
Effects of atorvastatin and mycophenolate mofetil (MMF) on atherosclerotic burden in LDLr.B6 and LDLr.Sle mice. A. Quantitative analysis of atherosclerotic lesion area in LDLr.B6 (left panel) and LDLr.Sle (right panel) mice. B. Representative sections of LDLr.Sle mice, stained with Oil-red-O. C. Serum cholesterol of LDLr.B6 (left panel) and LDLr.Sle (right panel). D. FPLC analysis of cholesterol lipoprotein distribution in serum of LDLr.B6 (left panel) and LDLr.Sle (right panel). Means±SEM are represented. *p<0.05 by one-way analysis of variance (ANOVA).
LDLr.B6 and LDLr.Sle mice both responded to atorvastatin-containing therapies by demonstrating reduced serum cholesterol (figure 1C). Therefore, despite significantly reduced cholesterol in LDLr.Sle mice, atorvastatin failed to significantly reduce atherosclerosis. Circulating cholesterol in both populations was not altered by MMF monotherapy. FPLC analysis revealed that the reduction in total cholesterol is associated with decreases in the LDL and very-low-density lipoprotein (VLDL) fractions (figure 1D). The ability to lower LDL and VLDL was dependant on atorvastatin treatment in LDLr.B6 and LDLr.Sle mice. These data suggest that, unlike SLE-resistant LDLr.B6 mice, and similar to preliminary studies in humans, lupus-mediated atherogenesis does not necessarily benefit from LDL-lowering treatment alone.
Effect of atorvastatin and MMF on atherosclerotic lesion composition
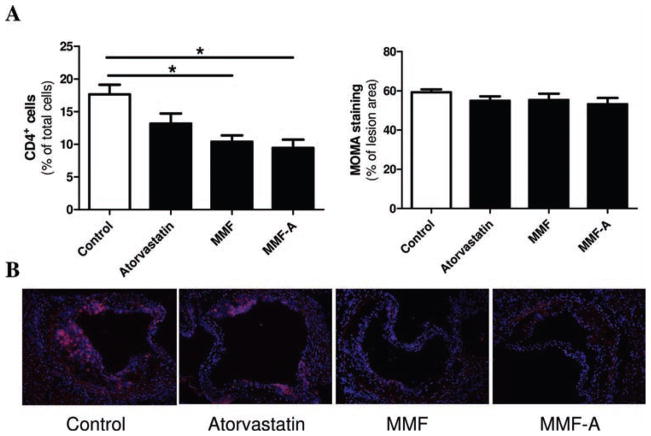
Considering MMF and atorvastatin exerted differential effects on the atherosclerotic process in SLE-susceptible animals, we decided to focus on LDLr.Sle mice specifically. To determine whether treatment modifies the immune cell composition of the plaque, immunohistochemical analysis specific for CD4+ T cells and macrophages was performed. This analysis revealed that treatment with either MMF monotherapy or MMF in combination with atorvastatin (MMF-A) lowered the number of CD4+ T cells in the lesions of LDLr.Sle mice by 41.2% and 46.3%, respectively (figure 2A, left panel and B). There was no significant difference in lesion macrophage content between any of the different treatments (figure 2A, right panel). Because atorvastatin alone did not significantly reduce atherosclerosis or affect the number of CD4+ T cells infiltrating the lesion in lupus-susceptible mice, we further investigated the effects of MMF treatment on the immune compartment of treated mice.
Figure 2.
Effects of atorvastatin and mycophenolate mofetil (MMF) on atherosclerotic plaque phenotype in LDLr.Sle mice. A. Quantitative analysis of aortic roots of LDLr.Sle mice stained with CD4 (left panel, n=7 per group) and monocyte and macrophage antibody 2 (MOMA-2) (right panel, n=6 per group). B. Representative sections of lesions of LDLr.Sle mice stained with CD4. Means±SEM are represented. *p<0.05 by one-way analysis of variance (ANOVA).
Effect of MMF on lupus activity
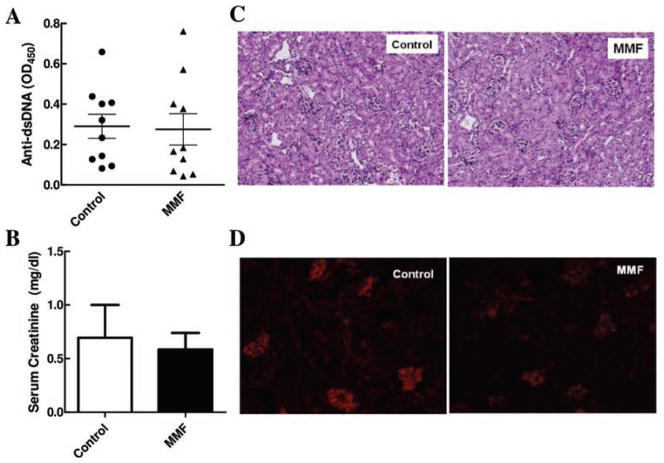
To determine whether MMF-mediated effects on atherogenesis are secondary to altered SLE disease, several parameters of lupus activity were analysed. MMF did not reduce anti-dsDNA antibody titres in LDLr.Sle mice (figure 3A). No significant differences in serum creatinine levels between the control or treatment group were observed (figure 3B). In addition, MMF treatment did not result in changes in kidney pathology or deposition of immunoglobulin complexes (figure 3C,D). These results suggest that, at the studied time point, treatment with MMF does not significantly affect kidney function in LDLr.Sle, and that changes in atherosclerosis are not an indirect effect of improved renal disease.
Figure 3.
Effects of mycophenolate mofetil (MMF) on lupus activity in LDLr.Sle mice. A. Serum titres of anti-double-stranded DNA (dsDNA) antibodies (n=≥6 mice per group). B. Serum creatinine levels (n=8 mice per group). C. Representative Jones stained sections of kidneys of LDLr.Sle mice. D. Representative frozen kidney sections stained for Ig deposition. Means±SEM are represented. *p<0.05 by t test.
Effect of MMF on peripheral immunity
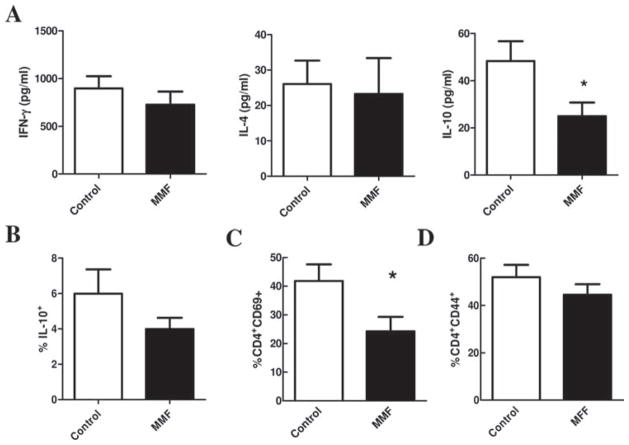
In order to evaluate the effects of MMF treatment on peripheral T cell responses in LDLr.Sle mice, splenocytes from control and MMF-treated mice were stimulated with plate bound CD3 and CD28 and cytokine secretion was measured in the supernatants by Milliplex assay. As shown in figure 4A, MMF treatment of LDLr.Sle mice did not affect IFNγ or IL-4 secretion. In contrast, MMF treatment significantly reduced the ability of T cells to secrete IL-10. Consistent with this, intracellular staining for IL-10 of T cells upon PMA/ionomycin stimulation revealed a trend toward decreased IL-10 producing cells in the CD4+ T cell population (figure 4B). We also assessed whether treatment of lupus-susceptible mice with MMF reduced activation markers on splenic CD4+ T cells. MMF treatment of LDLr.Sle mice significantly reduced the proportion of CD4+ T cells expressing the early activation marker CD69 (figure 4C), but did not have an effect on the CD44-expressing CD4+ T cell population (figure 4D).
Figure 4.
Effects of mycophenolate mofetil (MMF) on immunity in the periphery in LDLr.Sle mice. A. Levels of interferon (IFN)γ (left), interleukin (IL)-4 (middle) and IL-10 by splenocytes stimulated with CD3 and CD28. B. Proportion of IL-10-producing CD4+ T cells in the spleen of control and MMF-treated mice. Proportion of (C) CD69+ and (D) CD44+ T cells in the spleen of control and MMF-treated mice. Means±SEM are represented. *p<0.05 by t test.
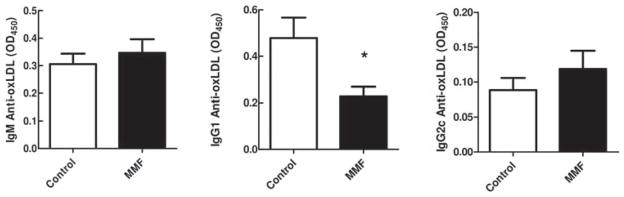
Antibody responses to atherogenic stimuli in MMF-treated mice
Antibody responses to oxLDL are a hallmark of atherosclerotic disease in the context of hyperlipidaemia. The contribution of this antibody response to the process of atherogenesis is unclear. Also, it is not known whether MMF affects the production of oxLDL antibodies. To determine whether MMF treatment of LDLr.Sle mice affects antibody responses to modified lipoprotein, we measured serum antibody titres to oxLDL by ELISA. As shown in figure 5, MMF did not influence the immunoglobulin (Ig) M (left panel) or IgG2c response (right panel) to oxLDL but reduced IgG1 titres (middle panel), suggesting that MMF downmodulates the IgG-associated T helper 2 (Th2) response to modified lipoprotein in hyperlipidaemic SLE mice, while the more natural IgM response is intact.
Figure 5.
Effect of atorvastatin and mycophenolate mofetil (MMF) on oxidised low-density lipoprotein (oxLDL) antibodies in LDLr.Sle mice. Serum titres of IgM-oxLDL (left panel), IgG1-oxLDL (middle panel) and IgG2a/c-oxLDL (right panel). Means±SEM are represented. *p<0.05 by t test.
DISCUSSION
In the current study we show that atorvastatin monotherapy inhibits atherogenesis in LDLr.B6 mice but not in the context of lupus. Despite significantly lowering circulating levels of cholesterol (primarily reductions of the atherogenic lipoproteins VLDL and LDL), atorvastatin failed to significantly reduce lesion area in LDLr.Sle mice. In contrast, MMF provided robust protection against atherogenesis in LDLr.B6 as well as LDLr.Sle mice. These data indicate that the aetiology of atherosclerosis in the context of SLE may be distinct from general atherosclerosis where lipid lowering appears sufficient to significantly decrease disease progression.
The finding that MMF-based treatment, which represents a more specific immunomodulatory intervention, was in fact able to reduce atherogenesis in LDLr.Sle mice, suggests that lymphocyte-mediated perturbations of the immune system play a relatively large role in lupus-mediated atherogenesis than the classical risk factor of increased LDL. In support of this hypothesis, we found that MMF treatment reduced the activated CD4+ CD69+ T cell population, as well as CD4+ T cell infiltration to the lesion. Interestingly, MMF-mediated downregulation of the cytokine response was limited to a reduction in IL-10 secretion by T cells. Dysregulation of IL-10 responses are a prominent feature of SLE and polymorphisms in the IL-10 gene correlate with susceptibility to SLE in human populations.14–18 Moreover, increased levels of IL-10 in SLE correlate with disease activity in humans.19–21 Consistent with this correlation, therapies that inhibit secretion of IL-10 in lupus-susceptible mice result in decreased SLE-associated pathologies.22, 23 In this study, however, MMF-mediated decreases in IL-10 did not appear to affect anti-dsDNA titres or kidney pathology. It is possible that this is due to the fact that the time point studied is not associated with overt renal disease, or that IL-10 secretion by other cellular subsets not affected by MMF treatment is more important in modulating autoantibody production and kidney pathology in SLE.24 While IL-10 has been shown to be atheroprotective in mouse models of hyperlipidaemia, the specific contribution of IL-10-secreting CD4+ T cells to the development of atherosclerosis in the context of SLE remains to be determined.25–28
In addition to MMF having effects on T cells, we observed a significant decrease in B cell production of IgG1 specific for the prototypic atherosclerosis antigen, oxLDL. At the same time, anti-oxLDL IgM levels remained unchanged by MMF treatment, leading to an increase in the oxLDL IgM:IgG ratio. Recent studies have suggested that B cells may be pathogenic for atherosclerosis via their production of T cell-dependent IgG.29, 30 Moreover, Kyaw, et al. demonstrated that while IgG may be atherogenic, naturally secreted IgM against oxLDL may be atheroprotective.30 Interestingly, a recent study demonstrated that patients with lupus have significantly higher atherosclerotic plaque burden compared to controls with lower anti-phosphorylcholine (anti-PC) IgM and higher anti-PC IgG titres.31 As it has been demonstrated that modified PC is the target protein antigen for oxLDL, these data demonstrate an inverse correlation between IgM and atherosclerosis. Therefore, as supported by our current study, one could hypothesise that a skew toward a more natural IgM response to oxLDL in MMF-treated animals might contribute to reduced atherosclerosis. It remains to be seen whether this is true in patients with lupus.
It is important to distinguish whether the beneficial actions of MMF are a result of a direct protective effect on the atherosclerotic process or secondary to diminished lupus disease activity. We observed that MMF treatment did not affect parameters associated with autoimmunity in SLE, as assessed by serum titres of anti-dsDNA antibodies, serum creatinine levels, kidney pathology and immunoglobulin deposition. This indicates that either these treatments do not affect SLE-associated kidney disease in the context of hyperlipidaemia, or that kidney disease in the control and experimental group was at an early stage. Indeed, one important consideration is that our model was designed to examine the effects of SLE on atherosclerosis before overt kidney disease is present. This may be one explanation for the lack of treatment effect on immunoglobulin deposition and gross kidney pathology. Because the control and MMF-treated group had a similar kidney phenotype, we can conclude that MMF-mediated reduction in atherosclerosis is due to modulation of the immune response and not dependent on renal function.
Altogether, our results demonstrate that MMF treatment, but not atorvastatin, reduces the atherosclerosis burden in SLE mice. Our data suggests that MMF slows down the progression of atherosclerosis by inhibiting CD4+ T cell activation and infiltration to the atherosclerotic lesion. This T cell phenotype was accompanied by a reduction in IgG1 serum titres to oxLDL. Overall, these results provide strong evidence to suggest that treatment of patients with SLE with MMF alone or in combination with statin treatment may prove beneficial in the prevention of atherosclerotic vascular disease.
Acknowledgments
Funding This work was supported by a fellowship from the International Atherosclerosis Society (SIL), American Heart Association Scientist Development Award (YVMF) and NIH grant R01HL088364 and R01HL089310 (to ASM).
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Perl A. Pathogenic mechanisms in systemic lupus erythematosus. Autoimmunity. 2010;43:1–6. doi: 10.3109/08916930903374741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.McMahon M, Hahn BH. Atherosclerosis and systemic lupus erythematosus: mechanistic basis of the association. Curr Opin Immunol. 2007;19:633–9. doi: 10.1016/j.coi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Leuven SI, Franssen R, Kastelein JJ, et al. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford) 2008;47:3–7. doi: 10.1093/rheumatology/kem202. [DOI] [PubMed] [Google Scholar]
- 5.Sherer Y, Zinger H, Shoenfeld Y. Atherosclerosis in systemic lupus erythematosus. Autoimmunity. 2010;43:98–102. doi: 10.3109/08916930903374527. [DOI] [PubMed] [Google Scholar]
- 6.Sarzi-Puttini P, Atzeni F, Gerli R, et al. Cardiac involvement in systemic rheumatic diseases: An update. Autoimmun Rev. 2010;9:849–52. doi: 10.1016/j.autrev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Petri MA, Kiani AN, Post W, et al. Lupus Atherosclerosis Prevention Study (LAPS) Ann Rheum Dis. 2011;70:760–5. doi: 10.1136/ard.2010.136762. [DOI] [PubMed] [Google Scholar]
- 8.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 9.Gibson WT, Hayden MR. Mycophenolate mofetil and atherosclerosis: results of animal and human studies. Ann N Y Acad Sci. 2007;1110:209–21. doi: 10.1196/annals.1423.023. [DOI] [PubMed] [Google Scholar]
- 10.van Leuven SI, van Wijk DF, Volger OL, et al. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis. 2010;211:231–6. doi: 10.1016/j.atherosclerosis.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Stanic AK, Stein CM, Morgan AC, et al. Immune dysregulation accelerates atherosclerosis and modulates plaque composition in systemic lupus erythematosus. Proc Natl Acad Sci USA. 2006;103:7018–23. doi: 10.1073/pnas.0602311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel L, Rudofsky UH, Longmate JA, et al. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–29. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 13.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143:103–12. [PubMed] [Google Scholar]
- 14.Chong WP, Ip WK, Wong WH, et al. Association of interleukin-10 promoter polymorphisms with systemic lupus erythematosus. Genes Immun. 2004;5:484–92. doi: 10.1038/sj.gene.6364119. [DOI] [PubMed] [Google Scholar]
- 15.Eskdale J, Wordsworth P, Bowman S, et al. Association between polymorphisms at the human IL-10 locus and systemic lupus erythematosus. Tissue Antigens. 1997;49:635–9. doi: 10.1111/j.1399-0039.1997.tb02812.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin YJ, Wan L, Huang CM, et al. IL-10 and TNF-alpha promoter polymorphisms in susceptibility to systemic lupus erythematosus in Taiwan. Clin Exp Rheumatol. 2010;28:318–24. [PubMed] [Google Scholar]
- 17.Rosado S, Rua-Figueroa I, Vargas JA, et al. Interleukin-10 promoter polymorphisms in patients with systemic lupus erythematosus from the Canary Islands. Int J Immunogenet. 2008;35:235–42. doi: 10.1111/j.1744-313X.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 18.Sobkowiak A, Lianeri M, Wudarski M, et al. Genetic variation in the interleukin-10 gene promoter in Polish patients with systemic lupus erythematosus. Rheumatol Int. 2009;29:921–5. doi: 10.1007/s00296-008-0776-4. [DOI] [PubMed] [Google Scholar]
- 19.Chun HY, Chung JW, Kim HA, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27:461–6. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara E, Gourley MF, Lee S, et al. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379–85. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 21.Park YB, Lee SK, Kim DS, et al. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–8. [PubMed] [Google Scholar]
- 22.Ishida H, Muchamuel T, Sakaguchi S, et al. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305–10. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalechman Y, Gafter U, Da JP, et al. Delay in the onset of systemic lupus erythematosus following treatment with the immunomodulator AS101: association with IL-10 inhibition and increase in TNF-alpha levels. J Immunol. 1997;159:2658–67. [PubMed] [Google Scholar]
- 24.Blenman KR, Duan B, Xu Z, et al. IL-10 regulation of lupus in the NZM2410 murine model. Lab Invest. 2006;86:1136–48. doi: 10.1038/labinvest.3700468. [DOI] [PubMed] [Google Scholar]
- 25.Caligiuri G, Rudling M, Ollivier V, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 26.Pinderski LJ, Fischbein MP, Subbanagounder G, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–71. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 27.Pinderski Oslund LJ, Hedrick CC, Olvera T, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–53. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 28.Potteaux S, Esposito B, van Oostrom O, et al. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–8. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 29.Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–87. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyaw T, Tay C, Khan A, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–19. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 31.Anania C, Gustafsson T, Hua X, et al. Increased prevalence of vulnerable atherosclerotic plaques and low levels of natural IgM antibodies against phosphorylcholine in patients with systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R214. doi: 10.1186/ar3193. [DOI] [PMC free article] [PubMed] [Google Scholar]