Abstract
Calcium (Ca2+) and phosphate (PO43−) homeostasis are coordinated by systemic and local factors that regulate intestinal absorption, influx and efflux from bone, and kidney excretion and reabsorption of these ions through a complex hormonal network. Traditionally, the parathyroid hormone (PTH)/vitamin D axis provided the conceptual framework to understand mineral metabolism. PTH secreted by the parathyroid gland in response to hypocalcemia functions to maintain serum Ca2+ levels by increasing Ca2+ reabsorption and 1,25-dihydroxyvitamin D [1,25(OH)2D] production by the kidney, enhancing Ca2+ and PO43− intestinal absorption and increasing Ca2+ and PO43− efflux from bone, while maintaining neutral phosphate balance through phosphaturic effects. FGF23 is a recently discovered hormone, predominately produced by osteoblasts/osteocytes, whose major functions are to inhibit renal tubular phosphate reabsorption and suppress circulating 1,25(OH)2D levels by decreasing Cyp27b1-mediated formation and stimulating Cyp24-mediated catabolism of 1,25(OH)2D. FGF23 participates in a new bone/kidney axis that protects the organism from excess vitamin D and coordinates renal PO43− handling with bone mineralization/turnover. Abnormalities of FGF23 production underlie many inherited and acquired disorders of phosphate homeostasis. This review discusses the known and emerging functions of FGF23, its regulation in response to systemic and local signals, as well as the implications of FGF23 in different pathological and physiological contexts.
I. INTRODUCTION
The parathyroid hormone (PTH)-vitamin D axis has provided the basis for our conceptualization of bone and mineral homeostasis, but recent discovery of the fibroblast growth factor (FGF)23 bone-kidney axis regulating vitamin D metabolism and renal phosphate handling have led to new insights into physiology and pathophysiology of mineral metabolism.
Comprehensive reviews of vitamin D metabolism and PTH functions have been published previously in this journal (83). Briefly, the principal function of the PTH-vitamin D axis is to maintain serum calcium levels in a narrow range by stimulating 1,25-dihydroxyvitamin D [1,25(OH)2D] production and decreasing urinary calcium excretion by the kidney. PTH also increases calcium efflux from bone. PTH secretion is predominately regulated by the calcium-sensing receptor (CASR) located in the parathyroid gland, which responds to decrements in serum ionized calcium by increasing the secretion of PTH, an 84-amino acid peptide that targets PTHR1 G protein-coupled receptors that are highly expressed in the renal tubules and osteoblasts/osteocytes in bone. PTH stimulates the production of 1,25(OH)2D in the proximal tubule by increasing CYP27b1 and increases calcium reabsorption in the distal tubule through regulation of TRPV5 (31). In bone, PTH increases calcium and phosphate efflux through stimulation of RANKL by osteoblasts, which in turn stimulates osteoclast-mediated bone resorption. In addition, increased 1,25(OH)2D production by the kidney targets the small intestines to increase absorption of both calcium and phosphate. The combined effects of efflux of calcium from bone, conservation of calcium by the kidney, and increased dietary absorption of calcium restores serum calcium to normal. The increased phosphate efflux from bone and influx from the gastrointestinal track is balanced by PTH effects to decrease renal tubular phosphate reabsorption to maintain neutral phosphate balance.
In contrast, the FGF23-bone-kidney axis is part of newly discovered biological systems linking bone to other organ functions through a complex endocrine network that is integrated with the PTH/vitamin D axis and which plays an equally important role in health and disease. The discovery that osteoblasts and osteocytes are the principal site for FGF23 production and secretion identified bone, not only as the major reservoir for calcium and phosphate, but as an endocrine organ that communicates with other organs involved in mineral homeostasis. FGF23 secreted by bone targets the kidney to regulate renal phosphate handing and vitamin D metabolism (225). The FGF23 bone/kidney axis has at least two physiological functions: 1) to provide a phosphaturic signal emanating from bone to coordinate bone phosphate flux due to alterations in bone turnover and mineralization with renal conservation of phosphate and 2) to provide a counterregulatory hormone to protect the organism from adverse effects of excessive vitamin D exposure by FGF23-mediated suppression of 1,25(OH)2D production and increased catabolism by the kidney. FGF23 may also have other functions to regulate phosphate and parathyroid gland functions, but additional knowledge is needed to fully understand the inconsistent data regarding regulation of FGF23 by phosphate and the physiological importance of a possible PTG-bone axis involving reciprocal regulation of PTH and FGF23.
II. FGF23 ORIGIN AND STRUCTURE
A. The FGF Family
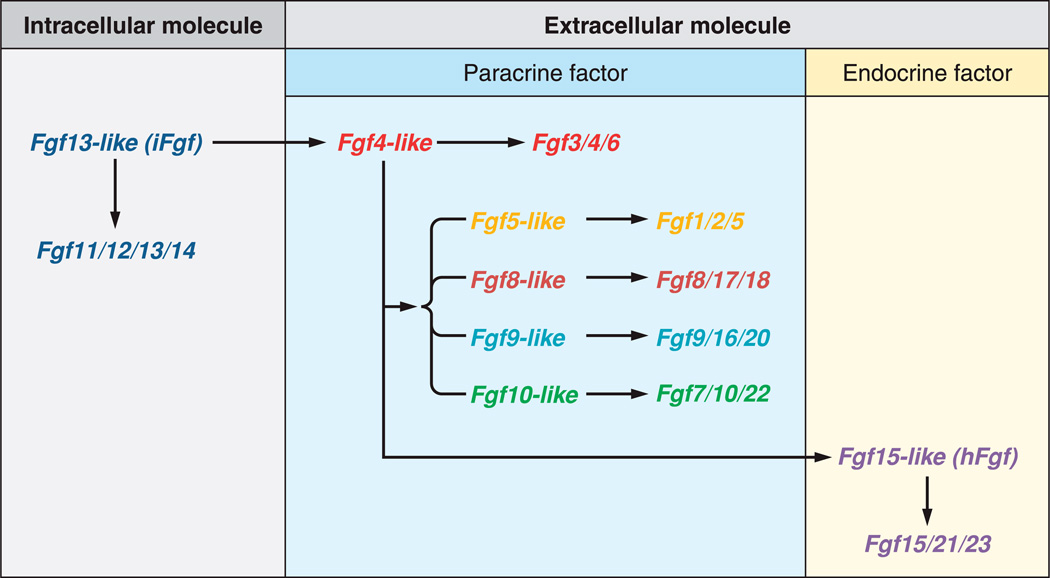
Although FGFs are involved in diverse functions including development, repair, or metabolism, all derive from an Fgf13-like ancestral gene and share a conserved ~120-residue core structural domain with ~30–60% identity which provides the basis for their classification. The human/mouse Fgf gene family comprises 22 members from Fgf1 to Fgf23. Fgf15 and Fgf19 are orthologs in vertebrates (80), with Fgf15 being absent in human and Fgf19 absent in mouse. The FGF family can be divided into seven phylogenetic subfamilies composing three groups according to their action mechanisms (81): the intracellular, the canonical, and the hormone-like Fgf genes (FIGURE 1).
FIGURE. 1.
Functional evolutionary history of ancestors of the mouse Fgf gene family. [From Itoh et al. (75), with permission from John Wiley and Sons.]
The intracellular FGF group includes the Fgf11/12/13/14 subfamily. These FGFs act as intracellular signaling molecules in an FGF receptor (FGFR)-independent manner (52, 223). The canonical FGF group includes the Fgf1/2/5, Fgf3/4/6, Fgf7/10/22, Fgf8/17/18, and Fgf9/16/20 subfamilies. Canonical FGFs mediate their biological responses as extracellular proteins by binding to and activating cell surface tyrosine kinase FGFR with heparin/heparan sulfate as a cofactor. They act as local signaling molecules in an autocrine/paracrine manner (80, 81, 201). The hormone-like or endocrine FGF group includes the Fgf19/21/23 subfamily. In contrast to the canonical FGF group, hormone-like FGFs act systemically as endocrine factors. However, they also mediate their response through FGFR-dependent mechanisms.
B. Hormone-like FGFs: The Endocrine Subfamily
In contrast to intracellular and canonical FGFs identified in invertebrates and vertebrates, hormone-like FGFs, Fgf15/19, Fgf21, and Fgf23, are vertebrate specific. The ancestral Fgf15-like gene of hormone-like FGFs was generated from the ancestral Fgf4-like gene of canonical FGFs by gene duplication early in vertebrate evolution. Later, Fgf15/19, Fgf21, and Fgf23 were generated from the ancestral Fgf15-like gene by genome duplication events. Canonical FGFs have a heparin-binding site necessary for the stable binding of FGFRs and local signaling. In contrast, during their evolution, hormone-like FGFs acquired endocrine functions by reduction of the heparin-binding affinity and presence of a novel COOH terminus that permits activation of FGF receptors in the absence of heparin (79). Specifically, a conformational change occurs mainly in the β10–β12 region containing the residues accounting for the heparin affinity (51). On the one hand, the weak heparin binding affinity of the endocrine FGFs prevents them from being captured in the extracellular matrix and thus allows them to function as circulating endocrine factors. On the other hand, the reduced affinity for heparin/heparin sulfate also prevents direct interaction between endocrine FGFs and FGFRs (232). Instead of heparin, endocrine FGFs require alternate cofactors to mediate their effects through FGFRs. Many tissues express one or more FGFR isoforms that potentially function as receptors for FGFs. Thereby, the expression of the cofactor in a tissue determines the target organ of any endocrine FGF to control metabolism and ensure hormonal specificity (44).
FGF15/19 requires βKlotho as a cofactor (97, 111). Fgf15/19 regulates bile acid metabolism in the liver. There is still controversy regarding the role of βKlotho as the FGF21 cofactor. In vitro data suggested that βKlotho is essential for its activity (153). But this was not demonstrated in vivo: FGF21 signals are still transduced in the absence of βKlotho, βKlotho does not precipitate FGF21, and the FGF21 knockout mice do not reproduce the βKlotho knockout phenotype (203). Fgf21 regulates lipid metabolism in the white adipose tissue. Finally, FGF23 requires the αKlotho cofactor (207). FGF23 regulates serum phosphate and active vitamin D levels. The function of FGF23 and its regulation by local and systemic factors are the focus of the present review.
C. FGF23
FGF23 is a member of the FGF19 subfamily of endocrine FGFs, which also includes FGF15/19 and FGF21. FGF23 shows the highest degree of homology with FGF21. FGF23 was first identified in the ventrolateral thalamic nucleus of the mouse brain (225), and its importance was revealed in patients with autosomal dominant hypophosphatemic rickets (ADHR) (2). The missense mutation of the Fgf23 gene resulting in ADHR confers resistance to the proteolytic cleavage of the FGF23 protein and consequently increased serum levels of full-length active FGF23 and disease development. Fgf23 is mainly expressed by osteocytes and osteoblasts in bone, but it is also expressed by salivary glands, stomach, and at much lower concentrations by other tissues, including skeletal muscle, brain, mammary gland, liver, and heart.
The Fgf23 gene is located on the human chromosome 12 and mouse chromosome 6 and extends over 8.5 kb. It is composed by 3 exons separated by 2 introns and encodes a 32-kDa glycoprotein containing 251 amino acid residues. The protein comprises a 24 amino acids hydrophobic signal sequence, an NH2 terminal of 154 amino acids containing the FGF core homology region, and a characteristic 73 amino acids COOH-terminal domain. After cleavage of the 24 amino acids signal sequence, and O-glycosylation by UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyl-transferase 3 (GALNT3), the mature protein 25-FGF23-251 is secreted into the circulation. In the bloodstream, the FGF23 protein circulates in two distinct forms: a full-length mature form (25-FGF23-251) and a shorter form (25-FGF23-179) lacking the unique 73-amino acid COOH-terminal tail (7, 226). The shorter form arises from proteolytic cleavage at the 176RXXR179 site, which follows the β10–β12 region of the FGF core homology region of FGF23 (51, 180, 217). Only the full-length form of FGF23 is active, since the COOH-terminal domain is essential for interaction with the cofactor αKlotho and downstream activation of FGFR signaling (51). O-glycosylation of FGF23 occurs in the 162–228 region (180) overlapping the 176RXXR179 cleavage site, and this posttranslational modification appears to protect FGF23 from cleavage by subtilisin-like proprotein convertases when using recombinant peptides in vitro (85).
Fgf23 mutant mice have been created to determine the function of FGF23. Overexpression in transgenic animals or the parenteral administration of FGF23 to rodents suppresses phosphate reabsorption and inhibits the synthesis of 1,25(OH)2D in the proximal renal tubules (9, 102, 182). Conversely, FGF23-null mice or humans with homozygous missense mutations in FGF23 develop severe hyperphosphatemia, elevated 1,25(OH)2D levels, and soft-tissue calcifications (120, 178, 188).
III. FGF23 FUNCTION
A. Klotho and FGF Receptor Complexes
1. Klotho and Tissue Specificity
Klotho genes encode cofactor proteins imparting the tissue-specific action of heparin-independent endocrine FGFs. βKlotho determines the organ targets for FGF15/19. The βklotho gene encodes a single-pass transmembrane protein expressed in adipose tissue, liver, and pancreas. Mice deficient in βklotho have overlapping phenotypes with mice lacking FGF15 or FGFR4.
αKlotho was discovered while studying the phenotype of transgenic mice overexpressing the rabbit type I sodium-proton exchanger (95, 96). Indeed, one animal displayed a particular phenotype resembling aging, caused by the insertional mutation of the transgene in the promoter region of what will be later identified as the Klotho gene. The homozygous animals for the inserted transgene had a shorter life span and precociously developed pathologies related to aging including osteoporosis (86), hypogonadotropic hypogonadism, arteriosclerosis, skin atrophy, pulmonary emphysema (78, 169), neurodegenerative (5, 146), and auditory syndromes (84, 197).
The five exons of αklotho gene encode a 1,014-amino acid single pass, type I transmembrane, and β-glycosidase-like protein with β-glucuronidase activity. The αklotho protein shares 41% amino acid identity with βklotho. αKlotho is predominantly expressed in the kidney and the epithelium of the choroid plexus in the brain (108). In addition to these tissues, low expression of αklotho is also reported in the pituitary gland, placenta, skeletal muscle, urinary bladder, aorta, pancreas, testis, ovary, and colon (13, 96). Knock-in of the lacZ gene downstream of the translational initiation codon of αklotho also shows expression of αklotho in the parathyroid gland and sinoatrial cells of the heart (181, 196).
The target organs for FGF23 are defined by the coexpression of the membrane form of αKlotho and FGFRs (98, 207). The importance of the membrane protein αKlotho in FGF23 signaling is illustrated by both human and mouse genetic disorders where loss of αKlotho results in end-organ resistance to Fgf23, but abnormalities resemble Fgf23 deficiency (71, 75, 96, 173). Subsequent studies have identified αKlotho as being the necessary cofactor for FGF23, forming complexes with FGFRs and increasing their affinity for FGF23 (98, 207).
2. Soluble klotho
Although the exact functions of soluble Klotho are yet to be defined, circulating Klotho can arise from increased gene transcription of the alternatively spliced secreted isoform or from ectodomain shedding of membrane extracellular domain of full-length αKlotho. The extracellular domain of the αKlotho protein is cleaved and secreted into the blood, cerebrospinal fluid (CSF), and urine by ADAM10 and ADAM17, two members of the A Desintegrin and Metalloproteinase (ADAM) family (26). In addition, αKlotho gene encodes a truncated, secreted form derived from alternative RNA splicing (133). Compared with the transmembrane form, this truncated gene product does not have the second internal repeat of the extracellular domain, the transmembrane domain, or the intracellular domain (133, 185). It only encodes the NH2-terminal half of αKlotho with its extracellular domain. The relative contribution of ectodomain shedding and alternative splicing to the circulating Klotho levels remains uncertain.
Soluble Klotho acts as a humoral factor (72), potentially through an unknown cell-surface Klotho receptor, and as an enzyme (25, 202) regulating several cell surface glycoproteins. Indeed, secreted αKlotho inhibits insulin- and insulin-like growth factor I (IGF-I)-induced autophosphorylation of insulin and IGF-I receptor in vitro (99, 218). This effect is consistent with an anti-aging role for klotho since downregulation of IGF-I signaling pathway extends lifespan (34, 67). A well-established role of the secreted protein is to inhibit internalization of cell-surface calcium channel “transient receptor potential cation channel, subfamily V, member 5” (TRPV5), primarily responsible for the Ca2+ entry in transepithelial Ca2+ reabsorption in the kidney (24, 25). Klotho is indeed coexpressed with TRPV5, the Na+/Ca2+ exchanger 1 (NCX1) and calbindin-D28K (a vitamin D-sensitive intracellular Ca2+ transporting protein) in a specialized region of the distal convoluted tubules where transepithelial Ca2+ reabsorption is actively regulated. This colocalization is important for the homeostatic control of Ca2+. Indeed, mice lacking TRPV5 display diminished renal Ca2+ reabsorption, which causes severe hypercalciuria. The secreted form of Klotho also potentiates the action of 1,25(OH)2D on renal reabsorption, as Klotho stabilizes TRPV5 on the membrane by hydrolyzing the extracellular sugar moieties of TRPV5. Similarly, soluble klotho also stabilizes calcium channel “transient receptor potential cation channel, subfamily V, member 6” (TRPV6), which is expressed in the intestinal and mammary epithelial cells (124). Soluble Klotho also regulates Ca2+ homeostasis by controlling PTH secretion (75), but these findings have not yet been confirmed. Moreover, intravenous infusion of soluble Klotho caused hypophosphatemia and phosphaturia, suggesting a phosphaturic role for the circulating form (69) independent of FGF23 signaling. Finally, Klotho is reportedly regulating “renal outer medullary potassium channel” (ROMK1) in the kidney (24).
Finally, emerging evidence reveals that Klotho deficiency is an early biomarker for chronic kidney disease (CKD) (70). Indeed, Klotho deficiency is associated with progression and chronic complications in CKD including vascular calcification and cardiac hypertrophy (3, 88, 227) in animal and human studies, and replacement of soluble Klotho and/or forced overexpression (59, 141, 210) protects the kidney from renal injuries, preserves kidney function, and suppresses renal fibrosis.
3. FGF receptors
The mammalian FGFRs are encoded by four distinct genes (Fgfr1–Fgfr4). The ectodomain of prototypical FGFRs consists of three Ig-like domains (D1–D3). A major alternative mRNA splicing event within the D3 of FGFR 1 to 3 generates “b” and “c” isoforms, which have distinct FGF-binding specificities. An additional splicing event generates shorter FGFR1–3 isoforms lacking D1 and/or D1–D2 linker.
FGF23 binds to multiple FGFRc isoforms in vitro, but has low affinity to the receptors (Kd 200–700 nM) and cofactors such as heparin/heparan sulfate do not function as with classical FGF/FGFR interactions to activate FGF signaling (12, 228). Rather, the unique feature of FGF19 family of FGF is that they require Klotho gene for FGFR activation. The COOH terminus of FGF23 forms a trimeric complex with αKlotho and the c isoforms of FGFR 1, 3, and 4. The ability of αKlotho to bind to FGFR2 is lower than with the other FGFRs. Binding of αKlotho to FGFRs changes their affinity to FGFs. FGF23 binds to an FGFR-αKlotho complex with much higher affinity than FGFR alone (98, 207), and FGF23 signaling is maximum when binding to FGFR1c-αKlotho complexes (207).
The hypothesized mechanism for FGF23 bioactivity with αKlotho is the recruitment of FGFRs to form heteromeric complexes that signal through the mitogen-activated protein kinase (MAPK) cascade (94, 207). By interacting with FGFR-αKlotho complexes, FGF23 initiates downstream signaling events through a variety of intracellular signaling proteins including FRS2, Gab1, Shc, PLC, or STAT1. These are phosphorylated in response to FGFR activation leading to a possible mechanism for differential and/or additive gene expression and suggest differential responses according to the additive involvement of multiple FGFRs (201).
B. Renal Targets
The major consequences of FGF23 excess are hypophosphatemia, aberrant vitamin D metabolism, impaired growth, and rickets/osteomalacia (9, 45, 102). Inversely, ablation of FGF23 in mice results in hyperphosphatemia, excess 1,25(OH)2D, and soft tissue calcifications (120, 178). Thus most of the known physiological function of FGF23 to regulate mineral metabolism can be accounted for by actions of this hormone on the kidney. On the one hand, the reduction of circulating 1,25(OH)2D levels in response to FGF23 excess is due to the regulation of the anabolic and catabolic events through inhibition of 25-hydroxyvitamin D 1-α-hydroxylase (Cyp27b1) and stimulation of 1,25-dihydroxyvitamin D 24-hydroxylase (Cyp24a1) in the proximal tubules (9, 102, 114, 179, 183). Cyp27b1 and Cyp24a1 are the renal enzymes respectively responsible for the synthesis of the bioactive form of vitamin D and the initiation of the degradation of the bioactive form of vitamin D into calcitroic acid. On the other hand, the hypophosphatemia induced by FGF23 excess is mediated by the inhibition of the solute carrier family 34 member 1 (SLC34a1, Npt2a, NaPi2a) and member 3 (SLC34a3, Npt2c, NaPi2c) sodium-dependent phosphate cotransporters in the renal proximal tubules.
1. Vitamin D metabolism
The best characterized physiological function of FGF23 is to act as a vitamin D counterregulatory hormone (117). Prior to the discovery of FGF23, it was assumed that phosphate regulation occurred as a secondary action of PTH and 1,25(OH)2D. In hypophosphatemic disorders caused by FGF23 excess, 1,25(OH)2D levels are abnormally suppressed for the degree of hypophosphatemia, which should increase 1,25(OH)2D levels. FGF23 reduces 1,25(OH)2D levels due to complex effects on Cyp27b1 and Cyp24a1 to decrease the production and increase the catabolism of 1,25(OH)2D (76). Studies demonstrate that increased FGF23 inhibits Cyp27b1 and stimulates Cyp24a1 expressions, which is opposite from what one would anticipate in the presence of high circulating PTH levels and concomitant hypophosphatemia (9, 177, 178).
Interestingly, the stimulatory effect of FGF23 on Cyp24a1 expression (177) and the reduction of serum 1,25(OH)2D levels are vitamin D receptor (VDR)-dependent mechanisms (76). The effects of FGF23 on Cyp27b1 are not consistent throughout the available literature. Injection of recombinant FGF23 to wild-type mice results in a dose-dependent decrease in renal abundance of Cyp27b1 and exerts a direct action on Cyp27b1 gene expression in human and mouse renal proximal tubule cells via an ERK1/2-dependent mechanism (159). Paradoxically, increased Cyp27b1 mRNA expression has been reported in association with FGF23 excess (43, 231), however, with a message to functional protein translational defect. In the Hyp mouse, murine homolog of human X-linked hypophosphatemic rickets (XLH) which displays excess FGF23, defects in translation of Cyp27b1 message have also been reported (28, 199). Taken together, these studies suggest that the mechanism of action of FGF23 on the enzymes that regulate 1,25(OH)2D production and degradation may differ from acute to chronic FGF23 excess (9, 136, 177, 178). Finally, FGF23 action on vitamin D metabolism is not dependent on a functional 1,25(OH)2D-VDR system, as treatment of VDR null mice with FGF23 further decreased hypophosphatemia due to reduced renal and intestinal phosphate absorption, accompanied by decreased NaPi2a, NaPi2b, and Cyp27b1 expression (142, 183).
2. Phosphate reabsorption
The kidney plays a central role in phosphate homeostasis. It adjusts urinary excretion of phosphate according to phosphate intake and maintains the serum phosphate concentration within a narrow range. Phosphate is reabsorbed almost exclusively in the renal proximal tubule through a transcellular pathway. The limiting step of this transepithelial transport system is the entry of phosphate at the apical domain of proximal tubular cells. This process requires sodium- dependent phosphate cotransporters that use the inward sodium gradient established and maintained by the activity of the Na+-K+-ATPase pump. There are three types of sodium-dependent phosphate cotransporters in renal proximal tubular cells: the type 1 cotransporter (NPT1) is an anion carrier that does not specifically mediate phosphate transport; the type 2 cotransporter family includes three carriers, NPT2a (encoded by the SLC34a1 gene), NPT2b (encoded by the SLC34a2 gene), and NPT2c (encoded by the SLC34a3 gene); and the type 3 comprises two transporters, PiT1 (encoded by the SLC20a1 gene) and PiT2 (encoded by the SLC20A2 gene).
It has been shown that FGF23 suppresses the expression of NPT2a and NPT2c and consequently can increase urinary phosphate excretion (47, 177). NPT2a is central to renal phosphate reabsorption and phosphate balance, and found almost exclusively in the apical membrane of renal proximal tubular cells. NPT2c is the isoform expressed at the brush-border membrane of the renal proximal tubular cells. Due to FGF23 effects on vitamin D metabolism (see above), and PTH (see below), it has been hypothesized that FGF23 regulation of NPT2 may be due to the reduction of serum 1,25(OH)2D levels, and/or increased PTH. However, these actions are vitamin D independent (76, 183), and effects of FGF23 on serum phosphate can be observed in parathyroidectomized rats (177).
Whereas FGF23 effects on the Npt2c isoform appear to be variable, continuous exposure to recombinant FGF23 was shown to cause increased renal phosphate clearance resulting from decreased renal expression of NPT2a (9, 10, 102, 173, 177, 179, 183). The phosphaturic effect of FGF23 is further convincingly demonstrated in vivo. For instance, transgenic mice overexpressing Fgf23 have severe urinary phosphate wasting due to the suppression of renal Npt2 expression and/or activity (9, 182). Inversely, Fgf23 null mice characterized by severe hyperphosphatemia and ectopic soft tissue calcification display increased renal expression of Npt2a (101, 187). Genetically restoring the systemic actions of FGF23 in Fgf23 null mice reversed hyperphosphatemia to hypophosphatemia and prevented ectopic calcification (32).
C. Mechanisms for FGF23 Function in the Kidney
Interactions between αKlotho and FGFR are necessary to mediate FGF23 signaling, but the mechanisms underlying FGF23 bioactivity in vivo are unclear. First, at least three different FGFR have shown increased affinity for FGF23 when complexed with αKlotho (see sect. IIIA2). Second, the limiting cofactor αKlotho predominately localizes to the renal distal convoluted tubule (DCT), and FGF23 biological responses on NPT2 isoforms and vitamin D metabolizing enzymes are observed within the proximal tubules (PT) (114, 117, 179). Thus uncertainty exists regarding the physiologically relevant FGFR for FGF23 in the kidney and the precise tubular segments that are targeted by FGF23.
1. Relevant FGF receptor(s) for FGF23 signaling
Although FGF23 binds to FGFR1c, FGFR3c, FGFR4, and but not FGFR2c in vitro (96–98), there is strong support for FGFR1c:αKlotho being the relevant target for FGF23 in the kidney (119, 207). First, FGFR1:Klotho complexes have been identified as the principal binding partner for FGF23 (207). Second, neither loss of Fgfr3 nor Fgfr4 rescues the effects of FGF23 excess, hypophosphatemia, and aberrant vitamin D metabolism in the Hyp mice (119). Third, the conditional deletion of Fgfr1 in the kidney abolishes the phosphaturic effects of recombinant FGF23 administration (47). However, administration of recombinant FGF23 to FGFR4−/− caused a smaller decrease in serum phosphorus levels compared with wild-type or FGFR3−/− control mice (47). Additionally, in our studies, FGF23 levels were further elevated in Hyp/Fgfr3−/− and Hyp/Fgfr4−/− mice (119), consistent with end-organ resistance to FGF23 caused by loss of FGFR3 or FGFR4. Moreover, combined loss of FGFR3 and FGFR4 in Hyp mice partially corrected the hypophosphatemia and Npt2-dependent transport defect in the proximal tubule, and increased the production of 1,25(OH)2D in wild-type mice (107).
Taken together, these data suggest that 1) FGFR1 may be the principal FGF23 receptor mediating FGF23 phosphaturic effects; 2) renal FGF23 effects are mediated through multiple FGF receptors and FGFR1, FGFR3, and FGFR4 may have redundant roles; and 3) FGFR3 and -4, rather than FGFR1, mediate FGF23 effects on vitamin D metabolism.
2. Distal to proximal tubular paracrine feedback mechanism
The mechanisms underlying the differential effects of FGFRs to regulate proximal tubular phosphate and vitamin D metabolism are not clear, since both FGF23 binding and signaling pathways do not differ between FGFR1, -3, and -4 (98). FGFR3 is expressed in the PT (21, 119), whereas FGFR1, -3, and -4 are expressed in the distal tubules (119). However, the limiting cofactor αKlotho, as mentioned earlier, is predominately expressed in the distal tubule (108), and exogenous FGF23 administration in mice induces phospho-ERK1/2 only within the DCTs (38). Recent studies show weak presence of αKlotho protein and mRNA in the proximal tubule (69); however, these studies did not determine if the detected transcripts encode for the membrane and/or secreted forms. Ex vivo studies of proximal tubular segments or cell lines have demonstrated variable effects of exogenously added FGF23 to inhibit NPT2 (165, 179). Interpretation of these findings is confounded by the use of nonphysiological amounts of FGF23 and the authenticity of the proximal tubular phenotype in cell culture models, which may be contaminated with distal tubular cells and/or undergo dedifferentiation (15, 165, 166, 224, 228).
Independently of these findings, the highest levels of the FGFR:αKlotho complexes are in the distal tubules, whereas the biological actions of FGF23 are observed in the proximal tubules (108), which theoretically excludes a major direct effect of FGF23 on proximal tubules. Alternatively, FGF23 actions on the proximal tubule may be indirect, possibly through FGF23 stimulation of the distal tubule and release of paracrine factors that regulate proximal tubule function (i.e., a “distal-to-proximal tubular feedback mechanism”) among which soluble Klotho protein might be the ideal candidate. This mechanism is rendered possible in vivo, by the close proximity of proximal and distal cells, and is supported by studies showing that αKlotho can be released into the circulation from the distal tubule by either ectodomain shedding or secretion of an αKlotho isoform lacking the transmembrane domain. Accordingly, FGF23 decreases the expression of αKlotho by the kidney, thereby creating complex feedback pathways for regulating phosphate and calcium metabolism (75, 128). Overexpression of αKlotho causes phosphaturia (69, 145), and forced expression of αKlotho in the proximal tubule cells or cell-free membrane vesicles decreases the insertion of Npt2a into the membrane (69). This supports the hypothesis of secreted Klotho mediating some of the effects of FGF23. Distinguishing between direct and indirect effects of FGF23 on renal tubular function requires additional studies.
Indeed, hereditary hypophosphatemia and hyperparathyroidism (HHH) is caused by a promoter region translocation that increases αKlotho expression and its circulating levels (145). However, this results in both elevated FGF23 and PTH (17), and it is hypothesized that αKlotho might directly regulate PTH secretion through its maintenance of cell surface Na+-K+-ATPase activity (75). This makes the interpretation of the animal models findings more complex and suggests that αKlotho phosphaturic effects are indirectly mediated through stimulation of PTH and FGF23. Moreover, the hypothesis that soluble αKlotho sequestrates Npt2a cotransporter independently of FGF23 (69) does not explain the FGF23-mediated suppression of Npt2a mRNA expression observed in states of excess FGF23, and is inconsistent with recent data showing that serum αKlotho levels are normal in patients with XLH (22). Furthermore, the Fgf23 and αKlotho double-knockout (Fgf23−/−/αKlotho−/−) mouse model provide compelling evidence that αKlotho does not have an FGF23-independent role in the regulation of systemic phosphate and vitamin D homeostasis, as their phenotype is similar to the single, Fgf23−/− or αKlotho−/−, mutant mice with regard to phosphate and vitamin D metabolism (148). If the distal to proximal feedback mechanism appears to be the only plausible explanation for FGF23 renal effects (FIGURE 2A), αKlotho does not seem to be the paracrine factor, responsible for conveying FGF23 signaling from the distal to the proximal tubules.
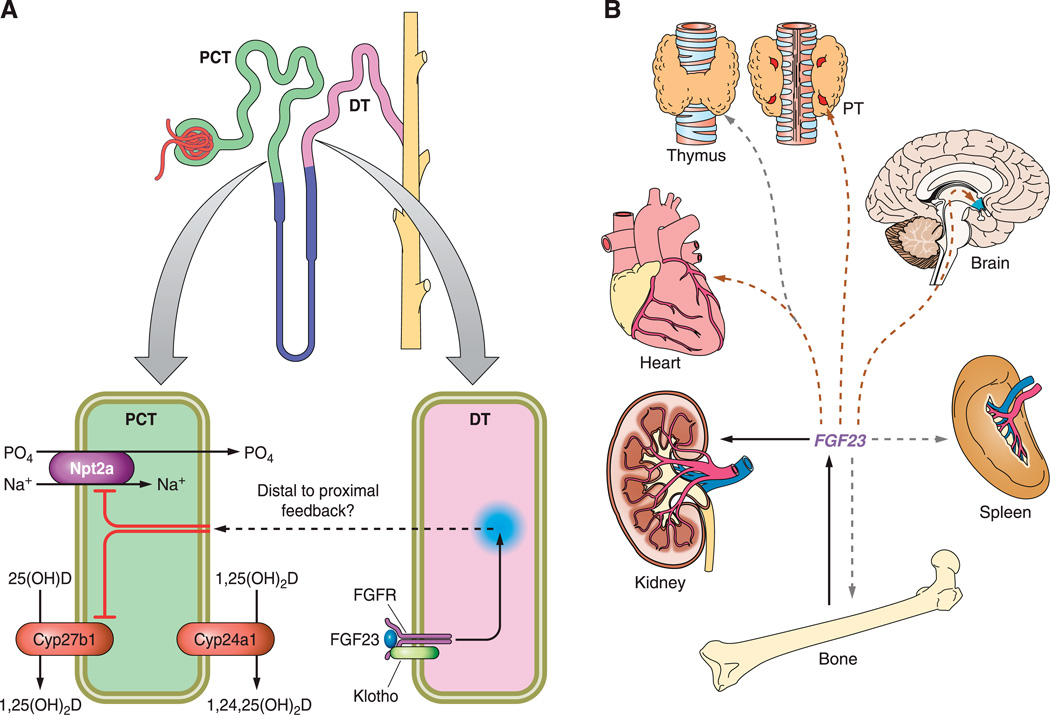
FIGURE. 2.
Renal and extrarenal functions of FGF23. A: hypothetical distal to proximal feedback mechanism: FGF23 activates FGFR/Klotho complexes in the renal distal tubules (DT) leading to two predominant events in the proximal convoluted tubules (PCT): the inhibition of expression of Npt2a and the inhibition of Cyp27b1. As a consequence, phosphate reabsorption and 1,25(OH)2D production are respectively decreased. Additionally, the increase in Cyp24a1 expression contributes to lowering 1,25(OH)2D levels due to increased catabolism of 1,25(OH)2D. B: extrarenal targets of FGF23 are tissues that express FGFR and Klotho, including kidney, but also parathyroid gland (PTG), heart, and brain (brown arrows) or tissues that express the FGFR alone such as thymus, spleen, or bone (gray arrows), indicating possible paracrine effects of FGF23. Possible hormonal regulation loops are yet to be discovered.
D. Extrarenal Targets
Rescue experiments of FGF23−/− mice by transgenic expression of human FGF23 in osteoblasts tend to demonstrate that FGF23 acts in a systemic manner (32), rather than as an autocrine/paracrine factor. As previously discussed, it appears that FGF23 primarily serves as a systemic factor to regulate phosphate homeostasis and vitamin D metabolism, but FGF23 may also directly signal through extrarenal FGFR/Klotho complexes (FIGURE 2B). Although less described, FGF23 also may regulate other genes in both the proximal and distal tubule, in addition to renal effects on phosphate cotransporters and vitamin D metabolism enzymes. Among them, some are likely to mediate FGF23 effects on other organs that do not express Klotho. Human or mouse genetic disorders where loss of Klotho results in end-organ resistance to Fgf23 and abnormalities resembling Fgf23 deficiency (71, 75, 96, 173) tend to plead against Klotho-independent effects. However, as FGF23 is capable of binding FGF receptors in absence of membrane Klotho, although with low affinity, it is plausible that activation of FGF receptors also occurs in presence of high FGF23 levels in organs where Klotho is absent (off-target effects). Finally, it is also plausible that another cofactor, yet undiscovered, may bind and activate FGF receptors.
1. Effects on parathyroid gland
The parathyroid glands express FGF receptors and Klotho, and the acute administration of recombinant FGF23 results in increments in Egr1 expression in parathyroid tissue in mice (207), but the role of FGF23 on the parathyroid gland in normal physiology is not clear (20, 96, 108). On the one hand, recent studies indicate that FGF23 negatively regulates PTH mRNA expression and protein secretion in vitro (92), and that FGF23 suppresses PTH secretion in vivo (13). On the other hand, patients with CKD typically exhibit secondary hyperparathyroidism associated with high serum FGF23 levels, which contradicts the ability of FG23 to suppress PTH secretion. In addition, overexpression of FGF23 in transgenic mice causes secondary hyperparathyroidism (9, 102). These contradictory results may be attributed to local and systemic confounding factors. For instance, Klotho/Fgfr1 expression is decreased in the parathyroid glands of patients and rats with advanced CKD (46, 91), and the functionality of the complexes is also impaired (46). Moreover, Klotho may also have an FGF23-independent role by facilitating PTH secretion through maintenance of membrane Na+-K+-ATPase activity in the setting of hypocalcemia (75), although this mechanism has been recently disputed (131). Nevertheless, given that FGF23 suppresses Klotho expression, whereas 1,25(OH)2D positively regulates it (206), increased FGF23 and hypovitaminosis D should impair Klotho-driven PTH secretion, in addition to considerably reducing FGF23 signaling, which may explain why the PTH resist the putative inhibitory effects of FGF23 in CKD. Furthermore, low 1,25(OH)2D and subsequent hypocalcemia could also be responsible for secondary hyperparathyroidism. Nonetheless, serum FGF23 correlates to PTH in predialysis CKD patients (176, 213) and in patients with early CKD (213) when levels of phosphate and calcium are maintained within normal range. Moreover, a strong positive correlation between FGF23 and PTH is also found in XLH subjects (22) who are normocalcemic and display low to normal levels of 1,25(OH)2D. On the contrary, Fgf23 null mice display low PTH levels (178) consistent with a stimulatory effect of FGF23 of PTH secretion.
Finally, Fgf23 null mice overexpressing human recombinant FGF23 display increased PTH (32) despite normal calcium and 1,25(OH)2D, and hypophosphatemia, consistent with the hypothesis that FGF23 acts directly on the parathyroid gland to induce PTH production. Taken together, these results suggest that FGF23 and PTH may form a regulatory loop (refer to sect. IVA) similar to FGF23-vitamin D loop, but some of the FGF23-PTH associations could be attributed to alterations of other systemic and local factors.
2. Effects on skeleton
Physiological phosphate balance is of crucial biological importance to skeletal mineralization, and as a master regulator of phosphate homeostasis, FGF23 is bound to affect bone metabolism, cellular function, and mineralization. Similarly, by regulating 1,25(OH)2D and PTH, the most described hormonal regulators of bone, and subsequent calcium changes due to 1,25(OH)2D and PTH, FGF23 affects bone development and function. Although FGF23 does not act independently of Klotho in the systemic regulation of vitamin D and phosphate homeostasis (148), and despite reported Klotho absence from mineralized tissues, there is a debate regarding whether FGF23 has direct effects or the bone changes are to be attributed to phosphate, 1,25(OH)2D, and PTH. Studies of FGF23 in human on genetic and acquired diseases and those using animal models have demonstrated that both under- and overexpression (2, 120, 177, 187) of FGF23 result in impairments in bone metabolism. Overexpression of FGF23 in mice causes hypophosphatemia, reduced 1,25(OH)2D levels, and rickets/osteomalacia (182). The bones exhibit widened and disorganized growth plates and reduced bone mineral density. Although the defective skeletal mineralization observed in patients and animal models with FGF23 excess is likely a consequence of low phosphorus and vitamin D values, FGF23 treatment of primary calvarial osteoblasts from wild-type mice (186) and osteoblast-like cells (175) leads to an inhibition of mineralization. Consistent with these findings, studies in animal models and in cell culture suggest that FGF23 has also a direct effect on bone (208, 209). Overall, these results suggest that excess of FGF23 can negatively affect bone mineralization. On the opposite, FGF23 deficiency results in severe hyperphosphatemia, hypervitaminosis D, and increased circulating calcium (178, 187). These animals also display a severe bone phenotype, characterized by a disorganized growth plate lacking hypertrophic chondrocytes and decreased mineralized bone mass with increased osteoid (178, 193). Ablation of vitamin D or vitamin D signaling (62, 188) and decreasing the circulating levels of phosphate by dietary (193) or genetic approaches (186) significantly improved the abnormal phenotype associated with lack of Fgf23 activities. However, skeletal abnormalities observed in Fgf23−/− mice including the decrease in hypertrophic chondrocytes in the growth plate, the increased mineral deposition adjacent to the growth plate, and the osteomalacic phenotype were found to be similar to Fgf23−/−/NaPi2a−/− compound mutants, despite the significantly reduced serum phosphate levels. These findings (186) suggest that Fgf23 may be an essential factor necessary for bone development, although the increased 1,25(OH)2D serum levels alone may be responsible for osteomalacia in Fgf23−/− mice. Indeed, studies have convincingly demonstrated that rats treated with high doses of 1,25(OH)2D have impaired bone mineralization (35, 220). Since deletion of VDR partially rescues the bone phenotype of FGF23 null mice (62, 188, 191), the direct effects of FGF23 on bone may represent nonspecific effects, comparable to other FGFs. However, rescue of FGF23 null mice by transgenic overexpression of human recombinant FGF23 does not rescue the bone phenotype, despite normalization of 1,25(OH)2D and calcium levels, which suggest that FGF23 has a cell autonomous effect (32). Alternatively, as both FGF receptors (100, 212), specifically FGFR1 and FGFR4, and Klotho (96) are expressed during myogenesis, skeletal FGF23 effects could be mediated through the muscular sites. Despite reported FGF23 expression in the skeletal muscle (2), none, to our knowledge, has studied FGF23 implication in myogenesis.
3. Effects on other organs
FGF23 action on organs other than kidney and parathyroid remain unstudied, with the exception of bone, mainly because current data support that FGF23, even at supraphysiological concentrations, likely has no effect without the presence of Klotho. However, little is known about FGF23 effects on organs that do express Klotho such as the brain (108) and the cardiovascular system (13, 96, 181, 196). The lack of studies in these areas is likely due to the masking of toxic effects of hyperphosphatemia and elevated 1,25(OH)2D levels that are admitted to be primarily responsible for increased mortality in absence of FGF23 signaling (62, 186, 191).
Theoretically FGF23 may also have central effects on FGFR/Klotho complexes in the choroid plexus and pituitary where FGF23 is also expressed. Despite the fact that in stages with very high FGF23 such as end-stage renal disease (ESRD), cognitive dysfunction is highly prevalent in patients when compared with the general population (39, 174), no direct relationship was established between FGF23 and cognitive impairement.
Understanding the extrarenal functions of FGF23 is of particular importance in patients with renal failure (103, 211) for whom FGF23 is a marker of early cardiovascular changes (139). Despite established Klotho expression in heart and aorta (181, 196), FGF23 effects were believed to be indirect or off-target effects only occurring at supraphysiological concentrations. Nonetheless, FGF23 is positively associated with left ventricular mass index and increased risk of having left ventricular hypertrophy (57, 68, 104, 138, 140).
Additional abnormalities are associated with FGF23, such as abnormalities in glucose homeostasis, growth retardation, abnormalities in thymic function, and ageing phenotypes (62, 120, 178, 187), consistent with a broader role for FGF23. However, many of the toxic effects of Fgf23 deficiency are indirect consequences of the concurrent elevated 1,25(OH)2D actions, since generating compound FGF23 null and either Cyp27B1 or VDR null mice, results in the disappearance of abnormal findings and soft tissue calcifications (62). However, both Fgf23−/− and Klotho−/− (148, 155) showed thymic atrophy and a reduced number of splenocytes, indicating that FGF23 signaling could influence hematopoiesis. Given that FGF23 is physiologically expressed in the thymus and that FGF23 is elevated in plasma cell dyscrasias (190), this suggests that FGF23 may well play a role in lymphopoiesis and hematopoiesis. Moreover, high doses of FGF23 can induce proliferation of murine bone marrow-derived pro-B cell lines (228), since Klotho is reported absent in lymphatic organs including bone marrow, thymus, and spleen (148).
IV. FGF23 REGULATION
A. Systemic Regulators of FGF23
1. Promoter activation by 1,25(OH)2D
1,25(OH)2D is the most important systemic factor regulating FGF23. The administration of 1,25(OH)2D increases FGF23 levels, while the disruption of 1,25(OH)2D pathways reduces circulating FGF23 in mice (117, 166). Increased 1,25(OH)2D targets the gastrointestinal tract to increase calcium and phosphate absorption. Increments in calcium along with 1,25(OH)2D target the parathyroid gland to suppress PTH, which in turn targets the kidney to increase urinary calcium excretion to maintain neutral calcium balance. However, lowering of PTH levels decreases phosphate excretion and would potentially result in positive phosphate balance from vitamin D-mediated increase in gastrointestinal phosphate absorption if not for compensatory elevations of FGF23, which also suppresses 1,25(OH)2D to counter the increase in vitamin D (117). This constitutes a classical hormonal loop: increased 1,25(OH)2D→increased FGF23→decreased 1,25(OH)2D. The expression of FGF23 is regulated by both VDR-dependent and VDR-independent signaling. Stimulation of the 1,25(OH)2D-VDR pathway induces the expression of FGF23, as evidenced by increased FGF23 levels after 1,25(OH)2D administration. In line with these findings, VDR null mice showed undetectable FGF23 levels (183, 229). In addition, normalization of serum calcium and phosphate levels by dietary means increased FGF23 levels in VDR null mice, indicating that FGF23 expression is also regulated by a VDR-independent pathway (102, 128, 177, 182).
2. Regulation by Phosphate
As of today, the effects of phosphate on FGF23 remain unclear. Unlike calcium, which has a calcium sensing receptor (CaSR) that permits the sensing and tight control of calcium levels, a phosphate sensor has not been identified and the regulation of serum phosphate levels is not so tightly controlled. Phosphate loading in mice increases FGF23 levels (158), but the magnitude of the phosphate regulation of FGF23 is small compared with the effects of 1,25(OH)2D and the importance of dietary phosphate in regulating FGF23 in humans is conflicting (41, 152). In humans, it was shown that serum FGF23 was regulated by dietary phosphate (41), whereas a subsequent study has shown that humans consuming diets containing increasing amounts of phosphate displayed decreased FGF23 concentration. Overall, in humans, the changes of secreted FGF23 are quite variable and modest when measured after high- or low-phosphate diets of long duration (18, 103, 158). In rodents, high-phosphate diet increases circulating FGF23, but this seems to be a 1,25(OH)2D-VDR dependent mechanism, as dietary phosphate failed to increase FGF23 expression in absence of VDR (183). This might indicate that in case of low or high vitamin D, the determinant factor for FGF23 is 1,25(OH)2D (152). FGF23 levels are also elevated in renal failure, and the degree of elevation correlates with the degree of hyperphosphatemia (211). In these settings, phosphate restriction failed to lower elevated FGF23 levels in patients with CKD (77). Use of phosphate binders in combination with dietary phosphate restriction sufficient to lower urinary phosphate excretion were less effective than expected in reducing FGF23 levels. Among the phosphate binders, only sevelamer hydrochloride, a binder known to provide an acidic load which may alter bone phosphate flux, mildly lowered FGF23 levels without significant changes in serum calcium or phosphate levels in humans (156). Similar studies involving uremic rats showed that the administration of sevelamer required 2 wk to achieve reductions in PTH and FGF23, whereas correction in hyperphosphatemia occurred rapidly (147). A high phosphorus diet was shown to enhance, and a low phosphorus diet to inhibit, the elevation of serum FGF23 levels in nephrectomized rats, but this result was obtained after 4 wk of dietary treatment (166). Altogether these data show that FGF23 may vary in animals and patients without any changes in serum phosphate levels, suggesting that phosphate load, rather than plasma levels, should be considered for regulation of FGF23. If true, this also suggests that circulating phosphorus levels do not adequately reflect phosphorus balance and that serum phosphate is not the major regulator of FGF23, at least in CKD. Additionally, low calcium intake is also associated with hypophosphatemia and elevated FGF23 levels in the absence of vitamin D deficiency in African and Asian populations (160). However, neither extracellular calcium nor phosphate directly stimulates FGF23 expression in osteoblast cultures (117).
3. Regulation by PTH
As mentioned in section IIID1, a controversy is emerging regarding the interactions between PTH and FGF23. A PTH-FGF23 feedback loop (increased PTH → increased FGF23 → decreased PTH) challenges the simply “FGF23 counterregulatory hormone for 1,25(OH)2D” hypothesis. The effect of PTH to increase FGF23 expression is now well established. Indeed, FGF23 is increased in primary hyperparathyroidism (87), in Jansens’ metaphyseal chondrodysplasia caused by activating PTH/PTHrp receptor mutations (16), and parathyroidectomy results in decrease in FGF23 in CKD (170). Moreover, PTH directly stimulates FGF23 gene expression in vitro (105). The ability of PTH to stimulate FGF23, however, is context dependent. For example, PTH failed to directly stimulate FGF23 production or FGF23 promoter activity in ROS17/2.8 osteoblast-like cells (117), or in calvarial culture (167). Furthermore, PTH null mice on Hyp background (8) and patients with hypoparathyroidism (54) display increased FGF23 levels, despite low levels of PTH. The mechanism underlying the variable effects of PTH on FGF23 gene expression is not known, but may involve VDR-dependent pathways. In this regard, vitamin D- and vitamin D receptor-deficient mice show abnormally low levels of FGF23 despite severe hyperparathyroidism (76, 168), and injection of vitamin D into PTH-deficient mice restores FGF23 production (117). Altogether, this indicates that PTH effects on FGF23 may be dependent on vitamin D and mineral status. Indirect effects to stimulate FGF23 could be mediated through PTH-mediated increases in 1,25(OH)2D or the presence of cofactors modulating PTH effects. The apparent ability of PTH to increase or decrease FGF23 might reflect the differential anabolic and catabolic effects of PTH on bone remodeling. In this regard, intermittent administration of PTH leading to net increments in bone formation results in reduced FGF23 levels (168), consistent with the need to conserve phosphate. In contrast, continuous administration of PTH that leads to catabolic effects on bone might be predicted to stimulate FGF23, which would help eliminate the increased phosphate efflux from bone.
4. Other systemic regulators
Elevated 1,25(OH)2D is closely correlated to increased fat mass in humans (49, 93) and mice (135, 205) and accompanied by a sustained decrease in 25(OH)D, which will in turn deepen the hyperparathyroidism and amplify its effects on the skeleton. Recently, it has been shown that leptin, a hormone secreted by white adipose tissue that acts centrally on hypothalamus and peripherally on other organs to control energy intake, expenditure, and bone homeostasis, stimulates FGF23 production in bone (205). Consistent with this, administration of intraperitoneal leptin in leptin-deficient ob/ob mice corrected abnormally elevated 1,25(OH)2D, calcium, and phosphate (134, 135). Furthermore, injection of FGF23 in leptin-deficient ob/ob mice corrected the over-production of 1,25(OH)2D, whereas addition of leptin to renal tubular cells did not modify CYP27b1 activity. Leptin as a marker of lipid metabolism should be found elevated together with FGF23 in pathologies with increased cardiovascular risks. This hypothesis is strengthened by the fact that in a recent study in humans (137), FGF23 has been associated with fat mass and dyslipidemia. In this context, bone appears by all standards as an endocrine organ (161) in a more complex model of multiorgan interaction, in which bone plays a central role to integrate both its endocrine functions in controlling energy and phosphate metabolism. As FGF23 is believed to be mainly a skeletal hormone, many of the factors regulating bone metabolism would impact upon FGF23 synthesis. Estrogen deficiency is a major cause of osteoporosis as estrogens play a key role in balancing bone remodeling directly (127, 163) and indirectly through modulation of PTH, calcium, and vitamin D signaling (110, 151, 198). Recent studies have found that estrogen treatment of ovariectomized rats caused hyperphosphaturia and hypophosphatemia due to the downregulation of the NaPi2a cotransporter in the renal proximal tubule and independently of changes in serum PTH levels (37). Consistently, estrogens correlated in a dose-dependent manner with the magnitude of the FGF23 increments, suggesting that estrogens may be a potent stimulator for FGF23 (23). Similarly, recent studies, although unpublished, reference glucocorticoid-regulated FGF23 production, in the context of pathogenesis of glucocorticoid-induced bone disease.
There is also evidence that the kidney may secrete factors or lead to other systemic effects that regulate FGF23, thereby closing a feedback loop. For example, FGF23 levels were slightly elevated in Hyp/Fgfr3−/− and Hyp/Fgfr4−/− mice (119) and more so in Hyp/Fgfr3−/−/Fgfr4−/− (107). This is consistent with end-organ resistance to FGF23 caused by loss of FGFR3 and FGFR4 and a feedback mechanism linking end-organ resistance to compensatory increments in FGF23 production by bone. Additionally, in CKD, also progressive increments in FGF23 occur in proportion to the degree of loss of renal function, again suggesting a compensatory mechanism. This factor is not phosphate, per se, since phosphate restriction fails to prevent the increments in FGF23 in CKD.
B. Bone Local Regulators of FGF23 Transcription
1. Regulation by PHEX and DMP1
Phosphate regulating gene with homologies to endopeptidases on the X chromosome (PHEX) is a 106-kDa protein member of the endothelin-converting enzyme family expressed by osteoblasts and osteocytes in bone. Inactivating mutation of Phex leads to increased Fgf23 gene transcription by bone (120). The conditional deletion of Phex in the osteoblast lineage in vivo is also sufficient to increase Fgf23 expression, suggesting that PHEX participates in the local mechanisms regulating Fgf23 (230). To date, the mechanisms whereby PHEX regulates Fgf23 gene transcription in bone are still unclear, and the relevance for Fgf23 production by osteoblasts and osteocytes is not known. Although an initial study suggested that PHEX processes FGF23 (15), subsequent studies failed to establish Phex-dependent cleavage of FGF23 (13, 53, 113). Screening of substrate phage libraries by us and others have identified that PHEX cleaves small peptides (19), but failed to identify a physiologically relevant substrate for PHEX (53). The ASARM peptide, a motif in MEPE and DMP1, is a substrate for PHEX in vitro (1). Additional data suggest that accumulation of ASARM as a consequence of inactivation of Phex can impair mineralization (129) and phosphate homeostasis (29). We have shown that ASARM binds to and inhibits PHEX activity against a synthetic substrate in vitro (115), but it is unlikely that ASARM from MEPE is responsible for stimulating FGF23 in Hyp, since ablation of MEPE fails to alter FGF23 expression in Hyp mice (30, 112).
Dentin matrix protein 1 (DMP1) is a 94-kDa member of the SIBLINGs extracellular matrix proteins. Similar to PHEX, DMP1 is expressed by osteoblasts and osteocytes in bone. Inactivation of DMP1 also leads to increased Fgf23 expression in bone (40, 121, 122). The main function of DMP1 is to regulate the mineralization of the extracellular matrix (48, 61). DMP1 exists as a latent protein that is cleaved into 37- and 57-kDa fragments by BMP1 or cathepsin B (195). The highly phosphorylated COOH-terminal 57-kDa fragment of DMP1 contains an RGD domain for integrin binding and an ASARM peptide for binding to PHEX. The NH2-terminal 37-kDa fragment of DMP1 is a proteoglycan with a chondroitin sulfate chain attached through Ser-74 that binds to proMMP-9 and may sequester growth factors (154).
The breakthrough in understanding Fgf23 transcriptional regulation in bone came through the comparative analysis of the Phex-mutant Hyp mouse and Dmp1 null mouse (130). The discovery that both mutations induce identical intrinsic abnormalities of mineralization and FGF23-dependent hypophosphatemia provided the initial insights into a bone kidney axis that coordinates bone mineralization and systemic phosphate homeostasis (162). Under physiological conditions, this coordination relies at least in part on the appropriate functions of PHEX and DMP1 to inhibit Fgf23 expression in bone and maintain bone mineralization status (FIGURE 3A). The loss of function of DMP1 or PHEX results in the intrinsic increase of Fgf23 by osteocytes (118). Several hypothetical mechanisms possibly leading to this increase have already been excluded. For example, PHEX does not cleave DMP1, indicating that other enzymes are required for DMP1 processing (125). Additionally, DMP1 does not regulate Fgf23 transcription directly by translocation to the nucleus (149), since the overexpression of DMP1 in mice does not decrease FGF23 levels below normal (126). Finally, the ASARM peptide resulting from the degradation of DMP1 (129) is not responsible for the elevation of FGF23, since the ablation of Dmp1 leads to increased FGF23 levels in Dmp1 null mice. Rather, there is stronger evidence that the proximate cause of increased FGF23 expression in osteocytes in Hyp and Dmp1 null mice is intrinsic to the bone milieu, and is mediated by the presence of unknown matrix-derived FGF23 stimulatory factors that are increased as a consequence of either Phex or Dmp1 mutations. In this regard, recent studies indicate that FGF23 is increased in callus during fracture healing, consistent with local matrix-derived FGF23-stimulating factors (50).
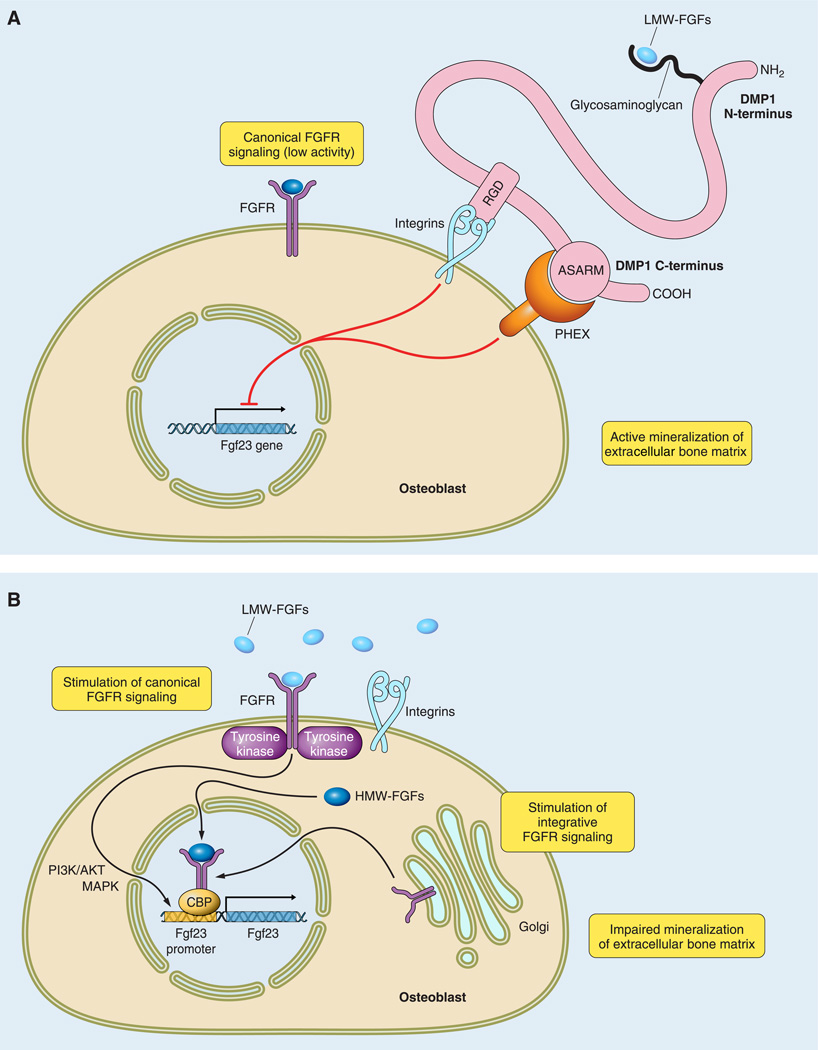
FIGURE. 3.
Predictive model for local regulation of Fgf23 transcription in bone. The COOH-terminal fragment of DMP1 binds to PHEX and integrins via the ASARM and the RGD motifs, respectively. A: in physiological conditions, this binding participates in maintaining low circulating FGF23 levels by inhibiting its transcription. B: absence of DMP1 or PHEX, due to inactivating mutations, leads to a pathological increase in Fgf23 transcription by stimulation of the FGFs/FGFRs pathway. Activation of the FGFs/FGRs pathway occurs when integrins are release from COOH-terminal DMP1 binding and low-molecular-weight FGFs (LMW-FGFs) are released from NH2-terminal DMP1 binding, thus allowing the formation of a complex between LMW-FGFs/integrins/FGFR. Alternatively, internalization of intracytoplasmic FGFR and high-molecular-weight FGFs (HMW-FGFs) also lead to increased Fgf23 transcription in absence of DMP1 and PHEX.
2. Regulation by FGFs/FGFRs pathway
FGFR-dependent signaling pathways have also emerged as important regulator of FGF23 expression in osteocytes. In this regard, osteoglophonic dysplasia (OGD), an autosomal dominant bone dysplastic disorder caused by activating mutations in FGFR1, also has hypophosphatemia and elevated FGF23 levels (216). FGFs/FGFR pathways may regulate FGF23 expression in bone via canonical, noncanonical, or intracrine pathways.
a) canonical
FGFR activation is mediated by secreted low-molecular-weight FGFs through binding to FGFR in the presence of heparin sulfate. We have identified by microarray gene expression analysis increments in FGF1 mRNA expression in Hyp and Dmp1 bone and have shown the ability of canonical activation of FGFR pathways by addition of recombinant FGF1 and FGF2 to osteoblast cultures stimulates Fgf23 promoter activity (221). These findings are consistent with the effects of long-term administration of FGF2 in vivo to induce hypophosphatemia and impair matrix mineralization (109, 150). FGFs are also stored in the extracellular matrix of bone through binding to heparin sulfate proteoglycans, and increased release of stored FGFs might also stimulate FGFRs. We have also shown that selective inhibition of FGFR blocks increments in FGF23 in Hyp-derived bone marrow stromal cell cultures (unpublished data), consistent with the loss of Phex function imparting FGFR-mediated regulation of FGF23 production. Collectively, these findings suggest that FGFs/FGFR activation is a central pathway regulating FGF23 expression in bone (FIGURE 3B).
b) noncanonical
FGFR-protein interactions facilitate FGFR activation. Noncanonical FGFR may serve to either sensitize or desensitize FGFR signaling. Klotho interaction with FGFRs is an example of noncanonical FGFR activation. The extracellular domain of Klotho is expressed by alternative splicing or secreted into the blood and urine by ectodomain shedding, making it theoretically possible that the extracellular domain of Klotho may activate FGFRs in the presence of FGF23, and creating a positive feedback loop. Klotho-dependent noncanonical activation of FGFR1 may explain the unexpected increase in serum FGF23 caused by translocations in humans that increase circulating Klotho levels and FGF23-mediated hypophosphatemic rickets (17). The ability of αvβ3 integrins to bind to FGF1 leading to FGFR activation is another example of noncanonical FGFR activation (144). We believe that this noncanonical FGFR activation is a strong candidate to explain the molecular mechanism whereby PHEX and DMP1 mutations regulate FGF23 expression, since DMP1 comprises the RGD and ASARM sequences allowing the binding with integrins and PHEX, respectively. This association might explain why some FGFR1 mutations lead to increased FGF23 and mutations at other sites in the receptor do not.
c) intracrine
The effects of FGFs that are independent of cell surface FGFR activation have also been described. FGF2 is produced as a high-molecular-weight isoform (HMW) and a low-molecular-weight isoform (LMW) by means of alternative usage of translation start sites in a single Fgf2 mRNA. Both HMW and LMW FGF2 isoforms are synthesized by osteoblasts. While the LMW forms are deposited in extracellular matrix where they are released to activate canonical FGFR pathways (36), HMW-FGFs localize to the nucleus to activate poorly defined “intracrine” pathways. Col1a1 (3.6 kb) promoter-driven HMW-FGF2 overexpression in mice causes hypophosphatemia and increases FGF23 expression (222), suggesting that intracrine functions of FGFs may also play a role in FGF23 regulation (FIGURE 3B). In contrast, overexpression of LMW-FGF2 under the control of the same promoter had increased bone mass with normal calcium/phosphate homeostasis (221).
The three mechanisms whereby FGFs/FGFR pathways might regulate FGF23 are not mutually exclusive. These observations implicate either canonical FGFR1 and/or the recently characterized integrative nuclear FGF receptor signaling in regulating FGF23 promoter activity in bone (33). We have proposed a hypothetical model to explain how mutations in Phex and Dmp1 lead to activation of FGFR through both canonical and noncanonical pathways.
3. Novel hypothetical concept linking transcriptional regulation of FGF23 by mineralization-dependent intrinsic matrix factors
There is growing evidence that another physiological function of FGF23 is to respond to changes in bone mineralization and turnover to adjust renal phosphate handling and balance the phosphate flux from bone. Bone is a buffer for minerals and can release calcium and phosphate into the circulation. Impaired mineralization would impair bone buffering capacity, leading to adaptive changes to excrete greater amounts of phosphate. Consistent with this possibility, there is an inverse relationship between FGF23 production by osteocytes and impaired mineralization.
a) primary defects in bone mineralization can regulate FGF23 production
Inactivating mutations of Enpp1, which more typically causes hereditary generalized arterial calcification of infancy (GACI), can also cause a variant of autosomal recessive hypophosphatemic rickets, characterized by hypophosphatemia and elevated FGF23 levels in some patients (106, 123). Enpp1 generates inorganic pyrophosphate (PPi), an essential physiological inhibitor of calcification, and substrate for alkaline phosphatase which converts it to Pi necessary for mineralization of bone. The inactivation of Enpp1 reduces the ratio of PPi to Pi, leading to increased mineralization of soft tissues. Treatment of GACI with bisphosphonates, however, causes hypocalcemia and elevated PTH in these patients, suggesting an unusual sensitivity of bone. Although there is no information on bone histology from GACI patients, studies of Enpp1 null mice indicate defective mineralization in long bones, which is postulated to occur because the PPi limits the availability of free Pi for mineralization of cortical bone (6). These findings suggest that primary physiochemical-mediated impairment of mineralization can somehow stimulate FGF23 expression in bone. Alternatively, the finding of hypophosphatemia and no evidence of aberrant vascular calcifications GACI patients with elevated FGF23 suggest a systemic pathway whereby ectopic soft tissue calcifications per se, or additional functions for Enpp1 are not yet discovered (106, 123). The regulation of FGF23 in Enpp1 null mice has not yet been evaluated, but might be informative regarding effects of impaired mineralization on FGF23 expression.
b) bone turnover can regulate FGF23 production
Low turnover bone disease, such as adynamic bone, leads to decreased phosphate buffering by bone, which could lead to increased production of FGF23, similar to defective mineralization. Consistent with this possibility, antiresorptive agent osteoprotegerin produced a profound reduction in bone resorption and formation in male and oophorectomized female mice, accompanied by an increase in serum levels of FGF23 (168). Theoretically, high rates of bone turnover would release calcium and phosphate from bone, leading to calcium-mediated suppression of PTH and elevated FGF23 preventing hyperphosphatemia. There is an association of increased FGF23 and plasma cell dyscrasias (190), but a formal assessment of the effect of increased osteoclastic mediated bone resorption of FGF23 expression in bone has not been performed. It is also possible that the discrepancies between PTH lack of a direct effect on FGF23 promoter activity and the apparent ability of intermittent versus continuous administration of PTH to respectively inhibit and stimulate FGF23 may be due to primary effects of PTH to affect bone remodeling. Also, the recent observation that leptin stimulates FGF23 might also be mediated through effects on bone remodeling and also points to the complex interplay between nutrition, fat, bone, and energy metabolism (205).
Altogether, the concept that bone mineralization and turnover might regulate the local production of FGF23 is an interesting hypothesis that explains the existing observations but requires experimental validation.
C. Posttranslational Regulation of FGF23
Circulating levels of biologically active full-length FGF23 are also regulated by furin-like proteases and the GalNAc transferase 3 (GALNT3), respectively, responsible for the proteolytic processing and initiation of O-glycosylation of FGF23. FGF23 contains an RXXR subtilisin-like proprotein convertase recognition sequence motif. The cleavage of the active full-length FGF23 protein at this site generates two inactive 180-amino acid NH2-terminal and 71-amino acid COOH-terminal fragments. This processing can be blocked by selective O-glycosylation of the cleavage site by GALNT3 (85). Indeed, mutations of GALNT3 or serine 71/glycine (S71G) and serine 129/phenylalanine (S129F) mutations of FGF23 at additional glycosylation sites result in hyperphosphatemic familial tumoral calcinosis (HFTC) (11, 189, 204). The defective O-glycosylation observed in HFTC is associated with hyperphosphatemia and massive ectopic calcifications and leads to low intact FGF23 levels with marked increase of processed COOH-terminal fragments in the circulation. The discovery of these mutations highly contributed to our understanding of the posttranslational regulation of FGF23. On the one hand, the discovery of the mutation of GALNT3 revealed the importance of O-glycosylation in preventing FGF23 from degradation by furin-like proteases (132), and on the other hand, the S71G and S129F mutations have shown evidence that O-glycosylation is essential and required for externalization of FGF23 (14, 85).
V. PHYSIOLOGICAL AND PATHOLOGICAL IMPLICATIONS OF FGF23
The discovery of FGF23 and its regulation and function, in addition to defining new physiological pathways and networks, has led to a new pathophysiological framework for classifying hereditary and acquired hypo- and hyperphosphatemic disorders, reconsideration of the treatment approaches to manage hereditary hypophosphatemic disorders, new insights into the pathogenesis of disordered mineral metabolism in chronic kidney disease, a mechanism for understanding the mechanism of hyperphosphatemia caused by various drugs, and identification of possible links between disordered mineral metabolism and cardiovascular mortality.
A. Physiological Implications of FGF23
Over 85% of the total body phosphate is stored in bone in both mineralized matrix and exchangeable pools. Phosphate moves in and out of bone (typically with calcium) in a coordinate fashion with bone mineralization and bone remodeling, the renal handling of phosphate, dietary absorption of phosphate, and acid-base status. In addition to extracellular phosphate being critical for mineralization of bone matrix, intracellular phosphate is critical for energy metabolism and intracellular signaling. Metabolic functions related to intracellular phosphate are most sensitive to hypophosphatemia, whereas excess phosphate overcomes the inhibitory effects of matrix Gla proteins and other factors to induce extraskeletal calcifications and untoward effects on the cardiovascular system and soft tissues leading to increased morbidity and mortality. The ability of excess phosphate to promote soft tissue calcifications is related to bone turnover, such that low bone turnover states are associated with increased vascular calcifications. The bone buffering capacity for phosphate is a difficult entity to quantify, but its effects on systemic phosphate homeostasis are indirectly observed in clinical disorders, such as severe hyperparathyroidism in chronic kidney disease, where excess efflux of phosphate from bone contributes to both hyperphosphatemia and serum calcium levels, and rapid reductions of PTH as occurs with parathyroidectomy results in profound hypophosphatemia and hypocalcemia due to increased uptake of calcium and phosphate in the “hungry bone syndrome.” Given the reservoir and buffering function of bone in phosphate homeostasis, and the toxicity of excess circulating phosphate, regulatory signals arising from bone to coordinate bone turnover and mineralization with systemic phosphate homeostasis would appear to be essential.
Excessive 1,25(OH)2D can exhibit toxicity that is mediated through its effects to increase the gastrointestinal absorption of calcium and phosphate and to stimulate RANKL in bone leading to increased osteoclastogenesis and efflux of calcium and phosphate from the skeleton. The increased calcium will suppress PTH through activation of the CASR in parathyroid glands. Reductions in PTH will reduce the renal production of 1,25(OH)2D through loss of PTH stimulation of CYP27b1. Reductions in PTH also result in loss of the tonic effects of PTH to increase renal phosphate restriction. To prevent hyperphosphatemia in the setting of increased 1,25(OH)2D and reduced PTH requires an additional regulator of renal phosphate handling.
There is much that remains to be discovered about FGF23 regulation and function. We have advanced the hypothesis that the principal physiological functions of FGF23 and the reason for its predominant expression in bone is that this hormone functions to protect the organism from the toxic effects of excess phosphate and 1,25(OH)2D. We propose that 1,25(OH)2D directly regulates FGF23 transcription in osteoblasts/osteocytes, but that phosphate, rather than directly modulating FGF23, has indirect effects that are mediated through the effects of phosphate on extracellular matrix mineralization. We also propose that bone formation and the bone buffering capacity of bone provides the mechanistic link between phosphate and regulation of FGF23 through the regulation of extracellular matrix factors involving the endopeptidase PHEX and the SIBLING protein dentin matrix protein 1 (DMP1). Finally, regulation of FGF23 is integrated with mineral and energy metabolism through cross-talk between PTH, 1,25(OH)2D, leptin, and secreted Klotho.
B. Hypophosphatemic Disorders Caused by Excess FGF23
There are four hereditary disorders caused by single gene mutations that are associated with increased FGF23 levels by bone in cells of the osteoblast lineage (typically osteocytes). These include 1) mutations of the RXXR site of FGF23 cleavage in autosomal dominant hypophosphatemic rickets (ADHR; OMIM no. 193100); 2) inactivating mutations of Phex, a cell surface endopeptidase, in XLH (OMIM no. 307800); 3) inactivation mutations of DMP1 in autosomal recessive hypophosphatemic rickets 1 (ARHR1; OMIM no. 241520); and 4) inactivation of ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), an enzyme that generates pyrophosphate, in autosomal recessive hypophosphatemic rickets 2 (ARHR2; OMIM no. 613312) (TABLE 1).
Table 1.
Comparative analysis of gene mutations leading to increased FGF23 expression in bone
| Factor for Comparison |
|||||||
|---|---|---|---|---|---|---|---|
| Hereditary hypophosphatemic disorders | ARHR2 | ARHR1 | XLH | OGD | None | MAS | ADHR |
| OMIM no. | 613312 | 241520 | 307800 | 166250 | None | 174800 | 193100 |
| Mutated gene | Enpp1 | Dmp1 | Phex | Fgfr1 | Fgf2-HMW | Gnas2 | Fgf23 |
| Type of mutation | Loss of function | Loss of function | Loss of function | Gain of function | Gain of function | Gain of function | Gain of function |
| Mouse homolog | Enpp1−/− | Dmp1−/− | Hyp | None | Tg-Fgf2-HMW | None | Tg-Fgf23 |
ARHR, autosomal recessive hypophosphatemic rickets; XLH, X-linked hypophosphatemic rickets; OGD, osteoglophonic dysplasia; MAS, McCune-Albright syndrome; ADHR, autosomal dominant hypophosphatemic rickets.
The biochemical hallmarks of excess FGF23 in these disorders are hypophoshatemia, inappropriately low or normal 1,25(OH)2D, and rickets and/or osteomalacia that mostly reflect the skeletal effects of hyphosphatemia. Hyperparathyroidism is often present in states of excess FGF23, likely due to FGF23-mediated reductions in 1,25(OH)2D. Inactivation of ENPP1 in ARHR2 differs from the one of infancy, presumably due to the failure to generate the mineralization inhibitor pyrophosphate. PPi is a substrate for alkaline phosphatase, which increases local Pi in bone. The paradoxical impaired mineralization of bone is likely due to a decreased local production of Pi by alkaline phosphatase due to reductions in the PPi substrate.
These four mutations are also providing insights into additional regulation mechanisms and physiological functions of FGF23. For example, the mutation in RXXR site points to metabolism of FGF23 as an important point of control of FGF23 levels; however, to date, the specific enzymes and physiological relevance of FGF23 degradation are not known. The mutations in XLH, ARHR1, and ARHR2 point to an important role of the extracellular matrix milieu in regulation of FGF23 expression in osteocytes. Studies of the Hyp and Dmp1 null mouse homologs of XHL and ARHR1 are uncovering pathways whereby PHEX and DMP1 interact to regulate both FGF23 expression and bone mineralization, whereas the alterations in the PPi/Pi ratio that controls bone mineralization provides additional support for a physiological role of FGF23 to coordinate bone phosphate buffering capacity with renal phosphate handling. The precise mechanisms whereby PHEX, DMP1, and ENPP1 regulate FGF23 expression in osteocytes are not known.
Another single gene missense mutation on Fgfr1 leads to constitutive activation in osteoglophonic dysplasia (OGD; OMIM no. 166250) (TABLE 1) and results in elevated FGF23 and hypophosphatemia, but with different skeletal abnormalities and additional clinical features. Additional evidence links local FGF pathway activation in osteoblasts with FGF23 production (refer to sect. IVB2). How Phex and Dmp1 mutations are linked to FGFR1 signaling in bone is an area of current investigations. However, these pathological abnormalities may provide a clue to a physiological function of FGF23, and a possible reason this phosphaturic hormone is predominantly produced in bone, which is to provide a pathway to coordinate bone phosphate buffering capacity related to bone mineralization and turnover with the renal handling of phosphate to maintain systemic phosphate homeostasis.
The hereditary disorder caused by activating mutation in Gnas1, which encodes the alpha subunit of stimulatory G protein, in McCune-Albright syndrome (OMIM no. 174800) and two sporadic/acquired disorders, including tumor-induced osteomalacia (TIO) and hypophosphatemic linear nevus sebaceous syndrome (OMIM no. 163200) (65), also result in elevated FGF23 and hypophosphatemia. GNAS1 leads to variable increase in FGF23 that is limited to fibrotic lesions of bone, whereas TIO is produced from mesenchymal derived tumors and linear nevus sebaceous syndrome (66) appears to have increased bone remodeling as a potential source of FGF23 (TABLE 1). These data suggest that high bone remodeling states may lead to increased FGF23 expression. However, activating mutations of PTH receptor 1 (PTHR1) observed in Jansen-type metaphyseal chondrodysplasia (OMIM no. 156400) are not reported to result in increased FGF23. Moreover, conditions associated with elevated PTH levels, such as secondary hyperparathyroidism in renal failure and pharmacological stimulation of bone remodeling with exogenous PTH, have variable effects on FGF23 expression. Parathyroidectomy suppresses FGF23 in uremic hyperparathyroidism, suggesting that high PTH in this setting stimulates FGF23 production. In addition, continuous PTH administration stimulates FGF23, whereas intermittent PTH administration does not. Since continuous PTH is catabolic to bone, and intermittent PTH is anabolic, this suggests that bone turnover can regulate FGF23. However, it is difficult to dissociate direct effects of PTH from indirect effects mediated by alterations in 1,25(OH)2D production. Indeed, Cyp27b1 null mice have low FGF23 levels in spite of very high PTH levels.
Finally, a translocation adjacent to αKlotho gene has been reported in a single individual leading to increased circulating αKlotho and FGF23 levels, hyperparathyroidism, and the metabolic bone disease hypophosphatemic rickets and hyperparathyroidism (OMIM no. 612089) (17). As noted above, FGF23 targets FGFR:Klotho complexes in cell membranes. The translocation leading to increased circulating levels of αKlotho suggests that circulating Klotho also may regulate pathways that stimulate FGF23 as well as PTH production.
Further research is needed to understand the interrelationships between bone remodeling, PTH, vitamin D, Klotho, and FGF23. Understanding the molecular mechanisms linking bone mineralization, vitamin D metabolism, and FGF23 production offers the potential to develop more effective and safe therapies for hereditary and acquired hypophosphatemic disorders. FGF23 levels are also proving to be important in diagnosing hereditary and acquired hypophosphatemic and hyperphosphatemic disorders. In addition, the link between 1,25(OH)2D and FGF23 has raised questions about the current use of active vitamin D analogs to treat XLH, which has recently been shown to further increase FGF23 in XLH patients (4, 74).
C. Hyperphosphatemic DDisorders DDue to Disruption of FGF23
In contrast to the FGF23-mediated hypophosphatemic disorders, there are also several hyperphosphatemic diseases caused by FGF23 deficiency including inactivating mutations of GALNT3 (HFTC), FGF23 (HFTC, refer to sect. IVC), and Klotho. All of these mutations lead to the clinical syndrome of hyperphosphatemic tumoral calcinosis (OMIM no. 211900), which is characterized by soft tissue and periarticular calcifications, hyperphosphatemia, and elevated serum 1,25(OH)2D levels, caused by the loss of functional FGF23 (71). Mutations of GALNT3 decrease the stability of FGF23, whereas Klotho mutations prevent complex formation with FGF23 and lead to decreased FGF23 signaling.
D. FGF23 in Nonhereditary Disorders
FGF23 may provide a new biological framework to understand the pathogenesis of metabolic bone and mineral disorders in chronic kidney diseases and how disorders of phosphate homeostasis may affect diverse extraskeletal functions, including cardiovascular disease, vascular calcification, and energy metabolism.
1. CKD
CKD leads to increased PTH and FGF23 and decreased circulating 1,25(OH)2D levels, hypocalcemia, hyperphosphatemia, bone disease, vascular calcifications, and cardiovascular morbidities, collectively referred to as chronic kidney disease-mineral and bone disorder (CKD-MBD) (143). The pathogenesis of CKD-MBD is traditionally viewed from the perspective of the PTH-vitamin D axis, and current treatments focus on suppressing PTH with active vitamin D analogs (215). Prior to the discovery of FGF23, secondary HPT during progression of CKD was thought to be due to a decline in Cyp27b1-mediated production of 1,25(OH)2D by the proximal tubule due to loss of renal mass (27). There is emerging evidence, however, that increased FGF23 is the initial event leading to reductions in 1,25(OH)2D and elevations of PTH in response to loss of renal function. Cross-sectional studies in humans show early FGF23 elevations in CKD in proportion to reduced GFR (56) and greater elevations in ESRD (56, 73, 211). FGF23 levels correlate with the degree of hyperphosphatemia (73, 211) and predict refractory hyperparathyroidism in patients with ESRD (90). Since FGF23 inhibits the production of and stimulates the catabolism of 1,25(OH)2D through its respective inhibition of Cyp27b1 and stimulation of Cyp24, the reduced levels of 1,25(OH)2D in CKD may not represent a true “vitamin D-deficient” state. Rather, FGF23-mediated suppression of circulating 1,25(OH)2D levels is an adaptive response, which protects against hyperphosphatemia through reduction of 1,25(OH)2D’s effects on gastrointestinal phosphate absorption. This active mechanism is supported by studies showing that treatment with FGF23 neutralizing antibodies prevents the decrease in serum calcitriol in rats with progressive CKD(60). Additionally, the decline in 1,25(OH)2D levels increases PTH production which acts in concert with FGF23 to stimulate phosphaturia. Treatment approaches to prevent the elevations of FGF23 may become the initial therapeutic focus. Treatment with paracalcitol further elevates FGF23 in ESRD (192). Since calcitriol increases FGF23, calcitriol sparing therapies may be warranted, such as combined low-dose paracalcitol and calcimimetics, which we show lowers FGF23 levels in ESRD patients (214, 215).
There are important gaps in our knowledge of FGF23 regulation and function in CKD. It is unknown how end-organ resistance to FGF23 due to loss of FGFR function and/or the presence of CKD lead to increased FGF23 production in bone and whether this upregulation of FGF23 represents a feedback loop between kidney and bone. New pathways for selective regulation of gene products by FGFR3, -4, and -1 (i.e., selective control of gene products by different FGFRs that may dissociate the regulation of phosphate transport from vitamin D metabolism as well as other functions) are yet to be fully explored.
2. Clarifying the Association between Elevated FGF23 and Mortality
As CKD progresses to ESRD, the progressive increase in FGF23 becomes maladaptive, possibly contributing to more rapid progress of renal failure (42), cardiac dysfunction (68), and vascular calcifications (82) and contributing to excessive morbidity and mortality (58). Analysis of cohorts with ESRD show that increased FGF23 is also associated with increased mortality, independent of serum phosphate levels (58). Elevated FGF23 may have untoward effects on mortality and cardiovascular outcomes in patients without end-stage renal disease (58, 139, 157). Increased FGF23 is associated with cardiovascular disease/mortality in elderly patients with “normal renal function” (139) and in patients with advanced CKD (219). FGF23 may also provide an explanation for the association between low bone remodeling and cardiovascular calcifications, since low osteocalcin expression, as a marker of bone turnover, is associated with high FGF23 and cardiovascular mortality (157). In addition, there is an association between elevations of FGF23 with hypertension and cardiac hypertrophy in patients with XLH (194). There is also an association between increased FGF23 with progression of renal fibrosis in CKD (42).
The mechanisms of the toxic effects of FGF23 are not clear. The association between elevated FGF23 and increased mortality is independent of phosphate levels in ESRD (58), suggesting other factors are involved. At this point, it is not clear whether the untoward effects of FGF23 are mediated by its suppression of Klotho expression by the kidney or other FGF23-regulated kidney-derived factors, or due to off-target effects of the very high levels of FGF23 found in CKD. There is emerging evidence that FGF23 suppresses kidney expression of ACE2, which unlike ACE, generates peptides, such as angiotensin-(1–7), that have vasodilatation and natriuretic effects to reduce blood pressure (55, 206). FGF23 upregulates midkine, a factor that is linked to increased ACE activity and hypertension in CKD (63). Thus FGF23 excess could impact on the cardiovascular system by suppressing Klotho or by enhancing the renin-angiotensin system (RAS) through multiple mechanisms.
3. TIO
TIO (also called oncogenic osteomalacia) is an acquired paraneoplastic disorder characterized by hypophosphatemia, osteomalacia, and aberrant vitamin D metabolism, similar to the hereditary hypophosphatemic disorders, but caused by increased production of FGF23 by benign tumors. FGF23 was cloned and characterized from tumors causing hypophosphatemic osteomalacia (179), and removal of the tumors results in decreased FGF23 levels (211). Serum FGF23 levels are useful for diagnosing TIO in patients with unexplained hyposphosphatemia, and octreotide sestabmibi scans can be helpful in identifying the site of occult benign mesenchymal neoplasms that secrete FGF23 (64).
4. Drug Treatment Effects
FGF23 bone kidney axis offers new explanations for hypophosphatemia and hyperphosphatemia observed in several other clinical settings. There are several drugs that cause hyper- or hypophosphatemia through modulating FGF23 expression. These include inhibitors of FGFR kinases being developed for cancer therapies that are reported to lead to hyperphosphatemia as well as intravenously injected iron preparations used to treat anemia (171, 172, 184) and glucocorticoids which cause hypophosphatemia. The mechanism of these effects is not entirely clear, but the effects of parenteral iron administration to cause hypophosphatemia through increased FGF23 might be mediated by inhibition of Phex (171) and/or bone mineralization. The effects of receptor tyrosine kinase inhibitors might be due to their effect to block the end-organ effects of FGF23. In addition, prednisone is associated with increased FGF23 as well as osteocytic osteolysis, possibly providing additional evidence for a link between bone mineralization and FGF23 expression (200).
VI. CONCLUSION
The discovery of FGF23 has changed our understanding of mineral metabolism by indentifying more complex cross-talk and endocrine feedback loops between the parathyroid gland, intestines, bone, and kidney to maintain systemic mineral homeostasis, energy metabolism, and bone health. A full understanding of the integrative functions of these hormonal networks remains to be elucidated, but the importance of FGF23 is revealed by the profound effects of its excess or deficiency on mineral homeostasis and the integration of many local and systemic factors to regulate FGF23 expression at the level of the osteocyte in bone. The main functions of FGF23 are its systemic effects to 1) act as a counterregulatory hormone for 1,25(OH)2D (117) and 2) coordinate renal phosphate handling to match bone mineralization (40, 115, 120, 164). Unequivocal evidence supporting a principal role in regulating vitamin D metabolism consists of 1,25(OH)2D regulation of FGF23 expression in bone and FGF23 dominant effects to regulate 1,25(OH)2D levels in states of FGF23 excess and deficiency. Evidence for a coupling between mineralization and FGF23 is more indirect, but is suggested by the effect of Dmp1 or Phex deficiency to increased FGF23 production by osteocytes in ARHR and XLH through local matrix factors (116) as well as other data linking alterations in bone turnover and phosphate flux with changes in FGF23. This coupling may regulate phosphate excretion by the kidneys to match the differing rates of phosphate deposition into bone (89, 117, 162). It is likely that many factors that regulate bone as well as energy metabolism will be discovered to also regulate FGF23 expression. Further studies are required to understand the molecular mechanisms whereby local alterations in the bone milieu and systemic factors regulate FGF23 expression in bone.
FGF23 likely has additional functions to regulate PTG function, but it is uncertain whether FGF23 inhibits or stimulates PTG function through the FGFR/Klotho complexes in the PTG. Also, whether the putative direct effects of PTH to regulate FGF23 expression in bone via transcriptional control of FGF23 expression or indirectly due to PTH-induced alterations in bone remodeling/phosphate flux remains to be determined. A proposed PTG-bone axis where PTH stimulates FGF23 and FGF23 inhibits PTH is paradoxical to the findings that PTH does not regulate FGF23 in the absence of 1,25(OH)2D (168) and the effects of FGF23 to suppress 1,25(OH)2D and stimulate PTH. An integrative hypothesis to explain these disparate findings needs to be developed. Similarly, FGF23 is clearly involved in the regulation of phosphate homeostasis, but unlike the tight coupling of changes in serum calcium with PTH secretion that is mediated through the calcium receptor CasR, the relationship between changes in phosphate and FGF23 is more variable and perhaps indirect. The molecular mechanism of phosphate regulation of FGF23 is not known and may involve phosphate effects on bone mineralization. Moreover, FGF23 is likely to have additional functions that are yet to be discovered. Indeed, the autocrine/paracrine effects of FGF23 on bone metabolism and other tissues where FGF23 is expressed in low abundance are poorly understood. Finally, the functions of FGF23 in the brain and pituitary are unknown, despite the fact that it was the first tissue where FGF23 was identified. There are also significant gaps in our understanding of FGF23 effects on the kidney. The apparent functional heterogeneity of FGFR isoform activation by FGF23, the tubular segments targeted by FGF23, and specific gene products regulated by FGF23 remain to be fully defined. Finally, the discovery of FGF23 has had a significant clinical impact, including a better understanding of how to diagnose and treat hereditary and acquired hypophosphatemic disorders, new insights into the pathogenesis of disordered mineral metabolism in chronic kidney disease, a new framework for understanding the mechanism of hyperphosphatemia caused by various drugs, and a molecular mechanism to link disordered mineral metabolism with increased cardiovascular mortality. However, much also remains to be discovered regarding the pathological significance of FGF23 in various diseases states, whether therapeutic strategies should consider the potential biological effects of altered FGF23 expression and if FGF23 itself will become a relevant therapeutic target for enhancing and/or inhibiting FGF23 function in various clinical settings.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- 2.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 3.Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 4.Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3:658–664. doi: 10.2215/CJN.04981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anamizu Y, Kawaguchi H, Seichi A, Yamaguchi S, Kawakami E, Kanda N, Matsubara S, Kuro-o M, Nabeshima Y, Nakamura K, Oyanagi K. Klotho insufficiency causes decrease of ribosomal RNA gene transcription activity, cytoplasmic RNA and rough ER in the spinal anterior horn cells. Acta Neuropathol. 2005;109:457–466. doi: 10.1007/s00401-004-0971-7. [DOI] [PubMed] [Google Scholar]
- 6.Anderson HC, Harmey D, Camacho NP, Garimella R, Sipe JB, Tague S, Bi X, Johnson K, Terkeltaub R, Millan JL. Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. Am J Pathol. 2005;166:1711–1720. doi: 10.1016/S0002-9440(10)62481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T, Fujita T. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:5523–5527. doi: 10.1210/jc.2005-0301. [DOI] [PubMed] [Google Scholar]
- 8.Bai X, Miao D, Goltzman D, Karaplis AC. Early lethality in Hyp mice with targeted deletion of Pth gene. Endocrinology. 2007;148:4974–4983. doi: 10.1210/en.2007-0243. [DOI] [PubMed] [Google Scholar]
- 9.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 10.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 11.Barbieri AM, Filopanti M, Bua G, Beck-Peccoz P. Two novel nonsense mutations in GALNT3 gene are responsible for familial tumoral calcinosis. J Hum Genet. 2007;52:464–468. doi: 10.1007/s10038-007-0126-5. [DOI] [PubMed] [Google Scholar]
- 12.Baum M, Schiavi S, Dwarakanath V, Quigley R. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int. 2005;68:1148–1153. doi: 10.1111/j.1523-1755.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergwitz C, Banerjee S, Abu-Zahra H, Kaji H, Miyauchi A, Sugimoto T, Juppner H. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94:4267–4274. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 16.Brown WW, Juppner H, Langman CB, Price H, Farrow EG, White KE, McCormick KL. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 19.Campos M, Couture C, Hirata IY, Juliano MA, Loisel TP, Crine P, Juliano L, Boileau G, Carmona AK. Human recombinant endopeptidase PHEX has a strict S1’ specificity for acidic residues and cleaves peptides derived from fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein. Biochem J. 2003;373:271–279. doi: 10.1042/BJ20030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010;21:1125–1135. doi: 10.1681/ASN.2009040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancilla B, Davies A, Cauchi JA, Risbridger GP, Bertram JF. Fibroblast growth factor receptors and their ligands in the adult rat kidney. Kidney Int. 2001;60:147–155. doi: 10.1046/j.1523-1755.2001.00781.x. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C, Olear E, Gundberg CM. Circulating levels of soluble Klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment and relationship to parathyroid status. J Clin Endocrinol Metab. 2010;95:352–357. doi: 10.1210/jc.2010-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrillo-Lopez N, Roman-Garcia P, Rodriguez-Rebollar A, Fernandez-Martin JL, Naves-Diaz M, Cannata-Andia JB. Indirect regulation of PTH by estrogens may require FGF23. J Am Soc Nephrol. 2009;20:2009–2017. doi: 10.1681/ASN.2008121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 26.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craver L, Marco MP, Martinez I, Rue M, Borras M, Martin ML, Sarro F, Valdivielso JM, Fernandez E. Mineral metabolism parameters throughout chronic kidney disease stages 1–5–achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham J, Gomes H, Seino Y, Chase LR. Abnormal 24-hydroxylation of 25-hydroxyvitamin D in the X-linked hypophosphatemic mouse. Endocrinology. 1983;112:633–638. doi: 10.1210/endo-112-2-633. [DOI] [PubMed] [Google Scholar]
- 29.David V, Martin A, Hedge AM, Drezner MK, Rowe PS. ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am J Physiol Renal Physiol. 2011;300:F783–F791. doi: 10.1152/ajprenal.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David V, Quarles LD. ASARM mineralization hypothesis: a bridge too far? J Bone Miner Res. 2010;25:692–694. doi: 10.1002/jbmr.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol. 2009;20:1693–1704. doi: 10.1681/ASN.2008080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLuca S, Sitara D, Kang K, Marsell R, Jonsson K, Taguchi T, Erben RG, Razzaque MS, Lanske B. Amelioration of the premature ageing-like features of Fgf-23 knockout mice by genetically restoring the systemic actions of FGF-23. J Pathol. 2008;216:345–355. doi: 10.1002/path.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunham-Ems SM, Lee YW, Stachowiak EK, Pudavar H, Claus P, Prasad PN, Stachowiak MK. Fibroblast growth factor receptor-1 (FGFR1) nuclear dynamics reveal a novel mechanism in transcription control. Mol Biol Cell. 2009;20:2401–2412. doi: 10.1091/mbc.E08-06-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupont J, Holzenberger M. IGF type 1 receptor: a cell cycle progression factor that regulates aging. Cell Cycle. 2003;2:270–272. [PubMed] [Google Scholar]
- 35.Erben RG, Kohn B, Weiser H, Sinowatz F, Rambeck WA. Role of vitamin D metabolites in the prevention of the osteopenia induced by ovariectomy in the axial and appendicular skeleton of the rat. Z Ernahrungswiss. 1990;29:229–248. doi: 10.1007/BF02023080. [DOI] [PubMed] [Google Scholar]
- 36.Fang MA, Glackin CA, Sadhu A, McDougall S. Transcriptional regulation of alpha 2(I) collagen gene expression by fibroblast growth factor-2 in MC3T3–E1 osteoblast-like cells. J Cell Biochem. 2001;80:550–559. [PubMed] [Google Scholar]
- 37.Faroqui S, Levi M, Soleimani M, Amlal H. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int. 2008;73:1141–1150. doi: 10.1038/ki.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20:955–960. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134:83–88. doi: 10.1016/0022-510x(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 40.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 42.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 43.Fujiwara I, Aravindan R, Horst RL, Drezner MK. Abnormal regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase activity in X-linked hypophosphatemia: a translational or post-translational defect. J Bone Miner Res. 2003;18:434–442. doi: 10.1359/jbmr.2003.18.3.434. [DOI] [PubMed] [Google Scholar]
- 44.Fukumoto S. Actions and mode of actions of FGF19 subfamily members. Endocr J. 2008;55:23–31. doi: 10.1507/endocrj.kr07e-002. [DOI] [PubMed] [Google Scholar]
- 45.Fukumoto S, Yamashita T. Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr Opin Nephrol Hypertens. 2002;11:385–389. doi: 10.1097/00041552-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 47.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–F291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey AL. Different forms of DMP1 play distinct roles in mineralization. J Dent Res. 2010;89:355–359. doi: 10.1177/0022034510363250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95:1595–1601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goebel S, Lienau J, Rammoser U, Seefried L, Wintgens KF, Seufert J, Duda G, Jakob F, Ebert R. FGF23 is a putative marker for bone healing and regeneration. J Orthop Res. 2009;27:1141–1146. doi: 10.1002/jor.20857. [DOI] [PubMed] [Google Scholar]
- 51.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, Matthews G, Rossi P, Ornitz DM, D’Angelo E. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron. 2007;55:449–463. doi: 10.1016/j.neuron.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo R, Liu S, Spurney RF, Quarles LD. Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab. 2001;281:E837–E847. doi: 10.1152/ajpendo.2001.281.4.E837. [DOI] [PubMed] [Google Scholar]
- 54.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 55.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 57.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci USA. 2007;104:2331–2336. doi: 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 61.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 2004;279:11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 62.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Hobo A, Yuzawa Y, Kosugi T, Kato N, Asai N, Sato W, Maruyama S, Ito Y, Kobori H, Ikematsu S, Nishiyama A, Matsuo S, Kadomatsu K. The growth factor midkine regulates the renin-angiotensin system in mice. J Clin Invest. 2009;119:1616–1625. doi: 10.1172/JCI37249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodgson SF, Clarke BL, Tebben PJ, Mullan BP, Cooney WP, 3rd, Shives TC. Oncogenic osteomalacia: localization of underlying peripheral mesenchymal tumors with use of Tc 99m sestamibi scintigraphy. Endocr Pract. 2006;12:35–42. doi: 10.4158/EP.12.1.35. [DOI] [PubMed] [Google Scholar]
- 65.Hoffman WH, Jain A, Chen H, Fedarko NS. Matrix extracellular phosphoglycoprotein (MEPE) correlates with serum phosphorus prior to and during octreotide treatment and following excisional surgery in hypophosphatemic linear sebaceous nevus syndrome. Am J Med Genet A. 2008;146:2164–2168. doi: 10.1002/ajmg.a.32395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffman WH, Jueppner HW, Deyoung BR, O’Dorisio MS, Given KS. Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet A. 2005;134:233–236. doi: 10.1002/ajmg.a.30599. [DOI] [PubMed] [Google Scholar]
- 67.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 68.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337:116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 69.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imai M, Ishikawa K, Matsukawa N, Kida I, Ohta J, Ikushima M, Chihara Y, Rui X, Rakugi H, Ogihara T. Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression. Endocrine. 2004;25:229–234. doi: 10.1385/ENDO:25:3:229. [DOI] [PubMed] [Google Scholar]
- 73.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 74.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010;95:1846–1850. doi: 10.1210/jc.2009-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 76.Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J. 2005;390:325–331. doi: 10.1042/BJ20041799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, Wolf M. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishii M, Yamaguchi Y, Yamamoto H, Hanaoka Y, Ouchi Y. Airspace enlargement with airway cell apoptosis in klotho mice: a model of aging lung. J Gerontol A Biol Sci Med Sci. 2008;63:1289–1298. doi: 10.1093/gerona/63.12.1289. [DOI] [PubMed] [Google Scholar]
- 79.Itoh N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 2010;342:1–11. doi: 10.1007/s00441-010-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 82.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009;24:948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 83.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 84.Kamemori M, Ohyama Y, Kurabayashi M, Takahashi K, Nagai R, Furuya N. Expression of Klotho protein in the inner ear. Hear Res. 2002;171:103–110. doi: 10.1016/s0378-5955(02)00483-5. [DOI] [PubMed] [Google Scholar]
- 85.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 86.Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104:229–237. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 88.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 89.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 90.Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–298. doi: 10.1038/ki.2009.466. [DOI] [PubMed] [Google Scholar]
- 91.Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K, Tsugawa N, Okano T, Kitazawa R, Fukagawa M, Kita T. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–238. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 92.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 93.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94:67–73. doi: 10.1210/jc.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 95.Kuro-o M, Hanaoka K, Hiroi Y, Noguchi T, Fujimori Y, Takewaki S, Hayasaka M, Katoh H, Miyagishi A, Nagai R. Salt-sensitive hypertension in transgenic mice overexpressing Na+-proton exchanger. Circ Res. 1995;76:148–153. doi: 10.1161/01.res.76.1.148. [DOI] [PubMed] [Google Scholar]
- 96.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 97.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwiatkowski BA, Kirillova I, Richard RE, Israeli D, Yablonka-Reuveni Z. FGFR4 and its novel splice form in myogenic cells: interplay of glycosylation and tyrosine phosphorylation. J Cell Physiol. 2008;215:803–817. doi: 10.1002/jcp.21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lanske B, Razzaque MS. Mineral metabolism and aging: the fibroblast growth factor 23 enigma. Curr Opin Nephrol Hypertens. 2007;16:311–318. doi: 10.1097/MNH.0b013e3281c55eca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 103.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 104.Larsson TE. The role of FGF-23 in CKD-MBD and cardiovascular disease: friend or foe? Nephrol Dial Transplant. 2010;25:1376–1381. doi: 10.1093/ndt/gfp784. [DOI] [PubMed] [Google Scholar]
- 105.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 106.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab. 2011;300:E508–E517. doi: 10.1152/ajpendo.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 109.Liang H, Pun S, Wronski TJ. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology. 1999;140:5780–5788. doi: 10.1210/endo.140.12.7195. [DOI] [PubMed] [Google Scholar]
- 110.Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- 111.Lin BC, Wang M, Blackmore C, Desnoyers LR. Liver-specific activities of FGF19 require Klotho beta. J Biol Chem. 2007;282:27277–27284. doi: 10.1074/jbc.M704244200. [DOI] [PubMed] [Google Scholar]
- 112.Liu S, Brown TA, Zhou J, Xiao ZS, Awad H, Guilak F, Quarles LD. Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol. 2005;16:1645–1653. doi: 10.1681/ASN.2004121060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 114.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 115.Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD. Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol. 2007;192:261–267. doi: 10.1677/joe.1.07059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu S, Tang W, Fang J, Ren J, Li H, Xiao Z, Quarles LD. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–1518. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 118.Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab. 2007;293:E1636–E1644. doi: 10.1152/ajpendo.00396.2007. [DOI] [PubMed] [Google Scholar]
- 119.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol. 2008;19:2342–2350. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 121.Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295:E254–E261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant. 2008;23:3397–3402. doi: 10.1093/ndt/gfn291. [DOI] [PubMed] [Google Scholar]
- 125.Lu Y, Qin C, Xie Y, Bonewald LF, Feng JQ. Studies of the DMP1 57-kDa Functional Domain both in vivo and in vitro. Cells Tissues Organs. 2009;189:175–185. doi: 10.1159/000151727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL, McKee MD, Drezner MK, Bonewald LF, Feng JQ. The biological function of DMP1 in osteocyte maturation is mediated by its 57 kDa C-terminal fragment. J Bone Miner Res. 2011;26:331–340. doi: 10.1002/jbmr.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 128.Marsell R, Krajisnik T, Goransson H, Ohlsson C, Ljunggren O, Larsson TE, Jonsson KB. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol Dial Transplant. 2008;23:827–833. doi: 10.1093/ndt/gfm672. [DOI] [PubMed] [Google Scholar]
- 129.Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martuseviciene G, Hofman-Bang J, Clausen T, Olgaard K, Lewin E. The secretory response of parathyroid hormone to acute hypocalcemia in vivo is independent of parathyroid glandular sodium/potassium-ATPase activity. Kidney Int. 2011;79:742–748. doi: 10.1038/ki.2010.501. [DOI] [PubMed] [Google Scholar]
- 132.Masi L, Beltrami G, Gozzini A, Ottanelli S, Amedei A, Falchetti A, Casentini C, Fossi C, Brandi M. A case of tumoral calcinosis-hyperphosphatemia due to a novel mutation in GALNT3 gene. J Bone Miner Res. 2009;24(Suppl 1) [Google Scholar]
- 133.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 134.Matsunuma A, Horiuchi N. Leptin attenuates gene expression for renal 25-hydroxyvitamin D3-1alpha-hydroxylase in mice via the long form of the leptin receptor. Arch Biochem Biophys. 2007;463:118–127. doi: 10.1016/j.abb.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 135.Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D3-1 alpha-hydroxylase and -24-hydroxylase in leptin-deficient, ob/ob mice. Endocrinology. 2004;145:1367–1375. doi: 10.1210/en.2003-1010. [DOI] [PubMed] [Google Scholar]
- 136.Meyer MH, Dulde E, Meyer RA., Jr The genomic response of the mouse kidney to low-phosphate diet is altered in X-linked hypophosphatemia. Physiol Genomics. 2004;18:4–11. doi: 10.1152/physiolgenomics.00210.2003. [DOI] [PubMed] [Google Scholar]
- 137.Mirza MA, Alsio J, Hammarstedt A, Erben RG, Michaelsson K, Tivesten A, Marsell R, Orwoll E, Karlsson MK, Ljunggren O, Mellstrom D, Lind L, Ohlsson C, Larsson TE. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31:219–227. doi: 10.1161/ATVBAHA.110.214619. [DOI] [PubMed] [Google Scholar]
- 138.Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, Larsson TE. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–3131. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 139.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 140.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 141.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838–843. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- 142.Miyamoto K, Ito M, Kuwahata M, Kato S, Segawa H. Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial. 2005;9:331–335. doi: 10.1111/j.1744-9987.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 143.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 144.Mori S, Wu CY, Yamaji S, Saegusa J, Shi B, Ma Z, Kuwabara Y, Lam KS, Isseroff RR, Takada YK, Takada Y. Direct binding of integrin alphavbeta3 to FGF1 plays a role in FGF1 signaling. J Biol Chem. 2008;283:18066–18075. doi: 10.1074/jbc.M801213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nabeshima Y. Toward a better understanding of Klotho. Sci Aging Knowledge Environ. 2006;2006:pe11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- 146.Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- 147.Nagano N, Miyata S, Abe M, Kobayashi N, Wakita S, Yamashita T, Wada M. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006;69:531–537. doi: 10.1038/sj.ki.5000020. [DOI] [PubMed] [Google Scholar]
- 148.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 150.Nauman EA, Sakata T, Keaveny TM, Halloran BP, Bikle DD. bFGF administration lowers the phosphate threshold for mineralization in bone marrow stromal cells. Calcif Tissue Int. 2003;73:147–152. doi: 10.1007/s00223-002-1033-6. [DOI] [PubMed] [Google Scholar]
- 151.Naveh-Many T, Almogi G, Livni N, Silver J. Estrogen receptors and biologic response in rat parathyroid tissue and C cells. J Clin Invest. 1992;90:2434–2438. doi: 10.1172/JCI116134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 153.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 155.Okada S, Yoshida T, Hong Z, Ishii G, Hatano M, Kuro OM, Nabeshima Y, Tokuhisa T. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int Immunol. 2000;12:861–871. doi: 10.1093/intimm/12.6.861. [DOI] [PubMed] [Google Scholar]
- 156.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moyses RM. Early control of PTH and FGF23 in normo-phosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 159.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 160.Prentice A, Ceesay M, Nigdikar S, Allen SJ, Pettifor JM. FGF23 is elevated in Gambian children with rickets. Bone. 2008;42:788–797. doi: 10.1016/j.bone.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 161.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Quarles LD. Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest. 2003;112:642–646. doi: 10.1172/JCI19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 166.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 167.Saji F, Shigematsu T, Sakaguchi T, Ohya M, Orita H, Maeda Y, Ooura M, Mima T, Negi S. Fibroblast growth factor 23 production in bone is directly regulated by 1α,25-dihydroxyvitamin D, but not PTH. Am J Physiol Renal Physiol. 2010;299:F1212–F1217. doi: 10.1152/ajprenal.00169.2010. [DOI] [PubMed] [Google Scholar]
- 168.Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology. 2009;150:4835–4845. doi: 10.1210/en.2009-0472. [DOI] [PubMed] [Google Scholar]
- 169.Sato A, Hirai T, Imura A, Kita N, Iwano A, Muro S, Nabeshima Y, Suki B, Mishima M. Morphological mechanism of the development of pulmonary emphysema in klotho mice. Proc Natl Acad Sci USA. 2007;104:2361–2365. doi: 10.1073/pnas.0607882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sato T, Tominaga Y, Ueki T, Goto N, Matsuoka S, Katayama A, Haba T, Uchida K, Nakanishi S, Kazama JJ, Gejyo F, Yamashita T, Fukagawa M. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:481–487. [PubMed] [Google Scholar]
- 171.Schouten BJ, Doogue MP, Soule SG, Hunt PJ. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem. 2009;46:167–169. doi: 10.1258/acb.2008.008151. [DOI] [PubMed] [Google Scholar]
- 172.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–2337. doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 173.Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–R779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- 174.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 175.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG. Fibroblast Growth Factor 23 (FGF23) and Alpha-Klotho Stimulate Osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89:140–150. doi: 10.1007/s00223-011-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shigematsu T, Kazama JJ, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, Fukagawa M. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44:250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 177.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 178.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 181.Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 182.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 183.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 184.Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, Fukumoto S, Sugimoto T, Fujita T. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–816. doi: 10.1016/j.bone.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 185.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 186.Sitara D, Kim S, Razzaque MS, Bergwitz C, Taguchi T, Schuler C, Erben RG, Lanske B. Genetic evidence of serum phosphate-independent functions of FGF23 on bone. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000154. e1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, Lanske B. Genetic ablation of vitamin d activation pathway reverses biochemical and skeletal anomalies in fgf-23-null animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Specktor P, Cooper JG, Indelman M, Sprecher E. Hyperphosphatemic familial tumoral calcinosis caused by a mutation in GALNT3 in a European kindred. J Hum Genet. 2006;51:487–490. doi: 10.1007/s10038-006-0377-6. [DOI] [PubMed] [Google Scholar]
- 190.Stewart I, Roddie C, Gill A, Clarkson A, Mirams M, Coyle L, Ward C, Clifton-Bligh P, Robinson BG, Mason RS, Clifton-Bligh RJ. Elevated serum FGF23 concentrations in plasma cell dyscrasias. Bone. 2006;39:369–376. doi: 10.1016/j.bone.2006.01.163. [DOI] [PubMed] [Google Scholar]
- 191.Stubbs J, Liu S, Tang W, Quarles LD. Role of hyperphosphatemia and 1,25(OH)2D3 in vascular calcifications and mortality in FGF23 null mice. J Am Soc Nephrol. 2006;17:689A. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 192.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 194.Stubbs JR, Quarles LD. Fibroblast growth factor 23: uremic toxin or innocent bystander in chronic kidney disease? Nephrol News Issues. 2009;23:33–37. [PubMed] [Google Scholar]
- 195.Sun Y, Prasad M, Gao T, Wang X, Zhu Q, D’Souza R, Feng JQ, Qin C. Failure to process dentin matrix protein 1 (DMP1) into fragments leads to its loss of function in osteogenesis. J Biol Chem. 2010;285:31713–31722. doi: 10.1074/jbc.M110.137059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 197.Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M. Age-dependent changes in the expression of klotho protein, TRPV5 and TRPV6 in mouse inner ear. Acta Otolaryngol. 2009:1–11. doi: 10.3109/00016480902725254. [DOI] [PubMed] [Google Scholar]
- 198.Ten Bolscher M, Netelenbos JC, Barto R, Van Buuren LM, Van der vijgh WJ. Estrogen regulation of intestinal calcium absorption in the intact and ovariectomized adult rat. J Bone Miner Res. 1999;14:1197–1202. doi: 10.1359/jbmr.1999.14.7.1197. [DOI] [PubMed] [Google Scholar]
- 199.Tenenhouse HS. Metabolism of 25-hydroxyvitamin D3 in renal slices from the X-linked hypophosphatemic (Hyp) mouse: abnormal response to fall in serum calcium. Cell Calcium. 1984;5:43–55. doi: 10.1016/0143-4160(84)90153-2. [DOI] [PubMed] [Google Scholar]
- 200.Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–16. doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 201.Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 202.Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279:9777–9784. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- 203.Tomiyama K, Maeda R, Urakawa I, Yamazaki Y, Tanaka T, Ito S, Nabeshima Y, Tomita T, Odori S, Hosoda K, Nakao K, Imura A. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci USA. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 205.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D(3) synthesis in leptin-deficient ob/ob mice. J Bone Miner Res. 2010;25:1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 206.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 207.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 208.Wang H, Yamamoto R, Tanne K, Aubin JE, Maeda N, Yoahiko Y. Adenovirus-mediated overexpression of FGF23 suppresses osteoblast development and matrix mineralization in fetal rat calvaria cell cultures. J Bone Miner Res. 2005;20:S128. [Google Scholar]
- 209.Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 210.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 212.Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- 213.Westerberg PA, Linde T, Wikstrom B, Ljunggren O, Stridsberg M, Larsson TE. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant. 2007;22:3202–3207. doi: 10.1093/ndt/gfm347. [DOI] [PubMed] [Google Scholar]
- 214.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wetmore JB, Quarles LD. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nat Clin Pract Nephrol. 2009;5:24–33. doi: 10.1038/ncpneph0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 218.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 219.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- 220.Wronski TJ, Halloran BP, Bikle DD, Globus RK, Morey-Holton ER. Chronic administration of 1,25-dihydroxyvitamin D3: increased bone but impaired mineralization. Endocrinology. 1986;119:2580–2585. doi: 10.1210/endo-119-6-2580. [DOI] [PubMed] [Google Scholar]
- 221.Xiao L, Liu P, Li X, Doetschman T, Coffin JD, Drissi H, Hurley MM. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J Biol Chem. 2009;284:3170–3182. doi: 10.1074/jbc.M804900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xiao L, Naganawa T, Lorenzo J, Carpenter TO, Coffin JD, Hurley MM. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J Biol Chem. 2010;285:2834–2846. doi: 10.1074/jbc.M109.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xiao M, Xu L, Laezza F, Yamada K, Feng S, Ornitz DM. Impaired hippocampal synaptic transmission and plasticity in mice lacking fibroblast growth factor 14. Mol Cell Neurosci. 2007;34:366–377. doi: 10.1016/j.mcn.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 224.Yamashita T, Konishi M, Miyake A, Inui K, Itoh N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:28265–28270. doi: 10.1074/jbc.M202527200. [DOI] [PubMed] [Google Scholar]
- 225.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 226.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 227.Yu J, Deng M, Zhao J, Huang L. Decreased expression of klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2010;391:261–266. doi: 10.1016/j.bbrc.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 228.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36:971–977. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 230.Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, Xie Y, Drezner MK. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yuan B, Xing Y, Horst RL, Drezner MK. Evidence for abnormal translational regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase activity in the hyp-mouse. Endocrinology. 2004;145:3804–3812. doi: 10.1210/en.2004-0192. [DOI] [PubMed] [Google Scholar]
- 232.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]