Abstract
We previously demonstrated the synergistic therapeutic effect of the cetuximab (anti-epidermal growth factor receptor [EGFR] monoclonal antibody, mAb)-trastuzumab (anti-HER2 mAb) combination (2mAbs therapy) in HER2low human pancreatic carcinoma xenografts. Here, we compared the 2mAbs therapy, the erlotinib (EGFR tyrosine kinase inhibitor [TKI])-trastuzumab combination and lapatinib alone (dual HER2/EGFR TKI) and explored their possible mechanisms of action. The effects on tumor growth and animal survival of the three therapies were assessed in nude mice xenografted with the human pancreatic carcinoma cell lines Capan-1 and BxPC-3. After therapy, EGFR and HER2 expression and AKT phosphorylation in tumor cells were analyzed by Western blot analysis. EGFR/HER2 heterodimerization was quantified in BxPC-3 cells by time-resolved FRET. In K-ras-mutated Capan-1 xenografts, the 2mAbs therapy gave significantly higher inhibition of tumor growth than the erlotinib/trastuzumab combination, whereas in BxPC-3 (wild-type K-ras) xenografts, the erlotinib/trastuzumab combination showed similar growth inhibition but fewer tumor-free mice. Lapatinib showed no antitumor effect in both types of xenografts. The efficacy of the 2mAbs therapy was partly Fc-independent because F(ab′)2 fragments of the two mAbs significantly inhibited BxPC-3 growth, although with a time-limited therapeutic effect. The 2mAbs therapy was associated with a reduction of EGFR and HER2 expression and AKT phosphorylation. BxPC-3 cells preincubated with the two mAbs showed 50% less EGFR/HER2 heterodimers than controls. In pancreatic carcinoma xenografts, the 2mAbs therapy is more effective than treatments involving dual EGFR/HER2 TKIs. The mechanism of action may involve decreased AKT phosphorylation and/or disruption of EGFR/HER2 heterodimerization.
Introduction
Pancreatic cancer is the fourth leading cause of cancer death both in men and women. Despite many efforts to improve the survival rate, currently, most patients with pancreatic cancer die within a year of diagnosis. When the tumor becomes symptomatic, 60% to 80% of the patients already have locally advanced or metastatic disease [1], thus allowing essentially only palliative therapy with a 5-year survival rate lower than 5% [2]. To find efficient treatments, translational research has investigated the molecular mechanisms implicated in pancreatic cancer development. Recent data suggest that a large number of genetic alterations affect only few major signaling pathways involved in pancreatic tumorigenesis, such as those associated with members of the epidermal growth factor receptor (EGFR) family [3] that can be targeted by novel therapies [4–6]. Dual inhibition of EGFR and HER2 was proposed for pancreatic carcinoma based on the observed overexpression of EGFR in 40% to 70% of these cancers, HER2 overexpression in a smaller subset of cases [7,8], and the major role played by EGFR/HER2 heterodimers [6]. Furthermore, the implication of EGFR and HER2 in the malignant phenotype of pancreatic cancer [4,9] paved the way for therapeutic strategies aimed at targeting both receptors.
Erlotinib, an EGFR tyrosine kinase inhibitor (TKI), was the first EGFR-targeted therapy approved by the US Food and Drug Administration for the treatment of pancreatic carcinoma. The combination of erlotinib with gemcitabine for pancreatic carcinoma was tested in a phase 3 clinical trial with modest survival benefit, which, was, however, better than the results obtained with cetuximab plus gemcitabine [10].
After US Food and Drug Administration approval of the combination of lapatinib, a dual EGFR/HER2 TKI, and capecitabine for the treatment of HER2 overexpressing advanced or metastatic breast carcinoma, three clinical studies to test the effect of lapatinib associated with chemotherapy in pancreatic carcinoma have been started. Two of these studies are ongoing clinical trials that assess the combination of lapatinib and capecitabine as a second-line treatment (NCT00881621) or in metastatic pancreatic carcinoma (NCT00962312). The third one is a phase 1 study that showed that a dosage of 1000 mg/d of lapatinib, in combination with gemcitabine and/or gemcitabine/oxaliplatin, induced no excessive toxicity in advanced pancreaticobiliary carcinoma [11].
We have recently shown that two therapeutic antibodies, the anti-EGFR cetuximab and the anti-HER2 trastuzumab, which are broadly used separately in the clinic, have a synergistic antitumor effect when used in combination. They significantly increased survival of mice xenografted with two different pancreatic carcinoma cell lines, whereas each antibody had a minor effect on its own [6]. Furthermore, this original dual EGFR/HER2 targeting strategy with cetuximab and trastuzumab (2mAbs therapy) was more efficient as first- and second-line treatment than the standard chemotherapy with gemcitabine [12].
In the present study, we compared the 2mAbs therapy (cetuximab/trastuzumab) to the combination of erlotinib and trastuzumab and to lapatinib alone in mice xenografted with two HER2low human pancreatic carcinoma cell lines. The 2mAbs therapy induced a marked and stable down-regulation of EGFR and HER2 and the downstream blockade of AKT phosphorylation in pancreatic carcinoma cells, whereas the other treatments had a more transient effect on receptor expression. Incubation of human pancreatic carcinoma cells with the two mAbs also induced a 50% disruption of EGFR and HER2 receptors heterodimerization and a crucial increase in homodimers.
Materials and Methods
Materials
The anti-EGFR mAbs cetuximab (human) and m425 (mouse) were purchased from Merck KGaA (Darmstadt, Germany) and Merck AG (Frankfurt, Germany), respectively. Trastuzumab and erlotinib were obtained from Roche Pharma AG (Grenzach-Wyhlen, Germany) and lapatinib was from GlaxoSmithKline (Hertfordshire, United Kingdom). The anti-HER2 mouse mAb FRP5 was kindly provided by N. Hynes (FMI, Basel, Switzerland). The F(ab′)2 fragments of cetuximab and trastuzumab were prepared by pepsin digestion as described [13]. Absence of undigested, intact mAbs was checked by SDS-PAGE.
Cell Lines and Culture
BxPC-3 and SKOV-3 cell lines were obtained from the ATCC (Rockville, MD). The Capan-1 cell line was kindly provided by Pr L. Buscail (Toulouse, France). All cell lines were maintained in the appropriate medium supplemented with 10% fetal calf serum, 50 U/ml penicillin, and 50 µg/ml streptomycin.
EGFR and HER2 Cell Surface Quantification
Cell surface expression of EGFR and HER2 in BxPC-3 and Capan-1 cells was quantified using the Quantitative Immuno-Fluorescence Indirect assay (QIFI kit; Dako, Copenhagen, Denmark) as described [14]. Briefly, cells were first labeled with anti-EGFR and anti-HER2 antibodies before adding an FITC-conjugated antimouse immunoglobulin G reagent (Sigma-Aldrich, St Louis, MO). The fluorescent standards (known calibration beads) were concurrently labeled with the same FITC-conjugated secondary antibody. The fluorescence intensity of the different standards was used to calculate the standard regression curve between fluorescence intensity and antigen density, expressed as the antibody binding capacity in molecules per cell. Samples were analyzed with a Coulter Epics XL-MCL Flow Cytometer (Beckman Coulter, Fullerton, CA).
Cell Proliferation Assay and IC50 Determination
The effect of the different TKIs on cell viability was evaluated using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium (MTS) and the electron coupling reagent phenazine methosulfate (PMS). Briefly, BxPC-3 and Capan-1 cells were cultured in 96-well microtiter plates at 10,000 cells/well. After 24 hours, cells were treated with TKI at concentrations ranging from 0.001 to 20 µg/ml for erlotinib or from 0.001 to 20 µM for lapatinib. After a 96-hour incubation, cells were exposed to MTS/PMS and incubated at 37°C for 2 hours. Absorbance was measured at 490 nm, and the half-maximal inhibitory concentration (IC50) values were calculated as the percentage of proliferating cells relative to untreated cells. All experiments were performed in triplicate.
EGFR and HER2 Dimer Analysis
EGFR/HER2 dimers were quantified using an antibody-based TR-FRET assay, as described [14]. Capan-1 or BxPC-3 cells were plated at 3 x 105 cells/well in 96-well sterile black microplates in Dulbecco modified Eagle medium (without phenol red) supplemented with 10% fetal calf serum and incubated overnight. Cells were treated with the two mAbs, TKIs alone, or erlotinib plus trastuzumab for 10 minutes at 37°C. After washing in KREBS buffer, cells were then fixed in 10% formalin for 2 minutes and washed once with KREBS buffer. Cells were labeled with 10 nM the anti-EGFR mAb m425 and 1 nM the anti-HER2 mAb FRP5 (both diluted in KREBS buffer), coupled to d2 (acceptor) and Lumi4 Tb cryptate (donor) dyes, respectively (Cisbio Bioassays, Bagnol-sur-Cèze, France) at 37°C for 6 hours. These two mouse mAbs are directed to different epitopes on the receptors than those targeted by the therapeutic antibodies trastuzumab and cetuximab, and thus, no interference was observed in the TR-FRET assay (data not shown). After four washes in KREBS buffer, the fluorescence of the Lumi4 Tb and d2 dyes was measured, respectively, at 620 and 665 nm emission (F665) (60-µs delay, 400-µs integration) on 337 nm excitation, on a Pherastar FS instrument (BMG Labtech, Offenburg, Germany).
The TR-FRET signal was expressed as ΔF665(%) = ΔF665 /F665Tb, with ΔF665 = F665c - F665Tb, as previously explained [14], and then data were presented considering the untreated sample as having 100% dimerization. The TR-FRET signal expressed as the percentage of dimers was correlated with the EGFR/HER2 heterodimer quantity normalized to the HER2 quantity. The 620-nm time-resolved fluorescence emission was correlated with the HER2 quantity. At the same time, the prompt fluorescence of the d2 dye was measured at 670 nm on a 620-nm excitation to quantify the EGFR receptors.
The same type of experiments was performed to detect EGFR homodimers and HER2 homodimers using 10 nM m425-Lumi4 Tb plus 10 nM m425-d2 and 1 nM FRP5-Lumi4 Tb plus 1 nM FRP5-d2, respectively. In the case of homodimers, the TR-FRET signal was correlated with the homodimer quantity normalized to the targeted receptor quantity.
Tumor Xenografts and Treatment Procedure
All in vivo experiments were performed in compliance with the French regulations and ethical guidelines for experimental animal studies in an accredited establishment (agreement no. C34-172-27). Six-week-old female athymic mice, purchased from Harlan (Le Malcourlet, France), were injected subcutaneously into the right flank with BxPC-3 (3.5 x 106), Capan-1 (10 x 106), or SKOV-3 (5 x 106) cells. Tumor-bearing mice were randomized in the different treatment groups (10 animals per group) when tumors reached a minimum volume of 50 mm3. Tumor volumes calculated by the formula: D1 x D2 x D3 /2. For survival comparison, mice were killed when tumor reached a volume larger than 1000 mm3.
Mice were treated with the 2mAbs (trastuzumab/cetuximab) therapy (ratio 1:1; 2 mg/kg of each mAb), trastuzumab (2 mg/kg)/erlotinib (100 mg/kg), or lapatinib alone (100, 200, or 300 mg/kg). Erlotinib and lapatinib were administrated daily through oral gavage, and antibodies were given intraperitoneally twice a week. All animals were treated for 4 weeks.
To determine the implication of the Fc part of the antibodies, BxPC-3 cells were xenografted in the right flank of SCID/Beige mice (Charles River, L'Arbresle, France), which lack NK cells. Mice were treated with F(ab′)2 fragments of trastuzumab and cetuximab (ratio 1:1; 1.35 mg/kg of each fragment) daily (to compensate for their reduced half-life) for 4 weeks or with intact trastuzumab and cetuximab (ratio 1:1; 2 mg/kg) twice a week. The concentration of fragments was adjusted to 2 µM like for the intact antibodies. Tumor dimensions and body weight were measured twice weekly.
Western Blot Analysis
At days 2, 7, and 15 after the beginning of treatment, tumors were harvested and lysed with buffer (150 mM NaCl, 10 mM Tris pH 7.4, 1 mM EDTA, 1% Triton X-100) containing 2 mM phenylmethylsulfonyl fluoride, 100 mM sodium fluoride, 10 mM sodium orthovanadate, and one tablet of complete protease inhibitor mixture (Sigma, St Louis, MO). Proteins were separated on 7% or 10% SDS-PAGE gels under reducing conditions and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA), which were then saturated in phosphate-buffered saline (PBS) containing 0.1% Tween-20 and 5% nonfat dry milk. Membranes were incubated with the appropriate dilutions of polyclonal rabbit antihuman EGFR, -HER2, -ERK1/2, -AKT, and -phosphorylated ERK1/2 or -AKT antibodies (Cell Signaling Technology, Beverly, MA). The level of tyrosine phosphorylation in tumors was analyzed using the anti-phosphotyrosine 4G10 antibody. Immunoblots were normalized using an antibody directed to glyceraldehyde-3-phosphate dehydrogenase (Millipore).
Antibody-Dependent Cell Cytotoxicity Assay by Chromium Release
Briefly, 2 x 105 BxPC-3 cells (target) were incubated with 100 µCi of 51Cr (Perkin-Elmer, Boston, MA) and 10 µg/ml of whole antibodies or F(ab′)2 fragments at 37°C for 1 hour. After three washes, cells were cultured in 96-well plates. Ficoll-purified human peripheral blood mononuclear cells (EFS, Montpellier, France), as effectors cells, were added at various effector-target (E/T) ratios. After 4 hours of incubation (37°C at 5% CO2), 100 µl of supernatant/well was recovered, and radioactivity was measured using a gamma counter. Spontaneous release was determined by incubating target cells alone, and maximal release was obtained by lysis of the target cells using 0.1 M HCl. The percentage of specific lysis was calculated as 100 x [(experimental release - spontaneous release) / (maximal release - spontaneous release)]. All measurements were done in triplicates.
Statistical Analysis
A linear mixed regression model was used to determine the relationship between tumor growth and the number of days after implantation. The fixed part of the model included variables corresponding to the number of days after implantation and to the different groups. Interaction terms were built into the model. Random intercept and random slope were included to take into account the time effect. The coefficients of the model were estimated by maximum likelihood and considered significant at the 0.05 level.
Kaplan-Meier survival estimates were calculated from the date of the xenograft until the date of the event of interest (i.e., tumor volume of 1000 mm3) and compared using the log-rank test. The statistical analysis was performed using the STATA 11.0 software (StataCorp [2009] Stata Release 11, Statistical Software; StataCorp LP, College Station, TX).
The statistical analysis of the data from the TR-FRET assay was performed using the Prism GraphPad software (San Diego, CA).
Results
In Vitro, the HER2low Pancreatic Carcinoma Cell Lines BxPC-3 and Capan-1 Are Sensitive to the EGFR TKI Erlotinib and the Dual EGFR/HER2 TKI Lapatinib
To compare the effects of the 2mAbs therapy (cetuximab and trastuzumab) and those of the erlotinib/trastuzumab combination and of lapatinib alone, first we assessed the expression of EGFR and HER2 in the human pancreatic carcinoma cell lines BxPC-3 and Capan-1. EGFR expression was high in BxPC-3 cells (20.3 x 104 receptors/cell) and moderate in Capan-1 cells (2.7 x 104), whereas HER2 expression was low in both cell lines, with 1.1 and 1.6 x 104 receptors per cell, respectively (Table 1).
Table 1.
Expression Level of EGFR and HER2 Receptors and IC50 of TKI Lapatinib and Erlotinib on BxPC3 and Capan-1 Pancreatic Cell Lines.
| Cell Line | K-ras Status | Molecules/Cell (x10-4) | IC50 (µM) | ||
| EGFR | HER2 | Lapatinib | Erlotinib | ||
| BxPC3 | WT | 20.3 ± 1.4* | 1.1 ± 1.0 | 11.5 ± 0.9 | 37.5 ± 2.0 |
| Capan-1 | Mutated | 2.7 ± 0.4 | 1.6 ± 1.5 | 13.2 ± 1.1 | 41.4 ± 1.6 |
Values are mean ± SE.
Quantification was done using the QIFI kit. Antigen density was expressed as antibody-binding capacity in molecules per cell.
The two TKIs lapatinib and erlotinib showed different antiproliferative effects. The IC50 for lapatinib (dual EGFR/HER2 TKI) was low with values of 11.5 µM in BxPC-3 cells and 13.2 µM in Capan-1 cells. Conversely, the IC50 for erlotinib (EGFR TKI) was higher in both cell lines (37.5 µM in BxPC-3 and 41.4 µM in Capan-1 cells), despite a 10-fold difference in EGFR expression (Table 1).
Combined Erlotinib/Trastuzumab Treatment Inhibits Tumor Progression in Mice Xenografted with BxPC-3 Cells, but Not in Mice Xenografted with Capan-1 Cells
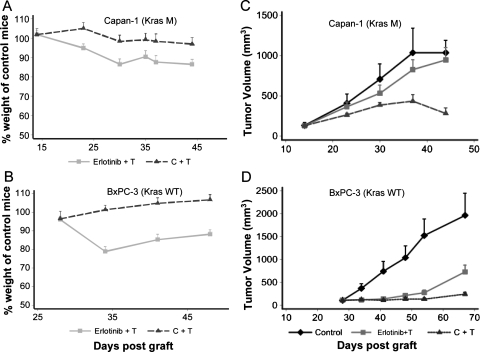
The efficacy of the dual anti-EGFR/HER2 strategy using erlotinib plus trastuzumab in comparison to the 2mAbs therapy (cetuximab/trastuzumab) was evaluated in mice xenografted (n = 10 for each group) with either BxPC-3 (wild-type K-ras) or Capan-1 (mutated K-ras), using 100 mg/kg erlotinib because a dose of 150 mg/kg was associated with important weight loss (data not shown). For trastuzumab, a 2-mg/kg dose was chosen on the basis of previous experiments [6]. No weight loss was observed in control animals and mice treated with the 2mAbs therapy, whereas the group treated with the erlotinib/trastuzumab combination showed weight loss (probably due to the TKI); however, weight was partially recovered by the end of the experiment. In Capan-1 model, the weight loss was slightly lower because the maximum loss was 13% if we compared the erlotinib/trastuzumab-treated mice to the control mice (Figure 1A). This value being 18% in the BxPC3 model (Figure 1B).
Figure 1.
Comparison of the antitumor activity of the 2mAbs therapy (cetuximab/trastuzumab) and of the erlotinib/trastuzumab combination in nude mice xenografted subcutaneously with Capan-1 and BxPC-3 human pancreatic carcinoma cells. The weight of the animals was followed up during treatment in the Capan-1 (A) and BxPC-3 (B) xenograft models. At days 14 (Capan-1 cells) (C) and 26 (BxPC-3 cells) (D) after graft, groups of 10 mice were treated with the two mAbs (ratio 1:1; 2 mg/kg of each mAb, twice a week), erlotinib (100 mg/kg; daily) plus trastuzumab (2 mg/kg, twice a week), or sterile PBS (daily). Results are presented as the mean tumor volume for each group. Bars, SD of the mean. C + T indicates cetuximab/trastuzumab; E + T, erlotinib/trastuzumab.
In mice xenografted with Capan-1 cells and treated with the 2mAbs therapy, tumor growth and volume were markedly reduced (mean tumor volume of 300 ± 30 mm3 at the end of the experiment) in comparison to controls (1000 ± 200 mm3, P < .001; Figure 1C). The therapeutic benefit was significant with a longer median survival than controls (34 days longer; P = .0107) and 20% survival (Table 2). In contrast, the erlotinib/trastuzumab combination had almost no effect on tumor growth (Figure 1A), with no tumor-free mice and no significant difference in median growth delay relative to controls (P = .25; Table 2).
Table 2.
Median Survival and Therapeutic Benefit of BxPC3 and Capan-1 Xenografted Mice Treated with TKI and/or Monoclonal Antibodies.
| Xenograft | Treatment | Median (day)* | Median (day)† | Tumor Free (%) |
| Capan-1 | Control | 38 | 0 | |
| Erlotinib + trastuzumab | 57 | +19 | 0 | |
| Lapatinib 200 mg/kg | 38 | 0 | 0 | |
| Lapatinib 300 mg/kg | 50 | +12 | 0 | |
| Cetuximab + trastuzumab | 72 | +34 | 2/10 (20%) | |
| BxPC3 | Control | 56 | 0 | |
| Erlotinib + trastuzumab | 79 | +23 | 0 | |
| Lapatinib 200 mg/kg | 56 | 0 | 0 | |
| Lapatinib 300 mg/kg | 56 | 0 | 0 | |
| Cetuximab + trastuzumab | 102 | +46 | 3/10 (30%) |
Median: days after graft where 50% of mice reach to a 1000-mm3 tumor volume.
Benefit = (median of the different treatments - median of the control group).
In mice xenografted with BxPC-3 cells, the 2mAbs therapy again induced a marked inhibition of tumor growth (Figure 1D) and a significantly higher delay of tumor progression compared with controls (P < .0001) (Table 2). Conversely, in this model, the erlotinib/trastuzumab combination also inhibited efficiently tumor growth (Figure 1B) with a significant therapeutic benefit of 23 days in comparison to controls (P < .001; Table 2). The therapeutic advantage of the 2mAbs therapy relative to the erlotinib/trastuzumab association was demonstrated by the significant (P = .05) difference of mean tumor volume (243 and 733 mm3, respectively) at the end of the experiment (64 days after graft) and in the number of tumor-free mice (3/10 and 0/10).
Unlike the 2mAbs Therapy, Lapatinib Has No Effect on Tumor Progression in Mice Xenografted with BxPC-3 or Capan-1 Cells
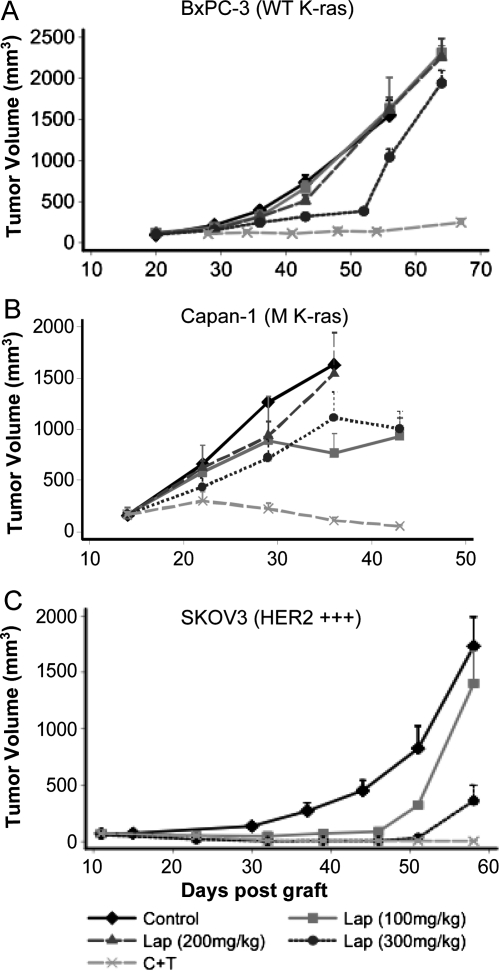
As a third strategy to simultaneously inhibit EGFR and HER2, we then evaluated the dual EGFR-HER2 TKI lapatinib. Mice xenografted with BxPC-3 or Capan-1 cells were treated with three relatively high doses of lapatinib (100, 200, and 300 mg/kg) orally twice per day or with the 2mAbs therapy (2 mg/kg) twice per week (n = 10 for each group). No weight loss or toxicity was observed with the three doses in the two xenograft models. Whereas 100 and 200 mg of lapatinib had no effect in both models, 300 mg/kg lapatinib was associated with a transient and moderate, but significant (P = .04) tumor growth inhibition in mice xenografted with BxPC-3 cells (Figure 2A) but not in those grafted with Capan-1 cells (Figure 2B). The efficiency of the 2mAbs therapy was again confirmed in the parallel experiments (Figure 2, A and B, and Table 2).
Figure 2.
Comparison of the effect of lapatinib (dual HER2/EGFR TKI) and of the 2mAbs therapy on tumor growth in nude mice xenografted subcutaneously with BxPC-3 (A), Capan-1 (B), or SKOV-3 (C) cells and randomized in different groups (n = 10 per group). At days 14 (Capan-1), 20 (BxPC-3), and 11 (SKOV-3 cells) after graft, mice were treated with lapatinib (100, 200, or 300 mg/kg, daily), the 2mAbs therapy (ratio 1:1; 2 mg/kg of each mAb, twice per week) or sterile PBS (daily). Results are presented as the mean tumor volume of each group. Bars, SD of the mean. C indicates cetuximab; Lap, lapatinib; T, trastuzumab.
To verify that the moderate effect of lapatinib was not due to the chosen regimen, we xenografted mice with SKOV-3 human ovarian carcinoma cells, known to strongly express HER2, and treated them with 100 or 300 mg/kg lapatinib orally twice a day or with the 2mAbs therapy for 4 weeks. In this xenograft model, lapatinib showed a significant dose-dependent inhibition of tumor growth (P < .0001 with 100 mg/kg and P < .0001 with 300 mg/kg) in comparison to untreated controls; however, rapid tumor escape was observed after the end of the treatment (day 44; Figure 2C). In contrast, in mice treated with the 2mAbs therapy, tumor growth inhibition was maintained until the end of the experiment (Figure 2C) with 80% of tumor-free animals confirming our previous observation [6].
Coinjection of Cetuximab and Trastuzumab F(ab′)2 Fragments Is Sufficient to Obtain the Antitumor Effects of the 2mAbs Therapy during Treatment
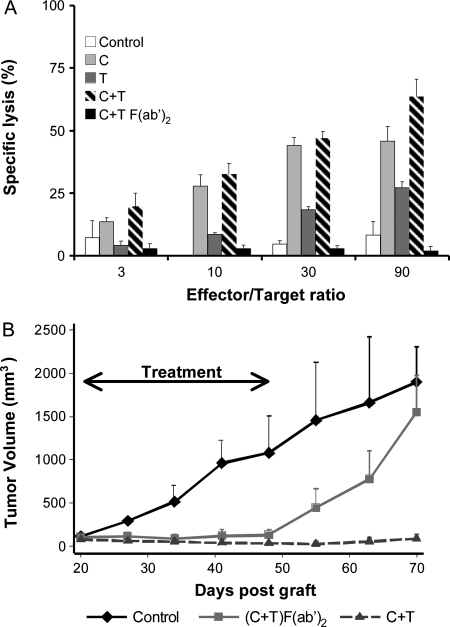
Unlike TKIs, mAbs can have Fc-dependent antitumor effects through activation of effector cells by antibody-dependent cell-mediated cytotoxicity (ADCC). To determine to which extent the antibody binding site reactivity with the receptors was implicated in the observed therapeutic synergy, F(ab′)2 fragments from cetuximab and trastuzumab were prepared, and their antitumor effect was tested in vitro and in vivo. As expected, intact mAbs induced an ADCC against 51Cr-labeled BxPC-3 tumor cells that was dependent on the effector/target cells ratio (Figure 3A). Because BxPC-3 cells express more EGFR than HER2 receptors at the cell surface, a higher percentage of lysis was observed with cetuximab than with trastuzumab, and its effect was comparable to what obtained using the two mAbs together. Conversely, the F(ab′)2 fragments obtained by pepsin digestion of the Fc of the two mAbs did not induce ADCC even in the presence of an excess of effector cells (Figure 3A).
Figure 3.
Comparison of the antitumor effect, in vitro and in vivo, of the 2mAbs therapy (cetuximab and trastuzumab) using intact mAbs or the F(ab′)2 fragments. (A) ADCC was assessed in vitro by incubating 51Cr-labeled BxPC-3 target cells with peripheral blood mononuclear cells (effector cells) at different concentrations in the presence of cetuximab and/or trastuzumab (whole mAbs) or their F(ab′)2 fragments. ADCC was determined by measuring the 51Cr released in the supernatant. (B) SCID/Beige mice bearing BxPC-3 pancreatic carcinoma xenografts were treated at day 20 after graft by daily coinjection of F(ab′)2 fragments of the two mAbs or twice per week with the intact mAbs. Results are presented as the mean tumor volume of each treated group. Bars, SD of the mean. C indicates cetuximab; double-head arrow, period of treatment; T, trastuzumab.
Then, highly immunodeficient SCID/Beige mice, which lack effector cells such as NK cells and monocytes (Croy BA), were xenografted with BxPC-3 cells and treated by daily injection, because of their shorter half-lives [15], of ADCC-inefficient F(ab′)2 fragments from the two mAbs, or biweekly injection of intact cetuximab or trastuzumab for 28 days. Similar significant tumor growth inhibition was obtained with the F(ab′)2 fragments and the intact mAbs during the treatment (Figure 3A), with a mean tumor volume of 200 ± 30 mm3 in comparison to 1100 ± 350 mm3 for the untreated control group at day 48 after graft (Figure 3B). However, tumor escape was observed immediately after the end of the treatment with the F(ab′)2 fragments, probably because of their very short half-life. Conversely, growth inhibition was still maintained 25 days after the end of the treatment with the intact mAbs.
Taken together, these results demonstrate that the effect of the 2mAbs therapy is, at least, partly Fc independent and, therefore, due to the induction of downstream signaling after the direct interaction of the antibodies with the receptors expressed at the tumor cell surface.
Only the 2mAbs Therapy Induces Long-term EGFR/HER2 Down-regulation and Inhibition of AKT Phosphorylation in Capan-1 Cell Xenografts
Downstream signaling from the EGFR/HER2 receptors activates several key signaling cascades, including the AKT and mitogen-activated protein kinase (MAPK) activation pathways [9]. To determine the in vivo mechanisms underlying the different efficacy of the various dual anti-EGFR/HER2 strategies, we analyzed EGFR/HER2 expression and AKT/MAPK phosphorylation in Capan-1 cells harvested from xenografted nude mice at different time points (days 2, 7, and 15) after the beginning of the mAb- and TKI-based treatments. The 2mAbs therapy induced a long-term and marked decrease in both EGFR and HER2 expression at all tested time points. Conversely, lapatinib was associated with a strong increase in HER2 expression at all time points. Treatment with erlotinib plus trastuzumab was followed by EGFR down-regulation mainly at day 2 after treatment, but not at later times, whereas the reduction in HER2 expression was more persistent, certainly due to trastuzumab (Figure 4A). The second marked and long-term effect of the 2mAbs therapy was the specific decrease in AKT phosphorylation, but not total AKT, at all tested time points (Figure 4A). In contrast, erlotinib/trastuzumab and lapatinib alone did not induce such effect (Figure 4A). No effect on MAPK phosphorylation and on the level of total MAPK was noted after the different treatments (Figure 4A). This result could be expected due to the mutated K-ras status of the Capan-1 cell line.
Figure 4.
(A) The 2mAbs therapy induces long-term EGFR and HER2 down-regulation and inhibition of AKT phosphorylation in Capan-1 pancreatic carcinoma cells. Mice bearing Capan-1 xenografts were treated with the 2mAbs (cetuximab/trastuzumab) therapy (ratio 1:1; 2 mg/kg of each mAb, twice a week), erlotinib (100 mg/kg, daily) plus trastuzumab (2 mg/kg, twice per week), or lapatinib (300 mg/kg, daily). Tumors were resected at day 2, 7, or 15 after the beginning of treatment. Cell lysates were analyzed by Western blot analysis for EGFR and HER2, total AKT, phosphorylated AKT, total MAPK, and phosphorylated MAPK expression. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. C + T indicates 2mAbs. (B) The 2mAbs therapy induces long-term inhibition of the overall tyrosine phosphorylation profile in Capan-1 tumor cells, whereas the tested TKIs did not. Mice xenografted with Capan-1 pancreatic carcinoma cells were treated with either the two mAbs therapy (ratio 1:1; 2 mg/kg of each mAb, twice per week), erlotinib (100 mg/kg, daily) plus trastuzumab (2 mg/kg, twice per day), or lapatinib (300 mg/kg, daily). Tumors were resected at day 2, 3, or 7 after beginning the mAbs therapy and at 1 hour, 6 hours, 7 days, and 15 days after the beginning of the treatment with erlotinib/trastuzumab or lapatinib. Cell lysates were analyzed by Western blot analysis for tyrosine phosphorylation (P-Tyr). C + T indicates cetuximab/trastuzumab; E + T, erlotinib/trastuzumab.
The 2mAbs Therapy Induces Long-term Inhibition of Tyrosine Phosphorylation Unlike the Two Other Dual Anti-EGFR/HER2 Treatments
The overall tyrosine phosphorylation profile of Capan-1 cells harvested from xenografted nude mice at days 2, 7, and 15 after the beginning of the different treatments was analyzed with Western blot analysis. The 2mAbs therapy induced long-term blockade of tyrosine phosphorylation from day 2 to day 7 in comparison to untreated controls (Figure 4B). Conversely, the erlotinib/trastuzumab combination and lapatinib alone induced only short-term inhibition, one hour after the beginning of treatment. These data support the notion of a prompt TKI effect on tyrosine phosphorylation in contrast to the persistent effect after the 2mAbs therapy.
Incubation of BxPC-3 Cells with Cetuximab and Trastuzumab Is Associated with Higher Disruption of EGFR/HER2 Dimerization and Homodimer Formation than Incubation with Erlotinib/Trastuzumab or Lapatinib
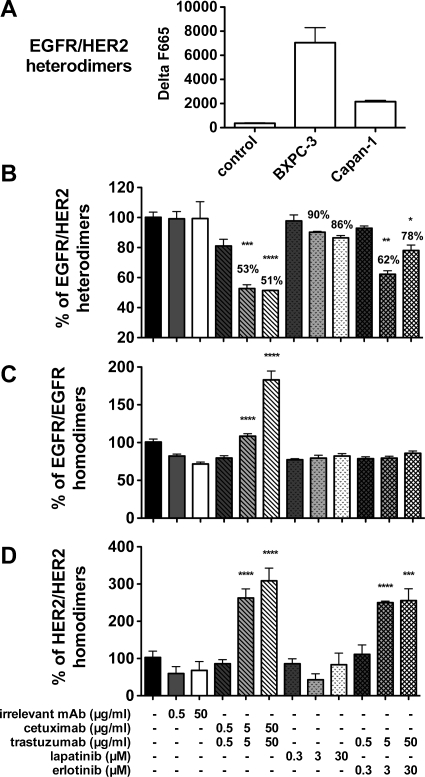
Using a recently described antibody-based TR-FRET assay that allows the detection of EGFR/HER2 heterodimerization [14], we analyzed the effect on EGFR/HER2 dimerization of a 10-minute incubation of BxPC-3 cells with cetuximab/trastuzumab, erlotinib/trastuzumab, or lapatinib alone. The BxPC-3 pancreatic carcinoma line was chosen for this analysis because it expresses more EGFR/HER2 heterodimers than the Capan-1 cell line, thus allows obtaining a better fluorescence signal to accurately follow heterodimer formation (Figure 5A). A significant dose-dependent disruption of EGFR/HER2 heterodimerization (approximately 50%) was observed when BxPC-3 cells were incubated with the two mAbs in comparison to untreated cells (P < .0001). The lower fluorescence signal was not due to steric hindrance by the two therapeutic mAbs because the labeled mAbs used in the FRET assay were directed to different EGFR and HER2 epitopes. Indeed, binding to the target cells and fluorescent signal (at 620 and 670 nm) of the two labeled mAbs were not inhibited by preincubation with the two mAbs. Incubation with erlotinib/trastuzumab or lapatinib also disrupted EGFR/HER2 heterodimerization but to a lower extent (38% maximal disruption, P < .001; and 14% maximal disruption, P > .05). Finally, preincubation with an irrelevant mAb did not influence dimer formation and fluorescence emission at 670 and 620 nm (Figure 5B). As expected, erlotinib alone did not disturb EGFR/HER2 heterodimerization at doses ranging from 30 nM to 30 µM, whereas trastuzumab alone induced a dose-dependent interference of EGFR/HER2 dimerization (up to 38% disruption at the dose of 5 µg/ml; data not shown). This suggests that the disruption of EGFR/HER2 heterodimerization induced by the erlotinib/trastuzumab combination is mainly due to the effect of trastuzumab. Preincubation with the two mAbs did not modify EGFR and HER2 expression. An increase in EGFR homodimers was observed after preincubation with the two mAbs, but not with the TKIs, in comparison to untreated cells. Conversely, HER2 homodimers were increased both by preincubation with the mAbs and with erlotinib/trastuzumab, mainly because of the effect of trastuzumab (data not shown).
Figure 5.
Effect of the 2mAbs (cetuximab/trastuzumab) therapy, of erlotinib plus trastuzumab, or of lapatinib alone on receptor heterodimerization and homodimerization using the antibody-based TR-FRET assay. (A) Quantification of EGFR/HER2 heterodimers in Capan-1 and BxPC-3 cells. (B) BxPC-3 cells were treated with increasing concentrations of cetuximab/trastuzumab (ratio 1:1), erlotinib and/or trastuzumab, or lapatinib for 10 minutes and then fixed in 10% formalin. Fixed cells were then incubated with d2-labeled anti-EGFR and lumi4-terbium-labeled anti-HER2 mAbs (directed against different epitopes than the ones recognized by cetuximab and trastuzumab) for 6 hours. Cells were washed, and the TR-FRET signals were measured at 665 nm in the TRF mode (60-µs delay, 400-µs integration) on 337 nm excitation to estimate the dimer level. The TR-FRET signal was expressed as ΔF665 (%) and then as the dimer percentage (see Materials and Methods). (C) Effect of the different therapies on EGFR or (D) HER2 homodimer formation. Data are mean ± SEM of three independent experiments performed in triplicate. ****P < .0001; ***P < .001, by two-way analysis of variance, with Bonferroni multiple-comparison post test (each treatment was compared with the irrelevant mAb treatment).
These results indicate that incubation of BxPC-3 cells with cetuximab/trastuzumab modifies the repartition of homodimers and heterodimers at the cell surface with disruption of EGFR/HER2 heterodimerization and increased homodimer formation.
Discussion
In previous studies, we demonstrated the synergistic effect of combining an anti-EGFR mAb (cetuximab) and an anti-HER2 mAb (trastuzumab) for pancreatic cancer treatment (2mAbs therapy). In the present study, we wanted to evaluate two other dual anti-EGFR/HER2 strategies involving TKIs (the combination of erlotinib with trastuzumab and the EGFR/HER2 TKI lapatinib) and compare them with the 2mAbs therapy. With up to 30% complete remission, the 2mAbs therapy (trastuzumab and cetuximab) had the greatest antitumoral effect in nude mice xenografted with two human HER2low pancreatic carcinoma cell lines, independently of their K-ras status. The therapeutic benefit of this treatment was associated with a marked and sustained down-regulation of EGFR/HER2 receptors as well as a downstream reduction of AKT phosphorylation. Moreover, the significant therapeutic benefit obtained with F(ab′)2 fragments of the two mAbs (Figure 3) clearly indicates that the observed antitumor effect must be due, at least in part, to the interaction of the binding part of the mAbs with the two receptors and the induced intracellular signaling [9]. This is in disagreement with the work by Clynes et al. [16] who, on the basis of the negative therapeutic effect of trastuzumab or anti-CD20 mAbs in Fcγ receptor KO mice, attributed most of the antitumor effect of the mAbs to an ADCC mechanism. However, these authors xenografted mice with HER2high BT474M1 tumor cells and treated them with trastuzumab alone. Although in our study the F(ab′)2 fragments were as efficient as the intact mAbs during the treatment period, tumors rapidly relapsed after the end of the treatment with fragments but not with intact mAbs. The sustained antitumor effect of intact mAbs suggests an implication of immune effector cells and was, therefore, very surprising because these experiments were performed in SCID/Beige mice, which lack NK cells, the major effector cells responsible for the ADCC mechanism. The continuous effect could thus be attributed to monocytes. The rapid tumor relapse after treatment with F(ab′)2 could also be explained by the very short half-life of the fragments owing to their lack of reactivity with the FcRn receptors [17]. Indeed, during the treatment period, the difference in half-life between mAbs and F(ab′)2 was compensated by the different schedules (twice a week for intact mAbs vs daily for F(ab′)2).
The erlotinib/trastuzumab combination induced marked tumor growth inhibition in mice xenografted with wild-type K-ras (BxPC-3 cells), but not with mutated K-ras (Capan-1 cells) pancreatic carcinoma cell lines, confirming the importance of the K-ras status in the response to EGFR/HER2-targeted therapy. Indeed, K-ras is considered a reliable biomarker of the efficacy of EGFR-specific antibody therapy in colorectal cancer patients [18], but its role as predictive biomarker remained to be clarified in patients treated with the EGFR-specific TKI erlotinib [19]. Moreover, the positive response of BxPC-3 xenografts to erlotinib/trastuzumab despite the low HER2 expression of these cells is very interesting because, previously, only HER2-overexpressing breast cancer cells were reported to respond to the combination of trastuzumab with gefitinib, another EGFR-specific TKI [20]. The limitation of the erlotinib/trastuzumab combination is its toxicity certainly due to the limited selectivity of erlotinib [21,22]. In our model, erlotinib-treated mice lost up to 17% of their weight and did not fully recover their initial weight 15 days after the end of the treatment.
HER3 expression is another parameter that is correlated with sensitivity to erlotinib in five human pancreatic cancer cell lines [23]. The erlotinib/trastuzumab-sensitive BxPC-3 cells strongly express HER3, whereas the erlotinib/trastuzumab-insensitive Capan-1 cells do not. In cells that strongly express HER3, erlotinib might indirectly inhibit HER3 phosphorylation by EGFR-induced transactivation [24]. Conversely, in HER3-negative/low pancreatic carcinoma cells, the HER3/PI3K/AKT pathway cannot be activated on EGFR/HER3 heterodimer formation, and thus, they are less dependent on EGFR-induced signaling and, consequently, not sensitive to erlotinib [23]. All these data suggest that EGFR/HER3 coexpression could be a predictor of erlotinib sensitivity in pancreatic cancer.
Lapatinib could have been an attractive alternative to the 2mAbs therapy because this TKI acts specifically and simultaneously on EGFR and HER2. Few preclinical studies have evaluated the potential of lapatinib for the treatment of pancreatic cancer, and they reported a synergistic effect when lapatinib was combined with 5-fluorouracil [25] or radiotherapy [26]. Furthermore, Safran et al. [11] demonstrated that patients with pancreatic cancer can be treated with the lapatinib/gemcitabine combination without toxicity. However, here we show that lapatinib on its own does not have any significant effect in mice xenografted with the HER2low Capan-1 and BxPC-3 (HER2low) pancreatic cancer cells. Conversely, the same doses of lapatinib efficiently inhibited tumor growth in mice xenografted with the HER2high SKOV3 human ovarian cancer cell line, confirming the correlation between high HER2 expression and therapeutic efficacy of lapatinib [27,28].
Half-life differences could explain, at least partially, the higher efficacy of the 2mAbs therapy relative to the treatment with TKIs, although this parameter was taken into account in the different regimens (twice a week for intact mAbs vs twice a day for the TKIs). This is illustrated by the tyrosine phosphorylation profile of Capan-1 cells in which a rapid but very short phosphorylation inhibition was induced by the erlotinib/trastuzumab combination and a persistent inhibition by the two mAbs.
The cell signaling pathways induced by the different treatment were investigated ex vivo in Caplan-1 (HER2low, K-ras mutated) pancreatic tumor cells isolated from treated nude mice. The long-term downregulation of EGFR and HER2 receptors after the 2mAbs therapy contrasted with the cell surface accumulation of HER2 receptors in cancer cells isolated from mice treated with lapatinib, as previously described in vitro and in vivo in HER2high breast cancer [29]. Ben-Kasus et al. [30] reported that the combination of HER2-specific antibodies had a stronger antitumoral effect through increased endocytosis and degradation of HER2 target receptors. Our results confirm and extend this observation because we show that concomitant EGFR and HER2 targeting has a clear antitumor effect and induces down-regulation of both EGFR and HER2 receptors. In contrast to anti-EGFR/HER2 strategies using TKIs, the 2mAbs therapy blocked phosphorylation of the AKT pathway, but did not affect MAPK activation, probably due to the K-ras mutated status of Capan-1 cells. K-ras mutations are known to result in impaired GTPase function, which locks K-ras in the GTP-bound “on” state, triggering downstream activation pathways, including particularly MAPK, and leading to a variety of cellular processes, including cell survival and mobility [31]. MAPK, therefore, could be an attractive therapeutic target, but a disappointing phase 2 trial of the MAPK inhibitor CI-1040 in pancreatic cancer stopped further development [32]. Because the combined inhibition of MAPK and EGFR using CI-1040 and gefitinib, respectively, was effective in a preclinical study on pancreatic cancer [33], the association of a MAPK inhibitor currently under clinical trial (e.g., GSK 1120212) with the 2mAbs therapy could improve our therapeutic strategy. Indeed, the recent study by Meira et al. [34] described a synergistic effect between the MAPK inhibitor PD98059 and cetuximab on cell proliferation and downstream signaling in cervical cancer cells.
Finally, we used our newly developed antibody-based TR-FRET assay [14] to analyze the importance of EGFR/HER2 heterodimer disruption by the different therapies. The 2mAbs therapy has a major effect with 49% disruption compared with 14% for lapatinib. This observation is of importance because cross talk between the four receptors of the HER family was shown to contribute to a more aggressive phenotype and to affect response to therapy in many cancers [35]. Furthermore, we observed a significant increase in homodimers in cells incubated with the two mAbs in comparison to untreated cells or after incubation with the TKIs. We can hypothesize that the two mAbs cetuximab and trastuzumab first perturb the ligand-induced heterodimerization process and then promote homodimer formation. Because of their bivalence, mAbs can favor the formation of receptor homodimers, which are preferentially internalized and degraded. As already described, EGFR homodimers are rapidly endocytosed in response to EGF stimulation and degraded [36], whereas HER2 undergo slow endocytosis, followed by recycle to the cell surface [37]. EGFR/HER2 dimer formation is thus impaired by EGF-induced endocytosis, resulting in extended activation [38] and increased heterodimer recycling at the cell surface [39].
In conclusion, in the present in vivo study, we demonstrate that the 2mAbs therapy, in which cetuximab is combined with trastuzumab, is more efficient than treatments with TKIs (erlotinib with trastuzumab or lapatinib alone). The efficacy of the 2mAbs therapy is based on the downstream inhibition of AKT phosphorylation and on EGFR/HER2 heterodimer disruption linked to an increase in EGFR and HER2 homodimer formation. These direct effects are associated with the involvement of immune cells to induce ADCC. These results support the ongoing clinical investigation on the effects of the 2mAbs therapy in patients with metastatic pancreatic carcinomas (independently of their K-ras status) and the rationale for using mAb combinations in therapeutic oncology.
Acknowledgments
The authors thank G. Heintz, S. Bousquié, and V. Garambois for excellent technical assistance M. Brissac and I. Aït-Arsa for help in performing the animal experiments.
Footnotes
C. Larbouret and this study were supported by Roche, France.
References
- 1.Azria D, Ychou M, Jacot W, Thezenas S, Lemanski C, Senesse P, Prost P, Delard R, Masson B, Dubois JB. Treatment of unresectable, locally advanced pancreatic adenocarcinoma with combined radiochemotherapy with 5-fluorouracil and cisplatin. Pancreas. 2002;25:360–365. doi: 10.1097/00006676-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Nelson NJ. Pancreatic cancer research matures. J Natl Cancer Inst. 2007;99:1432–1434. doi: 10.1093/jnci/djm180. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Zhang X, Parsons DW, Lin JCH, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol. 2009;6:412–422. doi: 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang ZQ, Buchsbaum DJ. Monoclonal antibodies in the treatment of pancreatic cancer. Immunotherapy. 2009;1:223–239. doi: 10.2217/1750743X.1.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larbouret C, Robert B, Navarro-Teulon I, Thèzenas S, Ladjemi MZ, Morisseau S, Campigna E, Bibeau F, Mach JP, Pèlegrin A, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13:3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–569. [PubMed] [Google Scholar]
- 8.Safran H, Steinhoff M, Mangray S, Rathore R, King TC, Chai L, Berzein K, Moore T, Iannitti D, Reiss P, et al. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol. 2001;24:496–499. doi: 10.1097/00000421-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 11.Safran H, Miner T, Resnick M, Dipetrillo T, McNulty B, Evans D, Joseph P, Plette A, Millis R, Sears D, et al. Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: a phase I study for advanced pancreaticobiliary cancer. Am J Clin Oncol. 2008;31:140–144. doi: 10.1097/COC.0b013e318145b9a5. [DOI] [PubMed] [Google Scholar]
- 12.Larbouret C, Robert B, Bascoul-Mollevi C, Penault-Llorca F, Ho-Pun-Cheung A, Morisseau S, Navarro-Teulon I, Mach JP, Pèlegrin A, Azria D. Combined cetuximab and trastuzumab are superior to gemcitabine in the treatment of human pancreatic carcinoma xenografts. Ann Oncol. 2010;21:98–103. doi: 10.1093/annonc/mdp496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germain C, Larbouret C, Cesson V, Donda A, Held W, Mach JP, Pèlegrin A, Robert B. MHC class I-related chain A conjugated to antitumor antibodies can sensitize tumor cells to specific lysis by natural killer cells. Clin Cancer Res. 2005;11:7516–7522. doi: 10.1158/1078-0432.CCR-05-0872. [DOI] [PubMed] [Google Scholar]
- 14.Gaborit N, Larbouret C, Vallaghe J, Peyrusson F, Bascoul-Mollevi C, Crapez E, Azria D, Chardès T, Poul MA, Mathis G, et al. Time-resolved fluorescence resonance energy transfer (TR-FRET) to analyze the disruption of EGFR/HER2 dimers: a new method to evaluate the efficiency of targeted therapy using monoclonal antibodies. J Biol Chem. 2011;286:11337–11345. doi: 10.1074/jbc.M111.223503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel CA, Bischof-Delaloye A, Mach JP, Pèlegrin A, Hardman N, Delaloye B, Buchegger F. Direct comparison of a radioiodinated intact chimeric anti-CEA MAb with its F(ab′)2 fragment in nude mice bearing different human colon cancer xenografts. Br J Cancer. 1993;68:684–690. doi: 10.1038/bjc.1993.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 17.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 18.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 19.da Cunha Santos G, Dhani N, Tu D, Chin K, Ludkovski O, Kamel-Reid S, Squire J, Parulekar W, Moore MJ, Tsao MS. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer. 2010;116:5599–5607. doi: 10.1002/cncr.25393. [DOI] [PubMed] [Google Scholar]
- 20.Normanno N, Campiglio M, De LA, Somenzi G, Maiello M, Ciardiello F, Gianni L, Salomon DS, Menard S. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with trastuzumab (Herceptin) on human breast cancer cell growth. Ann Oncol. 2002;13:65–72. doi: 10.1093/annonc/mdf020. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 22.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 23.Frolov A, Schuller K, Tzeng CWD, Cannon EE, Ku BC, Howard JH, Vickers SM, Heslin MJ, Buchsbaum DJ, Arnoletti JP. ErbB3 expression and dimerization with EGFR influence pancreatic cancer cell sensitivity to erlotinib. Cancer Biol Ther. 2007;6:548–554. doi: 10.4161/cbt.6.4.3849. [DOI] [PubMed] [Google Scholar]
- 24.Buck E, Eyzaguirre A, Haley JD, Gibson NW, Cagnoni P, Iwata KK. Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther. 2006;5:2051–2059. doi: 10.1158/1535-7163.MCT-06-0007. [DOI] [PubMed] [Google Scholar]
- 25.Komoto M, Nakata B, Nishii T, Kawajiri H, Shinto O, Amano R, Yamada N, Yashiro M, Hirakawa K. In vitro and in vivo evidence that a combination of lapatinib plus S-1 is a promising treatment for pancreatic cancer. Cancer Sci. 2010;101:468–473. doi: 10.1111/j.1349-7006.2009.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimple RJ, Vaseva AV, Cox AD, Baerman KM, Calvo BF, Tepper JE, Shields JM, Sartor CI. Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res. 2010;16:912–923. doi: 10.1158/1078-0432.CCR-09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusnak DW, Alligood KJ, Mullin RJ, Spehar GM, Arenas-Elliott C, Martin AM, Degenhardt Y, Rudolph SK, Haws TF, Jr, Hudson-Curtis BL, et al. Assessment of epidermal growth factor receptor (EGFR, ErbB1) and HER2 (ErbB2) protein expression levels and response to lapatinib (Tykerb, GW572016) in an expanded panel of human normal and tumour cell lines. Cell Prolif. 2007;40:580–594. doi: 10.1111/j.1365-2184.2007.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ, Finn RS. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res. 2010;16:1509–1519. doi: 10.1158/1078-0432.CCR-09-1112. [DOI] [PubMed] [Google Scholar]
- 29.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S, Arribas J, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Kasus T, Schechter B, Lavi S, Yarden Y, Sela M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc Natl Acad Sci USA. 2009;106:3294–3299. doi: 10.1073/pnas.0812059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji Z, Mei FC, Xie J, Cheng X. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem. 2007;282:14048–14055. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 32.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, Hamid O, Varterasian M, Asbury P, Kaldjian EP, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 33.Jimeno A, Rubio-Viqueira B, Amador ML, Grunwald V, Maitra A, Iacobuzio-Donahue C, Hidalgo M. Dual mitogen-activated protein kinase and epidermal growth factor receptor inhibition in biliary and pancreatic cancer. Mol Cancer Ther. 2007;6:1079–1088. doi: 10.1158/1535-7163.MCT-06-0448. [DOI] [PubMed] [Google Scholar]
- 34.Meira DD, de Almeida VH, Mororó JS, Nóbrega I, Bardella L, Silva RLA, Albano RM, Ferreira CG. Combination of cetuximab with chemoradiation, trastuzumab or MAPK inhibitors: mechanisms of sensitisation of cervical cancer cells. Br J Cancer. 2009;101:782–791. doi: 10.1038/sj.bjc.6605216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh R, Narasanna A, Wang SE, Liu S, Chakrabarty A, Balko JM, González-Angulo AM, Mills GB, Penuel E, Winslow J, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71:1871–1882. doi: 10.1158/0008-5472.CAN-10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpentier JL, White MF, Orci L, Kahn RC. Direct visualization of the phosphorylated epidermal growth factor receptor during its internalization in A-431 cells. J Cell Biol. 1987;105:2751–2762. doi: 10.1083/jcb.105.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Zhang L, Yeung TK, Chen X. Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol Biol Cell. 1999;10:1621–1636. doi: 10.1091/mbc.10.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson JC, Staros JV. Effect of ErbB2 coexpression on the kinetic interactions of epidermal growth factor with its receptor in intact cells. Biochemistry. 2002;41:8–14. doi: 10.1021/bi015839l. [DOI] [PubMed] [Google Scholar]