As evasive strategy, Francisella tularensis triggers TLR2-dependent NF-κB signaling resulting in development and activation of tolerogenic dendritic cells and regulatory T cells.
Keywords: NF-κB, lipopolysaccharide, neutrophils, Francisella tularensis, IL-10, TGF-β
Abstract
Tularemia is a vector-borne zoonosis caused by Ft, a Gram-negative, facultative intracellular bacterium. Ft exists in two clinically relevant forms, the European biovar B (holarctica), which produces acute, although mild, self-limiting infections, and the more virulent United States biovar A (tularensis), which is often associated with pneumonic tularemia and more severe disease. In a mouse model of tularemia, respiratory infection with the virulence-attenuated Type B (LVS) or highly virulent Type A (SchuS4) strain engenders peribronchiolar and perivascular inflammation. Paradoxically, despite an intense neutrophilic infiltrate and high bacterial burden, Th1-type proinflammatory cytokines (e.g., TNF, IL-1β, IL-6, and IL-12) are absent within the first ∼72 h of pulmonary infection. It has been suggested that the bacterium has the capacity to actively suppress or block NF-κB signaling, thus causing an initial delay in up-regulation of inflammatory mediators. However, our previously published findings and those presented herein contradict this paradigm and instead, strongly support an alternative hypothesis. Rather than blocking NF-κB, Ft actually triggers TLR2-dependent NF-κB signaling, resulting in the development and activation of tDCs and the release of anti-inflammatory cytokines (e.g., IL-10 and TGF-β). In turn, these cytokines stimulate development and proliferation of Tregs that may restrain Th1-type proinflammatory cytokine release early during tularemic infection. The highly regulated and overall anti-inflammatory milieu established in the lung is permissive for unfettered growth and survival of Ft. The capacity of Ft to evoke such a response represents an important immune-evasive strategy.
Introduction
Recognition of most bacterial pathogens triggers release of proinflammatory cytokines leading to activation of myeloid lineage cells (e.g., MΦ and DCs) and priming of antigen-specific type 1 CD4+ Th1 and CD8+ cytotoxic T cells. Typically, activation of MΦ and DCs results in release of Th1-type proinflammatory cytokines (e.g., TNF, IL-1β, IL-6, and IL-12). These immunomodulators enhance pathogen uptake and direct antimicrobial activities against them. One of the principal innate immune cells rapidly recruited to inflammatory foci and responsible for bacterial killing and clearance is the neutrophil. Neutrophils have an antimicrobial armamentarium that includes ROS, reactive nitrogen species, cationic peptides, and lytic enzymes. Another proinflammatory cytokine produced at sites of bacterial colonization is IL-17A, which synergizes with TNF, IL-1β, and IL-6 to further activate neutrophils and enhance their bactericidal activities [1].
In stark contrast to this classical Th1-type, proinflammatory response, the early phase (<72 h) of respiratory infection with Ft spp. holarctica (from which the attenuated LVS was derived) and spp. tularensis (e.g., SchuS4 strain) is typified by a lack of TNF, IL-1β, IL-6, and IL-12 [2–9]. However, the inflammatory milieu in the Ft-infected lung at this time is characterized by: the presence of active MMP9 [10]; degradation of ECM, which generates tripeptide fragments of Pro-Gly-Pro that are chemotactic for neutrophils [10]; an intense neutrophilic infiltrate [10]; and the production of IL-17A [5, 7, 11]. In addition, it has been reported that Ft induces PGE2 [5] and activation of DCs, resulting in release of IL-10 and TGF-β [2]. Despite the fact that all of the aforementioned immune responses are NF-κB-dependent, some studies suggest the bacterium has the capacity to actively block NF-κB signaling [12–16].
Telepnev et al. [12] proposed that infection with Ft blocks phosphorylation of IκB-α and p38-MAPK, thereby inhibiting TNF, IL-1β, and IL-12 production by mouse and human MΦ in response to the TLR4 agonist LPS. An extension of this work suggests that Ft initially triggers NF-κB signaling, which then is subsequently down-regulated, as bacteria escape into and replicate within the cytosol of MΦ [13]. Butchar et al. [14] suggest that Ft can subvert host responses and block cytokine production via induction of SOCS, specifically the family members SOCS1 and SOCS3, which can inhibit the NF-κB pathway. Shirey et al. [17] propose that Ft initially triggers a classical activation program in MΦ and then redirects their differentiation such that the cells become alternatively activated, typified by expression of arginase 1 and TGF-β rather than iNOS and TNF. Melillo et al. [15] suggest the basis for host cell suppression of proinflammatory cytokines is the capacity of Ft antioxidant enzymes to scavenge host-derived ROS. Such enzyme activity is thought to block signals required for MΦ cytokine production, including activation of PI3K and Akt phosphorylation, IκB-α degradation, and nuclear localization of NF-κB. Most recently, although contrary to what Melillo et al. [15] propose, Medina and coworkers [16] postulate that Ft restrains TLR2-triggered, proinflammatory responses via simultaneous activation of PI3K and downstream enhancement of MKP-1. In this scenario, the action of PI3K is thought to inhibit p38-MAPK-dependent, proinflammatory signals.
Clearly, a complete understanding of tularemia pathogenesis, particularly the mechanism whereby host cells respond to Ft in vitro, remains elusive. One fundamental caveat associated with the aforementioned studies is that due deference is not paid to the seminal finding by Hazlett et al. [18] and others [19–21]—that in vitro growth conditions have a profound qualitative and quantitative effect on the in vitro and in vivo host response to Ft LVS and SchuS4. Ft, cultivated under conditions that preclude HAd (e.g., growth in modified MHB, Thayer-Martin-based broth, or agar media) versus those that facilitate HAd (e.g., growth in BHI broth, Chamberlain′s defined medium, or replication within isolated MΦ or infected tissues), differs substantially; the former growth conditions impose upon the bacterium a proinflammatory phenotype that Ft fails to exhibit in vivo during natural infection. As the studies described above [12–16] were conducted with Ft grown under conditions that engender an aberrant, proinflammatory phenotype, the physiological relevance of the findings to tularemia pathogenesis and the interpretation of results with respect to host cell signaling events warrant re-evaluation.
Given that a broader understanding of tularemia pathogenesis can only be achieved once the basic immune processes, which underlie early disease development, are revealed, the present study had two objectives. First, we sought to clarify whether Ft actively blocks NF-κB signaling and if so, by what mechanism(s). Second, we sought to test an alternative hypothesis to explain the lack of TNF, IL-1β, IL-6, and IL-12 early during tularemic infection. Instead of blocking NF-κB signaling, we postulate that Ft triggers NF-κB-dependent development and activation of tDCs and Tregs to restrain Th1-type, proinflammatory cytokine release through elaboration of anti-inflammatory cytokines. The results presented herein detail the mechanism whereby Ft “side-steps” host cellular defenses to facilitate its nearly unfettered proliferation. We demonstrate that Ft has the capacity to drive the development and activation of tDCs and Tregs, thereby eliciting a predominantly anti-inflammatory host response following colonization of the pulmonary system. These findings should stimulate re-evaluation of the current paradigm regarding Ft-host response and provide a conceptual foundation for development of rational and effective immunotherapeutic strategies to combat this and potentially other bacterial respiratory pathogens.
MATERIALS AND METHODS
Bacteria
Ft LVS (ATCC 29684; American Type Culture Collection, Manassas, VA, USA) was kindly provided by Dr. Karen Elkins (U.S. Food and Drug Administration, Bethesda, MD, USA). Ft SchuS4, originally isolated from a human case of tularemia, was obtained from the U.S. Army Medical Research Institute for Infectious Diseases (Frederick, MD, USA). All experiments using SchuS4 were conducted within a Centers for Disease Control-certified Animal Biosafety Level-3/Biosafety Level-3 facility at Albany Medical College (Albany, NY, USA). The bacteria were cultured in modified MHB or BHI broth. A single colony picked from a MHB-agar plate was used to initiate a 5-ml MHB or BHI culture that was maintained for 12 h at 37°C while shaking at 220 RPM. These “starter” cultures were then used to inoculate (1:200) a 100-ml MHB or BHI culture, which was maintained for 12–16 h. Bacteria were harvested when cultures achieved an absorbance of 260 nm OD of 0.2, at which point, CFU/ml were determined by serial dilution and colony plating, as described elsewhere [3]. In addition, Ft LVS was recovered from culture supernatant following 24-h coincubation with mouse BM-derived MΦ (500 MOI). These MΦ-grown bacteria were used in comparative, in vitro cell-based studies along with their MHB- and BHI-grown counterparts.
Mice
WT C57BL/6 mice (purchased from National Cancer Institute, Bethesda, MD, USA) and congenic TLR2−/− animals were housed in the Animal Resources Facility at Albany Medical College. Food and water were provided ad libitum. All animal procedures conformed to the Institutional Animal Care and Use Committee guidelines. All experiments were conducted using equal numbers of male and female mice of 6–8 weeks of age.
In vitro cell culture and infection
Mouse RAW264.7 MΦ-like cells were cultured in DMEM, supplemented with 10% FBS, 2 mM Glutamax, 1 mM sodium pyruvate, and 25 mM HEPES in 5% CO2 at 37°C. Unless otherwise indicated, these standard culture conditions were used for all experiments. The RAW264.7 cells were stably transfected with the human ELAM (E-selectin) promoter (–760 to +60), driving expression of destabilized eGFP. Cells (5×105/ml) were unstimulated or stimulated for 6 h with Ft LVS (MOI of 100), LPS (100 ng), Pam3CSK (100 ng; a synthetic analog of a BLP), or purified rTul4 lipoprotein (100 ng). Following stimulation, cells were analyzed by flow cytometry for eGFP expression. To confirm that induced eGFP expression was strictly dependent on NF-κB activity, one set of cells was stimulated in the presence of 30 μM of the IKK inhibitor PTL. In another series of experiments, the capacity of RAW264.7 cells to be infected by and support the replication of Ft was evaluated as described previously [3].
Isolation and infection of BMDM and BM-derived DCs
BM cells were isolated from the femurs and tibias of 6- to 8-week-old mice and processed as described previously to enrich for BMDM [22]. For tolerance experiments, BMDM (5×105 cells/ml) were exposed to BHI-grown Ft (MOI of 10), LPS (100 ng/ml), or Pam3CSK (100 ng/ml) for 4 h. Cells then were washed twice with sterile PBS, received medium alone or were re-exposed to BHI-grown Ft (MOI of 100), LPS (500 ng/ml), or Pam3CSK (500 ng/ml), and incubated for an additional 24 h. Culture supernatants then were recovered and TNF levels measured by commercial ELISA (eBioscience, San Diego, CA, USA). For transcript analysis, BMDMs (5×105 cells/ml) were infected with MHB-, BHI-, and MΦ-grown Ft (MOI of 100 for each) for different periods of time, and total RNA was recovered and subjected to quantitative analysis of transcripts (i.e., socs1, socs3, tnf, and inos), as described elsewhere [22, 23]. Transcription of the arg1 gene was analyzed using the following oligonucleotide forward and reverse primers: 5′-ACCACGGGGACCTGGCCTTT-3′ and 5′-CCTGGCGTGGCCAGAGATGC-3′, respectively. All of the qPCR reactions were run in triplicate with no-template controls, and mean comparative threshold values were used for all of the calculations using 18S rRNA as an internal normalization control. Transcript levels for infected groups are presented as a fold change over their corresponding uninfected control group. A greater than twofold change, with respect to mock control, was considered significant.
DCs were generated by incubating BM precursor cells with mouse rFlt3L (50 ng/ml; R&D Systems, Minneapolis, MN, USA) for 9 days. Cells received fresh rFlt3L-containing medium every 3rd day. At the end of the culture period, nonadherent cells representing DCs were harvested and used in experiments. The dendritic phenotype of these cells was confirmed by flow cytometry-based examination of surface expression for CD11c, CD11b, CD8a, B220, and CR2 using commercial fluorochrome-conjugated antibodies (eBioscience). The DC population was >93% CD11c+ and expressed high levels of B220 but very low levels of CD8a, CD11b, and CR2. DCs (5×105 cells/ml) from WT and TLR2−/− mice were infected with MHB-, BHI-, and MΦ-grown Ft LVS or MHB- and BHI-grown Ft SchuS4 (MOI of 100 for each) for 24 h, and culture supernatants were analyzed for proinflammatory cytokines (i.e., TNF, IL-1β, and IL-6) by CBA (BD PharMingen, San Diego, CA, USA). Flow cytometric analysis was performed using a FACSArray flow cytometer (BDIS, San Jose, CA, USA). Data were acquired and analyzed using BD FACSArray software and FCAP Array software, version 1.0 (BDIS), respectively. Anti-inflammatory cytokines (i.e., IL-10 and TGF-β) were measured using commercial ELISA (eBioscience).
Measurement of NF-κB activation by TransAM NF-κB ELISA
Activation of NF-κB in BMDMs and DCs following in vitro infection with Ft grown in different media was assessed using a TransAM NF-κB ELISA kit (Active Motif, Carlsbad, CA, USA). Nuclear extracts were collected using a nuclear extraction kit (Active Motif) from cells stimulated with LPS (100 ng/ml) or Ft (MOI of 100), with and without prior addition of 30 μM PTL. Binding of the p65 subunit of NF-κB to a consensus-binding sequence (5′-GGGACTTTCC-3′) within target oligonucleotide-coating 96-well microtiter plates was detected by incubation for 1 h with primary antibody, followed by incubation with anti-IgG HRP conjugate and developing solution. The amount of p65 was quantified colorimetrically at 450 nm with a reference wavelength of 655 nm.
Western blot analysis of SOCS expression
Antibodies directed against SOCS1 and SOCS3 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and antibodies against β-actin were obtained from Bethyl Laboratories (Montgomery, TX, USA). Protein samples (25–100 μg, depending on the target) were resolved by SDS-PAGE, and Western blotting was performed as described elsewhere [22]. Specific signal was developed using the SuperSignal West Dura chemiluminescent substrate (Pierce Endogen, Rockford, IL, USA).
Infection of mice
All infection experiments used groups of three to five mice. Prior to i.n. inoculation, animals were deeply anesthetized via i.p. injection of a cocktail of Ketamine (20 mg/ml) and Xylazine (1 mg/ml). Following dilution in sterile PBS, 1 × 103 CFU of BHI-grown Ft LVS in a volume of 20 μl were instilled i.n. (10 μl/nare); actual dosages were confirmed by colony plating. Sham-inoculated controls received an equal volume of uninoculated BHI broth diluted in PBS. Killed mice were necropsied at various times PI, and lungs were perfused with PBS and excised aseptically. The smaller lobe of the lung was used for preparation of lung homogenate for bacterial counting and/or cytokine measurements as described previously [3], and the remainder was used for isolation of single cell suspensions for flow cytometry analysis or for histological evaluation. Tissues were processed using standard histological methods to obtain 5-μm-thick paraffin sections that were stained with H&E as described previously [3].
Bacterial burden and cytokine measurements
Portions of lung (20 mg) were suspended in 0.5 ml PBS containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) and were homogenized using a mechanical Mini Bead Beater (Biospec Products, Bartlesville, OK, USA) and sterile, inert Zirconia beads. Homogenates were processed, and quantification of bacterial numbers was performed as described previously [3]. Results are expressed as log10 CFU/ml. Lung homogenates were also assayed for the presence of proinflammatory and anti-inflammatory cytokines as described above. In addition, measurement of IL-17A was performed using a commercial ELISA kit (eBioscience).
Antibodies
For flow cytometry, mouse FITC-anti-CD4 (clone GK1.5), PE-anti-CD3 (clone 17A2), APC-anti-CD25 (PC61), PE-anti-FoxP3 (clone 150D), PE-anti-IgG1 isotype control (clone MOPC-21), APC-anti-IL-17A (clone TC11-18H10.1), APC-anti-rat IgG1 isotype control (clone RTK2071), FITC-anti-CD11b (clone M1/70), APC-anti-IL-10 (clone JES5-16E3), PerCP/Cy5.5-anti-Gr-1 (clone RB6-8C5), PerCP/Cy5.5-anti-CD49 pan NK cell (clone DX5), PE/Cy7-anti-CD11c (clone N418), PE-anti-F4/80 (clone BM8), PE/Cy7-rat IgG2b isotype control, and Pacific blue-anti-Gr-1 (clone RB6-8C5) were purchased from BioLegend (San Diego, CA, USA). Mouse FITC-anti-γδ TCR (clone GL-3), APC-anti-IL-17A (clone 17B7), APC-anti-TNF (clone MP6-XT22), APC-anti-rat IgG2a isotype control (clone 17-4321), PE-anti-CCR9 (CD199; clone CW-1.2), FITC-anti-CD103 (clone 2E7), and PE-anti-CD103 (clone 2E7) were purchased from eBioscience. APC-anti-mouse TGF-β1 (latency-associated peptide; clone 27232) was purchased from R&D Systems.
Flow cytometry
Single cell suspensions were prepared from the lungs of control and infected mice. Briefly, the lungs were cut into small pieces and suspended in 1 ml digestion buffer containing collagenase type I (Worthington Biochemical, Lakewood, NJ, USA) and rDNase I (Roche, Mannheim, Germany). Following digestion for 30 min at 37°C, the lung cells were passed through a cell strainer, collected by centrifugation (250 g, for 10 min), and resuspended in fluorescent assay buffer. For ICCS, lung cells were incubated with surface marker-specific antibodies for 30 min and placed in Fixation buffer (eBioscience). The fixed cells were then permeabilized with Permeabilization buffer (eBioscience) and incubated with cytokine-specific or isotype control antibodies for 40 min. The cells were gated on the basis of forward- and side-scatter characteristics and with respect to specific surface marker and/or cytokine expression. Specific cell populations are graphed as a percentage of the total cells recovered from uninfected lungs or infected lungs at various times. In addition to presenting the percentage of specific cell types observed, the total number of cells was calculated.
To characterize putative tDCs, CD11chighCD11blow cells were analyzed for surface expression of CD103 and CCR9. To identify Tregs, lung cells first were incubated with antibodies directed against CD4 and CD25, followed by intracellular staining with anti-FoxP3 or isotype control antibody. Multiparameter FACS analysis was performed on a LSRII instrument (Becton Dickinson, Franklin Lakes, NJ, USA), and data were compensated and analyzed using FlowJo software, version 7.6.1 (Tree Star, Ashland, OR, USA).
Statistical analysis
Where applicable, all results were expressed as mean ± sem from two or more independent experiments. Depending on the distribution of the dataset, comparisons between groups were made using a parametric ANOVA test with Tukey's post-test or a nonparametric Kruskal-Wallis test with Dunn's post-test. Differences between control and experimental groups were considered significant at α = 0.05 level. The relationship among the level of TGF-β, total number of Tregs, and the log of bacterial burden in the lungs was determined by linear regression analysis. The correlations for these measures in individual animals are presented for Days 0, 1, and 3 (during the exponential growth phase of the bacteria).
RESULTS
Ft triggers rather than blocks NF-κB-dependent signaling
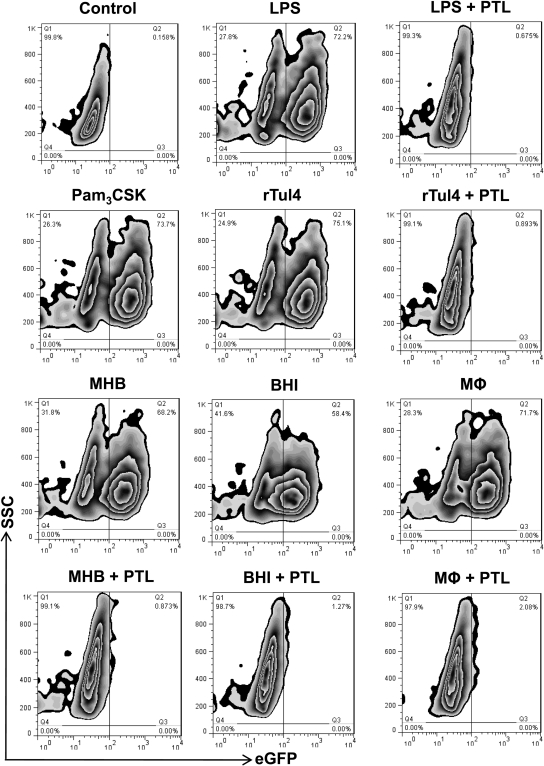
A number of studies have attributed the lack of Th1-type proinflammatory cytokines during early-phase tularemia to blockade of NF-κB signaling [12, 13, 15, 16]. However, this notion is discrepant with a number of immunopathogenic responses to the bacterium that are NF-κB-dependent, including activation of MMP9 and the production of IL-10, IL-17, TGF-β, and PGE2 [2, 5, 7, 10, 11]. To clarify whether Ft LVS has the capacity to activate or ablate NF-κB activity, a well-accepted and validated RAW264.7 reporter cell line was used. These cells, stably transfected with the human ELAM (i.e., E-selectin) promoter, driving expression of eGFP, were used to monitor the response to infection with MHB (i.e., non-HAd)- and BHI- and MΦ-grown (i.e., HAd) Ft. eGFP expression by this reporter cell line is strictly NF-κB-dependent and cannot occur if the signaling pathway is blocked. Following incubation with known TLR4 (i.e., LPS) and TLR2 (i.e., Pam3CSK and rTul4) agonists, the majority of RAW264.7 cells expressed eGFP (Fig. 1 and Supplemental Fig. 1A). Similarly, infection of cells with Ft LVS (regardless of means of cultivation) significantly increased NF-κB-dependent eGFP expression within just 6 h (the earliest time-point studied), and the percentage of positive cells was even greater at 24 h (data not shown). To confirm that expression of eGFP by Ft-infected cells was in fact driven by nuclear translocation of NF-κB, some cells were stimulated with TLR agonists or infected with bacteria in the presence of the IKK inhibitor PTL, which prevents release of NF-κB p50/p65 heterodimers from its IκB-α inhibitor, thus trapping it in the cytosol. In the presence of PTL, eGFP expression triggered by purified TLR agonists or Ft LVS was ablated completely (Fig. 1 and Supplemental Fig. 1A). To extend this finding to primary cells, the TransAM NF-κB p65 DNA-binding ELISA was performed using nuclear extracts from BMDMs incubated for 1 h with non-HAd- and HAd-Ft LVS at a MOI of 100. As seen in Supplemental Fig. 1B, regardless of growth conditions, Ft LVS stimulated the nuclear translocation of NF-κB. Similarly, activation of NF-κB was observed in Ft-infected DCs, irrespective of HAd status (data not shown). Furthermore, as in RAW264.7 cells, cellular activation of NF-κB in response to Ft was ablated completely in BMDMs (Supplemental Fig. 1B) and DCs by inclusion of PTL. As a control, the potential effect of different growth conditions on the capacity of Ft LVS to invade and replicate within cells was evaluated. HAd status failed to significantly influence the replication of bacteria within RAW264.7 cells or BMDMs (Supplemental Fig. 1C).
Figure 1. Ft induces activation of NF-κB signaling.
RAW264.7 cells (5×105/ml) were unstimulated or stimulated for 6 h with Ft LVS (MOI of 100), LPS (100 ng), Pam3CSK (100 ng), or purified rTul4 lipoprotein (100 ng). Ft was grown in liquid culture (i.e., MHB or BHI broth) or isolated from infected MΦ, as described in Materials and Methods. Following stimulation, cells were analyzed by flow cytometry for eGFP expression. To confirm activation of NF-κB (as reflected in induced GFP expression), one set of cells was stimulated in the presence of the IKK inhibitor PTL (30 μM). FACS results are representative of three independent experiments. SSC, Side-scatter.
Neither tolerance nor alternative activation of MΦ explains the inability of Ft to elicit TNF production
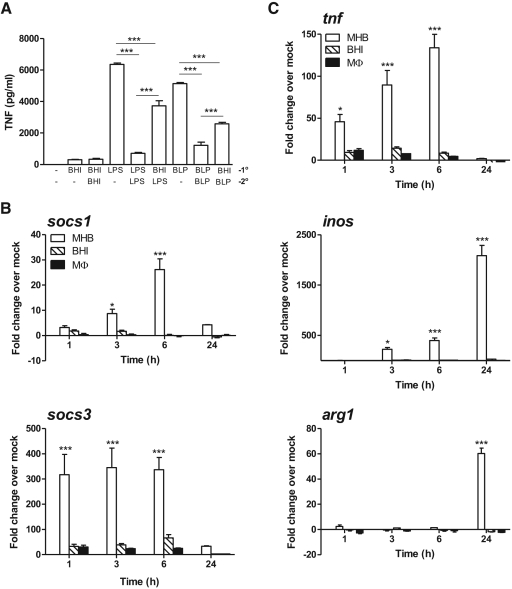
Induction of tolerance (i.e., blockade of NF-κB signaling) has been invoked as a possible explanation for the inability of Ft to stimulate TNF, IL-1β, IL-6, and IL-12 following in vitro infection of host cells or during early respiratory infection of mice [12–16]. Therefore, using a classic experimental design [22], we examined whether Ft has the capacity to tolerize cells against subsequent re-exposure and response to a homologous or heterologous TLR stimulus. BMDMs were exposed to a low-dose primary stimulus of BHI-grown Ft (MOI of 10), LPS (100 ng/ml), or BLP (100 ng/ml) for 4 h at 37°C. Cells were then washed twice and re-exposed to a higher homologous or heterologous stimulus, and TNF levels were measured after 24 h. LPS and BLP served as controls for canonical TLR4- and TLR2-tolerizing agonists, respectively. Cells whose primary and homologous stimuli were LPS or BLP exhibited an 89% and 76% reduction in TNF levels, respectively (Fig. 2A). In contrast, neither primary exposure alone nor primary plus re-exposure of cells to BHI-grown Ft elicited a TNF response above or below unstimulated controls. More importantly, primary exposure to Ft, followed by re-exposure to LPS or BLP, failed to ablate TNF release. The response of infected cells to secondary stimulation with the TLR4 or TLR2 agonist only diminished TNF levels by 41% and 50%, respectively. This reduction in TNF did, however, suggest that HAd-Ft could at least temper proinflammatory cytokine responses to TLR agonists. As such, we explored whether Ft activated SOCS, which target the NF-κB signaling cascade as a means of tolerizing MΦ to continued bacterial stimulation [22]. BMDMs were infected with MHB-, BHI-, or MΦ-grown bacteria, and total RNA was recovered from cells at different time-points. MHB-grown Ft induced an ∼30- and ∼300-fold increase in socs1 and socs3 transcript levels above baseline by 6 h PI, respectively (Fig. 2B). When cells were infected in the presence of the proteosome inhibitor MG132 and total cellular lysate analyzed by Western blot, this pattern of response was mirrored at the protein level (Supplemental Fig. 2). In contrast, HAd-Ft induced significantly lower transcription and translation of either of these negative regulators of NF-κB signaling. Nevertheless, the small amount of SOCS1 and SOCS3 protein produced may account for the HAd-Ft-induced reduction in TNF response to purified TLR agonists observed in Fig. 2A.
Figure 2. The inability of Ft to elicit TNF production does not reflect induction of tolerance, SOCS activity, or alternative activation of MΦ.
(A) BMDMs (5×105 cells/ml) were exposed to a low-dose primary (1°) stimulus of BHI-grown Ft LVS (MOI of 10), LPS (100 ng/ml), or BLP (100 ng/ml) for 4 h at 37°C. Cells were then washed twice and re-exposed to a higher homologous or heterologous secondary (2°) stimulus, and TNF levels were measured after 24 h. The values are expressed as mean ± sem from two independent experiments. (B and C) BMDMs (5×105 cells/ml) were infected with non-HAd (i.e., MHB-gown) or HAd (i.e., BHI- or MΦ-grown) Ft LVS (MOI of 100), and total RNA was recovered from cells after 1, 3, 6, and 24 h incubation. qPCR was used to determine transcript levels for sosc1, socs3, tnf, inos, and arg1. The values are expressed as mean ± sem from three independent experiments. *P < 0.05; ***P < 0.001.
Next, we tested whether HAd-Ft exhibited a greater capacity to induce an alternative rather than classical activation program in MΦ compared with their non-HAd counterparts. Following infection with MHB-grown bacteria, transcript levels for tnf, inos, and arg1 were elevated significantly above baseline over the course of 24 h (Fig. 2C). By comparison, BHI- and MΦ-grown organisms failed to induce transcription of these genes. Curiously, by 24 h of coincubation, the MHB-grown Ft simultaneously induced transcription of inos and arg1 as if BMDMs were receiving “mixed signals” from the bacterium as to whether a classical or alternative program should be initiated. Finally, another possibility to consider is whether HAd bacteria might block proinflammatory cytokine production through alteration of PI3K-AKT-p38-MAPK signaling, as suggested by other groups [15, 16]. In preliminary experiments, BMDMs were incubated with a PI3K inhibitor (LY294002), a general MAPK inhibitor (arctigenin), or a specific p38-MAPK inhibitor (SB202190) in a dose-escalation manner prior to infection with BHI- or MΦ-grown Ft. None of the inhibitors tested augmented or derepressed TNF production in response to HAd bacteria (data not shown). Thus, no evidence currently exists to implicate the PI3K-AKT-p38-MAPK pathway in modulating proinflammatory cytokine responses to HAd-Ft in vitro or during natural infection.
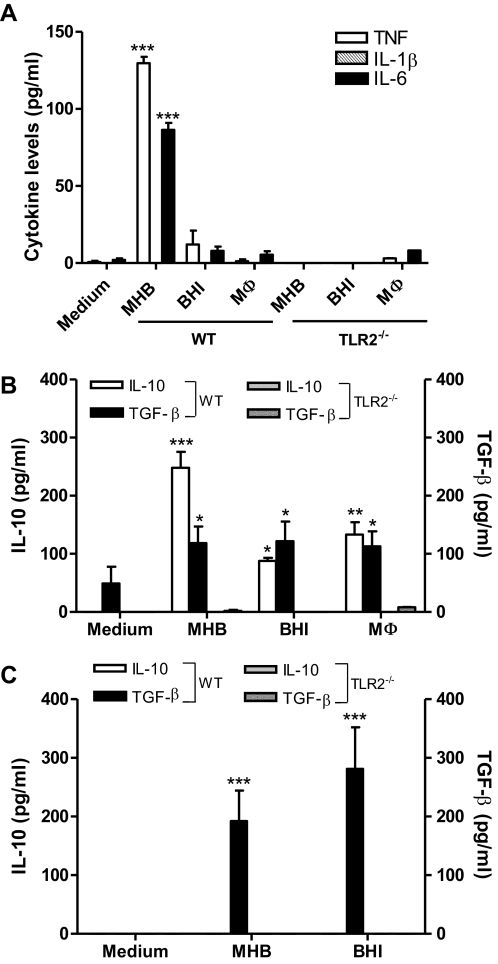
Ft LVS and SchuS4 elicit anti-inflammatory cytokines from DCs and do so in a TLR2-dependent manner
A number of studies have identified DCs as an initial target for infection by Ft and have implicated them in the process of bacterial dissemination as well [4, 24, 25]. It also is appreciated that TLR2 plays a critical role in tularemia pathogenesis [3, 26]. As such, it was of interest to determine to what extent TLR2 regulates cytokine production by DCs. Following in vitro infection with MHB-grown Ft LVS, DCs produced proinflammatory (i.e., TNF and IL-6 but not IL-1β) and anti-inflammatory (e.g., IL-10 and TGF-β) cytokines and did so in a TLR2-dependent manner (Fig. 3A and B). In contrast, BHI- and MΦ-grown LVS failed to elicit proinflammatory cytokines but did stimulate the release of IL-10 and TGF-β (Fig. 3B). These studies were then extended to characterize the anti-inflammatory cytokine response to Ft SchuS4 grown in MHB and BHI broth. As with LVS, this highly virulent Type A strain induced TGF-β in a strictly TLR2-dependent manner, irrespective of the medium used for its cultivation (Fig. 3C). However, MHB- and BHI-grown SchuS4 was unable to stimulate significant production of IL-10 by DCs. To what extent SchuS4-induced pro- and anti-inflammatory cytokine production by an isolated cell type(s) recapitulates host cell responses during early-phase respiratory tularemia is currently under investigation.
Figure 3. TLR2 is required for Ft LVS- and SchuS4-induced anti-inflammatory cytokine release from DCs.
DCs (5×105 cells/ml) from WT and TLR2−/− mice were infected with MHB-, BHI-, and MΦ-grown Ft LVS (A and B) or MHB- and BHI-grown SchuS4 (C; MOI of 100 for each) for 24 h; culture supernatants were analyzed for proinflammatory cytokines by CBA; and anti-inflammatory cytokines were measured using commercial ELISA. The values are expressed as mean ± sem from three independent experiments. *P < 0.05; **P < 0.01; and ***P < 0.001.
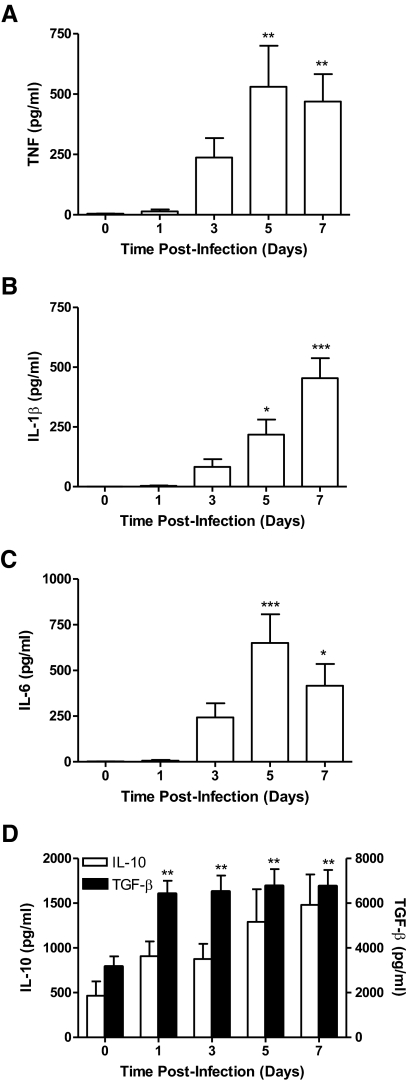
Infection with Ft results in temporally regulated production of pro- and anti-inflammatory cytokines
Despite the ability of Ft to trigger NF-κB signaling, as reported previously by us [3] and other groups [2, 4, 9, 27, 28], pulmonary infection fails to elicit early production (∼72 h) of classical Th1-type, proinflammatory cytokines such as TNF, IL-1β, IL-6, and IL-12. Appreciating the profound effect that HAd has on the cytokine-stimulatory capacity of Ft and the course of disease [18], mice were infected with BHI-grown Ft LVS, and the in vivo kinetics of cytokine production were evaluated. With respect to proinflammatory cytokine production, following i.n. inoculation of 1 × 103 CFU of HAd-Ft LVS, TNF, IL-1β, and IL-6 were only observed at 5 and 7 days PI (Fig. 4A–C). In contrast, TGF-β levels were elevated significantly above baseline within 24 h and continued to rise during the course of infection. IL-10 levels trended higher over the same period; however, they were not significantly different from uninfected controls (Fig. 4D).
Figure 4. Ft induces primarily anti-inflammatory cytokines during early respiratory infection.
Four groups of C57BL/6 mice were i.n.-infected with 1 × 103 CFU of BHI-grown Ft LVS and were killed at Days 1, 3, 5, and 7 PI. One group of sham-inoculated mice served as a control. The lung homogenates prepared from the uninfected lungs and infected lungs were analyzed for proinflammatory (A–C) and anti-inflammatory (D) cytokines. The values are expressed as mean ± sem from four independent experiments (n=14 total mice/group). *P < 0.05; **P < 0.01; and ***P < 0.001.
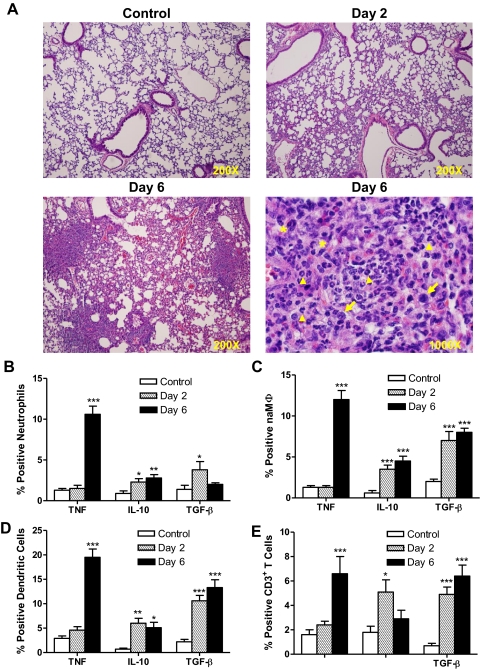
Next, we sought to determine whether the observed temporal disparity in proinflammatory cytokine production was reflected in tissue pathology. Accordingly, lungs recovered at early (Day 2) and late (Day 6) time-points PI were examined for gross and histological changes. Upon gross examination, the lungs of mice killed at Day 2 PI had no lesions, whereas lungs from mice infected for 6 days showed congestion and consolidation. Histological evaluation of H&E-stained lung sections revealed that infiltration of the parenchyma by polymorphonuclear cells and mononuclear cells is quite limited at Day 2 when compared with uninfected controls (Fig. 5A). At this time-point, cellular infiltrates are restricted to the basement membrane immediately beneath alveolar epithelial cells. Bronchiolar lumen also contained mild inflammatory exudates. However, on Day 6, focal areas of stellate necrosis and massive cellular infiltration were observed in the lungs. The inflammatory foci consisted of polymorphonuclear and mononuclear cells and occasional multinucleated giant cells (a pathologic indicator of severe intracellular parasitism). The necrotic areas were surrounded by zones of mixed cellular infiltrates, and thrombi were observed within small blood vessels.
Figure 5. Myeloid and lymphoid cells are a source of anti-inflammatory cytokines during early respiratory infection.
Two groups of C57BL/6 mice were i.n.-infected with 1 × 103 CFU of BHI-grown Ft LVS and killed on Days 2 and 6 PI. One group of sham-inoculated mice served as a control. (A) Histological evaluation of the lungs at Days 2 and 6 PI. Original magnification is ×200 or ×1000, oil immersion. Arrowheads, Polymorphonuclear cells; arrows, mononuclear cells; and *, multinucleated giant cells. (B–E) Lung cells were analyzed for ICCS by flow cytometry. The percentages neutrophils, naMΦ, DCs, and CD3+ T cells expressing TNF, IL-10, or TGF-β are shown. The values are expressed as mean ± sem from two independent experiments (n=11 total mice/group). *P < 0.05; **P < 0.01; and ***P < 0.001.
Having broadly characterized the pattern of cytokine production in lung tissue during the course of infection, we next wanted to identify specific cell types responsible for their release. To compare and contrast early- and late-phase intracellular cytokine profiles, mice were infected with 1 × 103 CFU of BHI-grown Ft, and animals were killed on Days 2 and 6 PI. Multiparameter flow cytometry was used to analyze lung cells that were stained for myeloid lineage markers (CD11b, CD11c, Gr1-1, and F4/80) or a lymphoid marker (CD3) in association with cytokine antibodies (TNF, IL-10, or TGF-β) and gated on specific phenotypic surface markers. Based on the expression of phenotypic markers, myeloid cells in the lungs were identified as neutrophils (CD11bhighGR1highF4/80lowCD11clow), naMΦ (CD11bhighGR1lowF4/80highCD11clow), or DCs (CD11blowGR1lowF4/80lowCD11chigh). As shown in Fig. 5, neutrophils (Fig. 5B), naMΦ (Fig. 5C), DCs (Fig. 5D), and CD3+ T cells (Fig. 5E) are generally a source of IL-10 and TGF-β during early- and late-phase disease (at Days 2 and 6, respectively), whereas production of TNF by these same cells was only observed at the later stage (Day 6). Among all of the inflammatory cells within the lung, DCs were the main source of IL-10 and TGF-β at Days 2 and 6, whereas later during infection, they also became the principal producer of TNF (Fig. 5D). Not only were increases in the percentage of cells expressing specific cytokines observed, but the total numbers of cytokine-expressing cells also increased significantly during the course of disease (Table 1).
Table 1. Total Number of Cells from Whole Lung Expressing Pro- and/or Anti-Inflammatory Cytokines during Early- and Late-Phase Respiratory Tularemia.
| Cells | TNF |
IL-10 |
TGF-β |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Day 2 | Day 6 | Control | Day 2 | Day 6 | Control | Day 2 | Day 6 | |
| Neutrophils | 2087 ± 340 | 2295 ± 711 | 231,141 ± 36,087a | 2162 ± 682 | 8180 ± 4370 | 68,120 ± 5956a | 3536 ± 1326 | 13,210 ± 6771 | 37,273 ± 5968a |
| naMΦ | 2904 ± 526 | 5019 ± 1210 | 382,143 ± 47,106a | 972 ± 377 | 17,528 ± 4143b | 143,938 ± 13,339a | 4123 ± 786 | 19,119 ± 3526b | 195,646 ± 16,722a |
| DCs | 3540 ± 684 | 10,581 ± 2436 | 171,924 ± 15,549a | 844 ± 227 | 4872 ± 1040b | 44,992 ± 6895a | 2862 ± 393 | 10,103 ± 2011b | 149,390 ± 21,525a |
| CD3+ T cells | 3003 ± 1363 | 8029 ± 745 | 35,340 ± 7921a | 3139 ± 567 | 17,802 ± 2044a | 3797 ± 679 | 765 ± 270 | 18,935 ± 5168a | 17,009 ± 2878a |
P < 0.001;
P < 0.05.
Ft rapidly stimulates a variety of cells to produce IL-17A in the lung
IL-17A is a member of the IL-17 family of proinflammatory cytokines produced within hours following epithelial cell injury or activation of PRRs [1]. Interestingly, microbial infection of mice results in early (within 4–8 h) production of IL-17A, which enhances neutrophil migration and production of IL-6 and other chemokines [29]. Although respiratory tularemia is characterized by a lack of Th1-type proinflammatory cytokines during the first 3 days, the induction of IL-17A, a precursor to development of Th1-biased immunity, has been observed as early as 2 days PI with Ft LVS [7]. To confirm and extend this observation, mice were infected with 1 × 103 CFU of Ft LVS, and IL-17A levels were found to be elevated nearly threefold above uninfected controls as early 1 day PI. Levels of IL-17A remained higher than baseline for the duration of the experiment (Fig. 6A). Next, it was determined what subsets of immune cells were responsible for producing IL-17A. Significant numbers of CD4+ (Fig. 6B) and CD4– (Fig. 6C) cells contained cytosolic IL-17A as early as 24 h PI. The percentage of cells expressing IL-17A is highest at 24 h PI and then wanes (as a result of infiltration of the lungs by additional inflammatory cells) over the 1st week of infection. In contrast, the total number of CD4+ and CD4– IL-17A+ cells in the lung steadily climbs throughout the course of disease. Among the CD4– cells expressing IL-17A, a significant percentage and number were γδ T cells (Fig. 6D), neutrophils (Fig. 6E), and Dx5+ NK cells (Fig. 6F). Interestingly, MΦ were not found to be a source of IL-17A in the Ft-infected lung (data not shown).
Figure 6. IL-17-producing cells were activated rapidly in the lungs of Ft-infected mice.
Four groups of C57BL/6 mice were i.n.-infected with 1 × 103 CFU of BHI-grown Ft LVS and killed at Days 1, 3, 5, and 7 PI. One group of sham-inoculated mice served as a control. (A) Levels of IL-17A were measured in the lung homogenates by ELISA. (B–F) Lung cells were analyzed for ICCS of IL-17A by flow cytometry. The percentages and total numbers of different cell types producing IL-17A are shown. The values are expressed as mean ± sem from four independent experiments (n=14 total mice/group). *P < 0.05; **P < 0.01; and ***P < 0.001.
Ft induces the development and activation of tDCs and Tregs in the lung
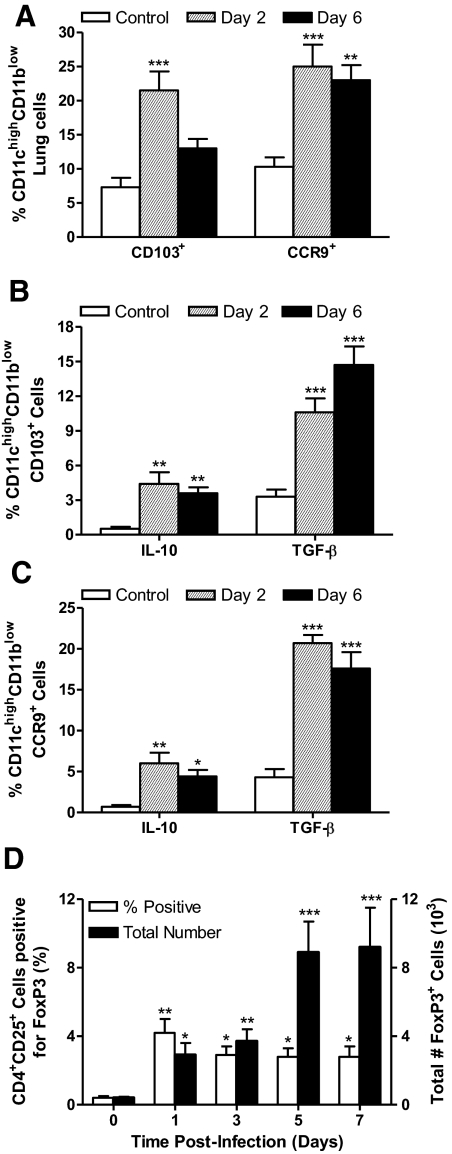
It is their differential and exclusive capacity to produce anti-inflammatory but not proinflammatory cytokines in response to HAd-Ft LVS and ShuS4, which suggests that the DCs activated during early-phase respiratory tularemia may be tolerogenic in nature [30]. To explore this possibility, multiparameter flow cytometry was used to characterize the CD11chighCD11blow cells recovered from uninfected mice and those infected with 1 × 103 CFU of Ft LVS for 2 or 6 days. At both time-points studied, there was a significant increase in the percentage of CD11chighCD11blow cells expressing CD103 or CCR9 (Fig. 7A and Table 2), two well-characterized tDC markers [31, 32]. Interestingly, dual staining for CD103 and CCR9 revealed that all CD103+ cells express CCR9, but the reverse was not true (data not shown). A statistically significant, albeit small, subset of CD11chighCD11blowCD103+ cells (Fig. 7B and Table 2) and CD11chighCCR9+ cells (Fig. 7C) expressed IL-10 and TGF-β. With regard to total cell numbers, CCR9+ tDCs expressed significantly more IL-10 and TGF-β at Days 2 and 6 PI than did cells from uninfected lungs (Table 2). Also, although not at Day 2, CD103+ tDCs did show a dramatic increase in expression of these anti-inflammatory cytokines by Day 6. The juxtaposition of tDCs, TGF-β, and IL-10 early during respiratory tularemia led to the hypothesis that naïve CD4+ T cells in the lung might acquire the phenotypic characteristics of Tregs within this anti-inflammatory milieu [30]. To test this notion, lung cells were recovered from uninfected and infected mice at multiple time-points and were analyzed for the presence of FoxP3. As seen in Fig. 7D, within the first 24 h of infection, the percentage of CD4+CD25+ cells expressing FoxP3 rises tenfold above uninfected control, as does the total number of Foxp3+ cells (sevenfold above uninfected control). Notably, the total number of Tregs resident in the lung increases substantially during the course of disease, such that by Day 6, there is a 23-fold increase in their total numbers. Increases in the percentage of FoxP3+ cells were also observed in the mediastinal LN and spleen of infected mice (data not shown).
Figure 7. Ft induces the development of pulmonary tDCs and Tregs.
Two groups of C57BL/6 mice were infected with 1 × 103 CFU of BHI-grown Ft LVS and killed at Days 2 and 6 PI. One group of sham-inoculated mice served as a control. (A) CD11chigh lung cells were evaluated by flow cytometry for surface expression of the tDC markers, CD103 and CCR9. CD11chighCD103+ cells (B) and CD11chighCCR9+ cells (C) were analyzed for IL-10 and TGF-β cytokine production by flow cytometry. (D) Tregs were identified on the basis of FoxP3 expression. Gating on CD4+ cells, the percentages, and total numbers of CD25+FoxP3+ cells were calculated. The values are expressed as mean ± sem from four independent experiments (n=14 total mice/group). *P < 0.05; **P < 0.01; and ***P < 0.001.
Table 2. Total Number of Cells from Whole Lung Expressing Pro- and/or Anti-Inflammatory Cytokines during Early- and Late-Phase Respiratory Tularemia.
| Cells | Surface marker |
IL-10 |
TGF-β |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Day 2 | Day 6 | Control | Day 2 | Day 6 | Control | Day 2 | Day 6 | |
| CD11chigh CD11blow |
7182 ± 1897 | 41,777 ± 3243a | 216,990 ± 15,645b | 185 ± 64 | 3250 ± 980 | 20,885 ± 3641b | 821 ± 178 | 6949 ± 1184 | 119,650 ± 14,815b |
| CD103+ | |||||||||
| CD11chigh CD11blow |
11,765 ± 1535 | 43,656 ± 4994a | 209,001 ± 15,285b | 231 ± 70 | 9927 ± 4102a | 44,011 ± 5582b | 1719 ± 395 | 23,069 ± 4692a | 103,137 ± 13,570b |
| CCR9+ | |||||||||
P < 0.05;
P < 0.001.
Development of tDCs and Tregs favors exponential bacterial growth and survival during early respiratory tularemia
Given the necessity for TNF, IL-1β, and IL-6 to enhance the activation and increase the bactericidal activities of neutrophils, we postulated that the presence of TGF-β and Tregs would not only block the production of such proinflammatory cytokines but also favor proliferation of Ft. The first 72 h of infection is typified by a four-log increase in bacterial burden within the lung of mice (Fig. 8A); however, whether a direct correlation between TGF-β and Tregs and the accumulation of Ft in tissues exists was unknown. Accordingly, for data collected during the bacterium's exponential growth phase, for each animal, we analyzed by regression the association among TGF-β, Tregs, and bacterial burden. The TGF-β concentrations (r2=0.67, and r2=0.77 for Days 1 and 3, respectively) and numbers of Tregs (r2=0.65, and r2=0.71 for Days 1 and 3, respectively) were significantly associated with variations in bacterial burden (Fig. 8B and C). That is, the animals with higher TGF-β levels and/or greater numbers of Tregs had higher bacterial burdens. This suggests a cause and effect relationship, wherein the anti-inflammatory effects of TGF-β and/or Tregs are regulating the extent of bacterial growth and survival. However, as TGF-β levels and the numbers of Tregs also share a linear relationship (Fig. 8D), this experiment cannot determine whether one or both of these factors are responsible for repressing control of Ft growth.
Figure 8. Levels of TGF-β, numbers of pulmonary Tregs, and Ft burden are interrelated features of early tularemia pathogenesis.
(A) Four groups of C57BL/6 mice were i.n.-infected with 1 × 103 CFU of BHI-grown Ft LVS and killed at Days 1, 3, 5, and 7 PI. One group of sham-inoculated mice served as a control. Bacterial burden was determined by colony plating and presented as log10 CFU. (B–D) Points represent individual animals, and lines are linear regression fits to data for each day. (B) The bacterial burden is positively correlated with TGF-β levels on Days 1 [r2=0.67; P=0.013; log(CFUs)=3.79+137(10−6)×TGF-β] and 3 [r2=0.77; P<0.01; log(CFUs)=6.37+82(10−6)×TGF-β]. (C) The bacterial burden is positively correlated with numbers of Tregs on Days 1 [r2=0.65; P=0.02; log(CFUs)=4.10+314(10−6)×Tregs] and 3 [r2=0.71; P<0.01; log(CFUs)=6.84+58(10−6)×Tregs]. (D) Numbers of Tregs are positively correlated with TGF-β levels at Days 0 (r2=0.91; P<0.001; Tregs=–231+0.254×TGF-β), 1 (r2=0.71; P<0.01; Tregs=–524+0.362×TGF-β), and 3 (r2=0.75; P<0.01; Tregs=–5982+1.17×TGF-β).
DISCUSSION
The two principal objectives of the present study were to clarify whether Ft blocks activation of NF-κB as a means of ablating proinflammatory cytokine production and if not, to test an alternative hypothesis to explain the lack of TNF, IL-1β, IL-6, and IL-12 early during tularemic infection. With regard to the first point, we present evidence that instead of ablating NF-κB signaling, infection of a RAW264.7 cell line and/or isolated primary BMDMs and DCs and mice by HAd-Ft results in the TLR2- and NF-κB-dependent activation of an anti-inflammatory response that likely restricts the early production of TH1-type proinflammatory cytokines. On the second point, substantive evidence is provided, suggesting that tDCs and Tregs sit at the “center” of this anti-inflammatory program and that IL-10 and TGF-β, not blockade of NF-κB signaling, facilitate bacterial growth and survival through diminution of neutrophil and MΦ antimicrobial effector functions.
Ft LVS and SchuS4 are capable of inducing NF-κB signaling, which directly stimulates the release of IL-10 and TGF-β in vitro and/or in vivo and does so in a strictly TLR2-dependent manner. A variety of cell types (i.e., neutrophils, MΦ, DCs, and CD3+ T cells) are induced to express these anti-inflammatory cytokines during the course of infection, whereas proinflammatory cytokines, such as TNF, IL-β, and IL-6, are only produced during late-phase disease or in vitro in response to MHB-grown (i.e., non-HAd) Ft. The restriction on secretion of Th1-type proinflammatory cytokines is a result of adaptation of Ft LVS and SchuS4 (data not shown) to its mammalian environment and perhaps the bacterium's capacity to evoke the effector functions of tDCs and Tregs. Ft-activated CD11chighCD11blow cells, observed during early- and late-phase disease, express CD103 and/or CCR9 (markers associated with tDCs [31, 32]) and are positive by intracytoplasmic staining for IL-10 and TGF-β. Although the exact origin and nature of these tDCs remain unknown, CD103+ [32] and CCR9+ [31] subsets have a demonstrated capacity to promote development of Tregs. To our knowledge, this is the first study to identify tDCs within an infectious disease model and to implicate their release of IL-10 and/or TGF-β in facilitating bacterial growth and survival.
A number of studies have reported that tDCs influence the expansion of Tregs through secretion of TGF-β and/or engagement of inhibitory receptors [33–36]. During early tularemic infection of the lung, given the juxtaposition of elevated levels of TGF-β and an increased frequency of tDCs, it is possible that resident, naïve CD4+ T cells are driven to acquire phenotypic characteristics of Tregs. Alternatively, the Tregs observed in the lung may have been generated “off-site” and then recruited to the inflammatory focus, a possibility that currently is under investigation. Regardless of their origin, Tregs are found in the lungs of Ft-infected mice as early as 24 h PI, where they persist and actually increase in total number during the course of disease. Although not tested in the present study, evidence in the literature shows that Tregs are also an important source of IL-10 and TGF-β [37]. Relevant to Ft burden in tissues and early tularemia pathogenesis, it is important to note that a wide range of pathogens, including bacteria [35, 38], viruses [39, 40], and helminthic parasites [41], promotes their own survival by activating DCs, which facilitates induction of Tregs and limits production of proinflammatory cytokines [42]. In so doing, the anti-inflammatory milieu, within which pathogens can proliferate, is augmented and perpetuated.
Despite the generally anti-inflammatory character of the lung for the first 72 h, recent studies report that by the 3rd day, IL-17A is detectable in the BAL fluid of Ft LVS-infected mice [6, 7]. IL-17A plays a role in host defense against Ft [6, 7], as well as other microbial pathogens [43]. Unlike with Klebsiella pneumoniae infection, wherein IL-17A not only enhances neutrophil migration but also production of IL-6 [44], the rapid production of IL-17A (within 24 h) in response to Ft reported herein is not associated with induction of IL-6. In fact, the lack of IL-6 (along with TNF and IL-1β) is what may undermine the effectiveness of innate immunity to Ft. This also likely explains why IL-17A does not play a profound role in controlling the growth of Ft until Day 4 PI [6] (when Th1-type proinflammatory cytokines are produced), and its inhibition decreases cumulative survival but does not shorten the mean time-to-death of mice [7].
As examples of how the findings presented herein might facilitate evaluation and in some cases, re-evaluation of the existing literature, it is instructive to focus attention on the consequences of using non-HAd Ft for in vitro cell-based studies [12, 13, 15, 16], mutation of sodB [45] and required for intracellular proliferation factor A (ripA) in Ft [8, 46], and antibody-based inhibition of TGF-β during infection with Ft SchuS4 [4]. To begin, irrespective of growth conditions (i.e., use of MHB or BHI broth or replication within MΦ), Ft LVS and/or SchuS4 have the capacity to trigger TLR2-dependent, NF-κB-mediated cytokine production. What distinguishes the cellular response is whether a combination of pro- and anti-inflammatory cytokines (as is the case with non-HAd bacteria) or only anti-inflammatory cytokines (as is the case with HAd bacteria) is produced. Contrary to evidence provided by Melillo et al. [15] and Medina et al. [16], using non-HAd-Ft, BHI- and MΦ-grown bacteria neither inhibit nor enhance PI3K signaling as a means of ablating cytokine release from host cells. The observation that NK-κB-dependent IL-10 and/or TGF-β are produced in vitro and in vivo following infection lends support to this notion. Thus, signaling cascades, purportedly initiated or blocked by non-HAd-Ft, appear not to be physiologically relevant to tularemia pathogenesis. Similarly, despite the ability of BHI-grown Ft to temper the release of TNF from MΦ in response to purified TLR4 and TLR2 agonists (an effect whose physiological relevance needs to be determined), HAd organisms exhibited a minimal capacity to induce SOCS1 and SOCS3 (well-established negative regulators of NF-κB signaling). HAd-Ft also appeared not to mediate a cytokine-modulatory effect through the PI3K/AKT/p38-MAPK cascade or induction of an alternative activation program in MΦ. Again, published literature offering tolerization, modulation of PI3K activity, or alternative activation as an explanation for the complete absence of TH1-type proinflammatory cytokines in in vitro cell-based assays and early during tularemic infection is based on use of non-HAd-Ft.
When considering the course of infection initiated by inoculation of mice with Ft genetically deficient for SodB, these mutants elicit a strong proinflammatory cytokine response from mouse and/or human MΦ (data not shown), replicate to lower numbers in infected tissues, and display a highly attenuated virulence phenotype in mouse survival studies [45]. Likewise, mutants lacking the ability to express RipA stimulate production of TNF, IL-1β, and IL-18 in vitro and in vivo and are limited in their capacity to replicate within infected tissues [8]. In light of the present study's findings, imbuing WT Ft with the ability to block NF-κB signaling through the action of SodB and/or RipA is less well-supported by evidence than the interpretation that these mutants are less able to establish an anti-inflammatory environment (i.e., typified by the presence of tDCs and Tregs and secretion of IL-10 and TGF-β) than their WT counterparts. In fact, a variety of other Ft mutants (i.e., katG [15], iglC [13], pyrF [47], tolC [48], flmF2 and flmK [49], and mglA [18]) are known to be more proinflammatory than their WT counterparts; it is unlikely that all of these genes encode immune-suppressive products that block NF-κB signaling. In many instances, the proteins encoded are internal components of the bacterium, so how they can impede signaling events in the host cell's cytosol remains to be explained and demonstrated in a well-controlled manner.
Finally, an elegant study performed by Bosio and coworkers [4] showed that respiratory infection of mice with 50 CFU of SchuS4 stimulated local and systemic release of TGF-β and no production of TNF and was associated with a dramatic four-log increase in bacterial growth within the first 72 h of infection (as was reported here using LVS). Upon treatment of infected mice with anti-TGF-β antibodies, an increase in TNF levels was inversely associated with decreased bacterial burden in the lung [4]. This relationship between pro- and anti-inflammatory cytokine production and bacterial growth and survival of SchuS4 is entirely consistent with the linear relationship among TGF-β, Tregs, and the tissue burden of Ft LVS, demonstrated in the present study. In fact, preliminary evidence from our lab suggests that administration of anti-TGF-β antibodies to HAd-Ft LVS-infected mice also significantly lowers the burden of bacteria in the lung (data not shown).
Collectively, the findings described above lend considerable support to the hypothesis that instead of blocking NF-κB signaling, Ft triggers NF-κB-dependent development and activation of tDCs and Tregs as a potential means of restraining Th1-type proinflammatory cytokine production early in the infectious disease process. By doing so, Ft establishes an anti-inflammatory milieu, in which to replicate unencumbered by potent antimicrobial innate immune responses. This body of work should stimulate re-evaluation of the field's understanding of mechanisms underlying Ft-host cell interactions as they relate to tularemia pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by U.S. Public Health Service grants AI076408, AI056320 (E.J.G.), AI075193 and AI056320 (T.J.S.). The authors thank Dr. Karsten Hazlett for critical review of the manuscript. All FACSArray, flow cytometry, and histology work was performed through the Center for Immunology and Microbial Disease Immunology Core and with the technical support of Yili Lin. The authors also thank Dr. Martha B. Furie (Stony Brook University, Stony Brook, NY, USA) for the generous gift of purified Ft Tul4 lipoprotein and Dr. Terry K. Means (Harvard Medical School and Massachusetts General Hospital, Boston, MA, USA) for the RAW264.7 ELAM-eGFP reporter cell line.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- APC
- allophycocyanin
- BDIS
- BD Immunocytometry Systems
- BHI
- brain heart infusion
- BLP
- bacterial lipoprotein
- BMDM
- bone marrow-derived monocyte
- CR2
- complement receptor 2
- ELAM
- endothelial cell leukocyte adhesion molecule
- FoxP3
- forkhead box P3
- Ft
- Francisella tularensis
- HAd
- host-adapted
- ICCS
- intracellular cytokine staining
- i.n.
- intranasal
- LVS
- live vaccine strain
- MΦ
- macrophage
- MHB
- Mueller-Hinton broth
- MMP9
- matrix metalloproteinase 9
- naMΦ
- nonalveolar macrophage
- Pam3CSK
- palmitoyl-3-cysteine-serine-lysine
- PI
- postinfection
- PTL
- Parthenolide
- qPCR
- quantitative PCR
- rFlt3L
- recombinant fetal liver tyrosine kinse 3 ligand
- rTul4
- recombinant Francisella tularensis Tul4
- SOCS
- suppressor of cytokine signaling
- SodB
- SOD B gene
- tDC
- tolerogenic DC
- Treg
- regulatory T cell
AUTHORSHIP
S.P., A.S., B.S., G.H.P., P.F.J., and T.R., performed research and analyzed data. S.P., A.S., B.S., E.J.G., and T.J.S. designed the research. S.P. and T.J.S. wrote the paper.
REFERENCES
- 1. Cua D. J., Tato C. M. (2010) Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 [DOI] [PubMed] [Google Scholar]
- 2. Bosio C. M., Dow S. W. (2005) Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175, 6792–6801 [DOI] [PubMed] [Google Scholar]
- 3. Malik M., Bakshi C. S., Sahay B., Shah A., Lotz S. A., Sellati T. J. (2006) Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect. Immun. 74, 3657–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosio C. M., Bielefeldt-Ohmann H., Belisle J. T. (2007) Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178, 4538–4547 [DOI] [PubMed] [Google Scholar]
- 5. Woolard M. D., Hensley L. L., Kawula T. H., Frelinger J. A. (2008) Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of γ interferon-positive T cells. Infect. Immun. 76, 2651–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin Y., Ritchea S., Logar A., Slight S., Messmer M., Rangel-Moreno J., Guglani L., Alcorn J. F., Strawbridge H., Park S. M., Onishi R., Nyugen N., Walter M. J., Pociask D., Randall T. D., Gaffen S. L., Iwakura Y., Kolls J. K., Khader S. A. (2009) Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Markel G., Bar-Haim E., Zahavy E., Cohen H., Cohen O., Shafferman A., Velan B. (2010) The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis. PLoS ONE 5, e11176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang M. T., Mortensen B. L., Taxman D. J., Craven R. R., Taft-Benz S., Kijek T. M., Fuller J. R., Davis B. K., Allen I. C., Brickey W. J., Gris D., Wen H., Kawula T. H., Ting J. P. (2010) Deletion of RipA alleviates suppression of the inflammasome and MAPK by Francisella tularensis. J. Immunol. 185, 5476–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma J., Mares C. A., Li Q., Morris E. G., Teale J. M. (2011) Features of sepsis caused by pulmonary infection with Francisella tularensis Type A strain. Microb. Pathog. 51, 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malik M., Bakshi C. S., McCabe K., Catlett S. V., Shah A., Singh R., Jackson P. L., Gaggar A., Metzger D. W., Melendez J. A., Blalock J. E., Sellati T. J. (2007) Matrix metalloproteinase 9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularensis. J. Immunol. 178, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 11. Henry T., Kirimanjeswara G. S., Ruby T., Jones J. W., Peng K., Perret M., Ho L., Sauer J. D., Iwakura Y., Metzger D. W., Monack D. M. (2010) Type I IFN signaling constrains IL-17A/F secretion by gd T cells during bacterial infections. J. Immunol. 184, 3755–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Telepnev M., Golovliov I., Grundstrom T., Tarnvik A., Sjostedt A. (2003) Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-α and IL-1 from murine macrophages. Cell. Microbiol. 5, 41–51 [DOI] [PubMed] [Google Scholar]
- 13. Telepnev M., Golovliov I., Sjostedt A. (2005) Francisella tularensis LVS initially activates but subsequently down-regulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb. Pathog. 38, 239–247 [DOI] [PubMed] [Google Scholar]
- 14. Butchar J. P., Cremer T. J., Clay C. D., Gavrilin M. A., Wewers M. D., Marsh C. B., Schlesinger L. S., Tridandapani S. (2008) Microarray analysis of human monocytes infected with Francisella tularensis identifies new targets of host response subversion. PLoS ONE 3, e2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melillo A. A., Bakshi C. S., Melendez J. A. (2010) Francisella tularensis antioxidants harness reactive oxygen species to restrict macrophage signaling and cytokine production. J. Biol. Chem. 285, 27553–27560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medina E. A., Morris I. R., Berton M. T. (2010) Phosphatidylinositol 3-kinase activation attenuates the TLR2-mediated macrophage proinflammatory cytokine response to Francisella tularensis live vaccine strain. J. Immunol. 185, 7562–7572 [DOI] [PubMed] [Google Scholar]
- 17. Shirey K. A., Cole L. E., Keegan A. D., Vogel S. N. (2008) Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J. Immunol. 181, 4159–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hazlett K. R., Caldon S. D., McArthur D. G., Cirillo K. A., Kirimanjeswara G. S., Magguilli M. L., Malik M., Shah A., Broderick S., Golovliov I., Metzger D. W., Rajan K., Sellati T. J., Loegering D. J. (2008) Adaptation of Francisella tularensis to the mammalian environment is governed by cues which can be mimicked in vitro. Infect. Immun. 76, 4479–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carlson P. E., Jr., Horzempa J., O′Dee D. M., Robinson C. M., Neophytou P., Labrinidis A., Nau G. J. (2009) Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J. Bacteriol. 191, 6855–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carlson P. E., Jr., Carroll J. A., O′Dee D. M., Nau G. J. (2007) Modulation of virulence factors in Francisella tularensis determines human macrophage responses. Microb. Pathog. 42, 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loegering D. J., Drake J. R., Banas J. A., McNealy T. L., Mc Arthur D. G., Webster L. M., Lennartz M. R. (2006) Francisella tularensis LVS grown in macrophages has reduced ability to stimulate the secretion of inflammatory cytokines by macrophages in vitro. Microb. Pathog. 41, 218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahay B., Patsey R. L., Eggers C. H., Salazar J. C., Radolf J. D., Sellati T. J. (2009) CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog. 5, e1000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benhnia M. R., Wroblewski D., Akhtar M. N., Patel R. A., Lavezzi W., Gangloff S. C., Goyert S. M., Caimano M. J., Radolf J. D., Sellati T. J. (2005) Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of Lyme disease. J. Immunol. 174, 1539–1548 [DOI] [PubMed] [Google Scholar]
- 24. Bar-Haim E., Gat O., Markel G., Cohen H., Shafferman A., Velan B. (2008) Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog. 4, e1000211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall J. D., Woolard M. D., Gunn B. M., Craven R. R., Taft-Benz S., Frelinger J. A., Kawula T. H. (2008) Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 76, 5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cole L. E., Shirey K. A., Barry E., Santiago A., Rallabhandi P., Elkins K. L., Puche A. C., Michalek S. M., Vogel S. N. (2007) Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect. Immun. 75, 4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersson H., Hartmanova B., Kuolee R., Ryden P., Conlan W., Chen W., Sjostedt A. (2006) Transcriptional profiling of host responses in mouse lungs following aerosol infection with type A Francisella tularensis. J. Med. Microbiol. 55, 263–271 [DOI] [PubMed] [Google Scholar]
- 28. Mares C. A., Ojeda S. S., Morris E. G., Li Q., Teale J. M. (2008) Initial delay in the immune response to Francisella tularensis is followed by hypercytokinemia characteristic of severe sepsis and correlating with upregulation and release of damage-associated molecular patterns. Infect. Immun. 76, 3001–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Q., Martin R. J., Rino J. G., Breed R., Torres R. M., Chu H. W. (2007) IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 9, 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maldonado R. A., von Andrian U. H. (2010) How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 108, 111–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hadeiba H., Sato T., Habtezion A., Oderup C., Pan J., Butcher E. C. (2008) CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 9, 1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iliev I. D., Spadoni I., Mileti E., Matteoli G., Sonzogni A., Sampietro G. M., Foschi D., Caprioli F., Viale G., Rescigno M. (2009) Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58, 1481–1489 [DOI] [PubMed] [Google Scholar]
- 33. Chen W., Jin W., Hardegen N., Lei K. J., Li L., Marinos N., McGrady G., Wahl S. M. (2003) Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andersson J., Tran D. Q., Pesu M., Davidson T. S., Ramsey H., O′Shea J. J., Shevach E. M. (2008) CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-β-dependent manner. J. Exp. Med. 205, 1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johanns T. M., Ertelt J. M., Rowe J. H., Way S. S. (2010) Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 6, e1001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Periasamy S., Dhiman R., Barnes P. F., Paidipally P., Tvinnereim A., Bandaru A., Valluri V. L., Vankayalapati R. (2011) Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation with Mycobacterium tuberculosis. J. Infect. Dis. 203, 1256–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belkaid Y., Oldenhove G. (2008) Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity 29, 362–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKinley L., Logar A. J., McAllister F., Zheng M., Steele C., Kolls J. K. (2006) Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J. Immunol. 177, 6215–6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fulton R. B., Meyerholz D. K., Varga S. M. (2010) Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 185, 2382–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee D. C., Harker J. A., Tregoning J. S., Atabani S. F., Johansson C., Schwarze J., Openshaw P. J. (2010) CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J. Virol. 84, 8790–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grainger J. R., Smith K. A., Hewitson J. P., McSorley H. J., Harcus Y., Filbey K. J., Finney C. A., Greenwood E. J., Knox D. P., Wilson M. S., Belkaid Y., Rudensky A. Y., Maizels R. M. (2010) Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207, 2331–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Belkaid Y. (2007) Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7, 875–888 [DOI] [PubMed] [Google Scholar]
- 43. Curtis M. M., Way S. S. (2009) Interleukin-17 in host defense against bacterial, mycobacterial and fungal pathogens. Immunology 126, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Happel K. I., Dubin P. J., Zheng M., Ghilardi N., Lockhart C., Quinton L. J., Odden A. R., Shellito J. E., Bagby G. J., Nelson S., Kolls J. K. (2005) Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202, 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bakshi C. S., Malik M., Regan K., Melendez J. A., Metzger D. W., Pavlov V. M., Sellati T. J. (2006) Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J. Bacteriol. 188, 6443–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fuller J. R., Craven R. R., Hall J. D., Kijek T. M., Taft-Benz S., Kawula T. H. (2008) RipA, a cytoplasmic membrane protein conserved among Francisella species, is required for intracellular survival. Infect. Immun. 76, 4934–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Horzempa J., O′Dee D. M., Shanks R. M., Nau G. J. (2010) Francisella tularensis DpyrF mutants show that replication in nonmacrophages is sufficient for pathogenesis in vivo. Infect. Immun. 78, 2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Platz G. J., Bublitz D. C., Mena P., Benach J. L., Furie M. B., Thanassi D. G. (2010) A tolC mutant of Francisella tularensis is hypercytotoxic compared to the wild type and elicits increased proinflammatory responses from host cells. Infect. Immun. 78, 1022–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kanistanon D., Hajjar A. M., Pelletier M. R., Gallagher L. A., Kalhorn T., Shaffer S. A., Goodlett D. R., Rohmer L., Brittnacher M. J., Skerrett S. J., Ernst R. K. (2008) A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog. 4, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.