Abstract
Mantle Cell Lymphoma (MCL) is associated with a dismal prognosis. Recently, along with the improved understanding of the pathophysiology of this disease, new first line regimens have been established and in addition novel treatment options have entered the clinical arena. In consequence, prognosis of the disease has fortunately improved. We here focus on the rationale, current clinical knowledge and future concepts of Temsirolimus, an inhibitor of mTOR, in the treatment of MCL. At this time this drug has been shown to be effective as single agent for relapsed disease and early combination data show promising results. In addition, with a brief outline of other treatment options, we aim to guide at which place in the current treatment algorithms Temsirolimus can be integrated into the treatment of MCL patients.
Keywords: mTOR-inhibitor, mantle cell lymphoma, temsirolimus
Introduction
Mantle Cell Lymphoma (MCL) is a well defined subtype of B-cell non-Hodgkin Lymphoma and represents 5% to 10% of that entity with an incidence of 2–3/100.000.1 It is, apart from rare exceptions, characterized by a chromosomal translocation t(11;14) (q13;q32) with nuclear cyclin D1 overexpression. Typically, MCL occurs in elderly people with a median age of 65 years and a clear predominance of male patients.2 At time of diagnosis, most of the patients with MCL will typically show an already disseminated disease. The most common extranodal manifestations involve bone marrow, liver, spleen, the Waldeyer’s tonsillar ring, and the gastrointestinal tract,3 the last-mentioned occasionally resulting in first clinical symptoms.4,5 The severity of symptoms correlates with stage and disease dynamics. Several subtypes of mantle cell lymphoma with distinct disease courses have been established so far: an indolent subtype, very slow in progress and characterized by a benign course, is found in 10%–15% of patients. The most frequent subtype is the classical MCL with a medium rapid course, and the most aggressive variant is the blastoid subtype that is found in 10% of patients, with a frequently very dismal course. The “Mantle cell Lymphoma International Prognostic-Index” (MIPI) includes four independent prognostic factors of MCL (ECOG performance status >2, white blood cell count >6,7/nL, LDH level >245 U/L and age >60 years6) and is a simple method to estimate the individual risk associated with the disease. By applying this MIPI, patients can be stratified into 3 risk-groups. Basis for this stratification is the prospective median overall survival (OS: time period between diagnosis and death or therapy initiation and death, if appropriate): low risk (OS of 6 years), intermediate risk (OS of 4 years) and high risk (OS of 2 years). Despite the high response rates to induction therapy, cure is almost never achieved.7 The median overall survival has been found to be not more than 3 to 4 years, and the proportion of long-term survivors has been low.8 Only in recent years, significant therapy improvements have been achieved by the use of dose-intensive chemotherapy regimens and the introduction of monoclonal antibodies, so that—at least for younger patients—a median OS of more than 5 years can now be assumed.9,10
Therapy
Untreated MCL
The selection of appropriate therapy is an individual decision and depends on various parameters, particularly age, performance status, MIPI, patient’s wish etc. A watch-and-wait strategy can only be recommended in asymptomatic patients with a low tumor burden and should otherwise not be pursued.2 Recently, however, markers like SOX11 that may help to specify at an early stage those patients in whom a watch-and-wait strategy might be justified have been identified.11 Besides the rare cases of truly limited MCL, where no commonly accepted standard exists, systemic therapy is the standard clinical option for most of the patients with MCL at time of diagnosis.
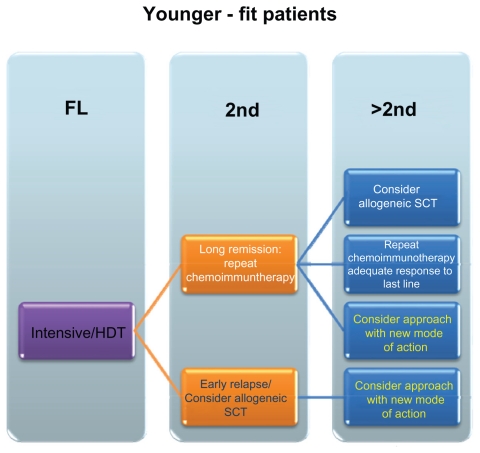
Currently, different treatment approaches are in use: conventional chemoimmunotherapy, dose-escalated chemoimmunotherapy and palliative care, using single agents for frail patients. Potential algorithms for younger and elder patients are outlined in Figures 1 and 2. In brief, for the treatment of younger patients CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy has been the treatment of choice for a long period. While the use of single-agent Rituximab has shown a merely moderate activity in MCL with an overall response rate of only 27%,12 a number of studies have by now demonstrated a benefit in median progression free survival (median time from initiation of treatment and disease progression) and median OS when the drug is combined with chemotherapy,2,13–15 and combination therapies are nowadays considered standard of care.
Figure 1.
Schematic overview of potential treatment approaches – younger/fit patients.
Abbreviations: FL, first line; HDT, high dose therapy; SCT, stem cell transplantation; 2nd, second line treatment; >2nd, higher than second line treatments.
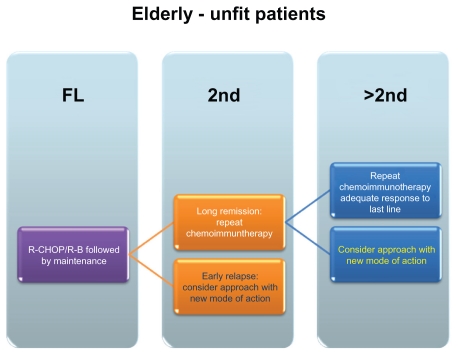
Figure 2.
Schematic overview of potential treatment approaches – elderly/unfit patients.
Abbreviations: R-CHOP, rituximab, cyclophosphamid, doxorubicin, vincristine, prednison; R-B, rituximab-bendamustine; FL, Firstline treatment; 2nd, second line treatment; >2nd, higher than second line treatments.
Furthermore, for younger/fit patients dose-intensified treatments have been proven beneficial. Two general strategies are adopted presently: one employing dose-escalated regimen like R-HyperCVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone, cytarabine, and methotrexate),16 or regimen that incorporate a consolidative high-dose therapy after an induction treatment. This induction frequently consisted of (R)-CHOP. Recently, however, several studies have been examining the effect of high-dose cytarabinosid implementation.9 A randomized trial of the European Mantle Cell Lymphoma Network has demonstrated the superiority of a high-dose cytarabinosid (ARA-C)-containing induction regimen like DHAP (dexamethasone, high-dose Ara-C, and cisplatin) to the use of CHOP for induction, indicated by a significant prolongation of progression free survival. Therefore to date – at least in Europe—a cytarabinoid-containing induction followed by high-dose consolidation with autologous stem cell transplantation represents the standard front-line-therapy in younger patients (<65 years).2,17–19
Appropriate combination regimens in elderly patients (>65years) or patients with comorbidities who are not qualified for dose-intensive therapy are the less intensive regimens like R-CHOP or, nowadays, bendamustine-rituximab (BR). The effect of bendamustine as first-line therapy especially in elderly patients with MCL has been shown in a study by Rummel et al with an improved complete remission (CR)-rate of 40,1% for BR versus 30,8% for R-CHOP and an improved PFS of 32.4 month versus of 22,4 month, respectively.20 Recent data support the use of Rituximab as maintenance therapy, at least in patients receiving conventional-dose chemoimmunotherapy (Kluin-Neelemans et al. oral presentation, EHA 2011). Patients that are unable to tolerate such an aggressive treatment are qualified for a single-agent therapy or a dose-reduced chemotherapy regimen with a palliative intent.17
Relapsed/Refractory MCL
For patients with relapsed disease, the choice of the most suitable therapy regimen depends on multiple parameters (Box 1), such as type of primary therapy, response to treatment, remission duration etc. For elderly patients or patients with long remissions to the last line of a chemoimmunotherapy, repetition of the initial or introduction of an alternative chemotherapy combination seems reasonable. In general, non-cross resistant regimens are frequently used, e.g., R-CHOP followed by BR or vice versa.
Box 1. Criteria for the selection of subsequent treatment.
Patient’s age and performance status
MIPI
Hematopoietic reserve
Tumor biology/histologic subtype
Patient’s wish
Approach(es) used in earlier treatment lines
Disease dynamics
Quality of remission induced
(MRD)
Duration of remission
So far, allogeneic stem cell transplantation is the only curative therapeutic approach in patients with advanced stage MCL based on a graft-versus-lymphoma effect. But because of the considerable morbidity and mortality associated with this therapy, only a subset of patients are suitable candidates for such an approach.18 Khouri et al reported on the introduction of a reduced-intensity conditioning in patients with relapsed MCL. The results of his study show a 100-day mortality of 0%, a CR-rate (no clinical evidence of disease or disease-related symptoms and spleen and liver non-palpable, without nodules) of 94% and a 3-year progression free survival (PFS: initiation of therapy until the occurrence of any disease progression or death) and median overall survival (OS) of 82% and 85%.18,21 Although no prospective data are available yet, the option of allogeneic stem cell transplantation should be evaluated in patients with relapsed disease after appropriate first-line therapy, and this especially holds true for young and motivated patients.
Besides these commonly proposed approaches, no general consensus exists on how to treat patients with relapsed MCL. Due to its high genetic instability, though, success rates and remission duration rapidly decrease during the course of disease, and patients have to be considered chemotherapy-refractory. Thus, additional options were and are urgently needed for these patients.
Due to the MCL’s well-defined pathophysiology, determining distinct therapeutic targets, a variety of candidate drugs has been identified and clinically developed, e.g., approaches like bortezomib as a proteasome inhibitor or thalidomide/lenalidomide as immunomodulatory agents.14,22 This review will focus on the results found for temsirolimus, a mTOR inhibitor, currently approved for the treatment of relapsed MCL in the EU.
mTOR-Inhibition
The mTOR pathway and pharmacologic mTOR-inhibition
The phosphatidylinositol-3-kinase (Pi3K) is among the most frequently affected pathways in malignancy. Additional members of this pathway include AKT, the negative regulator PTEN, and the mammalian Target Of Rapamycine (mTOR). These proteins are affected in various tumor entities, especially malignant lymphoma.23 mTOR itself regulates the translation of other oncogenes, for example cyclin A and c/EBPβ. In all of them, an increased activation of mTOR leads to cell proliferation, activation of growth and survival pathways, and inhibits autophagy.24
As described, in MCL an overexpression of Cyclin D1 results from the t(11;14). The gene (CCND1) coding for cyclin D1 consists of 5 exons which could be alternatively spliced in two different mRNAs leading to two different proteins: cyclin D1a and cyclin D1b.1 Cyclin D1a and cyclin-dependent kinases 4 and 6 (CDK 4/6) form a complex and thereby promote cell cycle entry. Both CDK4 and CDK6 are frequently overexpressed in patients with MCL, thereby perpetuating cell proliferation. 1 Although further studies have shown that isolated cyclin D1 overexpression is not sufficient to induce MCL in mice, it is considered a hallmark of disease development accompanied by additional events/ mutations.25 Cyclin D translation is mainly regulated via the PI3K/PTEN/Akt/mTOR pathway,26 As a consequence inhibition of elements of this pathway is an attractive therapeutic approach to counteract the cell cycle driving potential of cyclin D1.
Rapamycine – the first available mTOR-inhibitor— was isolated from the bacterium Streptomyces hygroscopius in 1970. To date, rapamycine is mainly used as immunosuppressive agent. However, already early studies have demonstrated its potential as an antitumor agent and its cytostatic properties, and it was the first molecule to inhibit the Pi3K/ AKT pathway. Rapamycine exerts its effect on the cell by ligating the 12 kDa FK506-binding protein (FKBP12). The complex of both inhibits the activity of mTOR through allosteric binding.27,28 The mTOR protein itself acts within 2 separate multiprotein signaling complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). While the rapamycin-sensitive mTORC1 complex is a key element of numerous signaling as described above, mTORC2, generally perceived as rapamycine insensitive,29 is involved in processes like cytoskeleton reorganisation. Lately, Sarbassov et al have shown that a prolonged use of rapamycin also leads to an inhibition of mTORC2 AKT and of the PI3K/AKT pathway in vitro. This can be explained as a consumption of free mTOR molecules, and thus the resulting ability to bind also mTORC2.30 The activation of mTOR leads to a phosphorylation of downstream targets like eIF-4EBP1 (Eukaryotic Translation Initiation Factor-4E-Binding Protein-1) and the ribosomal protein S6K1 (S6 Kinase 1), and this results finally in an increase in the translation of a subset of mRNAs that encode for proteins often associated with a proliferative response and with the transition from G1 to S-phase of cell cycle.31,32
In vitro studies using rapamycine in several MCL cell lines showed a reduction of cyclinD1 and the antiapoptotic proteins cFLIP, BCL-XL and Mcl-1 by pharmacological inhibition of the PI3K/AKT pathway.33 The reduction of cyclin D1 mRNA levels leads to a deficiency of active CDK4/cyclin D1 complexes. As rapamycine seems to affect primarily the stability of the transcript, a cytostatic rather than a cytotoxic effect was assumed.34 However, it has been found that some apoptotic processes are particularly evident in B-cells and rhabdomyosarcoma cells.35,36
In addition, mTOR inhibitors could synergize with other cytotoxic agents, such as vincristine, doxorubicin, bortezomib or rituximab, resulting in a pronounced inhibition of Raf-1, MAPK and mTOR.29 Temsirolimus also shows a synergistic antineoplastic effect in combination with vorinostat. It can be presumed that the addition of this histone deacetylase inhibitor exerted a proapoptotic effect with induction of autophagy in, for example, renal cancer cells.37,38 Autophagy could be observed in the development of acidic vesicular organelles.10
Clinical development of temsirolimus in MCL
Single agent temsirolimus
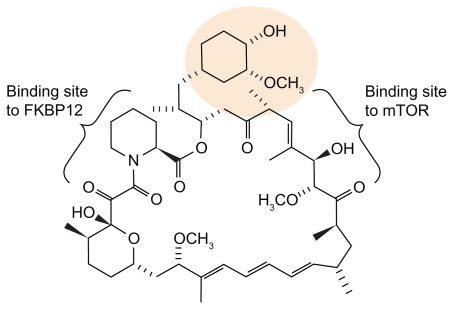
Temsirolimus is one of the mTOR–Inhibitors in clinical use that are currently available (others: Everolimus, Ridaferolimus). It is the water-soluble ester-derivative of rapamycine. All mTOR inhibitors display the same effect by binding mTOR via FKBP12 with a high specificity. The chemical structure of temsirolimus is shown in Figure 3. In several phase-I/II studies, pharmacokinetic properties of temsirolimus have been evaluated for the first time. In a dose escalation phase I trial in patients with advanced solid tumors using temsirolimus in doses from 7.5 to 220 mg/m2 as weekly 30-minute infusion, tolerability was demonstrated over a wide dose range. Reversible thrombocytopenia was noted as dose limiting toxicity (DLT) in this study. The most common drug-related adverse events were skin toxicity and mucositis/ stomatitis. No clinical immunosuppressive effects were observed.39 In addition, Atkins et al also conducted a dose escalation study in patients with advanced refractory renal cancer, employing 25 mg, 75 mg and 250 mg of temsirolimus.40 In fact, neither toxicity nor efficacy in both trials seemed to be significantly influenced by dose levels, and patients showed responses to treatment at all dose levels.40 As the measuring of pharmacokinetics based on body surface failed to demonstrate any superiority, too, further studies were conducted using a flat-dosing of temsirolimus.7
Figure 3.
Structure of temsirolimus.
Two Phase II Studies evaluated temsirolimus in different doses (250 mg/25 mg once weekly) as single agent in patients with relapsed or refractory MCL. The initial trial tested temsirolimus in a dose of 250 mg, similarly to trials in solid tumors that were active at the same time. In this study, Witzig et al reported on 35 patients. 34 patients with a median age of 70 years (range, 38–89years) were evaluable for analysis whereof 50% showed refractory disease, 91% had stage IV disease, in 69% 2 or more extranodal sites were found, with a median of 3 prior therapies (range 1–11) (rituximab (89%), alkylating agent (94%), or anthracycline (83%)). The overall response rate was 38%, with one complete remission (3%) and 12 partial remissions (35%). The median time to progression in all patients was 6.5 months (range, 2.9 to 8.3 months), for the 13 patients with a response the duration of response was 6.9 months (range, 5.2 to 12.4 months). Thrombocytopenia was one of the most frequent side effects and required a dose reduction in the majority of patients41 (Table 1).
Table 1.
Comparison of side effects observed in different phase II trials.
| % Phase II 250 mg | Phase II 25 mg | |||
|---|---|---|---|---|
| All grades | 3° or 4° | All grades | 3° or 4° | |
| Thrombocytopenia | 100 | 66 | 82 | 39 |
| Asthenia | 66 | 11 | 75 | 25 |
| Anemia | 66 | 26 | 15 | |
| Diarrhea | 77 | 11 | 4 | |
| Fever | NR | NR | NR | NR |
| Anorexia | 40 | 3 | 4 | |
| Mucositis | 71 | 6 | 39 | |
| Nosebleeds | NR | NR | NR | NR |
| Erythema/rash | 51 | 7 | 36 | |
| Infections | 63 | 26 | 32 | 15 |
As a consequence of the observed side effects, an additional phase II study tested 25 mg single agent temsirolimus, the dose approved for renal cell cancer. Again a heavily pretreated study population of 29 Patients was enrolled of whom 50% were refractory with a lack of complete or partial remission within 1 month to the last therapy. 27 Patients were evaluable for the analysis. The median age was 69 years (range, 51–85 years), 86% had stage IV disease, 71% showed 2 or more extranodal sites, and all had received a median of 4 prior therapies (range, 1–9) (rituximab (96%), alkylating agent (96%), or anthracycline (79%)). Similar to the initial trial’s outcome, an overall response rate of 41% was observed (1 complete response (3.7%) and 10 partial responses (37%)). For all patients, the median time to progression was 6 months, and the median duration of response for the 11 responders was 6 months (range, 1–26 months).42 Similar to the findings in other studies, primarily hematologic side effects were observed, and especially thrombocytopenia was the most common cause for dose reduction again.39,42
Owing to the promising results of the phase II trials, a randomized open label phase III study was performed to evaluate temsirolimus in two dosing levels compared with investigator’s choice therapy.43 Altogether, 162 patients with relapsed or refractory MCL were included. Patients were randomly assigned to three groups (54/54/53). Treatment consisted of temsirolimus 175 mg once weekly for three weeks, followed by either temsirolimus 75 mg, 25 mg weekly, or investigator’s choice treatment of options approved in advance (multiple conventional cytostatic regimes i.v. or p.o.; e.g., gemcitabine, fludarabine, thalidomide p.o.; alemtuzumab i.v.). Patients’ characteristics were well spread among all groups, the median age was 68 years (range, 44–87 years) in the temsirolimus 175/75 mg, 68.5 years (range, 43–85 years) in the 175/25 mg and 64.5 (range, 39–88 years) in the investigator’s choice cohort. The patients in the two different temsirolimus groups had received a median of three prior therapies whereas the patients in the investigator’s choice arm had been treated with a median of four prior therapies. Stage III and IV disease at baseline was 100%, 96% and 94% for the three groups: 175/75 mg, 175/25 mg and investigator’s choice, respectively (Table 2). The overall response rates were 22%, 6%, 2% in the three groups (175/75 mg, 175/25 mg and investigator’s choice), with complete remission rates of 2%, 0% and 2%. Partial remissions were induced in 20%, 6% and 0% of patients. The median time to progression (representing the primary objective of the trial) was 4.8 months for temsirolimus 175/75 mg, 3.4 months for temsirolimus 175/25 mg and 1.9 months for investigator’s choice group. This difference proved to be statistically significant.43
Table 2.
Response according to treatment arms of the randomized phase III trial.
| TEMSR 175/75 n = 54 |
TEMSR 175/25 n = 54 |
Invest choice n = 54 |
|
|---|---|---|---|
| Overall response rate | 22% | 6% | 2% |
| 95% CI | 11–13 | 0–12 | 0–5 |
| P-value | 0.0019 | 0.6179 | |
| Complete remissions, n | 1 | 0 | 1 |
| Partial remissions, n | 11 | 3 | 0 |
| Response median (95% CI), in month | 7.1 (4.1–NA) | 3.6 (3.2–10.6) | NA |
Apart from the observed differences in efficacy, pronounced hematologic toxicity was found to be associated with temsirolimus, and this again in a dose-dependent manner. In the different temsirolimus cohorts, rates of grade 3 thrombocytopenia were found in 63% (grade 4 3%) in the 175/75 and 39% (grade 4 0%) of the patients in the 175/25 mg dose group, respectively. Similarly, the neutropenia rates were higher in the 250 mg cohort (23% of patients had grade 3 and 6% of patients grade 4 thrombocytopenia), as compared with the 25 mg cohort (18% grade 3 and 0% grade 4).41,42 The grade 3 and 4 neutropenia rates were considerably higher in the investigator’s choice arm than in the temsirolimus arms. A somewhat higher rate of infectious complications was noted in the temsirolimus treatment arms, though. Further side effects included anemia, asthenia and gastrointestinal irritations.
Based on the results of this trial, temsirolimus has been approved for the treatment in the EU for patients with relapsed MCL.
Temsirolimus in combination therapy
Preclinical studies demonstrating additional, if not even synergistic effects build a strong rationale for the use of a combination of temsirolimus with conventional chemotherapy or monoclonal antibodies to improve the efficacy of single agent use.29 In detail, in vitro studies with rituximab showed an improved induction of apoptosis, complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) in lymphoma cell lines.44–46 On the other hand, rituximab seems to inhibit the pathway of extracellular signal-regulated kinase one and two (ERK1/2) and thus to interact with the PI3K pathway, the pathway also being altered by mTOR-Inhibitors. 47
Consequently, a phase II study was conducted to test the combination of temsirolimus 25 mg once weekly with the monoclonal antibody rituximab (375 mg/m2, once weekly). Drugs were given weekly for 4 weeks during the first cycle, then continued with a weekly dose of temsirolimus and a single dose of rituximab every second 28 day cycle. If a response could be observed after 6 cycles, up to 12 cycles were allowed. 71 patients with refractory or relapsed MCL were enrolled and 69 patients were available for analysis. The overall response rate was 59% with 13 complete remissions (19%) and 28 patients with a partial response (41%).48
In this trial, patients with rituximab-sensitive and -refractory disease were included, and appropriate cohorts were analyzed accordingly.49,50 Interestingly, in contrast to studies with rituximab in combination with conventional chemotherapy there was a smaller difference in response rates in responding patients between the two subgroups. The overall response rate in rituximab-sensitive patients was 63% (30 of 48) and 52% (11 of 21) for rituximab-refractory patients.48 Thus, we can assume that with this combination at least a partial restoration of Rituximab efficacy in refractory patients might be reached. In general, treatment-related grade 3 or 4 adverse events resembled the data obtained in mono-therapy trials, and again thrombocytopenia in 16 patients (23%) and neutropenia in 15 patients (21%) were the dominant side effects. Additionally, fatigue in 14% and pneumonia in 10% were noted.41,42,48
Although a number of combination trials are presently ongoing, no data have been published on the various trials testing mTOR inhibitor-combos yet. In brief, to date combinations of mTOR inhibitors like temsirolimus and everolimus have been tested in combinations with various chemotherapies (single agent: bendamustine, cladribine or combination regimen: CHOP, FC), immunomodulatory agents (lenalidomide), proteasome inhibitors (bortezomib) for MCL or MM or agents like PARP inhibitors or tyrosine kinase inhibitors like sorafenib, and it will be of great importance for the identification of optimal combination partners to compare the results of the different trials. Currently ongoing trials are listed in Table 3.
Table 3.
Ongoing clinical trials with treatments combining mTOR inhibitors in MCL.
| Registration number | Title | Combination type | Phase |
|---|---|---|---|
| NCT01076543 | Lenalidomide and Temsirolimus in Treating Patients With Relapsed or Refractory Hodgkin Lymphoma or Non-Hodgkin Lymphoma | Immunomodulatory agent | 2, recruiting |
| NCT01078142 | Temsirolimus, Bendamustine and Rituximab for Relapsed Follicular Lymphoma or Mantle Cell Lymphoma | Chemotherapy and antibody | 2, recruiting |
| NCT00787969 | Rituximab, Cladribine, and Temsirolimus in Treating Patients With Newly Diagnosed Mantle Cell Lymphoma | Chemotherapy and antibody | 2, recruiting |
| NCT01389427 | Bortezomib, Rituximab, and Dexamethasone With or Without Temsirolimus in Treating Patients With Untreated or Relapsed Waldenstrom Macroglobulinemia or Relapsed or Refractory Mantle Cell or Follicular Lymphoma | Proteasome inhibitor, antibody and steroid | 2, recruiting |
| NCT01381692 | Escalating Doses of Torisel in Combination With Three Chemotherapies Regimens: R-CHOP, R-FC or R-DHA for Patients With Relapsed/Refractory Mantle Cell Lymphoma (MCL). | Chemotherapy and antibody | 2, not yet recruiting |
| NCT01281917 | Study of Velcade and Temsirolimus for Relapsed or Refractory Non-Hodgkin Lymphoma | Proteasome inhibitor | 2, recruiting |
Current standard of care for MCL and potential role for temsirolimus
In recent years, widely accepted standard options for patients with untreated MCL have been established. But still no general approach for the selection of a specific therapy for second-line treatment has been found. In suitable patients with poor risk features, allogeneic transplantation is frequently offered, and in patients with long lasting remissions after prior therapy, a more conservative approach is often used, e.g., a second line of chemoimmunotherapy. However, if patients experience chemorefractoriness, which develops in a substantial proportion of patients, drugs functioning in an alternative way are attractive. Temsirolimus and other agents are frequently used in this clinical situation and have shown promising activity. Their clinical use should be prioritized if no sufficient response lasting for more than 6–12 months after the last line of combination treatment can be achieved, if the patients have been exposed to active drugs like anthracyclines and cytarabinosid or if any contraindications to the use of chemotherapy are given.
Currently available data do not allow the conclusion that single agent use or the combination with antibodies are the ideal use of this drug, as clinical data are missing to evaluate their value in earlier line or in combination therapy. The stimulating data of the combination with rituximab are a first indicator for the advantages of a future combination use of this drug, and currently a number of trials are ongoing to evaluate various combinations. It will be exceedingly interesting to see the results e.g., for the combination with immunomodulatory agents like lenalidomide or proteasome inhibitors like bortezomib, which have been shown to be effective as single agents in MCL (Table 3).
Among the other novel agents already in clinical use, the effectiveness of bortezomib as a potent, selective and reversible inhibitor of the 26S proteasome has been presented in a phase II-trial.51 But also in combination with rituximab or combined with conventional chemotherapy promising response rates have been shown, with a good cytotoxic activity and acceptable toxicity in relapsed MCL. Currently, several studies are going on that examine the use of bortezomib in first-line treatment of MCL. Additionally, there are several immunmodulatory drugs, especially thalidomid and lenalidomide both of which are agents with anti-angiogeneic, anti-inflammatory and immunomodulatory effective mechanism, with promising response rates in patients with relapsed MCL.2,22,52,53 Results of trials evaluating different novel agents are summarized in Table 4. However, it has to be kept in mind, that there is a great variability in patient selection within all these trials.
Table 4.
Comparison of the activity of novel treatment approaches in relapsed MCL. Survival data in relapsed/refractory mantle cell lymphoma in comparison.
| Phase | Med. pretx | ORR | PFS (months) | TTP (months) | OS (months) | Literature | |
|---|---|---|---|---|---|---|---|
| Temsirolimus (175/75) | II–III | 4 | 22% | 4.8 | 4.8 | 12.8 | 43 |
| Temsirolimus (175/25) | II–III | 4 | 6% | 3.4 | 3.4 | 9.7 | 43 |
| Bortezomib | II | 1* | 33% | 6.5 | 6.2 | 23.5 | 62 |
| Thalidomide + Rituximab | II | 1 | 81% | 20.4 | n.a. | 75% (estimated 3 year survival) | 52 |
| Lenalidomide | II | 4 | 53% | 5.6 | 6.5 | n.a. | 22 |
| Flavopiridol | II | 1* | 11% | 21.9 | n.a. | 63 |
Note:
Including untreated patients: med. pretx: median number of prior treatment lines.
In addition, a great number of monoclonal antibodies is currently investigated in preclinical and clinical trials. Thus, researches demonstrated promising response rates and long-term remission rates after monotherapy with blinatunomab in patients with relapsed MCL,54 and Advani presented promising response rates after application of a combination therapy with inotuzumab-ozogamicin and rituximab.55 Furthermore, drugs like PI3K inhibitors (CAL101), BTK inhibitors (PCI-32765) and Syk-inhibitors like fostamatinib have shown promising and sometimes impressive response rates. As reported, newer small molecules like the ATP-competitive mTOR kinase inhibitors (TKIs) bind to the ATP-binding site in the mTOR catalytic domain and inhibit both mTOR complexes consecutively.56,57 They may be even more efficient mTOR inhibitors. A combination of these drugs with and without chemotherapy and mTOR-inhibition seems attractive. However, the limited patient number combined with the number of available new agents requires a concerted action for the evaluation of these approaches.58 Importantly, overlapping toxicities may occur as well, and this requires the careful evaluation of potential combinations to avoid unnecessary complications, and a careful selection seems advisable.
Use of temsirolimus in clinical practice
The elimination of temsirolimus and its metabolites happens mainly via faeces. The mean half-life of the main metabolite, sirolimus,59 is approximately four times longer than that of temsirolimus and increases with dose. The incidence of adverse events is particularly correlated with the cumulative AUC.60 As the key enzyme in the metabolism is the cytochrome-P450-isoform CYP3A, inhibitors like conazoles, HIV protease inhibitors or grapefruit juice can reduce the metabolizing function and the resulting increased AUC level. On the other hand, CYP3A inductors like dexamethasone, AEDs or rifampicin may induce a faster metabolizing and elimination of temsirolimus and its metabolites, and Temsirolimus dose adjustment may be required to achieve sufficient drug levels.61 The typical side effect profile includes cytopenias, asthenia and gastrointestinal disorders, the last-mentioned can be reduced with preemptive treatment. In addition, one of the typical class effects of mTOR inhibitors is the induction of pneumonitis, and patients should be carefully evaluated if clinical symptoms suggest this diagnosis.
Today, the proven dose of 175/75 mg or the combination with Rituximab seems to be a valid option associated with a substantial remission rate. It seems advisable, even though we have no corresponding prospective data, to use a lower than the approved starting dose in patients with severely compromised bone marrow reserve in order to avoid treatment cessations. In this context, a currently active trial is challenging the value of the 175 mg initial dose, testing the 175/75 mg regimen vs. a 75 mg regimen (NCT01180049). Treatment with temsirolimus is applied on a weekly basis, and in general given until disease progression or the occurance of unacceptable toxicyt. In case of CR an individualized treatment decision if how long treatment should be continued has to be made. To date there are only anecdotic data about a re-exposition to Temsirolimus after treatment cessation.
Summary
Altogether, temsirolimus shows promising results in the therapy of patients with heavily pre-treated MCL, and especially responses in chemotherapy refractory patients underline its efficacy. Currently, the evaluation of the combination of temsirolimus with other agents is under progress, in order to improve its efficacy and/ or promote the drug among the available treatment algorithms.
Thus, this period can be of great consequence for patients with mantle cell lymphoma, providing a great number of promising novel approaches to the treatment of their disease, and the use of mTOR inhibitors has been one of the first successfully tested therapy approaches, broadening the armamentarium for the treatment of this challenging lymphoma subtype.
Footnotes
Conflicts of Interest
SK and SW do not have any disclosures; GH has received lecture fees and research funding from Pfizer and has served on advisory boards of this entity.
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011 Jan 6;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dreyling M, Hiddemann W. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology/the Education Program of the American Society of Hematology American Society of Hematology. 2009:542–51. doi: 10.1182/asheducation-2009.1.542. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer A, Salaverria I, Bosch F, Villamor N, Rozman M, Bea S, et al. Leukemic involvement is a common feature in mantle cell lymphoma. Cancer. 2007 Jun 15;109(12):2473–80. doi: 10.1002/cncr.22715. [DOI] [PubMed] [Google Scholar]
- 4.Cohen PL, Kurtin PJ, Donovan KA, Hanson CA. Bone marrow and peripheral blood involvement in mantle cell lymphoma. Br J Haematol. 1998 May;101(2):302–10. doi: 10.1046/j.1365-2141.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 5.Romaguera JE, Medeiros LJ, Hagemeister FB, Fayad LE, Rodriguez MA, Pro B, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003 Feb 1;97(3):586–91. doi: 10.1002/cncr.11096. [DOI] [PubMed] [Google Scholar]
- 6.Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008 Jan 15;111(2):558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 7.Schmidinger M, Berger W. Temsirolimus. Arzneimittel PROFIL Onkologie. 2007:1–18. [Google Scholar]
- 8.Martin P, Coleman M, Leonard JP. Progress in mantle-cell lymphoma. J Clin Oncol. 2009 Feb 1;27(4):481–3. doi: 10.1200/JCO.2008.19.5032. [DOI] [PubMed] [Google Scholar]
- 9.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008 Oct 1;112(7):2687–93. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermine O, Hoster E, Walewski J, Ribrag V, Brousse N, Thieblemont C, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and Autologous Stem Cell Transplantation (ASCT) is superior to 6 courses CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: results of the MCL younger trial of the european Mantle Cell Lymphoma Network (MCL net) ASH Annual Meeting Abstracts. 2010 Nov 19;116(21):110. [Google Scholar]
- 11.Fernandez V, Salamero O, Espinet B, Sole F, Royo C, Navarro A, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Research. 2010 Feb 15;70(4):1408–8. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 12.Ghielmini M, Schmitz SF, Cogliatti S, Bertoni F, Waltzer U, Fey MF, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK) J Clin Oncol. 2005 Feb 1;23(4):705–11. doi: 10.1200/JCO.2005.04.164. [DOI] [PubMed] [Google Scholar]
- 13.Lenz G, Dreyling M, Hoster E, Wormann B, Duhrsen U, Metzner B, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005 Mar 20;23(9):1984–92. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 14.Forstpointner R, Unterhalt M, Dreyling M, Bock HP, Repp R, Wandt H, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG) Blood. 2006 Dec 15;108(13):4003–8. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]
- 15.Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005 Oct 1;23(28):7013–23. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 16.Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005 Sep 10;23(26):6409–14. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 17.Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009 August 20;114(8):1469–76. doi: 10.1182/blood-2009-02-179739. [DOI] [PubMed] [Google Scholar]
- 18.Weigert O, Unterhalt M, Hiddemann W, Dreyling M. Mantle cell lymphoma: state-of-the-art management and future perspective. Leuk Lymphoma. 2009 Dec;50(12):1937–50. doi: 10.3109/10428190903288514. [DOI] [PubMed] [Google Scholar]
- 19.Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005 Apr 1;105(7):2677–84. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 20.Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005 May 20;23(15):3383–9. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 21.Khouri IF, Lee MS, Saliba RM, Jun G, Fayad L, Younes A, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol. 2003 Dec 1;21(23):4407–12. doi: 10.1200/JCO.2003.05.501. [DOI] [PubMed] [Google Scholar]
- 22.Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009 May;145(3):344–9. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010 Mar 16;17(3):249–61. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drakos E, Rassidakis GZ, Medeiros LJ. Mammalian target of rapamycin (mTOR) pathway signalling in lymphomas. Expert Rev Mol Med. 2008 Feb 4;10:e4. doi: 10.1017/S1462399408000586. [DOI] [PubMed] [Google Scholar]
- 25.Gladden AB, Woolery R, Aggarwal P, Wasik MA, Diehl JA. Expression of constitutively nuclear cyclin D1 in murine lymphocytes induces B-cell lymphoma. Oncogene. 2006 Feb 16;25(7):998–1007. doi: 10.1038/sj.onc.1209147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011 Mar 1;3(3):192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samad N, Younes A. Temsirolimus in the treatment of relapsed or refractory mantle cell lymphoma. Onco Targets Ther. 2010 Sep 7;3:167–78. doi: 10.2147/ott.s8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009 Dec;8(23):3831–7. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 29.Galimberti S, Petrini M. Temsirolimus in the treatment of relapsed and/ or refractory mantle cell lymphoma. Cancer Manag Res. 2010 Jun 28;2:181–9. doi: 10.2147/cmar.s7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006 Apr 21;22(2):159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005 Mar;69(1):79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. Journal of Cellular Physiology. 2005 Apr;203(1):144–55. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 33.Peponi E, Drakos E, Reyes G, Leventaki V, Rassidakis GZ, Medeiros LJ. Activation of mammalian target of rapamycin signaling promotes cell cycle progression and protects cells from apoptosis in mantle cell lymphoma. Am J Pathol. 2006 Dec;169(6):2171–80. doi: 10.2353/ajpath.2006.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivieres S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem. 1998 Jun 5;273(23):14424–9. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- 35.Muthukkumar S, Ramesh TM, Bondada S. Rapamycin, a potent immunosuppressive drug, causes programmed cell death in B lymphoma cells. Transplantation. 1995 Aug 15;60(3):264–70. doi: 10.1097/00007890-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Hosoi H, Dilling MB, Shikata T, Liu LN, Shu L, Ashmun RA, et al. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999 Feb 15;59(4):886–94. [PubMed] [Google Scholar]
- 37.Mahalingam D, Medina EC, Esquivel JA, 2nd, Espitia CM, Smith S, Oberheu K, et al. Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels. Clin Cancer Res. 2010 Jan 1;16(1):141–53. doi: 10.1158/1078-0432.CCR-09-1385. [DOI] [PubMed] [Google Scholar]
- 38.Yazbeck VY, Buglio D, Georgakis GV, Li Y, Iwado E, Romaguera JE, et al. Temsirolimus downregulates p21 without altering cyclin D1 expression and induces autophagy and synergizes with vorinostat in mantle cell lymphoma. Exp Hematol. 2008 Apr;36(4):443–50. doi: 10.1016/j.exphem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004 Jun 15;22(12):2336–47. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 40.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004 Mar 1;22(5):909–18. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 41.Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005 Aug 10;23(23):5347–56. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 42.Ansell SM, Inwards DJ, Rowland KM, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008 Aug 1;113(3):508–14. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009 Aug 10;27(23):3822–9. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 44.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994 Jan 15;83(2):435–55. [PubMed] [Google Scholar]
- 45.Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000 Jun;51(6):634–41. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 46.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998 Mar 1;91(5):1644–52. [PubMed] [Google Scholar]
- 47.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin’s lymphoma B cells by Rituximab. Cancer Res. 2004 Oct 1;64(19):7117–26. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 48.Ansell SM, Tang H, Kurtin PJ, Koenig PA, Inwards DJ, Shah K, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. Lancet Oncol. 2011 Apr;12(4):361–8. doi: 10.1016/S1470-2045(11)70062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Gnaoui T, Dupuis J, Belhadj K, Jais JP, Rahmouni A, Copie-Bergman C, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007 Aug;18(8):1363–8. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- 50.Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008 Dec;93(12):1829–36. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- 51.Bernstein ZP, Chanan-Khan A, Miller KC, Northfelt DW, Lopez-Berestein G, Gill PS. A multicenter phase II study of the intravenous administration of liposomal tretinoin in patients with acquired immunodeficiency syndrome-associated Kaposi’s sarcoma. Cancer. 2002 Dec 15;95(12):2555–61. doi: 10.1002/cncr.11009. [DOI] [PubMed] [Google Scholar]
- 52.Kaufmann H, Raderer M, Wohrer S, Puspok A, Bankier A, Zielinski C, et al. Antitumor activity of rituximab plus thalidomide in patients with relapsed/refractory mantle cell lymphoma. Blood. 2004 Oct 15;104(8):2269–71. doi: 10.1182/blood-2004-03-1091. [DOI] [PubMed] [Google Scholar]
- 53.Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2011 Jul;22(7):1622–7. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 54.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008 Aug 15;321(5891):974–7. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 55.Advani A, Coiffier B, Czuczman MS, Dreyling M, Foran J, Gine E, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010 Apr 20;28(12):2085–93. doi: 10.1200/JCO.2009.25.1900. [DOI] [PubMed] [Google Scholar]
- 56.Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009 Aug 1;69(15):6232–40. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 57.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATPcompetitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009 Mar 20;284(12):8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryce AH, Rao R, Sarkaria J, Reid JM, Qi Y, Qin R, et al. Phase I study of temsirolimus in combination with EKB-569 in patients with advanced solid tumors. Invest New Drugs. 2011 Sep 1; doi: 10.1007/s10637-011-9742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai P, Tsao R, Ruppen ME. In vitro metabolic study of temsirolimus: preparation, isolation, and identification of the metabolites. Drug Metab Dispos. 2007 Sep;35(9):1554–63. doi: 10.1124/dmd.107.014746. [DOI] [PubMed] [Google Scholar]
- 60.Boni JP, Leister C, Bender G, Fitzpatrick V, Twine N, Stover J, et al. Population pharmacokinetics of CCI-779: correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther. 2005 Jan;77(1):76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 61.Boni J, Leister C, Burns J, Cincotta M, Hug B, Moore L. Pharmacokinetic profile of temsirolimus with concomitant administration of cytochrome p450-inducing medications. J Clin Pharmacol. 2007 Nov;47(11):1430–9. doi: 10.1177/0091270007306957. [DOI] [PubMed] [Google Scholar]
- 62.Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009 Mar;20(3):520–5. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kouroukis CT, Belch A, Crump M, Eisenhauer E, Gascoyne RD, Meyer R, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003 May 1;21(9):1740–5. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]