Abstract
The number of circulating endothelial progenitor cells (EPCs) inversely correlates with cardiovascular risk and clinical outcome, and thus has been proposed as valuable biomarker for risk assessment, disease progression and response to therapy. However, current strategies for isolation of these rare cells are limited to complex, laborious approaches. The goal of this study was the design and validation of a disposable microfluidic platform capable of selectively capturing and enumerating EPCs directly from human whole blood in healthy and diseased subjects, eliminating sample pre-processing. We then applied the “EPC capture chip” clinically and determined EPC numbers in blood from patients with pulmonary arterial hypertension (PAH). Blood was collected in EDTA-tubes and injected into polymeric microfluidic chips containing microcolumns pre-coated with anti-CD34 antibody. Captured cells were immunofluorescently stained for expression of stem and endothelial antigens, identified and counted. The EPC capture chip was validated with conventional flow cytometry counts (r=0.83). The inter- and intra-day reliability of the microfluidic devices was confirmed at different time points in triplicates over 1–5 months. In a cohort of 43 patients with three forms of PAH (idiopathic/heritable, drug-induced, connective tissue disease), EPC numbers are ≈ 50% lower in PAH subjects vs. matched controls, and inversely related to two potential disease modifiers: body-mass-index and postmenopausal status. The EPC capture chip (5×30×0.05mm3) requires only 200μL human EDTA-blood and has the strong potential to serve as a rapid bedside test for the screening and monitoring of patients with PAH, and other proliferative cardiovascular, pulmonary, malignant, and neuro-degenerative diseases.
Keywords: progenitor cells, microfluidic device, pulmonary hypertension, biomarker, bedside test, diagnostics, biomedical engineering
Introduction
Peripheral blood contains a subtype of circulating, bone marrow-derived cells, called endothelial progenitor cells (EPCs) [1, 2]; EPC number inversely correlates with endothelial dysfunction and impairment of angiogenesis [1, 3], and has been suggested as a biomarker for cardiovascular disease [4–6]. Flow cytometry has been the method of choice for EPC analysis but is limited in routine use due to the need for laborious, non-automated preprocessing and the size and costs of equipment and reagents [5]. There is a need for a rapid bedside test that quantifies these rare progenitor cells as a means of assessing patient risk, response to therapy and prognosis, in conjunction with traditional clinical diagnostic methodologies. Here, we describe the design, validation and clinical utilization of a disposable, polymer-based microfluidic platform (“EPC capture chip”) which enables the isolation and detection of EPCs directly from human whole blood using surfaces coated with anti-CD34, followed by counterstaining with antibodies against characteristic EPC surface antigens, kinase insert domain (KDR) and CD31.
Based on our previous research with ovine EPCs [7] an advanced antibody-mediated microfluidic capture device was developed. While similar to previous designs for metastatic cells [8, 9], the device is distinct in it is much smaller in size (<7.5μL) and thus designed for capture of target cells from a single pass of a small volume of whole blood (200μL) – important in clinical pediatrics and small (transgenic) animal research. Moreover, the device allows parallel analysis of multiple cell types (besides EPCs) within a single blood sample.
Here, we report the design, validation, and clinical application of a disposable microfluidic platform capable of selectively capturing and enumerating EPCs (CD34+/KDR+ and CD34+/KDR+/CD31+/CD45−, so called “late” EPC) directly from whole blood in healthy and diseased subjects, i.e. patients with pulmonary arterial hypertension (PAH), thereby eliminating sample pre-processing. However, this study was not designed to comprehensively investigate the role of EPC and EPC function in PAH.
Numerous markers of EPC lineage have been proposed in the literature, sub-categorized into stem-cell makers (such as CD34, CD133, CD45, and c-kit) and endothelial-like markers (such as KDR, CD31, CD146, and von Willebrand factor) [2, 10]. However, the precise definition of what constitutes an EPC is the subject of an extensive debate [11, 12]. At present, the only EPC phenotype based on surface antigenic markers that provides strong and reproducible correlations across multiple studies on vascular damage and cardiovascular risk is CD34+/KDR+[13]. An additional phenotype that has recently been utilized in the literature is the inclusion of CD133 as a secondary stem cell marker [14], however, Timmermans et al. have recently questioned its utility as an EPC marker [10, 15]. Notably, the intersection of the CD34+/CD133+ and CD34+/KDR+ cell phenotypes (i.e. CD34+/CD133+/KDR+ cells) is known to be extremely rare [13] and, within the blood volumes used in the current investigation, no reliable enumeration of this rare cell type could be made (see Supplemental Results and Discussion).
Besides the controversy on the most accurate definition of EPCs (see above), there is currently an extensive debate on the role of EPCs in PAH; in particular it is discussed whether the number of circulating “EPCs” is actually decreased (CD34+/KDR+/CD31+, so-called “late EPCs”) or increased (CD34+/CD133+, so-called “early EPCs”) in PAH vs. healthy controls (see ref. [11, 12]). Several groups have demonstrated lower number of circulating CD34+ and CD34+/KDR+ cells versus controls [16–18] whilst other groups have reported an increase of CD34+/CD133+ and CD34+/CD133+/KDR+ cells [19, 20], or no change in CD34+/CD133+ cells[16, 21], in PAH patients compared to controls. Some of these apparently controversial findings may simply be explained by the different cell surface antigens targeted for EPC characterization (see discussion). Diller et al. [16] have characterized EPCs as CD34+/KDR+ cells and demonstrated that adult IPAH patients have reduced numbers of such circulating EPCs when compared with healthy controls. From a clinical perspective, it is important to note that a decreased number of EPCs was associated with worse hemodynamics [16], and that treatment with the phosphodiesterase type 5 (PDE5)-inhibitor sildenafil, led to a dose-dependent rise in EPC numbers [16].
Hence, we used the aforementioned most reproducible CD34+/KDR+ EPC phenotype [13] as the basis for our clinical study thereby aiming to establish a novel EPC capture chip as a new “bedside test.” Besides the technical advances, we demonstrate that EPC numbers (CD34+/KDR+, CD34+/KDR+/CD31+/CD45−) were ≈ 50% lower in patients with idiopathic/heritable PAH, but also in those with PAH associated with appetite suppressant use or connective-tissue disease, when compared with matched control subjects. The resulting EPC numbers were also shown to be inversely associated with two potential disease modifiers: body-mass-index and postmenopausal status.
Methods
Blood Collection
Whole blood was drawn from 14 healthy volunteers and 43 patients with pulmonary arterial hypertension (PAH; including idiopathic and heritable PAH, drug induced PAH, and PAH associated with connective tissue disease) and collected in EDTA-coated Vacutainer® tubes (Becton Dickinson, Franklin Lakes, USA). Subjects were recruited from the “research room” at the Pulmonary Hypertension Association’s 9th International Pulmonary Hypertension Conference and Scientific Sessions, Garden Grove, CA, USA, in June 2010. Approval from Stanford University School of Medicine and Northeastern University Institutional Review Boards was obtained and all study subjects provided written informed consent.
Microfluidic Device Design and Fabrication
The design and fabrication of the micropost array microfluidic devices followed previously described soft-lithography techniques [22]. First, a negative master was fabricated and assembled at the George J. Kostas Nanoscale Technology and Manufacturing Research Center at Northeastern University using conventional photolithography techniques. Briefly, a silicon wafer was coated with SU 8–50 photoresist to a thickness of approximately 43 μm. With the transparency overlaid, the wafer was exposed to 365 nm, 11 mW/cm3 UV-light from a Q2001 mask aligner (Quintel Co, San Jose, CA). Unexposed photoresist was then removed using SU 8 developer. Feature height was verified using a Dektak surface profiler (Veeco Instruments, Santa Barbara, CA).
Briefly, post array (Figure 1) devices consisting of 100 μm diameter post with a gap, edge-to-edge distance of 50 μm were fabricated. The posts were arranged in a hexagonal pattern, where three adjacent posts form an equilateral triangle pattern. The overall dimension of the device was 5 × 30 × 0.05 mm3, which results in a total volume of the channel of 7.5 μL. To form the polymeric chambers, poly(dimethylsiloxane) (PDMS; Sylgard 184, Dow Corning, Midland, MI) elastomer was mixed (10:1 ratio) and poured onto a negative master, degassed, and allowed to cure overnight. PDMS replicas were then removed; inlet and outlet holes were punched with a 19G blunt-nose needle. Prior to bonding PDMS replicates were extracted as described by Vickers et al. [23]. Replicas and glass microscope slides (25 × 75 × 1 mm3) were then exposed to oxygen-plasma and placed in contact to bond irreversibly.
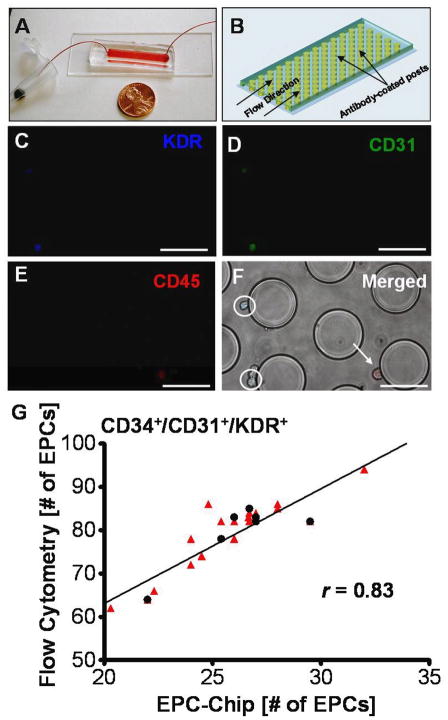
Figure 1.
Isolation of EPCs from human peripheral whole blood using a microfluidic platform. (A) The EPC capture chip requires the injection of 200μL of whole blood; shown alongside a US penny for comparison of device dimensions. (B) The microfluidic platform consists of a dense array of 100μm posts which are coated with anti-CD34 antibody. (C–E) Fluorescence micrographs of captured cells which express KDR (blue), CD31 (green) and CD45 (red). (F) Merged image with bright-field image of post-array, illustrating KDR+/CD31+/CD45− cells (circled) and a KDR−/CD31−/CD45+ cell (arrow). Scale bar = 100μm. (G) The EPC-chip cell capture was compared to traditional 3-color flow cytometry cell counts (CD34+/CD31+/KDR+). The red triangles (
 ) represent individual experiments (n=21 devices; 2–4 separate blood draws from 7 subjects) and the black circles (●) represent the median for each subject (n=7). The comparison illustrates an approximate 1:2.7 ratio in EPC number between microfluidic chip and flow cytometry. The solid line shows the linear regression fit of the median group (n=7). The Pearson correlation (r) was 0.83 (p=0.0196) for the narrower grouping using medians (n=7) and was 0.89 (p<0.001) for the large triplicate analysis grouping (n=21) where each red triangle represents the EPC number determined by one use of a microfluidic device. Within the 5% margin of error associated with flow cytometry [29] the curve fit was shown to cross the origin at approximately zero.
) represent individual experiments (n=21 devices; 2–4 separate blood draws from 7 subjects) and the black circles (●) represent the median for each subject (n=7). The comparison illustrates an approximate 1:2.7 ratio in EPC number between microfluidic chip and flow cytometry. The solid line shows the linear regression fit of the median group (n=7). The Pearson correlation (r) was 0.83 (p=0.0196) for the narrower grouping using medians (n=7) and was 0.89 (p<0.001) for the large triplicate analysis grouping (n=21) where each red triangle represents the EPC number determined by one use of a microfluidic device. Within the 5% margin of error associated with flow cytometry [29] the curve fit was shown to cross the origin at approximately zero.
Surface Modification
As described previously [22], the main steps in the surface modification protocol were (i) surface treatment with 4% (v/v) 3-mercaptopropyl trimethoxysilane (MPTS; Gelest, San Francisco, CA) solution, (ii) attachment of a coupling agent, 0.28% (v/v) N-[γ-maleimidobutyryloxy] succinimide ester (GMBS; Pierce Biotechnology, Rockford, IL), to the silane, and (iii) attachment of mouse anti-human CD34 protein (Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 0.01 mg/mL.
Microfluidic Device Flow Experiments
Whole EDTA-blood was directly flowed through the microfluidic device with functionalized posts at 10 μL/min to a total volume of 200 μL (20 min) using a Harvard Apparatus PHD2000 syringe pump (Harvard Apparatus, Holliston, MA). The blood was then rinsed from the device using PBS at a flow rate of 10 μL/min to a total volume of 100 μL (10 min) followed by a cell fixation using 4% (v/v) formaldehyde solution in PBS, again at 10 μL/min to a total volume of 100 μL (10 min). Formaldehyde solution was flushed out with PBS prior to staining for EPC-specific surface markers.
Immunofluorescent Staining
Following cell capture, rinse, and fixation, immuno-fluorescent staining was performed. Cells within the device were incubated for 10 min in the presence of primary antibodies against CD31 (PECAM-1; 1:100, Santa Cruz Biotechnology) conjugated to fluorescein isothiocyanate (FITC), CD45 (1:100, Santa Cruz Biotechnology) conjugated to phycoerythrin (PE), and kinase insert domain receptor (KDR/Flk-1; 1:100, Santa Cruz Biotechnology) with secondary antibody AlexaFluor350 (1:100, Invitrogen, Carlsbad, CA). All incubation steps were conducted at room temperature. Following incubation, devices were flushed with PBS and EPCs were counted via raster scanning with fluorescence microscopy (at 10× magnification) using a Nikon Eclipse TE2000 inverted microscope using fluorescein (480 ± 30 nm/535 ± 40 nm), rhodamine (540 ± 25 nm/605 ± 50 nm), and DAPI (360 ± 40 nm/460 ± 50 nm) excitation/emission filters.
Flow Cytometry
The blood was collected in EDTA-coated tubes and kept on ice. Flow cytometry-based EPC staining was performed within the 2 hours after blood collection. Peripheral blood mononuclear cells (PBMNC) were isolated from 100 μL aliquots of blood using 8.3 g/L ammonium chloride lysis buffer. A three color panel of antibodies was used to enumerate the EPCs: mouse anti-CD31-FITC (1:100; Santa Cruz), mouse anti-CD34-PE (1:100; Santa Cruz), and goat anti-KDR (1:100; Santa Cruz) along with donkey anti-goat PerCP secondary stain (1:500; R&D Systems, Minneapolis, MN) incubated at room temperature. Using a Beckman Coulter Cell Lab Quanta™ SC flow cytometer (Brea, CA), cells were processed for electronic volume, side scatter and the three fluorescent markers. Cell populations were quantified as an absolute number of live events that were CD31, CD34, and KDR positive (CD34+/KDR+/CD31+). Gating was conducted using the FlowJo™ gating software. A representative flow cytometry scatter plot for CD34+/CD31+/KDR+ cells is shown in Figure S1.
Statistical Analysis
Values from multiple experiments are expressed as mean±SEM unless stated otherwise. Using the Kolmogorov-Smirnov normality test we could show that the measured values were normally distributed. Statistical significance was determined via student t-test for side-by-side comparison or one-way analysis of variance (ANOVA), followed by Bonferroni post hoc test, for comparisons between multiple groups. Pearson correlations and scatter plots were used to study the association between flow cytometry and EPC capture on chip, and for EPC number association with age. The number of relevant subjects in each group is found in Table 1. A p value < 0.05 was considered significant. Reproducibility was tested with a Wilcoxon matched-pairs signed rank test (intra-day comparisons) and Kruskal-Wallis test (inter-day comparisons): A p-value close to one was considered a reproducible result.
Table 1.
Characteristic of Control Subjects and PAH Patients Enrolled.
| Characteristic | Control* | PAH* (Total) | Idiopathic/Heritable PAH† | Drug-Induced PAH‡ | PAH associated with connective tissue disease§ |
|---|---|---|---|---|---|
| N number | 6 | 43 | 28 | 4 | 11 |
| Age–years | 44.9 | 47.1 | 44.7 | 44.5 | 54.4 |
| Male Sex–no (%) | 1 (17) | 6 (14) | 6 (21) | 0 (0) | 0 (0) |
| Height–m | 1.66 | 1.64 | 1.65 | 1.66 | 1.62 |
| Weight–kg | 71.4 | 78.3 | 75.8 | 94.0 | 78.0 |
| BMI–kg/m2 | 25.7 | 28.8 | 27.7 | 34.2 | 29.5 |
| Race/Ethnicity | |||||
| -White | 6 | 38 | 26 | 3 | 9 |
| -Black | 0 | 1 | 1 | 0 | 0 |
| -Asian | 1 | 3 | 1 | 0 | 2 |
| -Hispanic | 0 | 0 | 0 | 0 | 0 |
| -Other | 0 | 1 | 0 | 1 | 0 |
Numbers are mean values or the number of subjects, as appropriate.
BMI, body-mass-index.
EPC-count (CD34+/KDR+) in larger control cohort (n=14; age 23–60yrs) and the total PAH cohort (n=43; age 19–77yrs.) showed no difference between genders
BMP-RII gene mutation, i.e. heritable PAH (n=1)
PAH associated with appetite suppressants (fenfluramine/phentermine; n=3); PAH associated with illegal drug use (n=1).
Systemic sclerosis (scleroderma; n=2), mixed connective tissue disease (MCTD; n=2), systemic lupus erythematodes (SLE; n=1), limited scleroderma (CREST; n=2), and scleroderma/MCTD (n=1).
Results
Development and Validation of the EPC Capture Chip
Briefly, peripheral blood was collected in EDTA tubes and directly injected into the polymeric microfluidic chips at a flow rate of 0.6 mL/h (Figure 1A–B). Following capture (CD34+ cells), cells were identified and enumerated via immunofluorescent staining (Figure 1C–F) for expression of CD31, KDR, and CD45 antigens. Data was tabulated for cell numbers which stained for (i.) KDR only (CD34+/KDR+), (ii.) for cells that stained for CD31 and KDR (CD34+/CD31+/KDR+), and (iii.) for cells that stained for CD31 and KDR whilst negative for CD45 (CD34+/CD31+/KDR+/CD45−) - all frequently described EPC phenotypes [1, 24, 25]. A comprehensive diagnostic readout was attained in approximately one hour, significantly faster than traditional techniques such as flow cytometry [5, 26], magnetic-bead based approaches [2], or colony forming cell assays [5, 27] (>2 hrs up to 5 days; see Table 2) To validate the efficiency and accuracy of the EPC-chip, CD34+/CD31+/KDR+ cell counts from 21 separate blood draws (7 control subjects) were directly compared to traditional 3-color flow cytometry measurements of the same three markers in the first set of experiments. As illustrated in Figure 1G, the comparison of EPC number on-chip with the flow cytometry measurements revealed a 1:2.7 ratio in EPC number (i.e. approx. 37% capture rate), with excellent correlation between the two techniques (r=0.83; p=0.0196) for the median values of 7 control subjects). In addition, we investigated the inter- and intra-day reliability of the microfluidic devices by measuring EPC number in five controls at different time points in triplicates over 1–5 months, and found control EPC numbers to be consistently 24–30/200μL whole blood with little fluctuation (see Figure S2 in the online data supplement).
Table 2.
Comparison of EPC capture chip with traditional EPC enumeration techniques
| EPC Capture Chip | Flow Cytometry# | Magnetic Bead-based Approaches† | Colony Forming Unit (CFU) Assay# | |
|---|---|---|---|---|
| Pre-processing | None |
|
|
|
| Volume Requirements | 0.2 mL whole blood | 0.1–2mL whole blood | ~0.5–50 mL whole blood | 5–10 mL whole blood |
| Processing Time | ~1 hr | ~2.5 hr | Variable (0.5–2 hrs) | 5 days |
| Post-processing | Manual enumeration via immunofluorescence | Gating according to FSC/SS/Markers | Manual counts under bright field microscope or followed by CFU assay or flow cytometry | Manual inspection and counting under phase contrast microscope |
| Additional Comments |
|
|
|
|
| Complete automation | Possible | Gates discriminated manually | Either manual counts under microscope or flow cytometric analysis required | Not possible as colonies discriminated by manual observation |
Endothelial Progenitor Cell Number Is Decreased In Patients With Pulmonary Arterial Hypertension
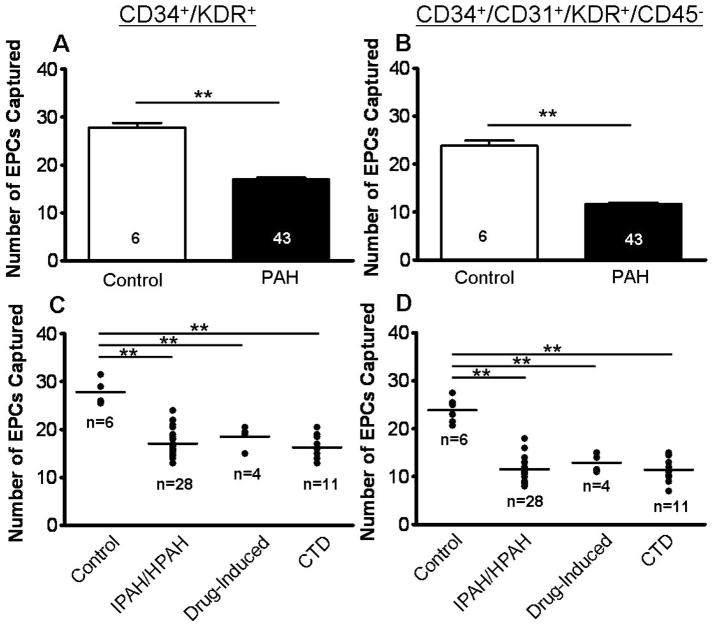
In the subsequent clinical study, the enrolled subjects included 43 patients diagnosed with PAH (6 males, 37 females; mean age of 47.1 yrs), and six age and gender-matched controls (1 male, 5 females; mean age of 44.9 yrs; see Table 3 and 4 for individual subject and control characteristics, respectively). PAH patients were further stratified into three distinct groups according to PAH subcategory [28], i.e. idiopathic and heritable PAH (IPAH/HPAH; n=28), drug-induced PAH (n=4; appetite suppressants), and PAH associated with connective tissue disease (n=11).
Table 3.
PAH patient characteristics
| ID | Age (yrs) | Gender (M/F) | Weight (kg) | BMI (kg/m2) | Diagnosis* | WHO Class# | Medication† |
|---|---|---|---|---|---|---|---|
| 1 | 41 | F | 59 | 20.9 | IPAH | 2 | BOS, SIL, NIF, WAR |
| 2 | 32 | F | 98 | 33.9 | IPAH | 3 | AMB, SIL, EPIV, O2 |
| 3 | 53 | M | 86 | 27.1 | IPAH | 2 | BOS, SIL |
| 4 | 37 | F | 71 | 26.7 | IPAH | 2 | AMB, TAD, TREPIN, FUR, THY, O2 |
| 5 | 59 | F | 86 | 31.6 | IPAH | 3 | AMB, SIL, TREPIN, FUR, ALD, WAR, SIM, IRO, OM3, PPI |
| 6 | 50 | F | 49 | 19.9 | IPAH | 2 | BOS, TAD, ASA, SIM, SER |
| 7 | 47 | F | 70 | 28.4 | IPAH | 2 | BOS, TREPIV, HCT, WAR, ESC, GAB |
| 8 | 19 | F | 54 | 21.9 | IPAH | 2 | SIL, TREPIV, FUR, ALD, DIG, WAR, IRO, PPI |
| 9 | 23 | F | 100 | 44.4 | IPAH | 2 | SIL, TREPIV, BUM, ALD, WAR, MON |
| 10 | 26 | M | 102 | 28.9 | IPAH | 2 | BOS, SIL, TREPPO, FUR, DIG, WAR |
| 11 | 60 | F | 56 | 22.7 | IPAH | 2 | TREPPO, FUR, ALD, WAR, DIP, LOV, FLU |
| 12 | 59 | F | 95 | 34.9 | IPAH | 2 | SIL, TREPIV, FUR, ALD, WAR, IRO, GAB |
| 13 | 74 | F | 84 | 29.8 | IPAH | 2 | SIL, THY, WAR, study drug, CL |
| 14 | 50 | F | 102 | 36.1 | IPAH | 2 | BOS, SIL, FUR, ALD, WAR, FLU, FEX, PPI |
| 15 | 60 | F | 75 | 31.2 | IPAH | 2 | AMB, TRI, ALD, NAP |
| 16 | 44 | F | 61 | 23.8 | IPAH | 2 | EPIV, ALD, FLU, BUP, GAB |
| 17 | 63 | F | 59 | 26.2 | IPAH | 2 | SIL, TREPIN, VAL, O2 |
| 18 | 38 | F | 78 | 26.1 | IPAH | 2 | BOS, TAD, TREPIV, FUR |
| 19 | 49 | F | 86 | 30.5 | IPAH | 2 | AMB, AML, TREPIN, TOR, HCT, PRA, PPI, O2 |
| 20 | 31 | F | 70 | 28.4 | IPAH | 1 | AMB, SIL, TREPIN, DIG, O2 |
| 21 | 33 | F | 64 | 22.7 | IPAH | 1 | NIF, WAR |
| 22 | 29 | M | 73 | 23.8 | IPAH | 1 | TAD |
| 23 | 52 | M | 102 | 32.2 | IPAH | 2 | SIL, AML, WAR, ASA, O2 |
| 24 | 51 | M | 81 | 27.1 | IPAH | 3 | EPIV, FUR, LIS, TRA, TRAM, HYD, CAR, DES |
| 25 | 43 | M | 95 | 31.7 | IPAH | 3 | BOS, TAD, EPIV, FUR, ALD, WAR |
| 26 | 31 | F | 68 | 22.2 | IPAH | 2 | PGI, WAR, ESC, GAB, PPI |
| 27 | 34 | F | 51 | 22.7 | IPAH | 2 | BOS, SIL, TREPSC, FUR, DIG, IRO, HYD, PRE |
| 28 | 63 | F | 48 | 18.8 | HPAH | 2 | SIL, DIL, BUM, WAR, SIM, INS, MSI |
| 29 | 50 | F | 107 | 37.9 | CTD | 2 | BOS, SIL, DIL, FUR, WAR, O2 |
| 30 | 62 | F | 64 | 26.3 | CTD | 2 | SIL, MTX, PPI |
| 31 | 77 | F | 72 | 27.1 | CTD | 2 | BOS, NIF, HCT, THY, PRED, MTX, OM3 |
| 32 | 58 | F | 70 | 27.3 | CTD | 2 | TAD, HCT, WAR, IRO |
| 33 | 40 | F | 82 | 26.8 | CTD | 3 | BOS, SIL, AML, TREPIV, FUR, ALD, PRED, LOS, CLO, ATE |
| 34 | 66 | F | 83 | 31.2 | CTD | 3 | BOS, SIL, FUR, ALD, THY, LIS, PRED, AZA, PPI, PAR, O2 |
| 35 | 50 | F | 60 | 26.0 | CTD | 2 | AMB, AML, FUR, ALD, DIG, ALL, COL, URS, O2 |
| 36 | 35 | F | 86 | 32.4 | CTD | 2 | BOS, NIF, TREPIN, PRED |
| 37 | 51 | F | 54 | 23.4 | CTD | 2 | BOS, SIL, DIL, THY, PRED |
| 38 | 52 | F | 104 | 38.2 | CTD | 2 | BOS, SIL, TREPIN, FUR, BUM, THY, INS, DUL, PRE |
| 39 | 57 | F | 84 | 30.9 | CTD | 2 | NIF, TREPIV, MTX, PRED, FA, PPI |
| 40 | 50 | F | 95 | 35.8 | APAH | 3 | SIL, EPIV, FUR, BUM, DIG, FFI, MET, PAR, PPI, O2 |
| 41 | 57 | F | 63 | 23.7 | APAH | 3 | BOS, SIL, EPIV, FUR, DIG, WAR, O2 |
| 42 | 40 | F | 98 | 32.0 | APAH | 2 | SIL, EPIV, WAR, MED |
| 43 | 31 | F | 120 | 45.2 | APAH | 3 | TREPIV, FUR, WAR, THY, HYD, PAN, GAB, O2 |
Clinical Classification of Pulmonary Hypertension (Dana Point 2008) as described in ref. [36].
Abbreviations: IPAH, idiopathic PAH (Dana Point PH category 1.1); HPAH, heritable PAH (Dana Point PH category 1.2.1); CTD, PAH associated with connective tissue disease (Dana Point PH category 1.4.1); APAH, PAH due to drug use (Dana Point PH category 1.3)
WHO Classification: 1–4.
Medications (prostanoids are in bold): ALD = aldactone PO; ALL = allopurinol PO; AMB = ambrisentan PO; AML = amlodipine; ASA = acetylsalicylic acid; ATE = atenolol (beta blocker) PO; AZA = azathioprine PO; BOS = bosentan PO; BUM = bumetanide PO; BUP = bupropion PO (norepinephrine-dopamine reuptake inhibitor); CAR = carisoprodol (muscle relaxant); CEL = celecoxib PO; CLO = clonidine PO; COL = colchicin PO; DES = desvenlafaxine (serotonin-norepinephrine reuptake inhibitor); DIG = digoxin PO; DIL = diltiazem; DIP = dipyridamole; DUL = duloxetine PO (serotonin-norepinephrine reuptake inhibitor); EPIV = epoprostenol IV; ESC = escitalopram PO (SSRI); EPSC = epoprostenol SC; FA = folic acid PO; FEX = fexofenadine PO; FFI = fenofibrate PO; FLU = fluoxetin PO (SSRI); FUR = furosemide (Lasix) PO; GAB = gabapentin PO; HCT= hydrochlorothiazide PO; HYD = hydrocodone; IBA = ibandronate (biphospate) PO; INS = insulin; IRO = iron PO; LIS = lisinopril PO; LOS = losartan (AT1 rec antagonist) PO; LOV = lovastatin PO; MED = medodrine; MET = metoprolol PO (beta bocker); MON = montelukast inhal.; MSI =metformin and sitagliptin; MTX = methotrexate PO; NAP = naproxene PO; NIF = nifedipine; O2 = oxygen by nasal canula; OM3=omega-3-acid ethyl esters PO; PAR = paroxetine PO (SSRI); PGI = prostanoid (unspec.); PPI = proton pump inhibitor PO; PRA = pravastatin PO; PRE = pregabalin; PRED = prednisone PO; RIS = risedronate (biphosphonate) PO; SD = PH study drug; SER = sertraline PO (SSRI); SIL = sildenafil PO; SIM = simvastatine PO; TAD = tadalafil PO; THY=levothyroxine PO; TOR = torsemide PO; TRA = trazodone (serotonine anatagonist and reuptake inhibitor, SARI); TRAM = tramadole; TREPIV = trepostinil IV; TREPIN = trepostinil inhal.; TREPPO = trepostinil PO; TREPSC = trepostinil subcutaneously; TRI = triamterene PO; URS = ursodiol PO; VAL = valsartan PO (AT1 rec antagonist); VEN = venlaxafine PO (serotonin-norepinephrine reuptake inhibitor); WAR = warfarin (Coumadin) PO
Table 4.
Characteristics of control subjects
| ID | Age (yrs) | Gender (M/F) | Weight (kg) | BMI (kg/m2) | PMHx* | WHO Class | Medication |
|---|---|---|---|---|---|---|---|
| 1 | 60 | F | 59 | 23.0 | Art. Hypertension | 1 | - |
| 2 | 40 | F | 91 | 31.5 | - | 1 | - |
| 3 | 39 | F | 66 | 24.2 | - | 1 | - |
| 4 | 36 | F | 57 | 22.3 | - | 1 | - |
| 5 | 54 | F | 77 | 26.6 | - | 1 | - |
| 6 | 48 | M | 79 | 26.4 | - | 1 | - |
PMHx: Past Medical History
Consistent with prior reports [16, 30], we found that circulating EPC numbers, as defined by the number of CD34+/KDR+ (double-labeled) or CD34+/CD31+/KDR+/CD45− (triple-labeled) cells, were significantly decreased in PAH patients vs. controls (p<0.001; Figure 2A–B). Cells captured on-chip prior to immunolabeling were defined as CD34+ cells (see Figure S3 in the online data supplement). The more stringent triple labeling excluded CD45+ bone marrow (BM) derived hematopoietic stem cells, and included only EPCs that were CD31+, thereby characterizing a more differentiated EPC phenotype typical for circulatory rather than BM-stationary EPCs [2]. This particular EPC phenotype (CD34+/CD31+/KDR+/CD45−) has also been termed “late-EPC” (also known as a late-outgrowth endothelial cell or endothelial colony-forming cell; for details on the significance of this sub-population see Supplemental Results and Discussion in the online data supplement). However, overall, the cell numbers for the two EPC phenotypes measured with the microfluidic chip (CD34+/CD31+; CD34+/CD31+/KDR+/CD45−) were very proportionate and in a similar range (Figure 2–4). The EPC number (CD34+/KDR+) did not differ significantly between genders within a larger set of control subjects (males: 28.5±1.0; females: 27.0±1.1 EPCs/200μL blood; p<0.05; n = 14; age range 23–60 yrs; data not shown) or among PAH patients (males: 16.9±1.0; females: 17.0±0.4 EPCs/200μL blood; p<0.05; n = 43; age range 19–77 yrs; see Table 1). A subgroup analysis by PAH subcategory revealed that not only PAH patients diagnosed with IPAH/HPAH, but also those with drug-induced PAH (due to appetite suppressants or illegal drugs), or PAH associated with connective tissue disease had comparably low EPC numbers that were approximately half the numbers in the healthy control subjects (Figure 2C–D).
Figure 2.
EPC number analysis according to PAH sub-group and EPC surface markers. Circulating endothelial progenitor cells (EPCs), defined as (A,C) CD34+/KDR+, or defined as (B,D) CD34+/CD31+/KDR+/CD45−, were enumerated on-chip. (A,B) Comparison of PAH patients to controls shows distinguishable reduction in overall EPC counts. (C,D) Stratification of PAH patients into subgroups, including idiopathic and heritable PAH (IPAH/HPAH), drug-inducted PAH, and PAH associated with connective tissue disease (CTD) illustrates that EPC numbers are comparable across PAH sub-classes. One-way ANOVA analysis. **p<0.001.
Figure 4.
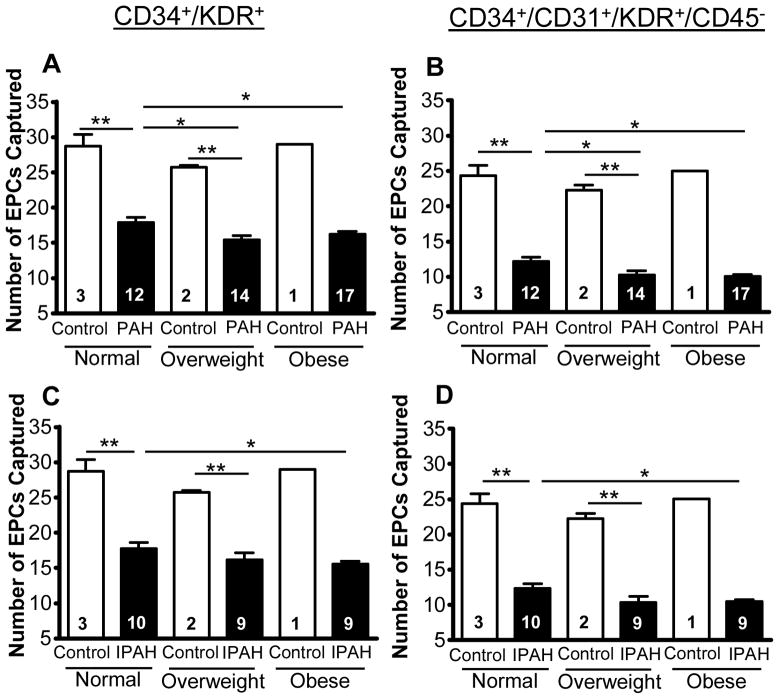
Body mass index (BMI) association with EPC counts in PAH patients. Circulating endothelial progenitor cells (EPCs), defined by expression of (A,C) CD34 and KDR, or defined by expression of (B,D) CD34, CD31 and KDR whilst not expressing CD45, in controls and PAH. Subjects were stratified by BMI for (A,B) PAH (n=43) and (C,D) IPAH patients (includes one HPAH patient; n=28) along with controls. Normal weights were considered as BMI = 18.5–24.9, overweight as BMI = 25–29.9, and obese as BMI ≥ 30 kg/m2. It was determined that EPC numbers decline with BMI in PAH patients for both (A) CD34+/KDR+cells and (B) CD34+/CD31+/KDR+/CD45− cells. Furthermore, for both (C,D) EPC phenotypes, EPC numbers were lower in obese IPAH patients when compared to IPAH patients with normal BMI. There was no significant difference in controls for all BMI subcategories, and no significant difference in overweight IPAH patients versus either obese or non-overweight IPAH patients. Comparison were made via one-way ANOVA analysis with Bonferroni post hoc test. *p<0.05; **p<0.001.
Association between EPC Number and Age in Control But Not PAH Patients
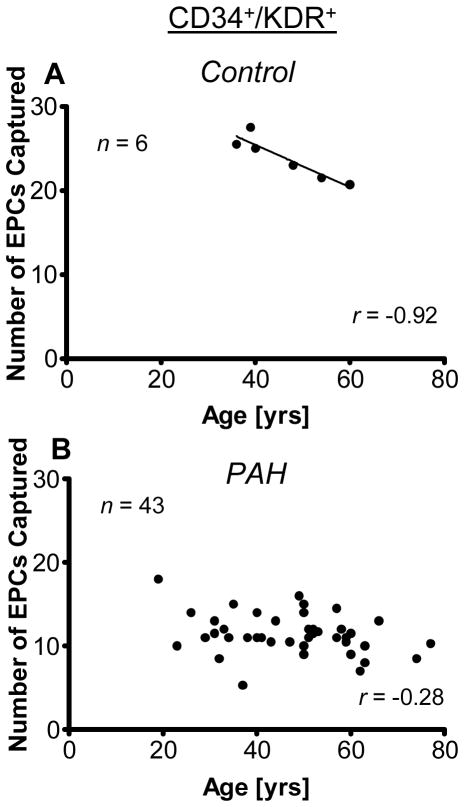
Interestingly, while EPC number (CD34+/CD31+/KDR+/CD45−) inversely correlated with age in control subjects (r=−0.93; p=0.008), there was not such an association in the cohort of enrolled PAH patients (r=−0.28; p=0.08; Figure 5). Thus, other modifiers must influence the EPC numbers to a greater extent than aging alone.
Figure 5.
Age association with EPC counts in controls subjects (A) and PAH patients (B). The number of circulating endothelial progenitor cells (EPCs), defined by expression of CD34 and KDR, inversely correlated with age in (A) controls (n=6; Pearson correlation coefficient r=−0.93 (p=0.008). However, (B) PAH patients (n=43) illustrated no such age-dependent decline with age (r=−0.28; p=0.08). Results with CD34+/CD31+/KDR+/CD45− cells illustrated a similar trend, where controls (n=6) showed a clear decline in cell numbers with age, but PAH patients (n=43) had no statistically significant correlation with age (r=−0.26; p=0.054).
EPC Number Is Inversely Associated With Two Potential Disease Modifiers: Postmenopausal Status And Body-Mass-Index
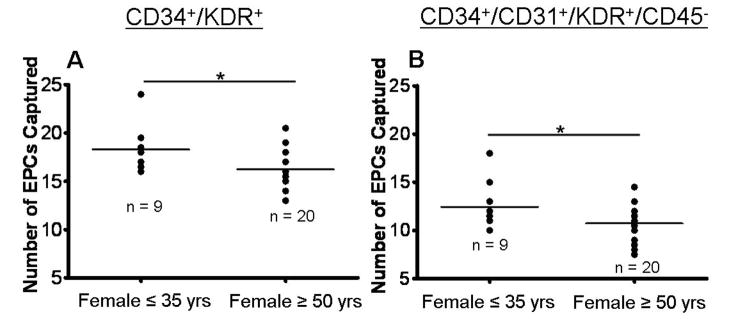
Because metabolic and hormonal factors such insulin resistance, dyslipidemia [31], mitochondrial dysregulation [32, 33], and imbalanced sex hormone composition (ratio) [34] are increasingly recognized as influential environmental factors and potential “second hits” in PAH development, we compared EPC number in pre- vs. postmenopausal women with PAH (Figure 3). Moreover, we measured EPCs within the IPAH/HPAH subgroup in males and females stratified by normal, mildly or greatly elevated body-mass-index (BMI; Figure 4). Interestingly, despite the lack of age-dependency of the EPC number in the PAH cohort, postmenopausal women with PAH (≥ 50 years of age) did have lower EPC numbers (CD34+/KDR+) than younger (premenopausal) affected females ≤ 35 years of age (18.3±0.8 vs. 16.3±0.4 EPCs/200 μL blood; p<0.05; Figure 3A); this difference was seen not only in CD34+/KDR+ but also in CD34+/CD31+/KDR+/CD45− (“late-EPC”) cell counts (12.4±0.9 vs. 10.7±0.4 EPCs/200 μL blood; p<0.05) (Figure 3B).
Figure 3.
Age association of EPC counts in female PAH patients. Circulating endothelial progenitor cells (EPCs) were enumerated on-chip. The number of EPCs, defined by the expression of (A) CD34 and KDR were lower in women over or equal to 50 years when compared to women younger or equal to 35 years of age. (B) This pattern was also illustrated in cells expressing CD34, CD31, KDR, and exclusion of CD45 (also called “late” EPCs or endothelial colony forming cells, ECFCs). Each point represents an individual patient’s EPC count and the bar represents the median value. A comparison between females younger than or equal to 35 yrs and patients over or equal to 50 yrs was made via a student t-test. *p<0.05.
Stratifying the data according to BMI for both the total PAH (n=43) and IPAH/HPAH (n=28) populations illustrates that higher BMI is associated with lower number of circulating EPCs, i.e., a predictor of clinical outcome. In the total PAH cohort, obese (16.2±0.4 EPCs/200 μL blood; p<0.05; BMI ≥ 30 kg/m2) and overweight (15.4±0.6 EPCs/200 μL blood; p<0.05; BMI = 25–29.9 kg/m2) PAH patients had a lower number of circulating EPC versus PAH patients with normal BMI (17.9±0.7 EPCs/200μL blood; BMI = 18.5–24.9 kg/m2) (see Supplementary Results and Discussion for subgroup analysis).
Discussion
We chose PAH for the first clinical application of the EPC capture chip for several reasons: (i) PAH is a prototype of proliferative cardiovascular diseases: Its pathobiology is characterized by endothelial cell death and progressive obliteration of the peripheral pulmonary arteries, and involves multiple signalling pathways which currently makes tailored PAH therapy extremely difficult. Novel PAH biomarkers that indicate disease severity, progression and prognosis of PAH and associated right ventricular (RV) dysfunction would be extremely helpful in guiding established and more experimental clinical therapies. (ii) The number of circulating EPCs represents a promising candidate biomarker for pulmonary vascular disease severity: Adult IPAH patients have reduced numbers of circulating EPCs when compared with healthy controls; a reduced number of EPCs (CD34+/KDR+) is associated with worse hemodynamics, and abnormally elevated concentrations of inflammatory markers, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP) [16], and asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor [16]. Heightened plasma ADMA levels are observed in PAH patients, and negatively correlate with hemodynamic performance and survival rates [35]. (iii) Pilot studies have demonstrated that autologous transplantation of EPCs is safe and leads to significant improvements in pulmonary hemodynamics, exercise capacity including 6-minute-walk-distance in children [36] and adults with PAH [37]. (iv.) EPC number indicates response to therapy: Treatment with the phosphodiesterase type 5 (PDE5)-inhibitor sildenafil, an established PAH medication, leads to a dose-dependent rise in EPC numbers in IPAH patients [16]. In separate studies, peroxisome proliferator-activated receptor-γ (PPARγ) treatment inhibit the negative effects of CRP on human EPC survival, differentiation, and function [30], and increase EPC number in culture [38]. Recently, we demonstrated that PPARγ agonists reverse PAH, right ventricular hypertrophy and pulmonary vascular remodeling in rodents [39] thereby revealing their potential as a new pharmacotherapy [40–42]. We [43] and others [44] have since shown that metabolic dysregulation such as insulin resistance (IR) [43] and dyslipidemia (low HDL-cholesterol [43, 44]) is more common in (female) PAH patients and associated with clinical worsening and poorer survival at six [43] and twenty [44] months follow up. Given our current results on the lower number of circulating EPCs in obese vs. non-obese, and post- vs. pre-menopausal PAH patients, and the previously described inverse relation between EPC number and hemodynamic status of PAH patients [16], it will be important to explore the impact of metabolic regulators such as PPARs [42], mitochochondrial regulatory proteins [33], micro RNAs [45, 46], and sex hormones [34, 47] on pulmonary vascular disease and associated right ventricular dysfunction.
There are several limitations to our study. Although most of the published competitive techniques solely use cell surface markers [13], it should be noted that the definition of a stem/progenitor cell optimally should be based on both surface markers and functional assays. Specifically, EPC characteristics including cell surface proteins have been shown to differ depending on the culture techniques and stage of differentiation in which the cells are isolated (CFU-Hill, early-EPCs, or late-EPCs; see Supplementary Results and Discussion for details). Among the existing EPC assays only the laborious colony forming unit (CFU) assays (processing time 5 days) give information on EPC function (see Table 2). Hence, as an EPC characterization tool, the described device is somewhat limited because the captured cell population is defined solely based on surface markers. However, as a practical diagnostic device, characterization of EPC function is secondary to quantifying a novel, reliable and validated cellular PAH biomarker such as EPC number that is inversely associated with the hemodynamic status of PAH patients and increased by the phospodiesterase inhibitor sildenafil [48]. This study was not designed to comprehensively investigate the role of EPC and EPC function in PAH; rather this report merely presents a novel EPC enumeration modality as a potential “bedside test” and facile alternative to the conventional laborious techniques.
Currently conflicting data exist in the literature on the relative EPC number in PAH patients [11, 12]. A number of studies have described a reduction in circulating EPCs when compared with healthy controls [16–18], consistent with our data presented herein. However, others have found an elevation [19, 20], or no difference [49] in EPC numbers in PAH patients versus healthy controls. A possible explanation for this discord in the literature may be attributed to the various methods and cell surface protein markers used to identify, isolate and quantify the cells, as well as possible differences in patient selection. Secondly, it is possible that “early” (immature) EPCs (CD133+) may be released from the bone marrow as an early adaptive response to pulmonary vascular injury [48]. We speculate that environmental factors such as inflammation [50], sex hormone dysbalance [34], insulin resistant state [31], and/or dyslipidemia [31, 44] subsequently inhibit the differentiation of the circulating EPCs from “early” to the “late” (mature) EPC (CD31+) phenotype. Such a biphasic EPC response may account for the different findings in patients with IPAH published to date [16–20, 49]. Nevertheless, the beneficial role of EPCs in PAH is supported by recent reports on the successful autologous transplantation of CD34+/KDR+ cells that lead to hemodynamic and clinical improvement in children [36] and adults [37] with IPAH.
In summary, the new EPC capture chip captures a significant percentage of EPCs in a single step, requiring minimal blood volume (200 μL) which is important for pediatric clinical care and small animal research. To the best of our knowledge, this is the first report on the application of a polymeric cell-affinity microfluidic diagnostic platform in cardiovascular disease and the first on the validation and clinical application of such a microfluidic device for the analysis of human circulating EPCs. In addition to patients with idiopathic PAH, those with PAH associated with either connective tissue disease or drug use (appetite suppressants) have about half the number of circulating EPCs relative to healthy, age and gender matched controls. Beyond the technical aspects, we found that the clinically relevant number of circulating EPCs (CD34+/KDR+, CD34+/KDR+/CD31+/CD45−) is inversely related to BMI and postmenopausal status in PAH patients. The novel EPC capture chip has the potential to become a practical, diagnostic tool in the risk assessment and clinical monitoring of patients with PAH and other cardiopulmonary and neurodegenerative diseases [51] as well as cancer [52], requiring only small blood volumes and no laborious pre-processing steps.
Supplementary Material
Acknowledgments
We thank the organizers and volunteers of the Pulmonary Hypertension Association’s Research Room, Garden Grove, CA, for their help with setting up the laboratory equipment.
Funding
This work was supported by NIH grant R01 EB009327 (S.K.M) and IGERT NSF/NCI grant NSF-DGE-0504331 (B.D.P.).
Footnotes
Potential conflict of interest: None
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Hristov M, Erl W, Weber PC. Endothelial progenitor cells - Mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 3.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 4.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 5.Van Craenenbroeck EM, Conraads VM, Van Bockstaele DR, Haine SE, Vermeulen K, Van Tendeloo VF, Vrints CJ, Hoymans VY. Quantification of circulating endothelial progenitor cells: a methodological comparison of six flow cytometric approaches. J Immunol Methods. 2008;332:31–40. doi: 10.1016/j.jim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 7.Plouffe BD, Kniazeva T, Mayer JE, Jr, Murthy SK, Sales VL. Development of microfluidics as endothelial progenitor cell capture technology for cardiovascular tissue engineering and diagnostic medicine. FASEB J. 2009;23:3309–3314. doi: 10.1096/fj.09-130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, Nanus DM, Giannakakou PA, Kirby BJ. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip. 2010;10:27–29. doi: 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asosingh K, Erzurum SC, Yoder MC, Tuder RM. Letter by Asosingh et al Regarding Article, “Circulating Endothelial Progenitor Cells in Patients With Eisenmenger Syndrome and Idiopathic Pulmonary Arterial Hypertension”. Circulation. 2009;119:E230. doi: 10.1161/CIRCULATIONAHA.108.800961. [DOI] [PubMed] [Google Scholar]
- 12.Diller GP, Bedard E, Wort SJ, Gatzoulis MA, van Eijl S, Ali O, Wilkins MR, Wharton J, Okonko DO, Howard LS, et al. Response to Letter Regarding Article, “Circulating Endothelial Progenitor Cells in Patients With Eisenmenger Syndrome and Idiopathic Pulmonary Arterial Hypertension”. Circulation. 2009;119:E231–E231. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34(−)/CD133(+)/VEGFR-2(+) endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:E20–E25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 15.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial Outgrowth Cells Are Not Derived From CD133+ Cells or CD45+ Hematopoietic Precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 16.Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bedard E, Gibbs JS, Bauersachs J, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117:3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, Schiavon M, Rea F, Avogaro A, Agostini C. Depletion of endothelial progenitor cells may link pulmonary fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2007;176:724–725. doi: 10.1164/ajrccm.176.7.724a. [DOI] [PubMed] [Google Scholar]
- 18.Zhu JH, Wang XX, Fu GS, Shang YP, Zhang FR, Chen JZ. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Resp Med. 2008;102:1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SAA, Xu WL, Licina L, Huang L, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol. 2008;172:615–627. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LSG, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, et al. Evidence of Dysfunction of Endothelial Progenitors in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smadja DM, Mauge L, Sanchez O, Silvestre JS, Guerin C, Godier A, Henno P, Gaussem P, Israel-Biet D. Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J. 2010;36:1284–1293. doi: 10.1183/09031936.00130809. [DOI] [PubMed] [Google Scholar]
- 22.Plouffe BD, Njoka D, Harris J, Liao J, Horick NK, Radisic M, Murthy SK. Peptide-mediated selective adhesion of smooth muscle and endothelial cells in microfluidic shear flow. Langmuir. 2007;23:5050–5055. doi: 10.1021/la0700220. [DOI] [PubMed] [Google Scholar]
- 23.Vickers JA, Caulum MM, Henry CS. Generation of hydrophilic poly(dimethylsiloxane) for high-performance microchip electrophoresis. Anal Chem. 2006;78:7446–7452. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- 24.Sales VL, Engelmayr GC, Jr, Johnson JA, Jr, Gao J, Wang Y, Sacks MS, Mayer JE., Jr Protein precoating of elastomeric tissue-engineering scaffolds increased cellularity, enhanced extracellular matrix protein production, and differentially regulated the phenotypes of circulating endothelial progenitor cells. Circulation. 2007;116:I-55–63. doi: 10.1161/CIRCULATIONAHA.106.6806637. [DOI] [PubMed] [Google Scholar]
- 25.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human Endothelial Progenitor Cells From Type II Diabetics Exhibit Impaired Proliferation, Adhesion, and Incorporation Into Vascular Structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 26.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protocols. 2007;2:805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leor J, Marber M. Endothelial Progenitors: A New Tower of Babel? J Am Coll Cardiol. 2006;48:1588–1590. doi: 10.1016/j.jacc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Erzurum S, Rounds SI, Stevens T, Aldred M, Aliotta J, Archer SL, Asosingh K, Balaban R, Bauer N, Bhattacharya J, et al. Strategic Plan for Lung Vascular Research: An NHLBI-ORDR Workshop Report. Am J Respir Crit Care Med. 2010;182:1554–1562. doi: 10.1164/rccm.201006-0869WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yen A. Flow cytometry: Advanced Research and Clinical Applications. CRC Press; Boca Raton, FL: 1989. [Google Scholar]
- 30.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 31.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, Michelakis ED. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med. 2010;2:44ra58. doi: 10.1126/scitranslmed.3001327. [DOI] [PubMed] [Google Scholar]
- 34.Christou HA, Khalil RA. Sex Hormones and Vascular Protection in Pulmonary Arterial Hypertension. J Cardiovasc Pharmacol. 2010;56:471–474. doi: 10.1097/FJC.0b013e3181fa8e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kielstein JT, Bode-Böger SM, Hesse G, Martens-Lobenhoffer J, Takacs A, Fliser D, Hoeper MM. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–1418. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- 36.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: Open-label pilot study. Ped Transpl. 2008;12:650–655. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang XG, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension - A pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Redondo S, Hristov M, Gumbel D, Tejerina T, Weber C. Biphasic effect of pioglitazone on isolated human endothelial progenitor cells: involvement of peroxisome proliferator-activated receptor-gamma and transforming growth factor-beta1. Thromb Haemost. 2007;97:979–987. [PubMed] [Google Scholar]
- 39.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 40.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansmann G, Rabinovitch M. The protective role of adiponectin in pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol. 2010;298:L1–2. doi: 10.1152/ajplung.00367.2009. [DOI] [PubMed] [Google Scholar]
- 42.Hansmann G, Zamanian RT. PPARgamma activation: a potential treatment for pulmonary hypertension. Sci Transl Med. 2009;1:12ps14. doi: 10.1126/scitranslmed.3000267. [DOI] [PubMed] [Google Scholar]
- 43.Zamanian RT, Hansmann G, Lilienfeld D, Rappaport K, Rabinovitch M, Reaven G, Doyle RL. Insulin resistance and pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;175:A713 (abstract). doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heresi GA, Aytekin M, Newman J, Didonato J, Dweik RA. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:661–668. doi: 10.1164/rccm.201001-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 Modulates the Expression of the Bone Morphogenic Protein Receptor Type II Through a Novel STAT3-microRNA Cluster 17/92 Pathway. Circ Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 46.Chan SY, Zhang Y-Y, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 Controls Mitochondrial Metabolism during Hypoxia by Repressing the Iron-Sulfur Cluster Assembly Proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tofovic SP. Estrogens and Development of Pulmonary Hypertension - Interaction of Estradiol Metabolism and Pulmonary Vascular Disease. J Cardiovasc Pharmacol. 2010;56:696–708. doi: 10.1097/FJC.0b013e3181f9ea8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diller GP, Thum T, Wilkins MR, Wharton J. Endothelial Progenitor Cells in Pulmonary Arterial Hypertension. Trends Cardiovasc Med. 2010;20:22–29. doi: 10.1016/j.tcm.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Smadja DM, Gaussem P, Mauge L, Israel-Biet D, Dignat-George F, Peyrard S, Agnoletti G, Vouhe PR, Bonnet D, Levy M. Circulating Endothelial Cells A New Candidate Biomarker of Irreversible Pulmonary Hypertension Secondary to Congenital Heart Disease. Circulation. 2009;119:374–381. doi: 10.1161/CIRCULATIONAHA.108.808246. [DOI] [PubMed] [Google Scholar]
- 50.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, Growth Factors, and Pulmonary Vascular Remodeling. J Am Coll Card. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Lee ST, Chu K, Jung KH, Park HK, Kim DH, Bahn JJ, Kim JH, Oh MJ, Lee SK, Kim M, et al. Reduced circulating angiogenic cells in Alzheimer disease. Neurology. 2009;72:1858–1863. doi: 10.1212/WNL.0b013e3181a711f4. [DOI] [PubMed] [Google Scholar]
- 52.Dome B, Timar J, Ladanyi A, Paku S, Renyi-Vamos F, Klepetko W, Lang G, Dome P, Bogos K, Tovari J. Circulating endothelial cells, bone marrow-derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: From biology to therapy. Crit Rev Oncol Hematol. 2009;69:108–124. doi: 10.1016/j.critrevonc.2008.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.