Abstract
Objective
Although diabetes mellitus is implicated in susceptibility to infection, the association of diabetes with the subsequent course and outcome is unclear.
Design and setting
Retrospective analysis of two multicenter cohorts. We determined the association of pre-existing diabetes on the host immune response, acute organ function, and mortality in patients hospitalized with community-acquired pneumonia (CAP) in the GenIMS study (n=1895) and on mortality following either CAP or non-infectious hospitalizations in the population-based cohort study, Health ABC (n=1639).
Measurements
Mortality rate within first year, risk of organ dysfunction, and immune responses, including circulating inflammatory (tumor necrosis factor, interleukin-6, interleukin-10), coagulation (Factor IX, thrombin-antithrombin complexes, antithrombin), fibrinolysis (plasminogen-activator inhibitor-1, and D-dimer), and cell-surface markers (CD120a, CD120b, HLA-DR, TLR-2 and TLR-4).
Results
In GenIMS, diabetes increased mortality rate within first year after CAP (unadjusted hazard ratio [HR]=1.41, 95% confidence interval [CI]=1.12–1.76, p=0.002), even after adjusting for pre-existing cardiovascular and renal disease (adjusted HR=1.3, CI=1.03–1.65, p=0.02). In Health ABC, diabetes increased mortality rate within first year following CAP hospitalization, but not after hospitalization for non-infectious illnesses (significant interaction for diabetes and reason for hospitalization [p=0.04]; HR for diabetes on mortality over first year after CAP 1.87, CI=0.76–4.6, p=0.16 and after non-infectious hospitalization=1.16, CI=0.8–1.6, p=0.37). In GenIMS, immediate immune response was similar, as evidenced by similar circulating immune marker levels in the emergency department and during the first week. Those with diabetes had higher risk of acute kidney injury during hospitalization (39.3% vs. 31.7%, p=0.005) and they were more likely to die due to cardiovascular and kidney disease (34.4% vs. 26.8% and 10.4% vs. 4.5%, p=0.03).
Conclusions
Pre-existing diabetes was associated with a higher risk of death following CAP. The mechanism is not due to an altered immune response, at least as measured by a broad panel of circulating and cell surface markers, but may be due to worsening of pre-existing cardiovascular and kidney disease.
It is a long-standing medical axiom that diabetes mellitus is a risk factor for infection.1,2 However, once infection occurs, the effect of diabetes on mortality is less clear. Some studies suggest diabetes is associated with higher mortality after an infection, but others show no association.3–7 Prior studies that showed the association between diabetes and higher mortality may be confounded by higher prevalence of pre-existing chronic conditions, such as chronic kidney disease (CKD) or cardiovascular disease.8–10
If diabetes indeed is associated with higher mortality after infection, underlying mechanisms are unknown. Several mechanisms could explain these survival differences. For instance, animal and human models of infection suggest that immune abnormalities in diabetes, such as higher pro-inflammatory,11,12 pro-coagulant, and anti-fibrinolytic activity,13,14 and higher expression of pathogen recognition cell-surface receptors,15,16 could worsen during acute illness and increase mortality. An alternate mechanism for higher mortality among diabetics is increased risk of acute organ dysfunction due to higher chronic disease burden.
We therefore examined two multicenter observational cohort studies to understand the effect of diabetes on the host immune response and outcomes of pneumonia. We analyzed a cohort of patients with community-acquired pneumonia (CAP) enrolled in the Genetic and Inflammatory Markers of Sepsis (GenIMS) study, and the subgroup of the population-based cohort, Health, Aging, and Body Composition (Health ABC) study, who required hospitalization. In both cohorts, we tested the hypothesis that pre-existing diabetes is associated with increased mortality within first year, independent of pre-existing chronic diseases. We then determined whether higher mortality is attributable to CAP per se by comparing survival differences over the first year in Health ABC between CAP and non-infectious hospitalizations. Finally, in GenIMS we tested the hypothesis that survival differences between those with and without diabetes were due to differences in immune response and a higher risk of acute organ dysfunction during infection.
METHODS
Subjects and design
We analyzed subjects enrolled in the GenIMS cohort to assess differences in mortality, organ dysfunction, and immune response. GenIMS is a prospective multicenter observational cohort of subjects with CAP enrolled in emergency departments (ED) of 28 academic and community hospitals in four US regions, including southwestern Pennsylvania, Connecticut, southern Michigan, and western Tennessee. Eligibility criteria are shown in Table 1. Of the 2320 subjects enrolled, we excluded 288 patients because they were discharged from the ED and an additional 137 patients because the clinical team ruled out CAP during the first three days of hospitalization, thus restricting the analysis to the remaining 1895 subjects.
Table 1.
Characteristics, eligibility criteria, and methods to determine clinical and outcome measures in Genetic and Inflammatory Markers of Sepsis (GenIMS) and Health, Aging, and Body Composition (Health ABC) cohorts.
| Variable | GenIMS study | Health ABC study |
|---|---|---|
| Inclusion criteria | Age >18 years and clinical and radiologic diagnosis of community-acquired pneumonia |
70–79 year community-dwelling older adults |
| Exclusion criteria | Transfer from another hospital, discharge from an acute care hospital within the previous ten days, diagnosis of pneumonia within the previous 30 days, chronic dependency on mechanical ventilation, cystic fibrosis, active pulmonary tuberculosis, admission for palliative care, prior enrollment in the study, incarceration, or pregnancy. |
Actively treated cancer, severe cognitive impairment, or plans of leaving the area within three years. |
| Enrollment period | 2001–2003 | 1997–1998 |
| Ascertainment of outcome measures | ||
| All-cause mortality | By study nurses during hospitalization and by National Death Index after hospital discharge |
Semi-annual telephone follow-up, notification of death by proxy, spouse, relative, or friend, and review of obituaries in local newspaper. Once a death was reported, a copy of the death certificate was obtained in all cases to confirm death, and the next of kin or a pre-designated proxy was interviewed |
| Cause specific mortality | National Death Index codes | Not analyzed due to small sample size |
| Severe sepsis | Defined as pneumonia and organ dysfunction, based on the International Consensus Criteria34 |
Not assessed |
| Comorbid conditions | ||
| Diabetes mellitus | Self-report and review of medications (use of oral hypoglycemic medications or insulin) |
Self-report and review of medications (use of oral hypoglycemic medications or insulin) or fasting glucose ≥126 mg/dl35 |
| Cardiovascular disease | Self-report and review of medications | Self-report and review of medications |
| Chronic kidney disease | Self report and pre-hospitalization creatinine measurements, if available. We also used creatinine measurements available during hospitalization to exclude chronic kidney disease |
Creatinine measurements at enrollment and using the MDRD criteria36 |
To assess whether higher mortality is attributable to CAP, we analyzed subjects enrolled in the Health ABC study, a population-based observational cohort of 70–79 year old well-functioning participants. Health ABC participants were enrolled from the same geographic regions as GenIMS, including southwestern Pennsylvania (Pittsburgh) and western Tennessee (Memphis). Of the 3075 enrolled, we analyzed 1645 (53.5%) subjects who were hospitalized at least once during the first five years of follow-up. We excluded an additional six subjects whose diabetes status was not known, restricting the analysis to the remaining 1639 subjects. Methods to ascertain hospitalization have been described previously.17 Briefly, we assessed outcomes after the first major hospitalization following enrollment, comparing mortality rate within first year among those initially hospitalized for CAP and non-infectious illnesses (see Appendix). For each cohort, the Institutional Review Boards at each site approved the study. Informed consent was obtained from participants or next of kin for GenIMS and from participants for Health ABC.
Outcome and clinical variables
For GenIMS and Health ABC cohorts, the primary outcome variable was all-cause mortality rate within first year (Table 1). We examined several additional secondary outcome variables in GenIMS. We examined cause-specific mortality using National Death Index codes. The validity of this method has been described previously.18,19 To compare risk of developing acute organ dysfunction during hospitalization, we compared risk of severe sepsis (infection plus organ dysfunction) using Consensus criteria,20 and the risk of individual organ dysfunction for six organ systems. For acute kidney injury, we used the RIFLE criteria.21 The RIFLE criteria classifies acute kidney injury into three categories of severity using changes in serum creatinine and urine output (Risk, Injury, and Failure corresponding to mild, moderate, and severe kidney injury, respectively). Finally, we used measures of severity of illness at hospital presentation, including APACHE III and the Pneumonia Severity Index (PSI).22,23 Details of methods to assess comorbid conditions in both cohorts, including diabetes, are included in Table 1. Microbiologic characteristics were available only in GenIMS and assessed using blood and sputum cultures (see Online Repository).
Laboratory procedures
We assessed differences in the immune responses to infection in GenIMS during hospitalization between those with and without diabetes by comparing changes in circulating concentrations of biomarkers within inflammatory (tumor necrosis factor-α [TNF], interleukin [IL]-6, IL-10), coagulation (Factor IX, thrombin-antithrombin complexes [TAT], antithrombin), and fibrinolysis (plasminogen activator inhibitor [PAI]-1, and D-dimer) systems and expression of cell surface markers (CD120a and CD120b [signaling receptors for TNF], HLA-DR, and Toll-like receptor [TLR]2 and TLR4) on presentation to ED and over the first week. Details of sample collection and processing have been described previously.24
We used automated chemiluminescent immunoassay analyzer (IMMULITE, Diagnostic Products Corp., Los Angeles, CA, USA) to analyze TNF, IL-6, and IL-10. We analyzed coagulation and fibrinolysis markers in a random subset of 734 subjects by a commercial laboratory (Esoterix, Agoura Hills, CA, USA). Specific methods and kits used were: D-dimer, latex immunoassay (Diagnostica Stago, Parsippany, NJ, USA); PAI-1, bio immunoassay (Biopool Chromolize, Biopool International, Ventura, CA, USA); AT, chromogenic (BioMerieux, Rhône-Alpes, France); Factor IX, clot (BioMerieux); and TAT, ELISA (Behring, King of Prussia, PA, USA).
We analyzed cell-surface markers on ED presentation and on third and seventh day. We analyzed cell-surface markers in a subset of 624 subjects enrolled in hospitals located within 60 miles of the University of Pittsburgh because samples for cell-surface markers have to be analyzed within 48 hours. We obtained fluorochrome- or biotin-conjugated antibodies from eBioScience (San Diego, CA, USA) for TLR2, TLR4, and HLA-DR and from Invitrogen (Carlsbad, CA, USA) for CD120a and CD120b. We incubated these antibodies with whole blood, lysed the red blood cells, and then washed, fixed, and stored the remaining cells at −4 °C. We acquired cell-surface marker data within 48 hours of fixation on a BD FACSVantage SE flow cytometer (San Jose, CA, USA) and used BD CellQuest software. Additional details of the assays for all markers are included in the Online Repository.
Statistical analyses
We conducted univariate comparisons of clinical characteristics for subjects with and without diabetes using chi-square and student’s t-test and their non-parametric counterparts when necessary in both cohorts. We compared mortality rate within first year by constructing Kaplan-Meier survival curves for subjects with and without diabetes and used the Cox proportional hazards model. For the proportional hazards model, we confirmed that the hazard ratios (HR) were similar over different intervals. We constructed serial models, including unadjusted, adjusted for demographic characteristics (age, sex, and race), and adjusted for demographic characteristics and chronic diseases associated with diabetes, such as cardiovascular disease and CKD. To assess if mortality differences were attributable to CAP, we compared mortality in those hospitalized initially for CAP and other non-infectious illnesses in Health ABC. We tested for an interaction between diabetes and the reason for initial hospitalization on mortality rate within first year.
We assessed mechanisms of increased mortality in GenIMS. We compared risk of severe sepsis and individual organ dysfunction using logistic regression analyses. We compared differences in immune response to infection, comparing biomarkers and cell-surface markers in subjects with and without diabetes at ED presentation and during the first week of hospital stay. We used regression analysis with mixed models to account for correlation of these markers over time. We also compared inflammatory and coagulation biomarkers at hospital discharge in the subset that was discharged alive and appeared to have recovered clinically to compare resolution of immune response. We have previously shown that those with unresolved immune response, as evidenced by higher concentrations of biomarkers at hospital discharge, had higher mortality over first year after hospital discharge.25 To account for biomarkers that were truncated because they were below detection thresholds, we used Tobit models to compare biomarker concentrations.26 Due to the large number of comparisons for the cell surface markers, we reported p-values adjusted for false discovery rate at 0.05.27
RESULTS
Baseline characteristics of the GenIMS cohort
Table 2 shows pre-hospitalization characteristics for 1895 subjects enrolled in GenIMS. The average age of the cohort was 67 years and two-thirds had a history of smoking. Respiratory disease was the most common chronic disease and occurred in approximately a third of the cohort, and cardiovascular disease occurred in a fourth of the cohort. Diabetes occurred in 384 (20.3%) subjects. The prevalence of other chronic diseases, such as CKD, cancer, and HIV, was low (<5%).
Table 2.
Demographic and clinical characteristics of all subject, stratified by diabetes, enrolled in Genetic and Inflammatory Markers of Sepsis (GenIMS) and Health, Aging, and Body Composition (Health ABC) study
| Variable | GenIMS study (n=1895) |
Health ABC study (n=1645) |
||||
|---|---|---|---|---|---|---|
| All subjects | With diabetes (n=384) |
Without diabetes (n=1511) |
All subjects | With diabetes (n=299) |
Without diabetes (n=1340) |
|
| Demographics | ||||||
| Age, mean (sd, median) | 67.2, (16.8, 72) | 69.3, (14.5, 73) | 66.7 (17.3, 71) | 73.7 (2.8, 74) | 73.7 (2.8, 73) | 73.7 (2.8, 74) |
| Sex, female, n (%) | 910 (48.0) | 177 (46.1) | 733 (48.5) | 766 (46.7) | 132 (44.1) | 634 (47.3) |
| Race, white, n (%) | 1,529 (80.7) | 307 (80) | 1,222 (80.1) | 939 (57.2) | 127 (42.4) | 812 (60.6) |
| Health behaviors | ||||||
| Ever smoked, n (%) | 1,262 (66.6) | 244 (63.5) | 1,018 (67.4) | 997 (60.9) | 183 (61.6) | 814 (60.8) |
| Chronic health conditions | ||||||
| Respiratory disease, n (%) | 718 (37.9) | 148 (38.5) | 570 (37.7) | 320 (24.2) | 64 (27.4) | 256 (19.3) |
| Cardiovascular disease, n (%) | 488 (25.7) | 165 (42.9) | 323 (21.3)* | 438 (27.3) | 97 (35.9) | 341 (25.9)* |
| Chronic kidney disease, n (%) | 92 (4.8)† | 40 (10.4) | 52 (3.4)* | 403 (24.5) | 102 (34.1) | 301 (22.4)* |
| Cancer§, n (%) | 94 (5.0) | 22 (5.7) | 72 (4.8) | - | - | - |
| HIV§, n (%) | 37 (2.0) | 1 (0.3) | 36 (2.4)* | - | - | - |
p<0.05 for comparison among subjects with and without diabetes;
Chronic kidney disease (CKD) diagnosis was available in 1571 (82.9%) subjects in GenIMS. In the remaining 324 subjects, CKD could not be ruled out based on history and because their plasma creatinine values were elevated on the first day of hospitalization for pneumonia,
Health ABC excluded subjects with active cancer and none reported HIV.
Subjects with diabetes, on average, were three years older than subjects without diabetes, but results did not reach statistical significance. Those with diabetes were more likely to have CKD (10.4% vs. 3.4%, p<0.0001) and cardiovascular disease (42.9% vs. 21.3%, p<0.0001). HIV was more prevalent among those without diabetes (2.4% vs. 0.3%, p=0.02) and only 1 subject with diabetes had HIV. No differences were seen in the prevalence of smoking, cancer, and respiratory disease between the two groups.
Blood or sputum cultures to determine microbiologic etiology were obtained in 1606 (84.7%) subjects within 48 hours of ED presentation. An etiologic agent was identified in 186 (11.5%) subjects and this frequency was similar among those with and without diabetes (12.6% vs. 11.3%, p=0.5). Among subjects in whom an etiologic agent was identified, Gram-negative organisms were more common among those with diabetes, Gram-positive organisms were more common among those without, and no differences were seen in the frequency of mixed or anaerobic organisms (7.5% vs. 3.1%, 4.2% vs. 7.5%, 1% vs. 0.7% for Gram-negative, Gram-positive, and mixed or anaerobic organisms, respectively, p=0.001).
Diabetes was associated with higher acute organ dysfunction in GenIMS
At ED presentation, subjects with diabetes had greater illness severity, as evidenced by higher PSI scores (111.1 vs. 96.9, p<0.0001), higher frequency of subjects with diabetes in PSI Classes IV and V (68.7% vs. 53.6%, p<0.0001), and higher APACHE III scores (59.5 vs. 55.2, p<0.0001). When points for the physiology components of the APACHE III score were compared, those with diabetes had a higher score compared to those without diabetes (42.6 vs. 39.8, p<0.0001).
Subjects with diabetes had higher risk of severe sepsis compared to those without diabetes (34.6% vs. 29.7%, OR=1.25, 95% confidence intervals [CI]=0.99–1.58), but results were not statistically significant (p=0.06). This association was not statistically significant when adjusted for age, sex, race, and pre-existing cardiovascular disease and CKD (OR=1.23, 95% CI=0.96–1.59, p=0.09).
Diabetes increased the risk of acute kidney injury (30.2% vs. 22.9% for subjects with and without diabetes, p=0.007) and this association remained significant when adjusted for age, sex, and race (p=0.02). The association persisted when subjects with CKD were excluded (29.3% vs. 23.6%, p=0.05). The higher risk of acute kidney injury was mainly due to increased risk of moderate (RIFLE-I) kidney injury (7.6% vs. 3.6%), and only small differences were observed in the risk of mild (RIFLE-R) (10.9% vs. 9.6 %) and severe kidney injury (RIFLE-F) (11.6% vs. 9.6%). No differences were observed in the risk of respiratory, neurologic, cardiovascular, coagulation, and liver dysfunction (Table 3). Furthermore, those with and without diabetes were equally likely to require mechanical ventilation (6.8% vs. 7%, p=0.87) and intensive care unit (ICU) admission (14.1% vs. 16.5%, p=0.25) and only a small difference was seen in the median length of hospital stay (7.8 vs. 7.4 days, p=0.02).
Table 3.
Risk of severe sepsis and organ dysfunction among subjects with and without diabetes*
| Variable | With diabetes | Without diabetes | P value |
|---|---|---|---|
| Risk of severe sepsis, n (%) | 133 (34.6) | 449 (29.7) | 0.06 |
| Organ dysfunction, n (%) | |||
| Respiratory | 76 (19.8) | 254 (16.8) | 0.17 |
| Cardiovascular | 15 (3.9) | 60 (4.0) | 0.95 |
| Renal | 116 (30.2) | 346 (22.9) | 0.007 |
| Liver | 1 (0.3) | 11 (0.7) | 0.27 |
| Neurologic | 31 (8.1) | 83 (5.5) | 0.06 |
| Coagulation | 4 (1.0) | 19 (1.3) | 0.49 |
Diabetes was associated with higher mortality in GenIMS
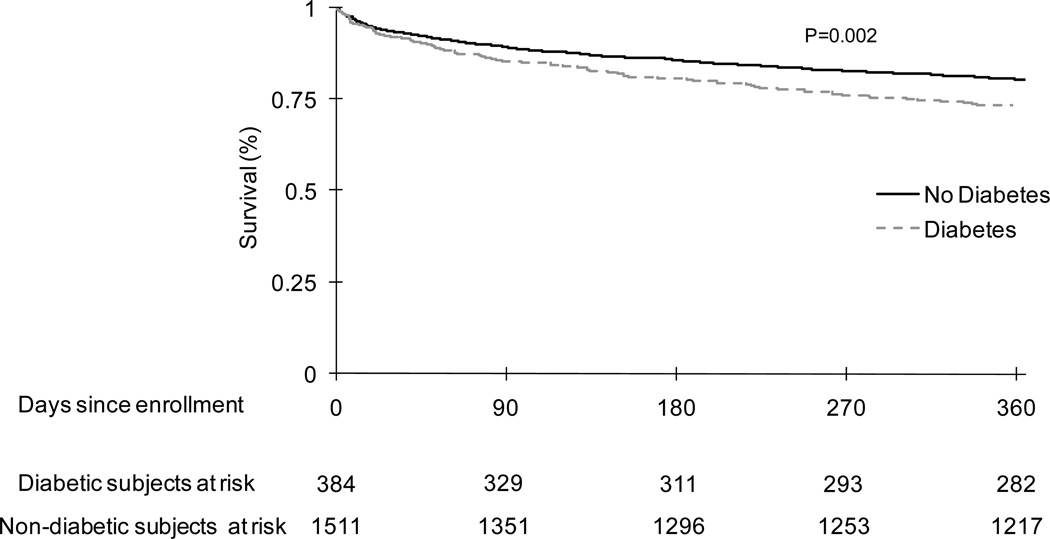
Subjects with diabetes had higher mortality rates over first year (unadjusted hazard ratio [HR] was 1.41, CI=1.12–1.76, p=0.002, Figure 1 and Table 4). The HR remained unchanged and the association remained statistically significant when adjusted for demographics (HR=1.32, CI=1.05–1.66, p=0.01) and additionally adjusting for higher burden of cardiovascular disease and CKD (HR=1.3, CI=1.03–1.65, p=0.02).
Figure 1.
Failure plots over 1 year showing higher risk of death for subjects with diabetes compared to those without diabetes following hospitalization for community acquired pneumonia in GenIMS.
Table 4.
Unadjusted and adjusted hazard ratios (HR) with 95% confidence intervals (CI) for mortality over 1 year for subjects with diabetes compared to those without using Cox proportional hazards model
| Variables | GenIMS study | Health ABC study |
|||||
|---|---|---|---|---|---|---|---|
| Pneumonia hospitalization | Pneumonia hospitalization | Hospitalization for non-infectious illness |
Interaction p value* |
||||
| HR with 95% CI | p value | HR with 95% CI | p value | HR with 95% CI | p value | ||
| Unadjusted | 1.41 (1.12–1.76) | 0.002 | 1.87 (0.76–4.6) | 0.16 | 1.16 (0.8–1.6) | 0.37 | 0.04 |
| Adjusted for age, race, sex |
1.32 (1.05–1.66) | 0.01 | 1.82 (0.72–4.62) | 0.2 | 1.08 (0.76–1.52) | 0.65 | 0.04 |
| Adjusted for age, race, sex, cardiovascular and kidney disease |
1.3 (1.03–1.65) | 0.02 | 1.8 (0.66–4.92) | 0.25 | 1.06 (0.75–1.51) | 0.7 | 0.09 |
p value for interaction between diabetes and the reason for initial hospitalization (pneumonia and non-infectious illness) on mortality over 1 year
The mortality at 1-year in individuals who developed acute kidney injury was higher compared to those who did not develop it, in both subjects with and without diabetes, but results did not reach statistical significance among subjects with diabetes (30.7% vs. 21.4%, p=0.08 and 28.7% vs. 14.3%, p<0.0001 in subjects with and without acute kidney injury stratified by presence and absence of diabetes).
Causes of death were different in those with and without diabetes (p=0.03). Deaths due to cardiovascular disease and kidney disease were higher among those with diabetes (34.4% vs. 26.8% and 10.4% vs. 4.5% for cardiovascular and kidney disease). Deaths due to cancer were lower among those with diabetes (12.5% vs. 26.5%), but no differences were observed in deaths due to infection, chronic respiratory disease, and other causes (15.6% vs. 15%, 16.7% vs. 14.7%, and 10.4% vs. 12.5% among those with and without diabetes for infection, chronic respiratory disease, and other causes, respectively).
Diabetes was associated with higher mortality after pneumonia compared to non-infectious illnesses in Health ABC
The clinical characteristics of subjects in Health ABC at enrollment are shown in Table 2. The average age was 73 years and approximately half were whites and females. The prevalence of diabetes was similar in Health ABC and GenIMS (n=299, 18.2% and n=384, 20.3% in Health ABC and GenIMS cohorts). Compared to GenIMS, the prevalence of respiratory disease was lower in Health ABC (24.2% vs. 37.9%), whereas CKD was more common in Health ABC (24.5% vs. 9.8%). Similar to GenIMS, subjects with diabetes enrolled in Health ABC had higher prevalence of CKD (34.1% vs. 22.4%, p<0.0001) and cardiovascular disease (35.9% vs. 25.9%, p=0.03).
An interaction was observed between diabetes and the reason for the initial hospitalization on mortality within the first year. In the unadjusted model, pre-existing diabetes was associated with higher risk of death after hospitalization for CAP compared to those hospitalized for non-infectious illnesses (HR=1.87, CI=0.76–4.6 and 1.16, CI=0.8–1.6 for those hospitalized for CAP and non-infectious illnesses, interaction p=0.04, [Table 4]). This interaction persisted in models adjusted for demographics (HR=1.82, CI=0.72–4.62 and 1.08, CI=0.76–1.52 for those hospitalized for CAP and non-infectious illnesses, interaction p=0.04) and additionally adjusted for pre-existing cardiovascular disease and CKD (HR=1.8, CI=0.66–4.92 and 1.06, CI=0.75–1.51 for those hospitalized for CAP and non-infectious illnesses, interaction p=0.09). Forty-one participants who were hospitalized initially for non-infectious illnesses were subsequently hospitalized for infections. Excluding these subjects did not alter the results and an interaction between diabetes, reason for hospitalization, and mortality within the first year persisted (unadjusted HR=1.87, CI=0.76–4.60, and 0.97, CI=0.66–1.41 for those hospitalized for CAP and non-infectious illnesses, interaction p=0.01).
Diabetes did not alter immune response after CAP hospitalization
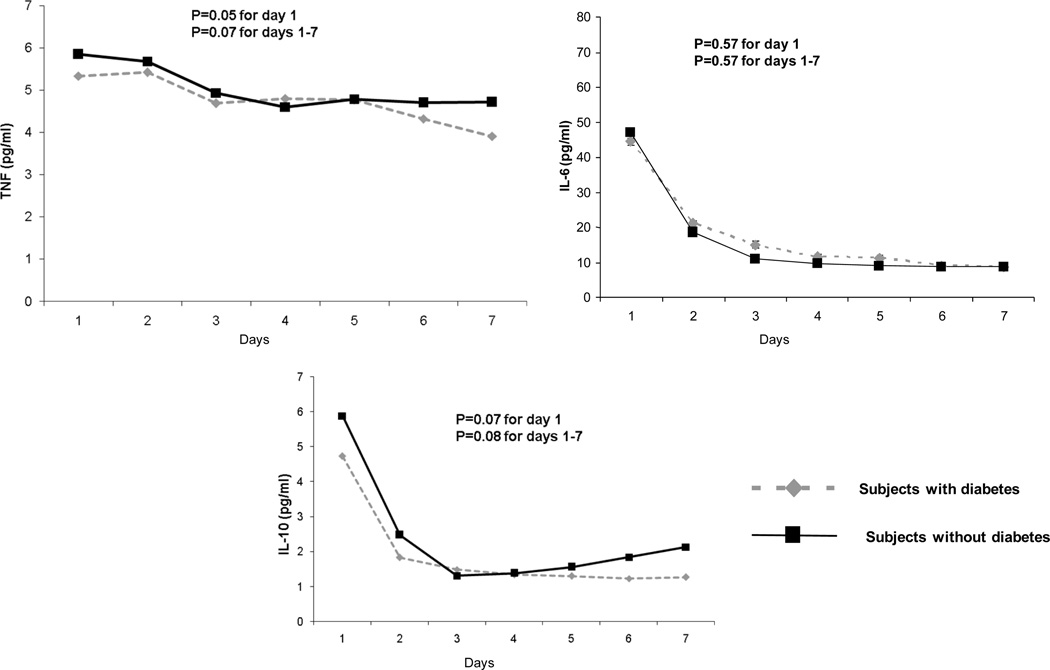
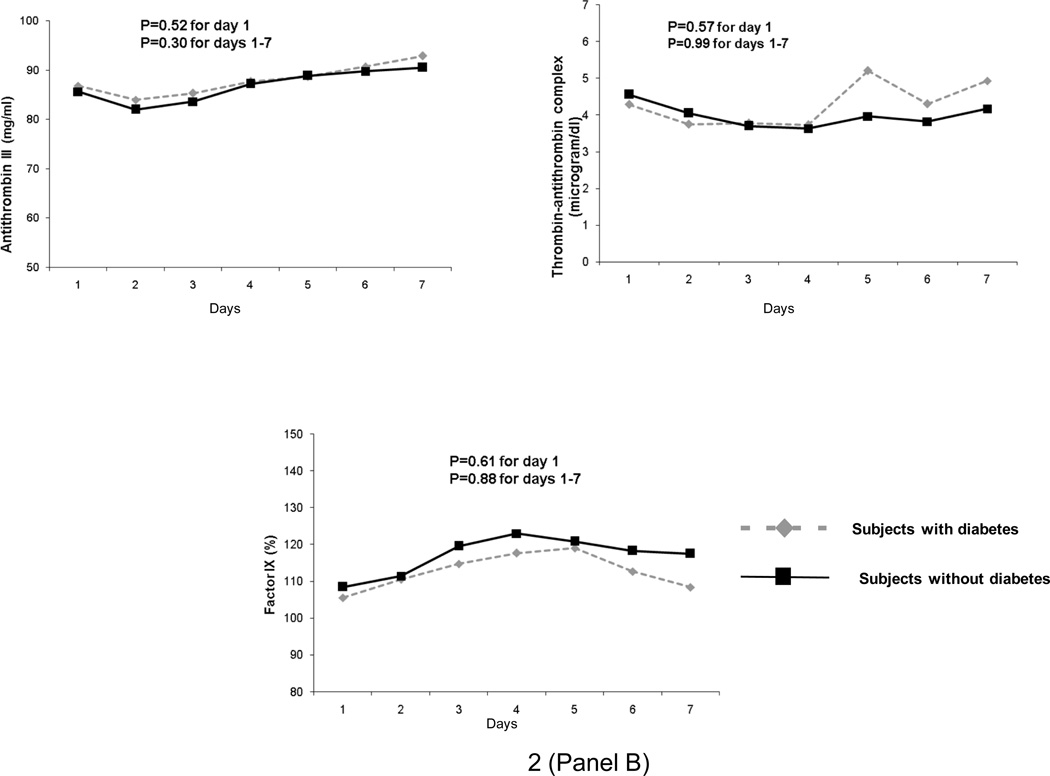
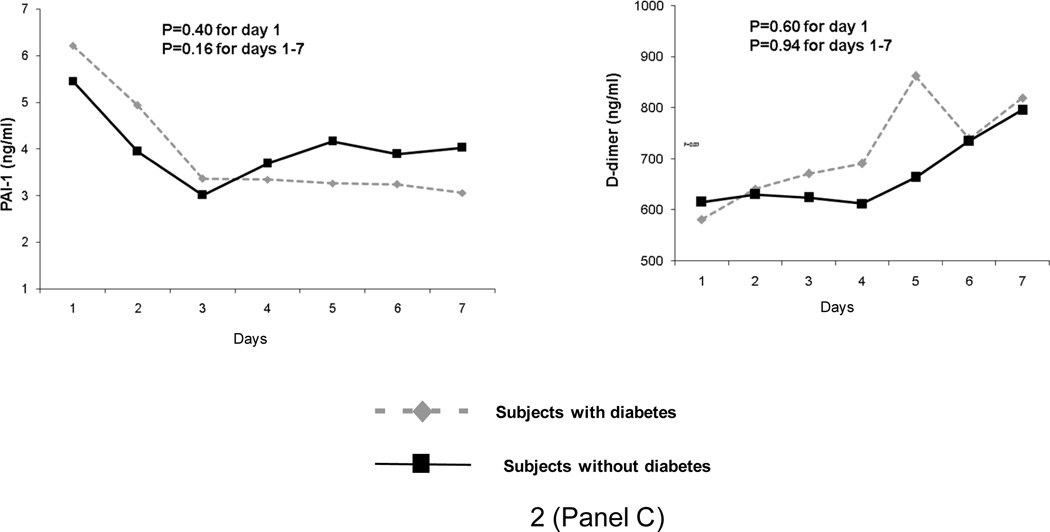
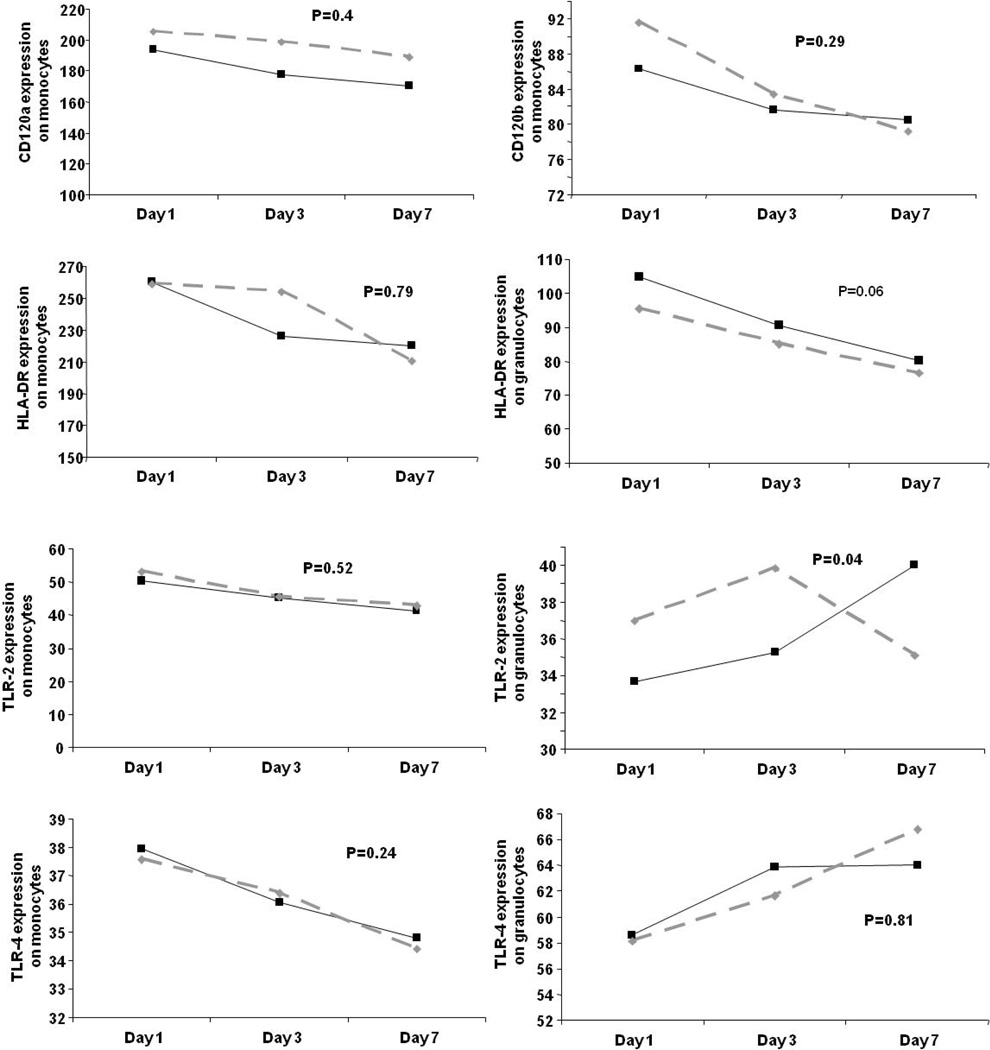
In GenIMS, the circulating concentrations of inflammatory (TNF, IL-6, and IL-10), coagulation (antithrombin, factor IX, TAT complexes), and fibrinolysis (PAI-1 and D-dimer) biomarkers were similar among subjects with and without diabetes at ED presentation and over first week of hospitalization (Figure 2). No differences were seen in expression of CD120a, CD120b, HLA-DR, and TLR4 and TLR2 expression on monocytes between those with and without diabetes (Figure 3). Expression of TLR2 on granulocytes was higher among subjects with diabetes on day 1 and 3, but higher among those without diabetes on day 7 (p=0.04). At hospital discharge, those with diabetes had higher IL-6 concentrations compared to those without, but these differences were small (8.67 vs. 7.41 pg/ml, p=0.03) (Table 5). No differences were observed in other biomarkers at hospital discharge.
Figure 2.
No differences were observed in geometric means of inflammatory (tumor necrosis factor [TNF], interleukin (IL)-6, and IL-10 shown in Panel A), coagulation (Factor IX, antithrombin, and thrombin-antithrombin [TAT) complexes shown in Panel B), and fibrinolysis (plasminogen activator [PAI]-1 and D-dimer shown in Panel C) biomarkers in 1427, 734, and 734 subjects with and without diabetes, respectively. Biomarkers were measured on presentation to the emergency department and over the first week of hospitalization and p values are shown for these comparisons.
Figure 3.
No differences were observed in cell surface markers on presentation to the emergency department and on third and seventh day in subjects with and without diabetes in 624 subjects. Values for cell surface markers were reported as the mean channel fluorescence (MCF) of cells positive for a given cell surface marker. P values are shown for comparisons over time. Only expression of TLR2 on granulocytes appeared to be higher among subjects with diabetes on day 1 and 3 and were higher in those without diabetes on day 7 (p=0.04).
Table 5.
Association between inflammatory, coagulation, fibrinolysis markers (geometric means with standard deviation) at hospital discharge stratified by diabetes among 1808 subjects discharged alive from the hospital
| Biomarker | With diabetes | Without diabetes | p-values |
|---|---|---|---|
| Inflammatory markers (pg/ml)* | |||
| TNF | 4.77 (2.12) | 4.73 (2.65) | 0.51 |
| IL-6 | 8.67 (6.83) | 7.41 (5.74) | 0.03 |
| IL-10 | 1 (18.45) | 1.24 (11.92) | 0.6 |
| Coagulation markers* | |||
| Factor IX activity (%) | 111.02 (1.30) | 119.14 (1.25) | 0.1 |
| Antithrombin (mg/ml) | 91.77 (1.04) | 91.50 (1.04) | 0.87 |
| TAT complexes (µg/ml) | 4.25 (3.70) | 3.58 (2.49) | 0.25 |
| Fibrinolysis markers (ng/ml)* | |||
| D-dimer | 623.79 (3.26) | 600.86 (2.92) | 0.67 |
| PAI-1 | 3.54 (4.27) | 3.72 (4.63) | 0.62 |
Inflammatory, coagulation, and fibrinolysis markers were available at hospital discharge in 1739, 892, and 892 subjects, respectively
Plasma glucose concentrations
Plasma glucose was available at ED presentation in most GenIMS subjects (n= 1795, 94.7%). Those with diabetes had higher median plasma glucose compared to those without diabetes (190 mg/dl, interquartile range (IQR)=136–256 mg/dl vs. 117 mg/dl, IQR=99–143 mg/dl, p<0.0001).
Regardless of diabetes, non-survivors at 1 year had higher median blood glucose on ED presentation compared to survivors, but differences were small (130 mg/dl, IQR=103–177 mg/dl vs. 122 mg/dl, IQR=102–158 mg/dl, p=0.02). Hyperglycemia at ED presentation (glucose >200mg/dl) was associated with higher mortality within first year (HR=1.31, CI=1.01–1.69, p=0.03). However, results did not reach statistical significance when adjusted for age, sex, and race (HR=1.27, CI=0.98–1.64, p=0.06). Furthermore, the HRs were different when the analyses were stratified by presence or absence of diabetes (HR=0.89, CI=0.6–1.32, p=0.57 in subjects with diabetes and HR=1.4, CI= 0.98–2.11, p=0.06 in subjects without diabetes).
DISCUSSION
We found that diabetes was common and present in 20% of subjects with CAP. Compared to subjects without diabetes, those with diabetes had higher mortality within the first year after CAP and a fourth died at 1 year. The higher mortality was not confounded by higher burden of pre-existing cardiovascular disease and CKD. Furthermore, diabetes was associated with higher mortality following hospitalization for CAP compared to hospitalization for non-infectious illnesses. These results suggest that higher mortality within the first year among individuals with diabetes is attributable to the pneumonia hospitalization. Diabetes did not modify the immune response in a broad panel of circulating inflammatory, coagulation, fibrinolysis, and cell surface markers, suggesting that differences in immune response are unlikely to explain survival differences. Based on patterns of organ dysfunction observed during the hospital course and cause-specific mortality, the higher incidence of acute kidney injury and acceleration of underlying cardiovascular disease may mediate higher mortality within the first year after CAP among subjects with diabetes.
Our results showing survival differences due to diabetes after CAP hospitalization has several strengths. First, the higher hazards of death due to diabetes were similar in two cohorts, though results did not meet statistical significance in Health ABC due to the small number of CAP events. Second, we assessed survival differences after the first hospitalization event in Health ABC to avoid confounding due to preceding non-infectious illnesses, such as acute myocardial infarction, which are common and may be associated with worse survival in diabetes.10 We also demonstrated an interaction between diabetes, reason for hospitalization, and mortality within the first year. Among those with diabetes, mortality was higher after CAP hospitalization compared to hospitalization for non-infectious illnesses. Third, survival differences persisted after adjusting for differences in chronic disease burden, such as cardiovascular disease and CKD. These results suggest that the higher mortality within the first year in diabetes can be attributed to the CAP hospitalization and cannot be explained by higher burden of pre-existing cardiovascular disease and CKD among those with diabetes.
We speculate that acceleration of pre-existing chronic disease may explain higher long-term mortality among those with diabetes. For instance, cardiovascular disease accounted for more than third of all deaths in individuals with diabetes. Pre-existing cardiovascular disease was more common among those with diabetes, which may be further accelerated by the acute infection. Early recognition or better management of atherosclerotic heart disease and concomitant risk factors, such as smoking and hyperlipidemia, may improve outcomes. We showed that diabetes was associated with higher risk of acute kidney injury, which was associated with higher risk of 1-year mortality in our study and prior studies.28 Acute kidney injury itself or its sequelae, chronic kidney disease, may lead to death by several mechanisms, including increased risk of cardiovascular disease and infections.
Our results may explain the conflicting results of prior studies which assessed the association between diabetes and survival after CAP. First, mortality was assessed at different time-points in prior studies. Our results suggest that, although the relative risk of death was similar at different time points, statistically significant differences are more likely to be seen in studies that assessed long-term outcomes due to fewer deaths at earlier time points.3 Second, we examined the effect of diabetes on outcomes, conditional upon developing pneumonia. Prior population-based studies that showed higher incidence of death due to infections in subjects with diabetes could not assess whether these differences were due to increased susceptibility to infections or worse outcomes after infection.5,6
We showed a 1.3-fold higher risk of acute kidney injury among diabetic subjects, even among subjects without history of chronic kidney disease. Mechanisms underlying increased risk of AKI after CAP among diabetics are unclear. We speculate that the higher risk of acute kidney injury in diabetic subjects could be due to higher prevalence of subclinical kidney disease prior to pneumonia or due to higher risk of developing contrast-induced nephropathy.29,30
Although immune dysfunction in diabetes is well recognized, the lack of a clear influence of diabetes on a broad panel of biomarkers and cell-surface markers at multiple time points during hospitalization for CAP is remarkable. We observed differences in expression of TLR2 on granulocytes over time between subjects with and without diabetes, but the direction of this difference was not consistent over time and uncertain in terms of biologic significance. We chose inflammatory, coagulation, and fibrinolysis biomarkers because these biomarkers are altered in diabetes 11,31 and in human endotoxemia models by hyperinsulinemia and hyperglycemia.12,14. We chose TLR2 and TLR4 because expression of these cell-surface markers and their downstream mediators are upregulated in diabetes 16 and they play an important role in host response to infection and sepsis.15 We performed serial measurements of these markers, including on presentation to the ED, when immune responses are least likely to be modified by therapeutic interventions. It is likely that diabetes may modify these markers prior to ED presentation or other mechanisms could be influenced by diabetes, such as alterations in neutrophil function, apoptosis, oxidative stress, and chemokines. However, the lack of effect of diabetes on the immune response in a broad panel of circulating biomarkers and cell-surface markers suggest that pre-existing diabetes does not influence the immediate host response to CAP, possibly because the responses elicited by pneumonia are much more profound than the relatively modest alterations produced by diabetes per se.
Our study has limitations. First, we assessed the effect of hyperglycemia, a potential mechanism of increased mortality, on ED presentation only, and serial glucose levels were not available. Second, we used different criteria to diagnose diabetes in both cohorts. Stringent criteria were used in Health ABC, including self-report and review of medication inventory, and fasting blood glucose in subjects who did not report diabetes. In GenIMS, we used a combination of self-report and review of medication inventory, and we may have misclassified some individuals as non-diabetic. The association between admission hyperglycemia and higher mortality only among those without diabetes in this cohort suggests that such a misclassification bias would skew the results towards the null and attenuate the hazards ratios between diabetes and long-term survival. Indeed, the HRs were slightly lower in GenIMS, suggesting that such a misclassification bias is unlikely to negate our results. Finally, we could not assess whether our results were confounded due to differences in microbiologic etiology between those with and without diabetes. Although cultures were obtained in most subjects, we identified an etiologic agent in a small subgroup. The low yield of cultures in our study is consistent with previous large studies of CAP patients32,33 and likely due to poor yield of current culture techniques. Larger studies will be necessary to understand differences in immune response for different etiologic agents.
In summary, once CAP occurs, those with diabetes were more likely to die over 1 year. The mechanism is unlikely to be due to alterations in immune response, at least as measured by a broad panel of circulating biomarkers and cell surface markers. The higher mortality may be due to worsening of pre-existing cardiovascular disease or higher risk of acute kidney injury.
Supplementary Material
ACKNOWLEDGMENT
We are indebted to the nurses, respiratory therapists, phlebotomists, physicians and other health care professionals who participated in GenIMS, as well as the subjects and their families who supported GenIMS and Health ABC study. A complete list of GenIMS investigators is available at www.ccm.upmc.edu/genims_investigators.
FUNDING
GenIMS was funded by NIGMS R01 GM61992 with additional support from GlaxoSmithKline for enrollment and clinical data collection, and Diagnostic Products Corporation for the cytokine assays. Health ABC was funded by NIA (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106), NHLBI (R01HL74104), and NIAID (27913 and 39482). Health ABC was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Dr. Yende is supported by K23 GM083215.
Appendix
List of non-infectious causes of hospitalization in Health ABC study
Myocardial infarction
Angina/other ischemic disease
Congestive heart failure
Carotid artery disease
Peripheral arterial disease
Stroke or cerebrovascular accident (CVA)
Transient ischemic attack
Chronic obstructive pulmonary disease (COPD)/Emphysema/Asthma
Upper gastrointestinal bleeding
Lower gastrointestinal bleeding
Abdominal hernia
Benign prostatic hyperplasia (BPH)
Gallbladder disease
Cancer
Depression
Dementia
Osteoarthritis
Fracture
Neoplasms (benign)
Endocrine, nutrition, metabolic diseases
Diseases of blood and blood forming organs
Mental disorders (not dementia and depression)
Diseases of nervous system, other than stroke
Diseases of circulatory system, other than myocardial infarction, angina, and congestive heart failure
Diseases of respiratory system, other than COPD, asthma, emphysema
Diseases of digestive system, other than bleeding
Diseases of genitourinary system, other than BPH
Diseases of skin
Diseases of musculoskeletal and connective tissue, other than osteoarthritis
Ill defined symptoms and signs
Injury and poisoning, excluding fractures
Footnotes
COMPETING INTERESTS AND LICENSING
None of the authors have any competing interests with respect to this manuscript.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Thorax and any other BMJPGL products to exploit all subsidiary rights, as set out in our license
REFERENCES
- 1.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 2.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 3.Falguera M, Pifarre R, Martin A, et al. Etiology and outcome of community-acquired pneumonia in patients with diabetes mellitus. Chest. 2005;128(5):3233–3239. doi: 10.1378/chest.128.5.3233. [DOI] [PubMed] [Google Scholar]
- 4.Kornum JB, Thomsen RW, Riis A, et al. Type 2 diabetes and pneumonia outcomes: A population-based cohort study. Diabetes Care. 2007;30(9):2251–2257. doi: 10.2337/dc06-2417. [DOI] [PubMed] [Google Scholar]
- 5.Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50(3):549–554. doi: 10.1007/s00125-006-0570-3. [DOI] [PubMed] [Google Scholar]
- 6.Valdez R, Narayan KM, Geiss LS, et al. Impact of diabetes mellitus on mortality associated with pneumonia and influenza among non-Hispanic black and white US adults. Am J Public Health. 1999;89(11):1715–1721. doi: 10.2105/ajph.89.11.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esper A, Moss M, Martin G. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13(1):R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Renal Data Systems. [accessed in December 2008];USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2007 Available at http://www.usrds.org and.
- 9.Prevalence of self-reported cardiovascular disease among persons aged >35 years with diabetes - United States, 1997–2005. MMWR. 2007;56(43):1129–1132. [PubMed] [Google Scholar]
- 10.Timmer JR, Ottervanger JP, Thomas K, et al. Long-term, cause-specific mortality after myocardial infarction in diabetes. Eur Heart J. 2004;25(11):926–931. doi: 10.1016/j.ehj.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Lu H, Raptis M, Black E, et al. Influence of diabetes on the exacerbation of an inflammatory response in cardiovascular tissue. Endocrinology. 2004;145(11):4934–4939. doi: 10.1210/en.2004-0737. [DOI] [PubMed] [Google Scholar]
- 12.Krogh-Madsen R, Moller K, Dela F, et al. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-{alpha}, and FFAs to low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2004;286(5):E766–E772. doi: 10.1152/ajpendo.00468.2003. [DOI] [PubMed] [Google Scholar]
- 13.Reverter JL, Reverter JC, Tassies D, et al. Thrombomodulin and induced tissue factor expression on monocytes as markers of diabetic microangiopathy: a prospective study on hemostasis and lipoproteins in insulin-dependent diabetes mellitus. Am J Hematol. 1997;56(2):93–99. doi: 10.1002/(sici)1096-8652(199710)56:2<93::aid-ajh4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Stegenga ME, van der Crabben SN, Blumer RME, et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112(1):82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 16.Devaraj S, Dasu MR, Rockwood J, et al. Increased Toll-Like Receptor (TLR) 2 and TLR4 expression in monocytes from patients with Type 1 diabetes: Further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93(2):578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yende S, Angus DC, Ali IS, et al. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007;55(4):518–525. doi: 10.1111/j.1532-5415.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 18.National Death Index. [Accessed December 2009]; http://www cdc gov/nchs/ndi htm.
- 19.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major U.S. mortality databases. Ann Epidemiol. 2002;12(7):462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 21.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 23.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 24.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yende S, D'Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177(11):1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein MP, Lin X, Boehnke M. A tobit variance-component method for linkage analysis of censored trait data. Am J Hum Genet. 2003;72(3):611–620. doi: 10.1086/367924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 28.Abosaif NY, Tolba YA, Heap M, et al. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. AmJ Kidney Dis. 2005;46(6):1038–1048. doi: 10.1053/j.ajkd.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 30.Ismail N, Becker B, Strzelczyk P, et al. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 1999;55(1):1–28. doi: 10.1046/j.1523-1755.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- 31.Graves DT, Liu R, Alikhani M, et al. Diabetes-enhanced inflammation and apoptosis--impact on periodontal pathology. J Dent Res. 2006;85(1):15–21. doi: 10.1177/154405910608500103. [DOI] [PubMed] [Google Scholar]
- 32.Metersky ML, Ma A, Bratzler DW, et al. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 33.Joshi N, Caputo GM, Weitekamp MR, et al. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341(25):1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 34.Levy MM, Fink M, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 35.Barzilay JI, Spiekerman CF, Wahl PW, et al. Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet. 1999;354(9179):622–625. doi: 10.1016/s0140-6736(98)12030-5. [DOI] [PubMed]
- 36.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modificationof Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 37.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.