Abstract
A defective skin epidermal permeability barrier (EPB) is responsible for a high mortality rate in premature infants, and is an important risk factor in inflammatory skin diseases such as eczema. We report here fast and accurate methods for measurement of EPB in animal models or in human patients using simple techniques that monitor diffusion of dyes (X-Gal or Lucifer Yellow) through the upper epidermis and measure transepidermal water loss (TEWL) resulting from a defective skin barrier. Accurate diagnosis and early detection of EPB defects in human patients are critical for effective treatment of certain classes of inflammatory skin diseases.
Keywords: Skin, EPB, TEWL, X-Gal diffusion, Lucifer Yellow
1. INTRODUCTION
The skin, which consists of the epidermis and underlying dermis, is a very attractive tissue in which to study the in vivo functions of genes that regulate the expression of proteins involved in the control of cellular proliferation and differentiation. It is the largest organ in the body comprising approximately 10% of body weight, and protects the body from dehydration and environmental insults through establishment of the protective epidermal permeability barrier (EPB). During embryonic development, the ectodermal cell layer covering the body develops into a stratified epidermis that is essential at birth, when the organism confronts the arid and toxic postnatal environments. Keratinocytes, an ectodermally derived cell type, form the proliferative, basal layer of the epidermis. Keratinocytes periodically withdraw from the cell cycle and commit to terminal differentiation, while migrating through the suprabasal layers. The outermost layer of the skin (stratum corneum) is composed of mechanically tough, dead, cornified cells (squames), which develop as a result of a complex terminal differentiation program, and provide vital physical and permeability barriers to vertebrates (1,2).
The epidermal barrier, which is composed of the cornified envelope, the cornified lipid envelope and extracellular lipids, is formed in a highly reproducible pattern during late stages of embryogenesis (3–5). Formation of the EPB requires the delivery of lipids and proteins, which are contained in lamellar granules (keratinosomes) present in keratinocytes of the granular layer, to the stratum corneum interstices, as well as the formation of high molecular weight polymers through the crosslinking of corneocyte envelope proteins (loricrin, involucrin, filagrin and other peptides) and packing of corneocytes by corneodesmosomes. Postnatally, the epidermal barrier is maintained by a complex epidermal differentiation program, which results in the constant production of the cellular and lipid components of the barrier [reviewed in (6)]. Epidermal homeostasis relies on a tightly regulated balance between keratinocyte proliferation and differentiation, the alteration of this leads to various skin diseases (7,8).
A defective EPB accounts for high mortality rate (>40%) in premature infants, and is an important feature of many inflammatory skin diseases such as eczema and psoriasis, affecting nearly 10% of the world population. Compromised barrier function in prematurely born infants or in patients with certain inherited skin diseases often results in dehydration and increased susceptibility to infections (9–12). Defects in protective skin barrier function(s) and minor lesions of the skin result in an increased transepidermal water loss (TEWL), altered skin pH and hydration.
(13) have developed an elegant and qualitative, whole-mount assay for skin permeability and showed that the assay measures the first stage of barrier formation. The barrier forms first at distinct epidermal sites then spreads across the epidermis as a moving front. It has been demonstrated that late stages of cornified envelope assembly accompany movement of the front. Hence the whole-mount permeability assays record developmental acquisition of a known, essential component of the adult barrier. The authenticity and utility of the assays was further validated by monitoring barrier formation after hormonal treatment (maternal glucocorticoid therapy) known to accelerate foetal barrier development. Similar patterned skin barrier acquisition in additional species confirmed that patterned change is probably a ubiquitous mode of epidermal differentiative change during mammalian development (13). Several laboratories, including us, have used those assays to demonstrate that barrier formation is indeed highly patterned during development (14,15,16).
Dye diffusion assays provide a rapid and cost-effective means of assessing EPB function in rodent models without the need of investing in additional equipment. Dye diffusion assays are most useful when the magnitude of EPB disruption is large. However, owing to the enhanced sensitivity of the technique, measurement of TEWL has become the preferred means for quantitative assessment EPB function, particularly when EPB dysfunction is subtle, as is likely the case in human dermatological diseases. For example, both Ctip2ep−/−and Gata-3ep−/− (conditional deletion of transcription factors Ctip2 and GATA-3, respectively, in epidermis) mice are able to exclude X-gal dye at ~E18.0 (before birth), suggesting normal EPB function. However, both lines of mice exhibit a significantly increased rate of TEWL at that stage compared to the control littermates (16,17). In these cases, dye diffusion assays and TEWL measurements appear at odds simply as a result of the differential sensitivities of these two techniques. In the present article, we briefly describe the various methodologies of determining EPB formation using mouse as a model system.
2. Methods
ICR or CD1 mice can be purchased from Jackson Laboratory (http://jaxmice.jax.org/) or Charles River Laboratories (http://ftp.criver.com). Ctip2L2/L2 and Brg1L2/L2 mice can be obtained from Mark Leid (Mark.Leid@oregonstate.edu) and Pierre Chambon (chambon@titus.u-strasbg.fr), respectively.
PBS (Dulbecco’s phosphate-buffered saline) (Cat. No. D5652),
potassium ferricyanide (Cat. No. 244023),
potassium ferrocyanide (Cat. No. P3289),
Lucifer Yellow CH (Cat. No. L0259),
toluidine blue O (Cat. No. T3260)
Hematoxylin solution A (Cat. No. 32897) are from Sigma-Aldrich ;
Diamidino-2-phenylindole dihydrochloride (DAPI) (Cat. No. 236276)
and X-Gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside) (Cat. No. 745740) are from Roche Diagnostics.
Tewameter® (Academ Inc., USA).
Zeiss Axio Scope fluorescent microscope with X-cite120 for four channels (green, red, blue, Hoecht) attached with an Axiocam monochromatic camera running with Axiovision 4.7.1 system software.
3. Methods
The X-Gal and Lucifer Yellow diffusion assays (described below), as well as the measurement of the TEWL, are routinely performed in laboratories around the world to detect skin barrier defects in mouse models. Using those techniques we have successfully established the roles of a transcriptional regulatory protein, Ctip2, and a member of the chromatin remodeling complex, Brg1, in epidermal terminal differentiation and skin barrier formation (14–16). Diagnostic TEWL is also routinely performed by dermatologists on patients with skin disorders, such as eczema and psoriasis, and the technique is also useful for evaluation of the efficacy of topical drug treatment on barrier recovery in the skin of these patients.
3.1 Embryos
ICR or CDI mice were time-mated within a 5-hour mating window (permeability assays, TEWL and EM studies).
The mid-point of the mating window designated gestational age zero.
Embryos were derived from random matings within a 4 day period and categorized according to gross morphology and barrier status.
Fetal gestational age was calculated (see Note 1) and pregnant dams were sacrificed by CO2 asphyxiation prior to recovery of the embryos.
3.2. Skin permeability assay
Assay 1
This assay depends on barrier-dependent access of 5-bromo-4-chloro-3- indolyl-β, D-galactopyranoside (X-gal) to untreated skin.
Unfixed, untreated, freshly isolated E18.5 embryos were rinsed in phosphate-buffered saline (PBS) and dried briefly.
Embryos were immersed in standard X-gal reaction mix (18), with pH adjusted to 4.5.
Embryos were incubated at 37°C for 8–10 hours, washed in PBS for 1–2 minutes and photographed.
EPB defect was assessed functionally depending on the diffusion of X-Gal dye into the embryos (Figure 1A, see Notes 2 and 5).
Figure 1. Epidermal permeability barrier defects in Ctip2−/− (germline deletion of gene encoding transcriptional regulator Ctip2), and CTIP2ep−/−(selectively lacking the gene for Ctip2 in epidermal keratinocytes) fetuses.
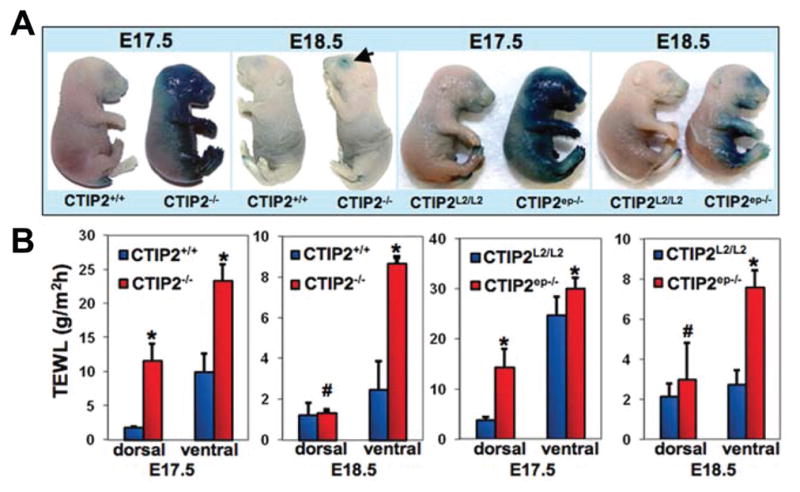
(A) X-gal diffusion assay performed on Ctip2+/+, CTIP2−/−, CTIP2L2/L2 and CTIP2ep−/− fetuses at E17.5 and E18.5 as indicated. (B) TEWL measurements from dorsal and ventral skin of CTIP2+/+, CTIP2−/−, CTIP2L2/L2 and CTIP2ep−/− mice at E17.5 and E18.5. (Plotted are mean measurements of three independent mice per genotype ± S.E.M.). * - p < 0.05, # - not statistically significant. Ctip2+/+ (wild type littermates) and CTIP2L2/L2 (with floxed Ctip2 allele) fetuses were used as controls. (Courtesy of Arup Kumar Indra and Mark Leid; adapted with permission from Golonzhka et al., 2009)
Assay 2
This assay modifies skin to permit barrier-dependent penetration by histological dyes such as toluidine blue or hematoxylin.
Unfixed, untreated embryos were incubated for 1–5 minutes in methanol and rinsed in PBS.
Embryos were incubated in 0.5% hematoxylin or 0.1% toluidine blue.
Embryos were embedded in agarose and photographed using a Zeis Stemi SV11 microscope with transmitted and surface illumination.
Scanned images were processed with Adobe Photoshop and the agarose background was removed.
EPB defects were evaluated depending on degree of dye penetration (see Notes 3, 4 and 5).
3.3. In vivo transdermal absorption of the fluorescent dye Lucifer yellow
E18.5 fetuses/embryos were restrained in Petri dishes with their backs in contact with 1 mM Lucifer Yellow (Sigma) in PBS (pH 7.4) at 37°C, as described (19).
After 1 hour of incubation, fetuses were sacrificed, frozen, and cryosectioned dorsoventrally at a thickness of 5 μm.
Sections were counterstained with 10 μg/ml DAPI (4′,6-diamidino-2-phenylindole dihydrochloride), dehydrated and mounted with DPX mounting medium.
Sections were analyzed by fluorescence microscopy and photographed using a Zeiss fluorescent microscope [see Figure 2 and Note 6].
Figure 2. Impaired skin barrier function in Brg1 ep−/−(c) mutant fetuses selectively lacking the gene for Brg1 (a member of the mammalian chromatin remodeling protein complex).
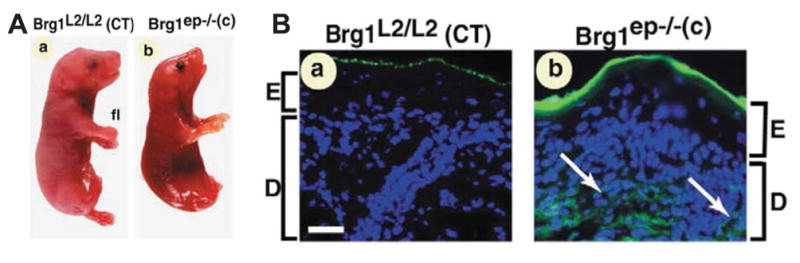
(A) Gross morphology of E18.5 Brg1 L2/L2 (control; a) and Brg1 ep−/−(c) fetuses (B) In vivo Lucifer yellow diffusion in (a) Brg1L2/L2 and (b) Brg1ep−/−(c) E18.5 fetuses. (Courtesy of Arup Kumar Indra and Pierre Chambon; reproduced and adapted with permission from Indra et al., 2005b).
3.4. Assessment of TEWL
TEWL is the most important and sensitive parameter for evaluating the integrity of the EPB in all conditions in laboratory mice, rats and in humans (see Notes 7 and 8).
Dorsal and ventral skin TEWL were determined on E17.5 and E18.5 embryos, neonatal or adult mice (after shaving to remove the hair) with a tewameter (Acaderm Inc., USA) equipped with a TEWL probe (see Figure 3).
Embryos, neonatal or adult mice were restrained on their dorsal or ventral side and TEWL on the skin surface was directly measured with the Tewameter®, which is the most accepted and best-selling TEWL measurement device worldwide [(Academ Inc., USA); see Note 9]
Mean values of six measurements per animal were determined.
Data were expressed in g/m2-h, as means ± s.e.m. from 3–4 animals [(Figures 1B and 3) see Note 10].
Figure 3. Measurement of TEWL using a tewameter.
The tewameter probe is touched on the skin surface and the readings are noted on the screen of a multiprobe adaptor (21) system.
In summary, assessment of EPB function is an extremely important technique for scientists, clinicians, and the cosmetological industry. It seems likely that the technology behind EPB measurement will continue to evolve and provide more sophisticated methods for the enhanced detection of compromised EPB function in mice and man. In addition, further research in this area will identify the biophysical properties of the skin barrier that regulate the relative permeabilities of small molecules, infectious agents, water, and gases across this surface, all of which are still poorly understood.
Acknowledgments
These studies were supported by grant AR056008 (AI) from the National Institutes of Health and by a NIEHS Center grant (ES00210) to the Oregon State University Environmental Health Sciences Center. Acknowledgements are due the copyright permission department of Development and Journal of Investigative Dermatology for allowing the reproduction and adaptation from previously published papers.
Footnotes
Estimated fetal gestational age (EGA, #98) was calculated from the time designated zero. For example, 16 days/5 hours after time zero was termed E16.5 or 16.5 days EGA.
X-Gal diffusion assay depends on barrier-dependent access of 5-bromo-4-chloro-3- indolyl-β, D-galactopyranoside (X-gal) to untreated skin. At low pH skin contains abundant endogenous β-galactosidase-like activity, which cleaves X-gal to produce a coloured precipitate (13–16).
The basis of the skin modification during dye diffusion (Assay 2) is unknown but is likely to involve extraction of polar lipid (20).
Skin permeability Assays 1 and 2 gave the same staining pattern, inferring that those techniques measure similar skin characteristics. Comparisons were performed on sagittal sides of a single embryo to eliminate variation arising from differences in developmental stage.
The EPB begins to develop on the dorsal aspect of developing fetuses at approximately E16.5, and spreads ventrally resulting in complete dye impermeability by E17.5 (13–16). Mice with impaired EPB development and/or function display increased staining which was assessed by either assay 1 or 2.
When visualized by fluorescence microscopy at E18.5 the flurescent dye Lucifer yellow labels the upper layer of the stratum corneum of control fetuses (19). In contrast, diffused staining was seen throughout the stratum corneum which extends down to the dermis and hypodermis of mutant fetuses with compromised EPB function [see Figure 2 (15,19)
The skin constantly loses water in form of vapor and that TEWL is accelerated by minor skin lesions and other factors that disrupt the EPB function. That has rendered the technique of measuring TEWL invaluable for both dermatological and cosmetological applications. In addition, TEWL measurment is widely used in occupational medicine, medical consultancy, observation of the newborn, and the food industry.
TEWL Measuring Principle and Methodology : The measurement of water evaporation is based on the diffusion principle in an open chamber. The open chamber measurement method is the only method available for assessing TEWL continuously, and in a non-invasive manner. Stable TEWL measurements can be made rapidly and with ease using a variety of instruments on the market such as a tewameter (Acaderm Inc., USA) or a vaporimeter (Delphin Inc., USA).
The TEWL probe of the Tewameter®, consists of two pairs of sensors to measure the humidity and temperature gradients in two different spacings. Based on the resulting humidity and temperature gradients the TEWL is automatically calculated and shown.
E18.5 control fetuses with normal skin barrier function display a TEWL value in the range of 1–2 g/m2-h on the dorsal side, and 5–10 g/m2-h on the ventral side using a tewameter or a vaporimeter (14–16). The difference in TEWL value (between the dorsal and ventral side) could be attributed to patterned barrier acquisition during embryogenesis when barrier formation is completed earlier on the dorsal side compared to the ventral side (13). E16.5—E17.5 fetuses in the early developmental stages of skin maturation and barrier formation, as well as E18.5 fetuses with an impaired EPB are expected to display significant higher values both on the dorsal and ventral side under identical conditions.
References
- 1.Byrne C, Hardman M, Nield K. Covering the limb--formation of the integument. J Anat. 2003;202:113–123. doi: 10.1046/j.1469-7580.2003.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays. 2002;24:789–800. doi: 10.1002/bies.10144. [DOI] [PubMed] [Google Scholar]
- 3.Koch PJ, Zhou Z, Roop DR. In: Skin Barrier. Elias PM, aKRF, editors. Marcel Dekker, Inc; New York: 2004. pp. 97–110. [Google Scholar]
- 4.Elias PM, Feingold KR. Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin Dermatol. 1992;11:176–182. [PubMed] [Google Scholar]
- 5.Koster MI. Making an epidermis. Ann N Y Acad Sci. 2009;1170:7–10. doi: 10.1111/j.1749-6632.2009.04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segre J. Complex redundancy to build a simple epidermal permeability barrier. Curr Opin Cell Biol. 2003;15:776–782. doi: 10.1016/j.ceb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Watt FM. Epidermal stem cells as targets for gene transfer. Hum Gene Ther. 2000;11:2261–2266. doi: 10.1089/104303400750035799. [DOI] [PubMed] [Google Scholar]
- 8.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 9.Cartlidge P. The epidermal barrier. Semin Neonatol. 2000;5:273–280. doi: 10.1053/siny.2000.0013. [DOI] [PubMed] [Google Scholar]
- 10.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- 14.Indra AK, Mohan WS, 2nd, Frontini M, Scheer E, Messaddeq N, Metzger D, Tora L. TAF10 is required for the establishment of skin barrier function in foetal, but not in adult mouse epidermis. Dev Biol. 2005a;285:28–37. doi: 10.1016/j.ydbio.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Indra AK, Dupe V, Bornert JM, Messaddeq N, Yaniv M, Mark M, Chambon P, Metzger D. Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes unveils two distinct developmental functions of BRG1 in limb morphogenesis and skin barrier formation. Development. 2005b;132:4533–4544. doi: 10.1242/dev.02019. [DOI] [PubMed] [Google Scholar]
- 16.Golonzhka O, Liang X, Messaddeq N, Bornert JM, Campbell AL, Metzger D, Chambon P, Ganguli-Indra G, Leid M, Indra AK. Dual role of COUP-TF-interacting protein 2 in epidermal homeostasis and permeability barrier formation. J Invest Dermatol. 2009;129:1459–1470. doi: 10.1038/jid.2008.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Guzman Strong C, Wertz PW, Wang C, Yang F, Meltzer PS, Andl T, Millar SE, Ho IC, Pai SY, Segre JA. Lipid defect underlies selective skin barrier impairment of an epidermal-specific deletion of Gata-3. J Cell Biol. 2006;175:661–670. doi: 10.1083/jcb.200605057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnerol C, Nicolas J-F. In: Guide to techniques in mouse development. Wasserman PM, aMLD, editors. Academic Press; San Diego: 1994. pp. 451–469. [Google Scholar]
- 19.Matsuki M, Yamashita F, Ishida-Yamamoto A, Yamada K, Kinoshita C, Fushiki S, Ueda E, Morishima Y, Tabata K, Yasuno H, et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase) Proc Natl Acad Sci U S A. 1998;95:1044–1049. doi: 10.1073/pnas.95.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wertz PW, Downing DT. Covalently bound omega-hydroxyacylsphingosine in the stratum corneum. Biochim Biophys Acta. 1987;917:108–111. doi: 10.1016/0005-2760(87)90290-6. [DOI] [PubMed] [Google Scholar]
- 21.de Pooter RF, Schmitt TM, de la Pompa JL, Fujiwara Y, Orkin SH, Zuniga-Pflucker JC. Notch signaling requires GATA-2 to inhibit myelopoiesis from embryonic stem cells and primary hemopoietic progenitors. J Immunol. 2006;176:5267–5275. doi: 10.4049/jimmunol.176.9.5267. [DOI] [PubMed] [Google Scholar]
- Steiner M, Aikman-Greed S, Dick FD. Side-by-side comparison of open chamber (TM 300) and closed chamber (Vapometer) TEWL. Skin Research and Technology. 2010;16:489–490. doi: 10.1111/j.1600-0846.2011.00509.x. [DOI] [PubMed] [Google Scholar]


