Abstract
Approximately 20%–25% of all breast cancers over express a key cell surface growth factor receptor known as HER2. HER2 plays a key role in cell growth and proliferation and is linked to worse clinical outcomes, making it a logical therapeutic target. The first HER2 targeted drug to be approved by the FDA, was the humanized monoclonal antibody trastuzumab, after it showed improvements in survival in the adjuvant setting, and delayed time to progression in the metastatic setting. Although highly effective, for reasons that are not clear, some patients display resistance to trastuzumab. Lapatinib is an oral, small molecule tyrosine kinase inhibitor, that inhibits both the HER1 ahd HER2 receptors and may be able to overcome trastuzumab resistance. Lapatinib is approved in the second line setting for use in combination with capecitabine or with letrozole. In this review, we will discuss the indications, concerns or any issues with regards to the drug.
Keywords: breast cancer, lapatinib, efficacy, tolerability
Introduction
Breast cancer is the commonest form of cancer in women worldwide and second leading cause of cancer-related death in North American women.1 Although treatment with chemotherapy, endocrine therapy and targeted therapy has significantly improved outcomes for women with early stage disease, overall survival for women with metastatic disease (MBC) remains poor with a five year survival rate of only 15% underscoring the need for novel therapeutic strategies.2
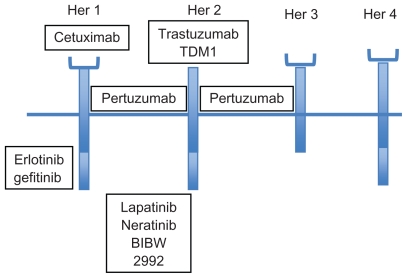
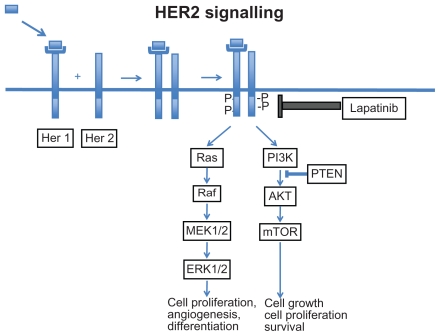
One of the major advances in the field of breast cancer has been the discovery that 20%–25% of breast cancers over express a key growth factor receptor known as the human epidermal growth factor receptor 2 or HER2.3,4 The main function of HER2 is to mediate cell growth, differentiation, and survival and as a result, tumors over expressing the HER2 receptor are more aggressive and have a poorer overall prognosis.5,6 HER2 belongs to a family of 4 closely related receptor tyrosine kinases: HER1 (EGFR, ErbB1), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4) (Fig. 1). These receptors generally have an N-terminus extra cellular ligand binding domain and a C-terminus cytoplasmic domain which exhibits tyrosine kinase activity. The HER2 receptor however has no known activating ligands and may be in a constitutively activated state or may only become active upon heterodimerization with other family members such as EGFR. Upon ligand binding the HER receptors homo- or hetero-dimerize with other members of the family. This stimulates the intrinsic intracellular tyrosine kinase activity, resulting in auto phosphorylation and activation of downstream signal transduction pathways such as the mitogen activated protein kinase (MAPK) pathway, and the phosphoinositide-3 kinase (PI3K)/Akt pathway. Activation of these pathways promotes cell proliferation, migration, angiogenesis and inhibition of apoptosis (Fig. 2).7,8 Therapeutic approaches, including monoclonal antibodies that interfere with ligand binding or receptor dimerization; and small molecules tyrosine kinase inhibitors (TKI) which block downstream signaling have all been shown to effectively inhbit the tumor proliferating effects of the HER receptors. Emerging evidence suggests that targeting the HER receptor by different approaches concurrently may even have additive antitumor effects.
Figure 1.
Human epidermal growth factor receptor family and targeted therapy.
Figure 2.
The Neo-Adjuvant Lapatinib and/or Trastuzumab Treatment Organization (Neo-ALTTO) study is a randomized, multicentre open-label phase III study of neoadjuvant lapatinib, trastuzumab and their combination plus paclitaxel in women with HER2/ErbB2 positive primary breast cancer. Her 2 Her 3 Her 4
Trastuzumab was the first HER2 targeted agent to enter clinical practice. It is an intravenously administered highly selective monoclonal antibody which targets the extra cellular domain of the HER2 receptor and has both preclinical and clinical antitumor activity. Trastuzumab was approved by the Food and Drug Administration (FDA) in 1998 after a landmark Phase III study showed significant improvement in response and survival when added to first line chemotherapy for the treatment of patients with HER2+ MBC.9 Trastuzumab is now also approved in the adjuvant setting after four large multicenter randomized trials (NSABP B-31, NCCTG N9831, HERA and BCIRG 006) showed significant improvements in disease free survival (DFS) and overall survival (OS) when trastuzumab was combined with chemotherapy. 10–13 Use of trastuzumab in HER2+ breast cancer is now standard of care both in the adjuvant and metastatic settings. Despite over expression of HER2, some patients display primary or acquired trastuzumab resistance for reasons that are not completely understood. Several mechansims of resistance have been proposed including the presence of a truncated HER2 receptor known as p95HER2 which lacks an extra cellular binding domain and is not only resistant to trastuzumab but may also be a predictor of worse outcome.14 Other mechanisms of resistance include up regulation and cross talk between pathways downstream of HER2 such as the Insulin-like growth factor (IGF-1) pathway;15 or deficiency of a key tumor suppressor gene known as PTEN which regulates the PI3K/Akt pathway or similarly activating mutations within the p110-alpha subunit of PI3Kinase;16,17 or failure of antibody activity. Nonetheless, at the clinical level, trastuzumab resistance remains a significant challenge. Research is ongoing to discover and develop novel HER2 targeted therapies that can overcome this resistance and slow or stop disease progression.
Lapatinib
Lapatinib ditosylate (GW 572016; Tykerb, Glaxo Smith Kline) monohydrate is an oral small molecule derivative of 4-anilinoquinazoline which targets the C-terminus tyrosine kinase domain of both the HER2 and EGFR receptors. Lapatinib reversibly attaches to and competes with ATP for binding to the intracellular adenosine triphosphate (ATP) binding site of the receptor. This inhibits both phosphorylation and activation of the downstream Ras-Raf- mitogen-activated protein kinase and PI3K-Akt signaling cascades resulting in cell cycle arrest, and increased apoptotic activity.18 Lapatinib is considered a potent inhibitor because of its slow dissociation half life of > 300 minutes causing longer inhibition of the receptors compared to other EGFR targeting quinazolines such as erlotinib (Tarceva) and gefitinib (Iressa), used commonly in the management of lung cancer.19 In BT474 HER2 over expressing breast cancer cell lines, lapatinib decreases HER2 and EGFR phosphorylation and blocks activation of the downstream ERK (extra cellular signal related kinase) and Akt pathways in a time and dose-dependent manner.20 Lapatinib also shows activity against trastuzumab-resistant cell lines where it decreased phosphorylation and activation of IGF-1 and s6 kinase-two pathways that may mediate trastuzumab resistance.21,22 Combining lapatinib and trastuzumab in HER2 over expressing cell lines, has further demonstrated additive or synergistic effects, which have now also been confirmed in the clinical setting. Studies are also ongoing to better understand what factors may predict for sensitivity or resistance to lapatinib. Resistance mechanisms including increased signalling via estrogen receptors, mutations within the HER2 receptor, hyper activation and PIK3Ca mutations of the PI3K pathway and over expression of other key receptors have been proposed.23–25
Clinically Lapatinib has now been evaluated in a number of studies and was approved by the FDA for use with capecitabine for HER2+ MBC, pretreated with prior anthracycline, taxane, and trastuzumab-containing regimens. In 2010, Lapatinib was also approved for use with letrozole for HER2+, hormone receptor positive MBC. In this review, we will focus on the clinical evaluation of lapatinib in terms of its efficacy and tolerability and will also discuss future directions.
Clinical Studies
Phase 1 and safety studies with lapatinib monotherapy
Two phase 1 studies were conducted to determine the safety, tolerability and pharmacokinetics of lapatinib administered as single and multiple dosing in healthy individuals. No serious adverse events were noted with either dosing schedule.26 The most common adverse events (AE) were headache, diarrhea, rash, cold symptoms, gastrointestinal symptoms, and elevated liver function tests. In a dose escalation study of 67 heavily pretreated patients with HER2 and/or HER1 over expressing metastatic cancers, Lapatinib was well tolerated at doses up to 1600 mg daily with clinical activity seen from 650–1600 mg daily, but most commonly between 900–1200 mg. At doses where clinical activity was observed, the main toxicities were grade 1 and 2 diarrhea (42%) and skin rash (31%). No grade 4 toxicities were reported and there was no cardiac toxicity. Diarrhea was linearly related to dose over the 500–1600 mg range, but not serum drug concentration, suggesting that Lapatinib may have direct toxic effects on the gut epithelium. Rash has been recognized as a common side effect of HER1 targeting agents, and has been proposed as a surrogate marker of efficacy; however this has not been confirmed and in this study there was no correlation between rash and efficacy. Pharmacokinetic studies showed peak serum concentrations occurred at a median of 3 hours after dose, and steady state levels were achieved at 6–7 days. Lapatinib is metabolized hepatically via CYP3A4 requiring dose reductions with hepatic dysfunction and dose modifications when used with other CYP3A4 A inducers/inhibitors such as antibiotics and antiepileptics. This dose escalation study was the first to show clinical activity of lapatinib. Four HER2+ trastuzumab pretreated MBC patients showed partial responses (PR) of median duration of 5.5 months and 10 other HER2+ MBC pts showed stable disease for > 6 months at a dose of 1200 mg daily.27
Another Phase 1 study involved 33 HER2 and/or EGFR over expressing metastatic cancers treated with Lapatinib. Four MBC pts had PRs and 11 pts with other cancers had stable disease (SD). Correlative studies showed responders had variable levels of inhibition of p-ErbB1, p-ErbB2, p-Erk 1/2, p-Akt, cyclin D1, transforming growth factor alpha (TGFalpha), and as well as increased tumor cell apoptosis. Increased pretreatment expression of ErbB2, p-ErbB2, Erk 1/2, p-Erk 1/2, Insulin like growth factor 1 (IGF-1), p70 S6 kinase, and TGFalpha all appeared to predict for clinical response, but this study was limited due to small sample size and therefore considered hypothesis generating.28 However, the concept of doing correlative studies to understand response or resistance to lapatinib will be critical to moving the field forwards and should be incorporated into all trials where possible.
Phase II Studies with Single Agent Lapatinib
Lapatinib monotherapy, 1500 mg/day, was studied in an open-label Phase II study in HER2+ (n = 140) and HER2− (n = 89) heavily pretreated MBC patients. The HER2+ group was defined as 3+ HER2 by immunohistochemistry (IHC), or 2+ by IHC and HER2 amplified by fluorescence in situ hybridization (FISH). The HER2+ group had a response rate by independent review of 1.4% and a clinical benefit rate (CBR) of 5.7%, where CBR was defined as (complete response (CR) + PR + SD for ≥ 24 wks). There were no responses in the HER2− group. Time to progression (TTP) was 9.1 wks (HER2+) vs. 7.6 wks (HER2−) and median survival (MS) was 29.4 wks vs. 18.6 wks respectively. Consistent with previous lapatinib studies, the most common AEs were diarrhea (59%), nausea (37%), and rash (32%).29
In another phase II study, 78 HER2+ MBC pts who had up to 2 prior trastuzumab containing regimens received lapatinib 1250 or 1500 mg once daily. Response rate was 5.1%, CBR was 9.0%, median TTP was 15.3 wks, and MS was 79 wks. The results of this study were likely better than the Burstein study due to the limitation on number of prior lines of therapy. Toxicities were similar (rash, diarrhea, nausea) and both doses of lapatinib were well tolerated.30 Given these encouraging phase II results with respect to both efficacy and tolerabilty, further combination studies with lapatinib were undertaken.
Combination of Lapatinib and Chemotherapy
In MBC the taxanes which stabilize microtubules are amongst the most active and frequently used agents. Combining the taxanes with trastuzumab in HER2+ disease has previously been shown to be effective and is standard first line treatment. In the seminal report by Slamon et al the addition of trastuzumab to chemotherapy increased response rates, prolonged duration of remissions, and lengthened survival vs. chemotherapy alone.9 Trastuzumab has also been combined effectively with other chemotherapy regimens, providing the basis for similar combination studies of lapatinib and chemotherapy.
Lapatinib and Paclitaxel
After a phase II study showed efficacy and tolerability of combining lapatinib and paclitaxel, a phase III double blind study of 579 HER2− and HER2 uncharacterized MBC pts was conducted. Patients received either paclitaxel (175 mg/m2 IV q3 wks) or the combination of paclitaxel and lapatinib 1500 mg daily. This trial was important because it served to demonstrate the importance of HER2 status in dictating response to Lapatinib. After central pathology review, 15% of pts who were confirmed to be HER2+ had a statistically higher objective response rate (60% vs. 36%, P = 0.027), and better TTP (8.1 vs. 5.8 mo, P = 0.011) with the combination compared with paclitaxel alone. HER2− patients on the other hand, did not benefit from the addition of lapatinib. Toxicity in the combination arm was significantly higher with rash, diarrhea, mucositis, vomiting and more fatal AE related to sepsis and diarrhea, the latter necessitating aggressive antidiarrheal management.31 In light of these results, a head to head trial in the first line setting comparing paclitaxel with lapatinib vs. paclitaxel with trastuzumab in first line HER2+ MBC was initiated. Correlative studies including pharmacogenomics and pharmacokinetics may add important information about what host and drug factors may impact on both toxicity and response to these regimens.
Lapatinib and Capecitabine
For patients progressing on the taxanes, second line treatment often consists of capecitabine, an oral prodrug of the DNA synthesis inhibitor 5- fluoruracil. A Phase I study of 45 pts combining lapatinib (1250 mg) with capecitabine (2000 mg/m2) given on days 1–14, showed, an AE profile to be no worse than either drug alone, and evidence of antitumor activity.32 This led to the pivotal randomized phase III study of 324 patients comparing lapatinib plus capecitabine vs. capecitabine alone.33 Unlike the prior paclitaxel + lapatinib study, eligible patients had to have HER2+ (3+ by IHC, or 2+ by IHC+ and FISH+) MBC progressing after an anthracycline, a taxane, and trastuzumab. Prior treatment with capecitabine was not permitted, but fluorouracil was allowed. Normal LVEF was required and central nervous system (CNS) metastases were permitted if clinically stable for at least 3 months after the discontinuation of corticosteroid and anticonvulsant therapy. The interim analysis showed the median TTP was 8.4 mo (combination) vs. 4.4 mo (mono therapy), representing a 51% reduction in the risk of disease progression (P < 0.001). The Objective response rate was 22% vs. 14%, which was statistically significant. Biomarker analysis confirmed that FISH confirmation of IHC HER2 positivity most accurately predicted for response to the combination. The main AE in the combination arm were diarrhea, hand-foot syndrome, nausea, vomiting, fatigue, and rash.33 On the basis of efficacy and absence of safety concerns, the data safety and monitoring committee recommended terminating enrollment, reporting the results, unblinding, and allowing 36 patients to cross over to receive lapatinib. Final analysis supported the TTP benefit and this trial ultimately led to the FDA approval of lapatinib plus capecitabine in trastuzumab resistant HER2+ MBC.34 Lapatinib plus capecitabine is currently under evaluation as first line therapy in HER2+ MBC. Another key finding of this study was the reduction of CNS metastases as first site of disease progression in patients receiving lapatinib. Unlike trastuzumab, lapatinib is a small molecule and as such may be able to penetrate the blood brain barrier better in the context of CNS metastases. As will be discussed, dedicated trials with Lapatinib are underway focusing on the issue of CNS metastases.
Lapatinib and Vinorelbine
For patients progressing on a taxane and capecitabine, vinorelbine (a vinca alkaloid) represents a well tolerated IV chemotherapy option administered on days 1 and 8 of a 21 days cycle. Efficacy and safety of Lapatinib with vinorelbine, in patients previously treated with taxanes and/or anthracyclines has recently been reported. Lapatinib 1250 mg daily, and Vinorelbine 25 mg/m2 was used in the first 6 patients but then reduced to Vinorelbine 20 mg/m2 after neutropenia was a found to be an issue. PRs were seen in 5/19 patients, SD in 8/19, and progression free survival (PFS) was 20 wks in a patient population who had a median 2 (range 1–4) prior chemotherapy regimens.35 Lapatinib plus Vinorelbine, is also being evaluated in earlier stage metastatic disease, and in one study being compared with lapatinib and capecitabine with an optional cross over at progression.36
Lapatinib and Gemcitabine/Cisplatin
Lapatinib plus the two drug regimen of Gemcitabine and Cisplatin has also been evaluated. In a phase 1 study, pretreated HER2+ MBC patients received Gemcitabine 1000 mg/m2 IV days 1 and 8, Cisplatin 25 mg/m2 days 1 and 8 and oral lapatinib 1000 mg continuously. In this small study of 19 patients Grade 3 or 4 hematologic toxicity, diarrhea, hepatic toxicity and mucositis were observed. Median PFS was 4 months, and CBR was 44%, suggesting that this may be an active regimen, but dosing may not be optimal for this heavily pretreated population.37
Lapatinib and other 2 or 3 Drug Combinations
There are several other studies of lapatinib in combination with 2 or 3 drug chemotherapy regimens. As illustrated above, the main consideration of these multidrug regimens may be one of tolerability, and so the best setting in which to evaluate these combinations may be in early stage disease where patients are less heavily pretreated.
The GeparQuinto was an open-label Phase III trial led by the German Breast Group evaluating 620 HER2+ patients in the neoadjuvant setting. Patients received epirubicin/cyclophosphamide (EC) followed by docetaxel in combination with either trastuzumab or lapatinib. Postoperatively, the trastuzumab group received an additional 6 months of trastuzumab while the lapatinib group received trastuzumab for 12 months. The primary endpoint was pathological complete (pCR) defined as the no invasive or noninvasive residual disease in the breast and nodes. In the trastuzumab arm pCR was 31.3% vs. 21.7% in the lapatinib arm (P < 0.03). Most common adverse events were gastrointestinal, blood disorders and infections. Discontinuation and dose reductions due to toxicity were more common in the lapatinib arm raising the question of whether this may have impacted on efficacy. Analysis of primary endpoint efficacy and safety findings is ongoing.38 Other early studies include a preoperative lapatinib + paclitaxel + gemcitabine study. This regimen was well tolerated so there are now plans for a Phase 2.39 Another Phase 1 study of Lapatinib plus Docetaxel, Carboplatin and T rastuzumab (TCarboH) in the adjuvant setting required Lapatinib dose reductions to 750 mg/day due to diarrhea, highlighting the fact that in chemotherapy combinations with lapatinib, diarrhea may be a dose limiting toxicity.40
Lapatinib and Hormonal Therapy
There is now a growing body of evidence to suggest that cross-talk exists between HER2 and estrogen receptors, and this cross talk may underlie trastuzumab resistance and provides the rationale for combining lapatinib with hormonal therapy. As an added benefit, both Lapatinib and hormonal therapy are oral, well tolerated, and have few overlapping toxicities.
Lapatinib in combination the aromatase inhibitor, letrozole, has been evaluated in a Phase I trial with 39 patients with hormone receptor positive MBC.41 Clinically relevant doses of lapatinib in combination with letrozole were well tolerated and did not result in pharmacokinetic interaction. In a phase III trial, postmenopausal women with hormone receptor positive MBC were randomized to lapatinib 1500 mg daily plus letrozole 2.5 mg daily or letrozole alone.42 In 219 HER2+ pts median PFS was 8.2 months in the letrozole-lapatinib group versus 3.0 months in the letrozole alone group (HR 0.71). CBR was significantly higher for the combination group 48% vs. 29% for letrozole alone. There was no improvement in PFS seen in the HER2− patients. In the combination arm, grade 3/4 diarrhea and rash were more common. Lapatinib is currently being tested in 2 phase II trials, in hormone resistant, estrogen receptor positive MBC, both as a single agent, and in combination with tamoxifen.
Lapatinib and Targeted Therapy
Lapatinib and trastuzumab
Perhaps one of the most exciting areas of drug development is the concept of combining targeted therapies without the need for chemotherapy. Several preclinical studies have shown that dual HER2 targeting, with lapatinib plus trastuzumab has a synergistic antitumor effect. In a phase 1 study of 54 pts, lapatinib plus trastuzumab was well tolerated at a dose of lapatinib of 1000 mg/day and weekly trastuzumab.43 This led to a Phase III study, in HER2+, MBC patients failing prior trastuzumab. There were 296 pts randomized to lapatinib plus trastuzumab or lapatinib alone. Despite being heavily pretreated and having disease progression on prior trastuzumab, the combination arm had improved PFS (HR 0.73) and CBR (24% vs. 12.4%) compared with lapatinib alone. There was also a nonsignificant trend towards improved OS with the combination. The results of this study confirm the preclinical anti-tumor benefits of a more complete HER2 blockade and offer a non-chemotherapy containing treatment option. The commonest AE in the combination were fatigue with diarrhea, and cardiac toxicity was no worse than each agent alone.44 In a separate study assessing the quality of life (QOL), which is a critical endpoint in studies of advanced disease, the QOL with the combination was comparable to Lapatinib alone.45 The encouraging results of these studies have led to the combination of lapatinib and trastuzumab being evaluated in both the neoadjuvant and adjuvant settings.
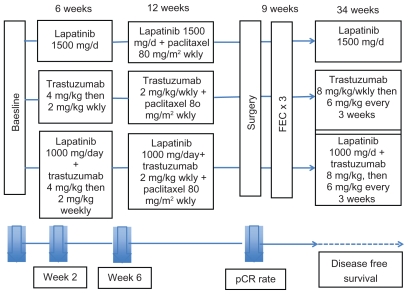
In the neoadjuvant setting the landmark NeoALTTO study randomized patients to lapatinib, trastuzumab or the combination of lapatinib plus trastuzumab for six weeks, followed by the same treatment plus paclitaxel for an additional 12 weeks until surgery (Fig. 3).46 The primary endpoint was pathological complete response (pCR) defined as no invasive cancer in the breast or only noninvasive in-situ cancer. Secondary endpoints included pCR in breast and lymph nodes (total pCR); objective response rate at week 6,% of patients with node-negative disease at surgery, rate of conversion to breast conserving surgery in all patients and in those with nonoperable disease at presentation, disease free survival (DFS) overall survival (OS) and safety and tolerability. The pCR rate was significantly higher (51.3%) for the combination compared to trastuzumab alone (29.5%) vs. lapatinib alone (24.7%). In terms of total pCR, again dual HER2 therapy appeared superior, with a trend towards better response in hormone receptor negative pts. Manageable toxicities, primarily diarrhea and liver enzyme alterations were increased in both lapatinib containing arms, and again there were no major cardiac toxicities. Overall this study supports preclinical results showing benefit of dual HER2 blockade. As well it illustrates the advantages of evaluating new agents in the neoadjuvant setting where there is potential for evaluating not only clinical response but also biological responses and potentially adding imaging correlates. The results of this study, are potentially practice changing, but raise a key question about the cost of using two targeted agents concurrently.
Figure 3.
Neo-ALTTO study design.
In the adjuvant setting, the ALTTO trial (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization) randomized early stage HER2+ pts to one of 4 treatment arms: trastuzumab alone for 52 wks, Lapatinib alone for 52 wks, trastuzumab for 12 or 18 wks, followed by a 6 wk break, followed by lapatinib for 28 or 34 wks, or lapatinib in combination with trastuzumab for 52 wks. The primary objective was to compare DFS between each of the lapatinib containing arms and trastuzumab alone. Secondary objectives included OS, time to recurrence, time to distant recurrence, safety and tolerability and incidence of brain metastasis. Each of these analyses would be conducted according to cmyc gene amplification, expression of PTEN, and p95HER2 domain. After an interim analysis, the lapatinib alone arm was discontinued because it was felt that it was unlikely to meet the pre-specified criteria to demonstrate noninferiority to trastuzumab in DFS. The trial has now closed to accrual with final results pending.
Lapatinib and Angiogenesis Inhibitors, mTOR Inhibitors
In two separate studies, Lapatinib has also been evaluated in combination with pazopanib, a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor and Bevacizumab, an antibody to VEGF respectively. In both studies toxicity was manageable and early clinical activity was seen. Building on this and the studies above, a triplet combination of lapatinib, trastuzumab and bevacizumab was undertaken. Again, there were no major toxicities and preliminary responses were seen in MBC resistant to both trastuzumab and lapatinib.47 Other combination strategies include lapatinib and the mTOR inhibitor everolimus. The mTOR pathway may play a role in trastuzumab resistance providing the rationale for this approach. Diarrhea, stomatitis and fatigue were the main dose limiting toxicities of this combination and the maximal tolerated dose was determined to be 1250 mg lapatinib, and 5 mg everolimus daily.48 Taken together, the encouraging results seen with these studies combining HER2 targeted therapies with other targets increases hope that non-chemotherapy containing regimens may prove to be both well tolerated and active in advanced disease.
Lapatinib and Brain Metastases
As mentioned, there is also interest in the role of lapatinib in managing CNS metastases. Due to its small size, lapatinib can theoretically cross the blood brain barrier whereas the larger trastuzumab molecule cannot. Although pre-clinical models did not show lapatinib crossed the intact blood-brain barrier to a significant degree, the blood-brain barrier may be more permissive in the setting of metastases.1
Lapatinib monotherapy was evaluated in a Phase II study of 39 HER2+ trastuzumab pretreated patients, with refractory brain metastases.49 One patient achieved a PR in the brain by RECIST (Response Evaluation Criteria in Solid Tumors) criteria and seven patients (18%) were progression free in both the CNS and non-CNS sites at 16 weeks. The most common AEs were diarrhea and fatigue. Brain metastases were also examined in another Phase II trial with pts who had CNS progression after cranial radiation.50 Objective responses were observed in 6% of 242 patients in the lapatinib group and in 20% of patients who received lapatinib and capecitabine. This study confirmed the modest antitumor activity of lapatinib and the additional response when combined with capecitabine. Another similar study showed the benefit of capecitabine and lapatinib in 81 HER2+ patients with brain metastases who were not pretreated with either lapatinib or capecitabine.51 Patients treated with lapatinib and capecitabine had a median overall survival benefit compared to patients treated with trastuzumab based therapies only, beyond brain progression (27.9 months versus 16.7 months respectively, P = 0.01). In the Landscape Phase II trial, HER2+ MBC patients with brain metastases before whole-brain radiotherapy were treated with lapatinib 1250 mg/day and capecitabine 2000 mg/m2. The CNS-OR rate was 67% (95% CI 51–81), with a median time from inclusion to response of 1.8 month. Median TTP was 5.5 months (95% CI 3.9–5.9) and median time to whole-brain radiotherapy was 8.3 months (95% CI 5.1–11.7). However, grade 3+ drug related toxicities were common, suggesting that dosing may need to be adjusted. Two combination studies with lapatinib for CNS metastases are also underway. The first Phase 1 study combines lapatinib with the chemotherapy temozolamide and early data shows good tolerability at all dose levels.52 The second Phase 1 study examined the combination of lapatinib and whole brain radiotherapy but did not meet its predefined criteria for feasibility and toxicity was a concern.53 Nonetheless, the ability of Lapatinib to penetrate the CNS offers an important step forwards, in a disease that can often be controlled well systemically but has a propensity for difficult to control CNS metastases.
Conclusions
There have been significant advances in the field of HER2+ breast cancer over the last decade, with several new agents targeting the HER2 pathway, but other related pathways also now becoming available. After trastuzumab, lapatinib, a small molecule tyrosine kinase inhibitor is the second HER2 targeted agent to be approved in HER2+ MBC: either in combination with capecitabine in second line or in combination with letrozole for hormone receptor positive disease. Unlike trastuzumab, lapatinib is oral, has less cardio-toxicity and may penetrate the CNS better. Several studies are ongoing to examine the role of lapatinib in additional settings including the neoadjuvant and adjuvant settings, in combination with other targeted agents including HER2 targeted agents as well as in the setting of CNS metastases. Perhaps most interesting and exciting, is the confirmation of preclinical studies that targeting the HER2 receptor by different approaches concurrently may be the most effective, raising the potential for a nonchemotherapy based treatment approach in breast cancer in the very near future.
In addition to lapatinib there are also a number of other molecular targeted agents being studied for use in breast cancer. Neratinib for example is an oral, irreversible pan-ErbB receptor TKI being evaluated in both early stage and advanced breast cancer.54 There is also emerging data for the treatment of trastuzumab-refractory disease with other novel agents including pertuzumab (receptor dimerization inhibitor), trastuzumab-DM1 (trastuzumab linked to maytansine chemotherapy), HSP90 and P13K pathway inhibitors.55
As more and more agents become available, further research will be needed to determine how best to sequence these agents manage toxicity and tailor treatments to an individual patient. There is also the question of dosing, because unlike classic cytotoxic agents, it is not clear that higher dose necessarily correlates with better efficacy. In the case of lapatinib, there is interest in the question of whether appearance of a rash correlates with efficacy and whether it is appropriate to titrate dosing according to rash. As we aim to move forwards in the field obtaining biopsies at the time of disease progression may need to be incorporated into the standard of care. This may result in a better understanding of the patterns of resistance and allow us to choose the most appropriate subsequent treatment. It is without doubt an exciting time in the field of HER2 positive breast cancer with two drugs already approved and several other novel agents currently in development in preclinical and clinical testing.
Footnotes
Author Contributions
Conceived and designed the experiments: SSS. Analysed the data: SSS, PR. Wrote the first draft of the manuscript: PR. Contributed to the writing of the manuscript: PR, SSS. Agree with manuscript results and conclusions: PR, SSS. Jointly developed the structure and arguments for the paper: PR, SSS. Made critical revisions and approved final version: SSS. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA. Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Facts and Figures. 2010. http://www.cancer.org/Cancer/BreastCancer/OverviewGuide/breast-cancer-overview-survival-rates.
- 3.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu protooncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 5.Seshadri R, Firgaira FA, Horsfall DJ, et al. Clinical significance of HER-2/ neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. JCO. 1993;11(10):1936–42. doi: 10.1200/JCO.1993.11.10.1936. [DOI] [PubMed] [Google Scholar]
- 6.Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. JCO. 1989;7(8):1120–8. doi: 10.1200/JCO.1989.7.8.1120. [DOI] [PubMed] [Google Scholar]
- 7.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 8.Olayioye MA, Graus-Porta D, Beerli RR, et al. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol. 1998;18(9):5042–51. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. NEJM. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. NEJM. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 11.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. NEJM. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 12.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. NEJM. 2011;365(14):1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. NEJM. 2006;354(8):809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 14.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. JNCI. 2007;99(8):628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 15.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8(6):215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. MCT. 2002;1(9):707–17. [PubMed] [Google Scholar]
- 18.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 19.Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64(18):6652–9. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 20.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. MCT. 2001;1(2):85–94. [PubMed] [Google Scholar]
- 21.Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab- resistant breast cancer cells: effects on insulin-like growth factor I signaling. MCT. 2007;6(2):667–74. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 22.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab- treated breast cancer cells. Cancer Res. 2006;66(3):1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. PNAS. 2006;103(20):7795–800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trowe T, Boukouvala S, Calkins K, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008;14(8):2465–75. doi: 10.1158/1078-0432.CCR-07-4367. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka Y, Mukohara T, Shimada H, et al. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21(2):255–62. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 26.Bence AK, Anderson EB, Halepota MA, et al. Phase I pharmacokinetic studies evaluating single and multiple doses of oral GW572016, a dual EGFR-ErbB2 inhibitor, in healthy subjects. Invest New Drugs. 2005;23(1):39–49. doi: 10.1023/B:DRUG.0000047104.45929.ea. [DOI] [PubMed] [Google Scholar]
- 27.Burris HA, III, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. JCO. 2005;23(23):5305–13. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 28.Spector NL, Xia W, Burris H, III, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. JCO. 2005;23(11):2502–12. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 29.Burstein HJ, Storniolo AM, Franco S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19(6):1068–74. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20(6):1026–31. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 31.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. JCO. 2008;26(34):5544–52. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu QS, Schwartz G, de Bono J, et al. Phase I and pharmacokinetic study of lapatinib in combination with capecitabine in patients with advanced solid malignancies. JCO. 2007;25(24):3753–8. doi: 10.1200/JCO.2007.11.1765. [DOI] [PubMed] [Google Scholar]
- 33.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. NEJM. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 34.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–43. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 35.Saip P, Eralp M, Ozkan M, et al. Phase 2 study of lapatinib in combination with vinorelbine in patients with ErbB2-amplified recurrent or metastatic breast cancer. ASCO Annual Meeting J Clin Oncol. 2011;29(Suppl) abstr e13079. [Google Scholar]
- 36.Janni W, Sarosiek T, Papadimitrou C, et al. A phase 2 randomized trial of lapatinib with either vinorelbine or capecitabine as first- and second-line therapy for ErbB2-overexpressing Metastatic Breast Cancer (MBC): Safety results. ASCO Annual Meeting J Clin Oncol. 2011;29(Suppl) abstr e11097. [Google Scholar]
- 37.Valero M, Ruiz-Borrego M, Salvador D, et al. Cisplatin, gencitabine, and lapatinib in patients with HER2-positive metastatic breast cancer: An experience in routine clinical practice. ASCO Annual Meeting J Clin Oncol. 2011;29(Suppl) abstr e11005. [Google Scholar]
- 38.Untch M, Loibl S, Bischoff J, et al. Lapatinib vs. Trastuzumab in Combination with Neoadjuvant Anthracycline-Taxane-Based Chemotherapy: Primary Efficacy Endpoint Analysis of the GEPARQUINTO STUDY (GBG 44). Proceedings of the SABCS; 2010; Abs [S3–1] [Google Scholar]
- 39.Park I, Lee K, Kang H, et al. A phase Ib study of preoperative lapatinib, paclitaxel, and gemcitabine combination therapy in women with HER2-positive early breast cancer. ASCO Annual Meeting J Clin Oncol. 2011;29(Suppl) doi: 10.1007/s10637-011-9759-5. abstr e11069. [DOI] [PubMed] [Google Scholar]
- 40.Bueno Hume C, Moreno-Aspitia A, Hillman D, et al. Safety and tolerability of lapatinib ditosylate given concurrently with docetaxel, carboplatin, and trastuzumab (TCHL) as part of adjuvant therapy for patients with HER2+ breast cancer: Pilot data from the North Central Cancer Treatment Group Trial N083E. ASCO Annual Meeting J Clin Oncol. 2010;28(Suppl) abstr 565. [Google Scholar]
- 41.Chu QS, Cianfrocca ME, Goldstein LJ, et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clin Cancer Res. 2008;14(14):4484–90. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 42.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. JCO. 2009;27(33):5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 43.Storniolo AM, Pegram MD, Overmoyer B, et al. Phase I dose escalation and pharmacokinetic study of lapatinib in combination with trastuzumab in patients with advanced ErbB2-positive breast cancer. JCO. 2008;26(20):3317–23. doi: 10.1200/JCO.2007.13.5202. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Amonkar MM, Sherrill BH, et al. Impact of lapatinib plus trastuzumab versus single-agent lapatinib on quality of life of patients with trastuzumab-refractory HER2+ metastatic breast cancer. Ann Oncol. 2011;22(12):2582–90. doi: 10.1093/annonc/mdr014. [DOI] [PubMed] [Google Scholar]
- 45.Burstein HJ, Blackwell KL, Wu Y, et al. Impact of lapatinib plus trastuzumab versus single-agent lapatinib on quality of life (QOL) of patients with trastuzumab-refractory HER2+ (ErbB2+) metastatic breast cancer (MBC). Proceedings of the Breast Cancer; 2008; Symposium-Abstract 154. [Google Scholar]
- 46.Baselga J, Bradbury I, Eidtmann H, Di Cosimo SCA. First results of the NeoALTTO trial (BIG 01-6/ EGF 106903): A phase III, randomized, open label, neoadjuvant study of lapatinib, trastuzumab, and their combination plus paclitaxel in women with HER2HER2-positive primary breast cancer. Proceedings of the SABCS; 2010; Abs [S3–3] [Google Scholar]
- 47.Falchook G, Moulder S, Wheler J, et al. Combination trastuzumab, lapatinib, and bevacizumab in HER2+ breast cancer and other malignancies. ASCO Annual Meeting J Clin Oncol. 2009;27(Suppl) abstr 244. [Google Scholar]
- 48.Hoban C, Hoering A, Synold T, et al. Phase I evaluation of lapatinib and everolimus in patients with advanced malignancies: Southwest Oncology Group trial S0528. ASCO Annual Meeting J Clin Oncol. 2009;27(Suppl) abstr 3553. [Google Scholar]
- 49.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. JCO. 2008;26(12):1993–9. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–9. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 51.Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22(3):625–30. doi: 10.1093/annonc/mdq434. [DOI] [PubMed] [Google Scholar]
- 52.De Azambuja E, Lemort M, Rossari J, et al. Phase I study of lapatinib (L) and temozolomide (T) combination for the treatment of progressive brain metastases (BM) in HER2-positive metastatic breast cancer patients (Pts) (LAPTEM, LAP 111172) ASCO Annual Meeting J Clin Oncol. 2010;29(Suppl) abstr 570. [Google Scholar]
- 53.Lin NU, MR, Younger W, et al. Phase I study of lapatinib (L) in combination with whole-brain radiation therapy (WBRT) in patients (pts) with brain metastases from HER2-positive breast cancer. ASCO Annual Meeting J Clin Oncol. 2010;28(Suppl):15 s. abstr 1154. [Google Scholar]
- 54.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. JCO. 2010;28(8):1301–7. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 55.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11(2):263–75. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]