Abstract
Retinoid X receptor (RXR) has been implicated in several neoplastic diseases. Previously, we have shown that RXR-α is downregulated in human and rodent colonic tumors, suggesting a potential target for colon cancer prevention (http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/colorectal-cancer-key-statistics). Experiments were designed to assess the chemopreventive efficacy of the selective RXR agonist bexarotene for the suppression of intestinal tumorigenesis in ApcMin/+ mice. Before the efficacy studies, we determined that the maximal tolerated dose in C57BL/6J mice was less than 400 ppm. For the efficacy study, 6-week-old male and female C57BL/6J-ApcMin/+ mice (nine mice per group) were fed diets containing 0, 30, and 60 ppm of bexarotene or 200 ppm of bexarotene for 80 days before intestinal tumors were evaluated. Dietary administration of 30 and 60 ppm of bexarotene suppressed the intestinal polyp formation by 38% (P < .015) and 60% (P < .0001) in males, respectively, and by 8.5% and 37% (P < .007) in females, respectively. Also, significant inhibition (50%–100%) of colonic tumor formation was observed in both male and female mice with bexarotene treatment. Administration of 200 ppm of bexarotene showed significant suppression of tumor formation (66%, P < .0001); however, it had significant toxicity. Intestinal tumors of bexarotene-fed mice showed significantly reduced expression of proliferating cell nuclear antigen (60%, P < .0001), cyclin D1, and cyclooxygenase 2 and increased RXR-α messenger RNA and uptake of oleate (34%, P < .01). Also, bexarotene-fed mice showed dose-dependent suppression of serum triglycerides (25%–72%, P < .0001) and inflammatory cytokines.
Introduction
Despite improved advances in early diagnosis and treatment, colon cancer remains the leading cancer problem in the United States and worldwide in both men and women. In recent years, the annual incidence rate of colon cancer in the United States has fallen slowly, but colon cancer remains the second most common cause of cancer-related deaths in the United States [1]. A significant increase is observed in the incidence of colorectal cancers in the developing world. Several agents have been and currently are being investigated for chemoprevention of colon cancer, including cyclooxygenase 2 (COX-2) inhibitors, tyrosine kinase inhibitors, and retinoids, to cite a few [2–6]. Although retinoids are promising chemopreventive agents in animals and humans, they are not generally used for cancer prevention because of their toxicity [7]. Naturally occurring and synthetic retinoids activate different retinoid receptors. All-trans retinoic acid (RA) binds only retinoic acid receptors (RARs), whereas 9-cis RA is an agonist for both RARs and retinoid X receptors (RXRs). The synthetic rexinoid bexarotene is a highly selective RXR agonist with low affinity for RARs [8]. It is evident that RXR plays vital roles in multiple signaling pathways, including in carcinogenesis through nuclear receptor family members. The loss of RXR function will likely negatively impact physiological roles of the nuclear receptors.
In adenomatous polyposis coli (APC) mutant mice, the nuclear accumulation of β-catenin leads to altered signaling, causing polyp formation. The degradation of cytoplasmic β-catenin was specifically induced by RXR-α but not by RAR-α [9]. RXR-α has unique characteristics that distinguish it from the other RAR and RXR subtypes [10,11]. It is the most abundant of the RXR subtypes, it mediates the heterodimerization of RARs with other members in the thyroid hormone nuclear transcription receptor family, it is essential for the functional activation of RARs by their ligands, and it has the ability to form homodimers with itself or heterodimers with other nuclear receptors such as peroxisome proliferator-activated receptor γ and vitamin D. In addition, RXR-α has been suggested to be a key player in the carcinogenesis of several types of cancer, including prostate, ovarian, skin, and leukemia [12–14].
Modulation of multiple cancer pathways by a single agent is an attractive approach for targeted therapy. Although significant progress has been made toward understanding RAR/RXR-mediated signaling pathways, the molecular mechanisms underlying the gene modulations caused by ligand-activated RXRs are highly complex and incompletely understood. Our previous studies with a natural RXR-α agonist, β-ionone, have shown inhibition of the proliferation of malignant colon cells and suppression of aberrant crypt formation in an azoxymethane (AOM)-induced rat colon carcinogenesis model [2]. To develop agents that will prevent cancer with increased efficacy by activating multiple pathways, we investigated the cancer preventive activity of the RXR-selective rexinoid, bexarotene (LGD1069; Targretin). In this study, we investigated the ability of bexarotene to prevent the development of small intestinal (SI) and colon tumors in ApcMin/+ mice. The results demonstrated that bexarotene significantly prevented the development of SI and colon tumors by modulating markers of tumor growth.
Materials and Methods
Chemicals
Bexarotene was kindly provided by the National Cancer Institute's chemopreventive drug repository (Rockville, MD). Primary antibodies anti-RXR-α (1:500) and anti-proliferating cell nuclear antigen (PCNA) were from Santa Cruz Biotechnology (Santa Cruz, CA); monoclonal and polyclonal horseradish peroxidase-conjugated secondary antibodies (1:1000) were from Santa Cruz Biotechnology. Multi-Analyte ELISArray kit was from SA Biosciences (Frederick, MD).
Breeding and Genotyping of ApcMin/+ Mice
All animal experiments were performed in accordance with the institutional guidelines of the American Council on Animal Care and were approved by the Institutional Animal Care and Use Committee at the University of Oklahoma Health Sciences Center (OUHSC). Male ApcMin/+ (C57BL/6J) and female wild-type littermate mice were initially purchased from The Jackson Laboratory (Bar Harbor, ME) as founders, and our own breeding colony was established in the OUHSC rodent barrier facility and genotyped according to the vendor's instructions. All mice were housed three per cage in ventilated cages under standardized conditions (21°C, 60% relative humidity, 12-hour light/12-hour dark cycle, 20 air changes per hour). All mice were allowed ad libitum access to the respective diets and automated tap water purified by reverse osmosis.
Diets
All ingredients for the semipurified diets were purchased from Bioserv (Frenchtown, NJ) and stored at 4°C before diet preparation. Diets were based on the modified American Institute of Nutrition (AIN)-76A diet. Bexarotene was premixed with a small quantity of diet and then blended into bulk diet using a Hobart mixer. Both control and experimental diets were prepared weekly and stored in a cold room. Agent content in the experimental diets was determined periodically in multiple samples taken from the top, middle, and bottom portions of individual diet preparations to verify uniform distribution. In this study, experimental diets were prepared with AIN-76A diet containing 0, 30, 60, or 200 ppm of bexarotene.
Determination of Bexarotene Maximum Tolerable Dose in C57BL/6J Mice
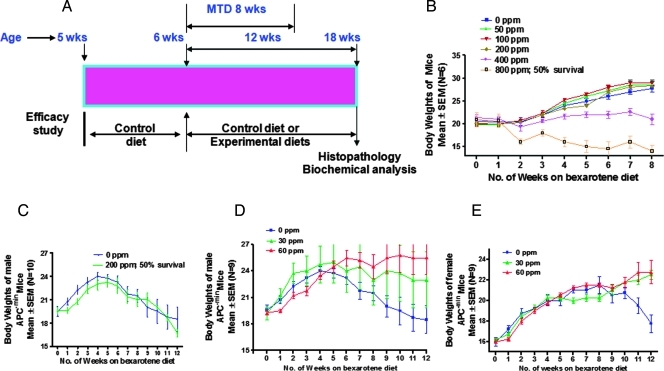
At 6 weeks of age, groups of male C57BL/6J mice (6/group) were fed the modified AIN-76A diet containing the various levels of bexarotene (50, 100, 200, 400, and 800 ppm; Figure 1A). Body weights were recorded twice weekly for 6 weeks. All animals were examined daily for any symptoms of toxicity. At the end of 8 weeks, all animals were killed, and colon, small intestine, stomach, liver, and kidney were examined grossly under a dissection microscope for any abnormalities. The maximum tolerable dose (MTD) is defined as the highest dose that causes no more than a 10% weight decrement compared with the appropriate control diet group and does not produce mortality or any external signs of toxicity that would be predicted to shorten the natural life span of the animal. These external signs of toxicity include roughened coat, ill-kept appearance, chromodacryorrhea, rhinitis, and prostration, to cite a few.
Figure 1.
(A) Experimental design for the evaluation of the chemopreventive efficacy of bexarotene administered in the diet from 6 weeks of age to the end of the experiment. AIN-76A was the control diet. The study was terminated after 80 days. For details about animals and treatments, see Materials and Methods. (B) C57BL/6J male mice were exposed to five different doses of bexarotene for 8 weeks to observe the MTD in mice. Changes in body weight in this period for control mice and mice on bexarotene diet are plotted in the graph. Bexarotene at 400 ppm caused significant body weight loss and that at 800 ppm was toxic, with 50% survival by the end of the study. (C) Changes in body weight over time in male ApcMin/+ treated with or without 200 ppm of bexarotene. Statistically significant differences in bodyweight between bexarotene-treated and control groups were observed. Bexarotene-treated animals were found to lose weight, and 50% survival was observed by the end of the study. (D) Changes in body weight over time for male ApcMin/+ mice treated with or without 30 or 60 ppm of bexarotene. Statistically significant differences in bodyweight between bexarotene-treated and control groups were observed. Bexarotene-treated animals were found to gain weight by the end of the study. (E) Changes in body weight over time for female ApcMin/+ mice treated with 0, 30, or 60 ppm of bexarotene. Differences in bodyweight gain by high-dose bexarotene-treated group versus control groups were observed.
Bioassay: Intestinal Tumorigenesis in ApcMin/+ Mice
The antitumor efficacy of bexarotene was assessed in male and female ApcMin/+ mice according to the experimental protocol summarized in Figure 1A. Five-week-old mice were randomized for age and average body weights in each group (nine ApcMin/+ mice in each group), and mice were fed the AIN-76A diet for 1 week. At 6 weeks of age, mice were fed control or experimental diets containing 0, 30, 60, or 200 ppm of bexarotene until termination of the study. Body weight, animal behavior, and food and fluid consumption were monitored weekly for signs of weight loss, lethargy, or decreased consumption that might indicate intestinal obstruction or anemia. Mice were checked routinely for any other abnormalities. After 12 weeks, all mice were killed by CO2 asphyxiation, blood was collected by heart puncture, and serum was separated by centrifugation and stored at -80°C until further analysis. This point in time was chosen to minimize the risk of intercurrent mortality caused by severe progressive anemia, rectal prolapse, or intestinal obstruction, which usually occurs among Min mice at older than 20 weeks of age. After necropsy, the entire intestinal tract was harvested, flushed with 0.9% NaCl, and opened longitudinally from the esophagus to the distal rectum. The tissue was flattened on filter paper to expose the tumors and briefly frozen on dry ice to aid visual scoring of tumors. The number, location, and size of visible tumors in the entire intestine were determined under a dissection microscope (x5). All tumors were scored and subdivided by location (duodenal, jejunal, and ileum and colon) and size (>2, 1–2, or <1 mm in diameter). This procedure was completed by two individuals who were blinded to the experimental group and the genetic status of the mice. Colonic and other SI tumors that required further histopathologic evaluation were fixed in 10% neutral-buffered formalin, embedded in paraffin blocks, and processed by routine hematoxylin and eosin staining. In addition, multiple samples of tumors from the small intestines were harvested and stored in liquid nitrogen for analysis of COX-2 expression levels.
Assessment of Serum Triglycerides and Packed Cell Volume
Triglycerides were quantified in the nonhemolyzed serum using Infinity Triglycerides Liquid Stable Reagent (Thermo Scientific, Middletown, VA) as per the manufacturer's instructions. For packed cell volume (PCV)/hematocrit measurement, blood was sampled by cardiac puncture with a 21-gauge needle attached to a 1-ml heparinized syringe and dispensed into a plastic microfuge tube on ice. Microhematocrit tubes containing ammonium heparin were then placed in the microfuge tube and centrifuged in a hematocrit centrifuge for 5 minutes.
Lipid Extraction, Methylation, and Gas Chromatography
Lipid extraction was performed by the addition of chloroform-methanol (2:1) to partition the lipid and nonlipid constituents from biopsy samples. This method was previously described by Bligh and Dyer [15]. Lipid esters were then methylated by adding anhydrous methanol in the presence of potassium hydroxide after drying down the lipid extract. Methyl esters were finally solubilized in hexane. Two microliters of the esterified lipid sample was separated by gas chromatography (GC2010; Shimadzu, Columbia, MD), and the relative abundance (expressed as percentages) of individual lipid constituents was determined within each sample. Standards containing known amounts of pure fatty acids were run under the same conditions and retention times of the lipid samples were matched with those of the standards.
Histopathologic Analyses
After the intestines were scored, a segment of the small intestine (beginning at the jejunum) and colon was fixed in 10% formalin for 24 hours and then Swiss rolled and paraffin embedded for pathologic analysis.
Immunohistochemistry
To evaluate the effect of bexarotene, we assessed the PCNA expression in intestinal tumor tissue sections along with RXR-α expression by immunohistochemistry, as described. Briefly, paraffin sections were deparaffinized in xylene, rehydrated through graded ethanol solutions to distilled water, and washed in phosphate-buffered saline (PBS). Antigen retrieval was carried out by heating sections in 0.01 M citrate buffer (pH 6) for 30 minutes in a boiling water bath. Endogenous peroxidase activity was quenched by incubating in 3% H2O2 in PBS for 5 minutes. Nonspecific binding sites were blocked using protein block for 20 minutes. Sections were then incubated overnight at 4°C with 1:500 dilutions of rabbit monoclonal antibody against PCNA and RXR-α (Santa Cruz Biotechnology). After several washes with PBS, the slides were incubated with secondary antibody for PCNA and RXR-α for 2 hours. The color reaction was developed with 3,3′diaminobenzidine, according to the manufacturer's instructions given in the kit supplied by Zymed Laboratories (Camarillo, CA). Substituted nonimmune rabbit immunoglobulins for primary antibodies were used as negative controls. Scoring using light microscopy at x400 magnification was performed by two investigators blinded to the identity of the samples. Cells with brown nuclei were considered positive. The proliferation index was determined by dividing the number of positive cells per polyp (upper, middle, and lower) and multiplying by 100.
RXR-α, COX-2, and Cyclin D1 Messenger RNA Expression by Reverse Transcription-Polymerase Chain Reaction
Total RNA from tumor samples was extracted using ToTALLY RNA Kit for isolation of total cellular RNA (Ambion, Foster City, CA) as per the manufacturer's instructions. Equal quantities of DNA-free RNA were used for reverse transcription reactions for making complementary DNA using SuperScript reverse transcriptase (Invitrogen, Grand Island, NY). Polymerase chain reaction (PCR) was performed for RXR-α, COX-2, and cyclin D1 using the following conditions: COX-2 denaturation at 94°C for 2 minutes, followed by 35 cycles at 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 1 minute. Oligonucleotide primer sequences used were as follows: 5′-CCTGTGCCTGATGATTGC-3′ (sense) and 5′-CGGTGAAACTCTGGCTAG-3′ (antisense). RXR-α denaturation was carried out at 94°C for 5 minutes, followed by 35 cycles at 94°C for 45 seconds, 60°C for 20 seconds, and 72°C for 1 minute. Oligonucleotide primer sequences used were as follows: 5′-CTTTGACAGGGTGCTAACAGAGC-3′ (sense) and 5′-ACGCTTCTAGTGACGCATACACC-3′ (antisense). Cyclin D1 denaturation was done at 94°C for 3 minutes, followed by 35 cycles at 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. The oligonucleotide primer sequences used for the cyclin D1 gene were as follows: 5′-ATGGAACACCAGCTCCTGTG-3′ (sense) and 5′-ACCTCCAGCATCCAGGTGGC-3′ (antisense). PCR was done using the Taq polymera se Master Mix (Qiagen, Valencia, CA). The PCR products were visualized and photographed under UV illumination.
Inflammatory Cytokines Assay
Determination of inflammatory cytokine levels in serum was evaluated by enzyme-linked immunosorbent assay (ELISA; SA Biosciences) as per the manufacturer's instruction. The mouse Inflammatory Cytokines and Chemokines Multi-Analyte ELISArray Kit analyzes a panel of 12 proinflammatory cytokines in serum all at once using an ELISA protocol under uniform conditions. The cytokines and chemokines included in this array are interleukin (IL) 1A, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-17A, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), granulocyte-colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Results are expressed as nanograms per milliliter of serum. Determination was carried out in triplicates from each sample.
Sample Size and Statistical Analyses
A least-squares regression statistical analysis (power α = 0.8; traditional 0.05 level test) was applied, using approximately 0.7 to 1.8 (mean) as an estimate for colon tumor multiplicity in ApcMin/+ mice based on previous studies [16] and estimating a approximately 25% to 75% decrease in SI polyps with bexarotene based on studies in other tumor types in other models [17]. For the tumor assay, a minimum of eight mice per group was determined to be adequate to identify a difference between control and treated mice of more than 25%. For the cellular and molecular outcome parameters, a sample size of three to six (depending on marker variability) per treatment was calculated to be adequate to produce effects that are statistically distinguishable. All results are expressed as means ± SE. Differences in body weights were analyzed by analysis of variance, and differences in tumor multiplicity and volume were determined by Student's t test. Differences were considered significant at the P < .05 level. All statistical analysis was performed in GraphPad Prism Software 5.0 (GraphPad Software, Inc, San Diego, CA).
Results
MTD and Overt Toxicity of Bexarotene
Wild-type mice fed 400 and 800 ppm of bexarotene showed a retardation of body weight gain. Notably, mice fed 800 ppm of bexarotene had only 50% survival (Figure 1B), and the remaining mice showed more than 25% body weight loss. Although mice fed 400 ppm of bexarotene showed body weight loss, this dose had no effect on the survival rates. In addition, both 400- and 800-ppm diets caused significant liver toxicity. Mice fed 200 ppm or less bexarotene showed no change in body weight or any observable toxicity by histopathologic analysis. This MTD bioassay suggests that the bexarotene MTD is more than 200 ppm or less than 400 ppm. Based on these studies, we selected 200 ppm of bexarotene as the highest dose for efficacy studies.
Efficacy and Toxicity of 200 ppm of Bexarotene in Male ApcMin/+ Mice
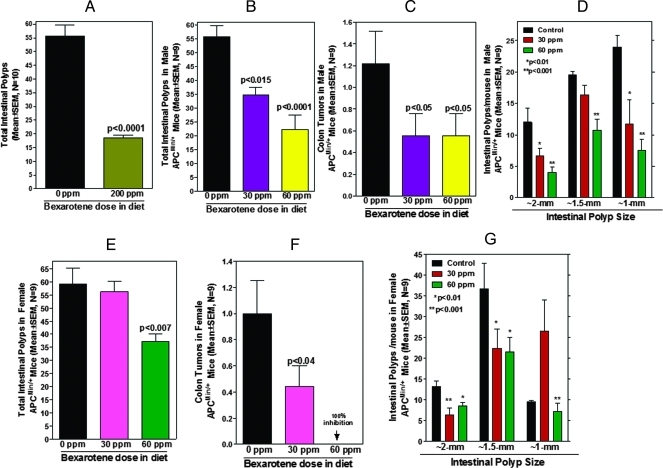
ApcMin/+ mice fed 200 ppm of bexarotene were observed to lose weight similarly to ApcMin/+ mice fed the control diet (Figure 1C). In contrast to wild-type mice (MTD assay), male ApcMin/+ mice fed 200 ppm of bexarotene showed significant toxicity and had approximately 50% survival by the end of the 12 weeks of bexarotene exposure. Despite the toxicity, the chemopreventive efficacy of 200 ppm of bexarotene was significant, with 67% inhibition of total SI polyps (55.7 tumors in control vs 18.6 tumors in treated mice, P < .0001; Figure 2A). Based on this information, we selected nontoxic doses of bexarotene (30 and 60 ppm) for further efficacy studies in both male and female ApcMin/+ mice.
Figure 2.
(A) Inhibition of total SI polyp formation in ApcMin/+ male mice by 200 ppm of bexarotene. Data are means ± SE of nine animals per treatment group. The control and treated groups are significantly different from one another (P < .0001). (B) Inhibition of total SI polyp formation in ApcMin/+ male mice by bexarotene (30 and 60 ppm). Data are means ± SE of nine animals per treatment group. Control and treated groups are significantly different from one another (P < .015 or P < .0001). (C) Average number of colon tumors per mouse in control and treated ApcMin/+ male mice. A significant (P < .05) inhibition of colon tumors was observed with both low-dose and high-dose bexarotene. Data are means ± SE of nine animals per treatment group. (D) Tumor sizes in the small intestine of ApcMin/+ male mice. Intestines were divided into sections and examined under a stereomicroscope; the size of the polyps was determined. Data are given as means ± SE of nine animals per treatment condition. Tumors greater than 2 mm in size and those 1 mm in size were suppressed/inhibited approximately 75% in high-dose bexarotene-treated mice. (E) Inhibition of total SI polyp formation in ApcMin/+ female mice by bexarotene (30 and 60 ppm). Data are means ± SE of nine animals per treatment condition. Control and treated groups are significantly different from one another (P < .007). (F) Average number of colon tumors per mouse in control and treated ApcMin/+ female mice. A significant (P < .04) inhibition of colon tumors was observed with low-dose bexarotene and 100% inhibition was observed with a high dose. Data are means ± SE of nine animals per treatment condition. (G) Tumor sizes in the small intestine of ApcMin/+ male mice. Intestines were divided into sections and examined under a stereomicroscope; the size of the polyps was determined. Data are given as means ± SE of nine animals per treatment condition. The numbers of tumors greater than 2 mm and those approximately 1 mm in diameter were decreased ∼75% in high-dose bexarotene-treated mice.
Efficacy of 30 and 60 ppm of Bexarotene in Male and Female ApcMin/+ Mice
ApcMin/+ mice fed 30 or 60 ppm of bexarotene showed somewhat higher body weights with no noticeable signs of toxicity compared to control ApcMin/+ mice. Also, mice fed 30 or 60 ppm of bexarotene diets showed less anemia than those fed a control diet. Statistically significant (P < .05) differences in body weights were observed between the dietary groups (Figure 1, D and E). As expected, control diet-fed ApcMin/+ mice began to lose body weight at approximately 12 weeks of age because of intestinal obstruction and progressive anemia. To assess the effects of bexarotene on intestinal tumor formation in APC mutant transgenic mice, we examined the polyp number and size in different regions of the small intestine and colon. Dietary administration of 30 and 60 ppm of bexarotene significantly suppressed total SI polyps in a dose-dependent manner in ApcMin/+ male and female mice, by 38.2% to 59.9% (P < .015 to P < .0001) or 8.5% to 36.9%, respectively (P < .007; Figure 2, B and E). The numbers of large (>2 mm) and small polyps were more significantly decreased with the high-dose treatment in male mice (Figure 2D). Male mice fed 30 or 60 ppm of bexarotene had 44% or more than 60% fewer polyps, respectively, with a diameter greater than 2 mm and 54% or 68% fewer polyps with a diameter of approximately 1 mm, respectively. Untreated female mice had fewer small polyps of approximately 1 mm than untreated male mice (∼8 vs 23 per mouse) but had similar numbers of intermediate or large polyps. In female, mice fed 60 ppm of bexarotene had approximately 35% suppression of polyps greater than 2 mm compared with control mice and 26% fewer polyps of approximately 1 mm (Figure 2G). In the female mice, 30 ppm of bexarotene caused a decrease in numbers of the larger polyps accompanied by an increase in numbers of the small approximately 1-mm polyps. This maybe due to inhibition of proliferation that halted the small polyps to increase in size by bexarotene treatment. Male mice treated with bexarotene (30 or 60 ppm) had a 50% reduction in the number of colonic tumors (control 1.22 vs treated 0.55 tumors per mouse, P < .05; Figure 2C). Female mice treated with 30 and 60 ppm of bexarotene showed approximately 50% and 100% reduction, respectively, in the colonic tumors (Figure 2F).
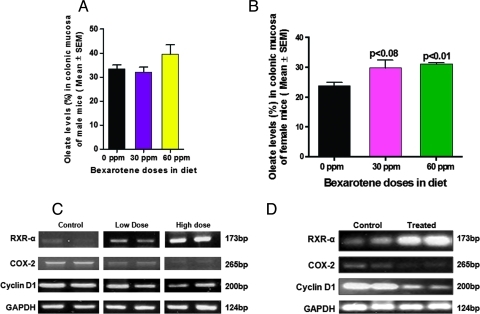
RXR-Mediated Increase in Oleate in Colonic Mucosa and Down-regulation of COX-2 Messenger RNA Expression in Response to Bexarotene
Because RXRs forms heterodimers with liver X receptor (LXR) receptors, which are known to regulate the levels of fatty acids in enterocytes, we performed an experiment to determine whether bexarotene treatment had any effect on fatty acids in colonic mucosa. Subtoxic doses of bexarotene caused in an increase in metabolically active oleate, an n-9 monounsaturated fatty acid, in colonic mucosa from male and female C57BL/6J mice. In females, both 30 and 60 ppm of bexarotene caused a significant (P < .08 and P < .01, respectively) increase in oleate with not much difference in other fatty acids (Figure 3B). In males, some increase in oleate was observed at 60 ppm, but it did not reach statistical significance (Figure 3A). The increase in oleate was associated with a concomitant reduction in COX-2 messenger RNA (mRNA) concentrations (Figure 3, C and D). These results correlate well with the inhibition of colon tumors in female ApcMin/+ mice.
Figure 3.
(A) Effects of bexarotene on oleate uptake in intestinal mucosa of male mice. A modest uptake of oleate was observed in colonic mucosa from male mice treated with 60 but not 30 ppm of bexarotene compared with that in the untreated animals. (B) Effects of bexarotene on oleate uptake in intestinal mucosa of female mice. A significant uptake of oleate (P < .008 to P < .01) was observed in the colonic mucosa from female mice mouse treated with 30 or 60 ppm of bexarotene compared with that in untreated animals. (C) Modulatory effects of bexarotene on RXR-α, COX-2, and cyclin D1 mRNA expression in SI polyps of treated and untreated ApcMin/+ male mice. A significant dose-dependent increased expression of RXR-α mRNA was observed on bexarotene treatment (30 and 60 ppm). A significant dose-dependent suppression of COX-2 and cyclin D1 mRNA was observed on bexarotene treatment in ApcMin/+ mice. (D) Modulatory effects of bexarotene on RXR-α, COX-2, and cyclin D1 mRNA expression in colon tumors of treated and untreated ApcMin/+ female mice. A significant dose-dependent increase in expression of RXR-α mRNA was observed on bexarotene treatment. A significant dose-dependent suppression of COX-2 and cyclin D1 mRNA was observed on bexarotene treatment (30 ppm) in ApcMin/+ mice.
Modulation of RXR-α in Intestinal Tumors
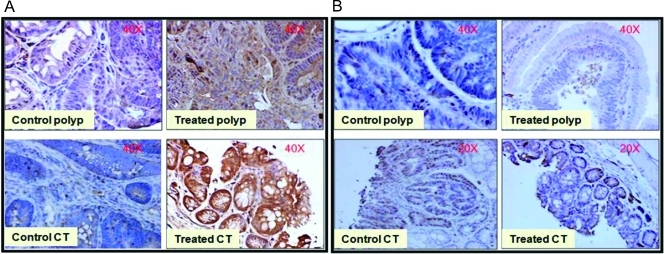
Expression levels of RXR-α, which are important in various signaling pathways of antiproliferation and apoptosis, were analyzed in SI polyps by reverse transcription-PCR and immunohistochemistry (Figures 3 and 4). A dose-dependent increase in RXR-α mRNA expression was observed in bexarotene-treated intestinal polyps (Figure 3, C and D). Dietary administration of 60 ppm of bexarotene resulted in a significant increase in RXR-α protein expression in intestinal polyps compared with control polyps as observed with immunostaining (Figure 4A). These results correlate with the down-regulation of COX-2 and uptake of oleate with bexarotene treatment (Figure 3).
Figure 4.
(A) Increase in RXR-α protein expression in the nucleus of bexarotene-treated intestinal tumors. Serial paraffin sections of intestinal tumors from ApcMin/+ were subjected to immunohistochemical analysis using an anti-RXR-α polyclonal antibody. Marked accumulation of RXR-α is clear in the nucleus of tumors in treated animals compared with that in tumors from control animals. Top panel is representative of SI polyps; bottom panel is representative of colon tumors. (B) Serial paraffin sections of small intestine and colon from ApcMin/+ mice were subjected to immunohistochemical analysis using an anti-PCNA monoclonal antibody. Intense positive staining for PCNA in the tumor region of control animals was observed. Staining for PCNA was decreased in the nuclei of tumors from bexarotene-treated animals. Top panel represents SI polyps; bottom panel represents colon tumors. Original magnifications, x20.
Effect of Dietary Administration of Bexarotene on SI Polyp Proliferative Index (PCNA) and Cyclin D1 mRNA Expression Levels
Figure 4 (A and B) demonstrates the effects of bexarotene on tumor cell proliferation as measured by PCNA overexpression. Qualitative microscopic examination of PCNA-stained sections showed a substantial decrease in PCNA-positive cells in the SI polyps from bexarotene-fed mice compared with the untreated controls. Bexarotene at 60 ppm significantly suppressed proliferation in the SI polyps compared with control polyps (Figure 4B). The quantification of PCNA staining (not shown in the figure) revealed 55.1 ± 5.4 (mean ± SEM) PCNA-positive cells in control polyps compared with 20.2 ± 4.4 (mean ± SEM) PCNA-positive cells in bexarotene-treated SI polyps, accounting for a decrease in the proliferation index by approximately 60%. The quantification of PCNA staining showed 60.6 ± 3.3 (mean ± SEM) PCNA-positive cells in control tumors compared with 30 ± 2.4 (mean ± SEM) PCNA-positive cells in colon tumors from bexarotene-treated mice, accounting for a decrease in the proliferative index by approximately 50%. Similarly, the expression of cyclin D1 mRNA levels showed a strong decrease in intestinal polyps of bexarotene-treated mice compared with untreated ApcMin/+ mice (Figure 3, C and D). The reduced expression of PCNA and cyclin D1 suggests a decreased tumor cell proliferation in treated mice.
Bexarotene Decreases Serum Triglycerides and Increases PCV
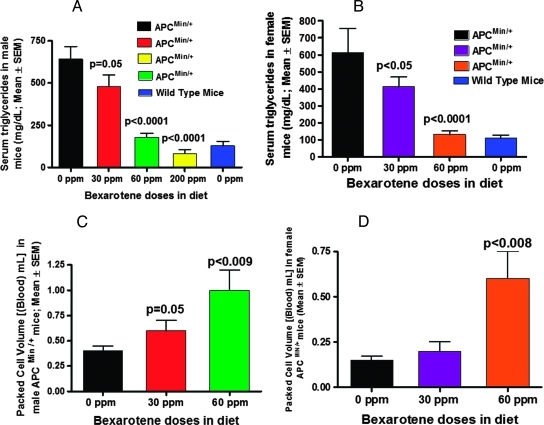
Untreated ApcMin/+ mice had a significant increase in serum triglyceride (TG) compared with control wild-type C57BL/6J mice. Both male and female ApcMin/+ mice fed bexarotene diets showed a dose-dependent suppression in serum TGs (25.2%, P < .01 or 32.9%, P < .001 for male and female, respectively, with 30 ppm; and 72%, P < .0001 or 90%, P < .0001 with 60 ppm; and 87% P < .0001 for male ApcMin/+ mice with 200 ppm of bexarotene; Figure 5, A and B). TG levels of ApcMin/+ mice fed high dose bexarotene were comparable to those of untreated wild-type mice (Figure 5, A and B). Also, we observed a dose-dependent increase in PCV in blood from bexarotene-fed male and female ApcMin/+ mice compared with control ApcMin/+ mice (Figure 5, C and D).
Figure 5.
(A) Dose-dependent decrease in TGs in male ApcMin/+ mice treated with bexarotene. The TG level in bexarotene-treated ApcMin/+ mice was significantly lower than that in the untreated animals (P = .005 to P < .0001). In the 200-ppm bexarotene-treated group, the TG levels were reduced to approximately 80% of levels observed in wild-type mice. (B) Dose-dependent decrease in TGs in female ApcMin/+ mice treated with bexarotene. The decrease to 40% to 85% of control was significant at P < .05 to P <. 0001. (C) PCVs of ApcMin/+ male control versus treated animals. A significant increase in PCV was observed in male mice treated with high-dose (60 ppm) bexarotene. (D) PCVs of ApcMin/+ female control versus treated animals. A significant increase in PCV was observed in female mice treated with 60 ppm of bexarotene.
Modulation of Inflammatory Cytokines
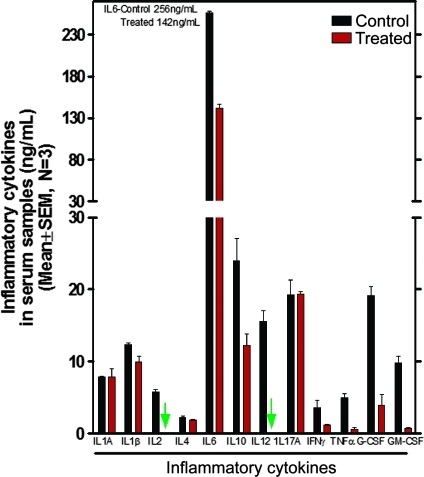
Expression levels of inflammatory or COX-2-inducing cytokines (IL-1β, IL-2, IL-6, IL-10, IL-12, IFN-γ, TNF-α, G-CSF, GM-CSF) are significantly (P < .05 to P < .0001) decreased in serum from bexarotene-treated (60 ppm) animals (Figure 6). GM-CSF was reduced by approximately 95%, and IL-2 and IL-12 were completely inhibited. No significant difference was observed in the expression levels of anti-inflammatory cytokine IL-1A or in IL-17A (Figure 6).
Figure 6.
Effect of bexarotene (60 ppm) on inflammatory cytokines in serum samples from treated and untreated ApcMin/+ mice as analyzed by ELISA. All assayed cytokines except IL-1A and IL-17 were decreased by bexarotene. IL-2 and IL-12 were undetectable in sera from treated animals.
Discussion
In this study, we demonstrated that a selective RXR agonist bexarotene inhibited intestinal tumorigenesis in ApcMin/+ mice, a model for human familial adenomatous polyposis. Bexarotene treatment caused a decrease in serum TGs and enhanced uptake of oleate, and caused tumor growth inhibition, suggesting that the RXR pathway is an important target for colon cancer prevention. The suppressive effect of bexarotene on the development of colonic tumors also was closely correlated with a decrease in proinflammatory cytokines. Although the value of the ApcMin/+ mouse as model for predicting efficacy of chemopreventive agents in humans is a subject of debate, results obtained in this model [18,19] did forecast the efficacy of COX inhibitors, such as sulindac and celecoxib, to retard adenoma recurrence in humans [20,21].
The expression of proliferation marker PCNA is significantly reduced in the treated polyp, with no effect on normal crypt (Figure 4). This could be due to tumor-specific suppression of proliferation by bexarotene, and it is well known that tumor cells that proliferate much faster contain very high levels of PCNA compared with normal crypt cells. Additional experiments would be needed to determine whether and to what extent bexarotene influences the normal crypt cell PCNA levels. The chemopreventive effect of bexarotene in the ApcMin/+ mouse was, to a certain extent, dose related. Bexarotene at 200 ppm, which reduced SI polyp number by approximately 75%, could not extend mouse survival because of significant toxicity; after 12 weeks of feeding, only 50% of the mice survived. However, lower subtoxic doses of bexarotene (30 and 60 ppm) significantly inhibited SI tumorigenesis by 38.2% and 59.9% (P < .015 to P < .0001) or 8.5% and 36.9% (P < .007), for male and female mice, respectively. It is intriguing that bexarotene showed better efficacy against SI tumors in males compared to female mice and, in contrast, better efficacy against colon tumors in female than in male mice. The reasons for these differences need further investigation. However, it is possible that hormonal differences or differences in bioavailability of bexarotene in male versus female mice might have played a role. The differences between male and female mice in the dose-response relationship suggest that the bexarotene dose must be optimized separately for males and females for optimal colon cancer chemoprevention. The recommended dosage of bexarotene is 300 mg/m2 per day clinically (cancer patients). Data reported by others suggest that dosages of retinoids up to 10 and 50 mg/kg per day used in mice (female MMTV-erbB2 transgenic mice) can achieve peak plasma levels that are similar to those from human trials using 200 to 400 mg/m2 per day [22–24], which is equivalent to approximately 6 to 10 mg/kg body weight per day. Thus, 60 ppm (∼10 mg/kg body weight per day) of bexarotene used in the present study should be very close to the clinical dosage (by kilogram body weight); however, it is much lower than the dosage used by others in previous studies in mice.
The regulation of growth and differentiation by rexinoids results from direct and indirect effects on gene expression mediated by the RAR and RXR nuclear receptors. RXRs form heterodimers with LXR receptors and regulate the levels of fatty acids [25]. Hence, in the presence of bexarotene, the increased expression of RXR and its interaction with LXR might have caused the increase in oleate in colonic mucosa. Kim et al. [26] showed that the expression of several rexinoid-regulated biomarkers is modulated in vivo in mammary glands from mice treated with bexarotene. Microarray analysis showed up-regulation of stearoyl-CoA desaturase (regulatory enzyme involved in the synthesis of the monounsaturated fatty acids palmitoleate and oleate) and down-regulation of COX-2. Bexarotene binding to RXR may induce the absorption of oleate, which affects the expression of COX-2, an important gene in colon tumor development. COX-2 is induced by a variety of mitogenic and inflammatory stimuli. It is well known that COX-2 expression is markedly elevated in colorectal cancer in both humans and rodents, and COX-2 plays an important role in cancer cell proliferation [27].
The COX-2 molecule could be a second messenger for transcriptional action of the fatty acid oleate as reported by Duplus et al. [28]. In the current study, COX-2 levels in colon polyps from ApcMin/+ mice were much greater than those in wild-type mice. Treatment with bexarotene increased oleate levels in colon mucosa, and this increase somewhat correlated with reduced colon tumors. Oleate is reported to lower cholesterol levels, thereby reducing atherosclerosis and the risk of cardiovascular disease. It reduces insulin resistance, thereby improving glucose maintenance, improves immune function, and provides protection against certain types of cancer [29]. Our findings also suggest that RXR may regulate COX-2 and, thus, the production of prostaglandins, which are known to play a key role in colon tumor development.
Epidemiologically, a positive association between hypertriglyceridemia and colorectal cancer development has been reported [30]. Also, an experimental rodent study showed a positive effect of serum TG on the development of aberrant crypt foci [31]. Niho et al. [32–34] and our laboratory [16] recently showed that a hyperlipidemic state is associated with intestinal polyp formation in Apc-deficient mice, and this was attributed mainly to down-regulation of lipoprotein lipase in Apc mutant mice. They observed that peroxisome proliferator-activated receptor ligands and lipoprotein lipase activator reduce serum TG levels and suppress intestinal polyp formation in Apc-deficient mice [32–34]. Other laboratory investigations also have shown that RXR-selective agonists increase the transcription of apolipoprotein C-III, possibly interfering with plasma lipid clearing [35]. Lipid metabolism and the intracellular transport of bioactive molecules are critical in the modulation of proliferation through interactions with nuclear receptors. Our finding that administration of bexarotene improved hyperlipidemia and suppressed intestinal polyp formation in the ApcMin/+ mice is consistent with these other findings. ApcMin/+ mice showed approximately six-fold higher serum TG levels compared to wild-type control mice, but both male and female ApcMin/+ mice fed bexarotene showed a dose-dependent suppression of serum TG levels. Importantly, decreased serum TG levels were highly correlated with decreased intestinal polyp number and tumor size induced by bexarotene.
The ability of bexarotene to interfere with tumorigenesis also could be due to the inhibition of inflammatory cytokines. We observed inhibition of IL-2, IL-12, IFN-γ, and other proinflammatory cytokines. Recent studies show that retinoids inhibit the production of several inflammatory cytokines by activated macrophages through functional interactions between their receptors (RXR and RAR) and nuclear factor-κB, a crucial transcription factor for IL-12 gene expression [36]. Furthermore, IL-1β has been shown to enhance the production of vascular endothelial growth factor through IL-2, which was shown to induce angiogenesis in colon cancer cells [37]. TNF-α and IL-1β are key cytokines involved in inflammation, immunity, and cellular organization [38]. In this study, the expression of TNFα and IL-1β proteins was substantially upregulated in the serum of control ApcMin/+ mice. Bexarotene treatment reduced the expression of TNFα and IL-1β protein in the serum of ApcMin/+ mice. Because the induction of COX-2 is regulated by TNFα and IL-1β, these cytokine effects may play important roles in tumor promotion. Thus, the suppression of TNFα and IL-1β expression by bexarotene may contribute to the low frequency of large tumors (size ∼1.5-2 mm) as observed in this study.
In conclusion, the dietary administration of bexarotene could effectively suppress intestinal tumor development in the ApcMin/+ mice by affecting multiple factors, including up-regulation of RXR-α, and by decreasing COX-2, inflammatory cytokines, and hyperlipidemia, all of which are involved in colon carcinogenesis. Collectively, these data suggest a role of bexarotene in chemoprevention of intestinal cancers.
Acknowledgments
The authors thank Julie Sando for her valuable suggestions and for editing the article. The authors also thank the OUHSC Rodent Barrier Facility staff for the valuable support in bioassay studies.
Footnotes
This work was supported by Public Health Service/National Institutes of Health, National Cancer Institute (NO1-CN 53300 and R01CA-109247).
References
- 1.American Cancer Society. [October 20, 2011]. Available at: http://www. cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/colorectal-cancer-key-statistics.
- 2.Naveena BJ, Indranie C, Altaf M, Steele VE, Rao CV. β-Ionone inhibits colonic aberrant crypt foci in rats, suppresses cell growth and induces retinoid X receptor a in human colon cancer cells. Mol Cancer Ther. 2008;7(1):1–10. doi: 10.1158/1535-7163.MCT-07-0529. [DOI] [PubMed] [Google Scholar]
- 3.Reddy BS, Hirose Y, Lubet R, Steele VE, Kelloff G, Paulson S, Seibert K, Rao CV. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60(2):293–297. [PubMed] [Google Scholar]
- 4.Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- 5.Kelloff GJ, Fay JR, Steele VE, Lubet RA, Boone CW, Crowell JA, Sigman CC. Epidermal growth factor receptor tyrosine kinase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1996;5(8):657–666. [PubMed] [Google Scholar]
- 6.Zheng Y, Kramer PM, Olson G, Lubet RA, Steele VE, Kelloff GJ, Pereira MA. Prevention of by retinoids of azoxymethane-induced tumors and aberrant crypt foci and their modulation of cell proliferation in the colon of rats. Carcinogenesis. 1997;18:2119–2125. doi: 10.1093/carcin/18.11.2119. [DOI] [PubMed] [Google Scholar]
- 7.Shalita AR. Mucocutaneous and systemic toxicity of retinoids: monitoring and management. Dermatologica. 1987;175:151–157. doi: 10.1159/000248878. [DOI] [PubMed] [Google Scholar]
- 8.Gottardis MM, Bischoff ED, Shirley MA, Wagoner MA, Lamph WW, Heyman RA. Chemoprevention of mammary carcinoma by LGD1069 (Targretin): an RXR-selective ligand. Cancer Res. 1996;56:5566–5570. [PubMed] [Google Scholar]
- 9.Easwaran V, Pishvaian M, Salimuddin, Byers S. Cross-regulation of β-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]
- 10.Bugge TH, Pohl J, Lonnoy O, Stunnenberg HG. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992;11:1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froeschlé A, Carnac G, Alric S, Montarras D, Pinset C, Rochette-Egly C, Bonnieu A. RXR alpha is essential for mediating the all-trans retinoic acid-induced growth arrest of C2 myogenic cells. Oncogene. 1996;12:411–421. [PubMed] [Google Scholar]
- 12.Dahiya R, Park HD, Cusick J, Vessella RL, Fournier G, Narayan P. Inhibition of tumorigenic potential and prostate-specific antigen expression in LNCaP human prostate cancer cell line by 13-cis-retinoic acid. Int J Cancer. 1994;59:126–132. doi: 10.1002/ijc.2910590122. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Zhang D, Zhang ZP, Soprano DR, Soprano KJ. Critical role of both retinoid nuclear receptors and retinoid-X receptors in mediating growth inhibition of ovarian cancer cells by all-trans retinoic acid. Oncogene. 1998;17:2839–2849. doi: 10.1038/sj.onc.1202208. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal C, Chandraratna RA, Teng M, Nagpal S, Rorke EA, Eckert RL. Differential regulation of human ectocervical epithelial cell line proliferation and differentiation by retinoid X receptor- and retinoic acid receptor-specific retinoids. Cell Growth Differ. 1996;7:521–530. [PubMed] [Google Scholar]
- 15.Bligh EG, Dyer WJ. A rapid of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed A, Janakiram NB, Li Q, Choi CI, Zhang Y, Vernon ES, Rao CV. Chemoprevention of colon and small intestinal tumorigenesis in ApcMin/+ mice by licofelone, a novel dual 5-LOX/COX inhibitor: potential implications for human colon cancer prevention. Cancer Prev Res. 2011;4:2015–2026. doi: 10.1158/1940-6207.CAPR-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Zhang Y, Hill J, Kim H-T, Shen Q, Bissonnette RP, Lamph WW, Brown PH. The rexinoid, bexarotene, prevents the development of premalignant lesions in MMTV-erbB2 mice. Br J Cancer. 2008;98:1380–1388. doi: 10.1038/sj.bjc.6604320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ, et al. Cyclooxygenase-2 over expression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- 19.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the Min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–5044. [PubMed] [Google Scholar]
- 20.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 21.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 22.Wu K, Kim HT, Rodriquez JL, Hilsenbeck SG, Mohsin SK, Xu XC, Lamph WW, Kuhn JG, Green JE, Brown PH. Suppression of mammary tumorigenesis in transgenicmice bythe RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002;11:467–474. [PubMed] [Google Scholar]
- 23.Howell SR, Shirley MA, Grese TA, Neel DA, Wells KE, Ulm EH. Bexarotene metabolism in rat, dog, and human, synthesis of oxidative metabolites, and in vitro activity at retinoid receptors. Drug Metab Dispos. 2001;29:990–998. [PubMed] [Google Scholar]
- 24.Rizvi NA, Marshall JL, Dahut W, Ness E, Truglia JA, Loewen G, Gill GM, Ulm EH, Geiser R, Jaunakais D, et al. A phase I study of LGD1069 in adults with advanced cancer. Clin Cancer Res. 1999;5:1658–1664. [PubMed] [Google Scholar]
- 25.Sanal MG. The blind men “see” the elephant—the many faces of fatty liver disease. World J Gastroenterol. 2008;14(6):831–844. doi: 10.3748/wjg.14.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H-T, Kong G, DeNardo D, Li Y, Uray I, Pal S, Mohsin S, Hilsenbeck SG, Bissonnette R, Lamph WW, et al. Identification of biomarkers modulated by the rexinoid LGD1069 (bexarotene) in human breast cells using oligonucleotide arrays. Cancer Res. 2006;66:12009–12018. doi: 10.1158/0008-5472.CAN-05-2515. [DOI] [PubMed] [Google Scholar]
- 27.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Duplus E, Glorian M, Forest C. Fatty acid regulation of gene transcription. J Biol Chem. 2000;275:30749–30752. doi: 10.1074/jbc.R000015200. [DOI] [PubMed] [Google Scholar]
- 29.Nicolosi RJ, Woolfrey B, Wilson TA, Scollin P, Handelman G, Fisher R. Decreased aortic early atherosclerosis and associated risk factors in hypercholesterolemic hamsters fed a high- or mid-oleic acid oil compared to a high-linoleic acid oil. J Nutr Biochem. 2004;15:540–547. doi: 10.1016/j.jnutbio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.McKeown-Eyssen GE. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev. 1994;3:687–695. [PubMed] [Google Scholar]
- 31.Koohestani N, Chia MC, Pham NA, Tran TT, Minkin S, Wolever TM, Bruce WR. Aberrant crypt focus promotion and glucose intolerance: correlation in the rat across diets differing in fat, n-3 fatty acids and energy. Carcinogenesis. 1998;19:1679–1684. doi: 10.1093/carcin/19.9.1679. [DOI] [PubMed] [Google Scholar]
- 32.Niho N, Takahashi M, Kitamura T, Shoji Y, Itoh M, Noda T, Sugimura T, Wakabayashi K. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090–6095. [PubMed] [Google Scholar]
- 33.Niho N, Takahashi M, Shoji Y, Takeuchi Y, Matsubara S, Sugimura T, Wakabayashi K. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPAR γ ligand. Cancer Sci. 2003;94:960–964. doi: 10.1111/j.1349-7006.2003.tb01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niho N, Mutoh M, Takahashi M, Tsutsumi K, Sugimura T, Wakabayashi K. Concurrent suppression of hyperlipidemia and intestinal polyp formation by NO-1886, increasing lipoprotein lipase activity in Min mice. Proc Natl Acad Sci USA. 2005;102:2970–2974. doi: 10.1073/pnas.0500153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vu-dac N, Gervois P, Torra IP, Fruchart JC, Kosykh V, Kooistra T, Princen HM, Dallongeville J, Staels B. Retinoids increase human Apo C-III expression at the transcriptional level via the retinoid X receptor. J Clin Invest. 1998;102(3):625–632. doi: 10.1172/JCI1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Na S, Kang BY, Chung SW, Han S, Ma X, Trinchieri G, Im S, Lee JW, Kim TS. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J Biol Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 37.Akagi Y, Liu W, Xie K, Zebrowski B, Shaheen RM, Ellis LM. Regulation of vascular endothelial growth factor expression in human colon cancer by interleukin-1b. Br J Cancer. 1999;80:1506–1511. doi: 10.1038/sj.bjc.6690553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]